Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There is no nobler goal than achieving the relief of pain and suffering. —Ronald Melzack
Pain is one of the most common symptoms experienced by children receiving palliative care and one of the most feared by children and their families. The severity of this symptom may increase with time, especially when the terminal phase is reached. Palliative therapeutics should generally be implemented once the underlying causative mechanisms have been established, because therapies directed at the primary cause may ultimately have a more effective outcome for symptom management.
The goals of this chapter are to describe the role of the interdisciplinary team (IDT), the importance of quality improvement, the epidemiology of pain in children with life-limiting illnesses, pain assessment and measurement, the myths surrounding pain management, and pain management guidelines. The reader is referred to other references for more detailed discussion of pediatric analgesic pharmacology.
In the context of living with a life-threatening illness, the experience of pain is potentially the result of a complex interplay among physical, psychological, social, spiritual, and other factors. The severity of a child's pain, for example, may be exacerbated by anxiety, depression, or suffering, which may be of the spiritual realm. All these factors must be considered, assessed, and treated in order to effectively alleviate suffering related to the experience of pain. Pain assessment and treatment may therefore be complex and access to an IDT may therefore be needed.
The World Health Organization (WHO) mandates that a certain standard of pain management be available to every child receiving palliative care irrespective of location. As an extension of the WHO document, individual countries and groups of countries are declaring standards of pain management related to pediatric palliative care. Quality of life is often related to a child's experience of pain and is therefore vital that the child receive excellent pain assessment and management. Successful pain management often needs the combined efforts of an IDT within the palliative care team of the medical, nursing, social work, play therapy, physiotherapy, occupational therapy disciplines, among others.
The degree to which the child or caretaker believes pain is well managed can influence other outcomes, such as where a family might choose to care for their child for the end-of-life phase. Poorly controlled pain can militate against a family caring for a child at home. Good pain control, on the other hand, can mean the difference between memories of distress and angst or memories that soften the inevitable transition from life to death. Communication and flexibility are foundation stones of good management of pain in children. These two factors provide firm foundations for all other building blocks in the construction of a successful pain management plan.
Whatever the source and intensity of the child's pain, communication is central to successful pain management. Communication among all parties involved in the child's care ensures continuity, concordance, and a feeling of safety for the child and family. A pediatric palliative care service supports families in their choice of location for end-of-life care. These locations can include home, hospital, or a pediatric in-patient hospice unit. Home may provide a sense of familiarity and comfort that may lessen the need for medications to manage physical pain. Conversely, hospital or hospice may provide a sense of security in having access to doctors and nurses and a variety of medications not necessarily available at home.
In the hospital, one role of the palliative care team is to provide pain management for the child in collaboration with the primary care team as well as supportive care to the family. Once the child and family leave the hospital, the palliative care nurse often establishes the link to community services and becomes the link between the family and the primary care team. Communication, consultation, and feedback to the family enhances a feeling of safety and ensures them that they have not been abandoned by health professionals who have been involved in the child's care for months or years. This communication can improve pain control and reduce unnecessary admissions to hospital.
The role of a pediatric palliative care team is often that of a consultant-liaison team who take responsibility for both symptom management and family support. A palliative care service that provides successful pain management can facilitate collaboration and a strengthening of professional relationships among the palliative care and other care teams within a hospital. It also diminishes the notion that palliative care teams are the harbingers of death. Just as important as dialogue between teams and the family is communication with the child. Time should be spent listening to the child, to his or her complaint of pain, in an effort to determine not only pain severity but the meaning of the pain to the child.
Successful pain management requires flexible care provision from all healthcare providers. Flexibility is needed when pain escalates and is difficult to manage; when parents try unproven therapies; when families suddenly choose to go for a family holiday providing little notice to clinicians; when the location for terminal care is changed at short notice. Flexibility is also needed by clinicians when extraordinary doses or unusual therapies are needed for pediatric symptom management.
Suffering as a contributing factor to pain must also be considered. Suffering is a multifaceted entity. Frequently this component of care may be helped by first trying to gauge an understanding of the meaning of suffering to each child and family. Some children, for example, may be experiencing great sadness at the knowledge of their impending death and the loss of their future. Others may be suffering because the family is silent about the progression of illness, and are unable to share their fears and anxieties with family members. For others it may be long periods of hospitalization and missing familiar environments or friends. Some children suffer from the loss of body image, from their inability to participate in sports or other activities, or the unrelenting nature of their symptoms. This suffering can amplify the experience of pain.
Support for parents includes education about anticipated potential symptoms. Knowledge will increase a sense of control. This in turn lessens anxiety and this may positively affect the child's pain experience. Competent and compassionate care can alleviate a child's pain and suffering. This can be achieved through a trusting, consistent, and honest relationship among all involved in providing care, and the family and the child. Because children are especially vulnerable and depend on adults to act as their advocates we must support the humane and competent treatment of pain and suffering at all times ( Table 31-1 ).
Peta was a 7-year-old girl with disseminated neuroblastoma metastatic to the long bones of her lower limbs and significant left-sided auxiliary lymphadenopathy causing mild lymphedema of the left arm. At the referral to palliative care, four months before her death, she had no pain complaint. Peta was the only child of Mark and Tricia. Her mother was 8 months' pregnant. The interdisciplinary palliative care team met the family and discussed its role in the management of symptoms and support with psychological, social, and spiritual issues, should they arise. While the parents' primary goal was for Peta to die free of pain at home, they expressed an interest in the possibility of Peta dying in an in-patient hospice facility if home care became too difficult. The team contacted the local pediatric in-patient hospice unit and facilitated Peta's admission with her family for respite care. The family were impressed by their visit and made a reservation for Peta to stay for respite care when the baby was born. During these admissions, professional relationships were developed with the palliative care social worker, nurses, doctors, and play therapist. One outcome was that the pediatric in-patient hospice unit was viewed as a viable alternative to home for terminal care.
Bone, auxiliary and left arm pain became symptom management issues during the last two months of life. Pain management became complex because of presumed infiltration of the left brachial plexus with tumor and significant lymphedema of the entire left arm. The bone pain was initially treated successfully with localized palliative radiation. Long-acting oral morphine and supplementary short-acting morphine for breakthrough pain and gabapentin as an adjuvant analgesic were prescribed for the management of neuropathic pain related to brachial plexus infiltration by tumor.
Helpful nonpharmacological interventions were lymphedema massage of the left arm and play therapy. Of all the clinicians on the interdisciplinary team, the child life therapist was the one with whom Peta most eagerly engaged. Play as part of an overall strategy for pain management was helpful because it permitted Peta to more easily express her feelings. The financial situation was difficult as the father became unemployed and the mother needed to give up her work to devote time to Peta. The family had great anxiety related to finances and the involvement of the palliative care social worker became critical.
Peta's pain severity rapidly increased during her last week of life. As the oral route of administration was becoming more difficult, the morphine dose was converted to the intravenous equivalent dose and administered as a continuous infusion with a bolus option using a patient controlled analgesia (PCA) pump via a central line. The palliative care nurse educated the family in the care and management of the PCA pump. She also educated the local community nurses by providing an in-service program and educational materials on pain management for children.
A brachial plexus block was considered for Peta's neuropathic pain. A consulting anesthetist's review of MRI studies and ultrasound examination indicated that, because of distorion of landmarks and tissue planes by tumor, it would not be technically feasible to position a brachial plexus catheter proximal (medial and rostral) to the site of tumor, as needed to provide analgesia. Unfortunately, Peta developed thrombocytopenia and no form of nerve block was possible. A drug switch to methadone was made, which substantially improved pain control. Peta's condition deteriorated quickly and she died peacefully and free of pain at home in the presence of her family; the family's wishes being fulfilled.
| Professional background | Contribution toward pain management |
|---|---|
| Pediatric palliative care physician | Primarily responsible for the assessment, diagnosis, and management of physical pain, including the prescription of pharmacologic, and non-pharmacologic management. May have a role in the alleviation of the psychological and existential components of pain. |
| Pediatric palliative care nurse | Primarily responsible for implementing and monitoring pain management through ongoing assessment and measurement. Also has a role in advocating for the child's pain management and a role in the alleviation of the psychological and existential components of pain. |
| Pediatric palliative care social worker | Primarily responsible for the social domain of care of the child and family, especially when affecting pain management. Has a major role advocating for the child and family, including pain management. |
| Pediatric palliative care child-life therapist | Primarily responsible for the use of play as a therapeutic means of self-expression. This may include the expression of pain severity and suffering through therapeutic play. |
| Pediatric palliative care spiritual care provider | Primarily responsible for assessing and managing the spiritual components of patient receiving palliative care and the existential suffering related to dying. Suffering related to the spirit may be related to the experience of physical pain. |
Internationally, there has been a rapid growth in the number of pediatric palliative care services. Many children's hospitals throughout the world have dedicated pain services, and in tandem with this development is the evolution of dedicated pediatric palliative care services. While the latter may be in a children's hospital or a pediatric department of a larger adult hospital, many pediatric palliative care services have evolved for children receiving care at home or in an in-patient hospice unit. The continued advancement of these important areas of pediatric clinical practice can be achieved only through the development of pediatric palliative care quality programs and improved research output in these areas.
Part of quality measurement involves the use of outcome measures. These may be clinical indicators, as in measures of clinical outcomes; process indicators such as measures of clinical processes; and qualitative reflective review. Outcome measures are designed to identify the rate of occurrence or non-occurrence of an event, which in turn reflects the care that was or was not provided. They are aggregated data from all patients receiving the service that can highlight areas that need improving, and can be used to measure the service against particular standards. Indicators can be collected over a number of settings and used as a way of benchmarking or comparing like services.
Clinical indicators concerning pain management at the end of life may include care planning and symptom management, patient and family education, and the provision of holistic care. For example, a high number of patients whose distressing symptoms were addressed may indicate a provision of high-quality education, symptom assessment, care planning and implementation. Conversely, a high number of children being admitted to hospital for symptom management at end of life may reflect a poor quality of symptom management at home.
Pain and other physical and psychological symptoms are highly prevalent in children at the end of life. The proxy report of nurses documented the symptoms of dying children, using a modified Memorial Symptom Assessment Scale. A mean of 11.1 ± 5.6 symptoms was documented per child. At least half of the children had six symptoms; the most frequent ones being lack of energy, pain, drowsiness, skin changes, irritability, and extremity swelling. Lack of energy was the most distressing symptom for nearly one-third of the children. Nervousness, worry, and dysesthetic extremities were notably distressing, although not frequent. Most children were described in the health professionals' notes as being “always comfortable” to “usually comfortable” in the last week (64%), day (76.6%), and hour (93.4%) of life. A retrospective chart review documented the signs and symptoms occurring at the end of life in 28 children dying from cancer in Japan. All children experienced anorexia, 82.1% had dyspnea, and 75% had pain. Other symptoms included fatigue (71.4%), nausea and/or vomiting (57.1%), constipation (46.4%), and diarrhea (21.4%). This symptom profile parallels that of the North American reviews of the symptoms of dying children.
Despite the predominance of treatment-related pain, a number of children have pain related to a tumor, despite the initial response of their pain to treatment. One-third of the pain experienced by patients in the hospital setting was tumor-related pain, but less than 20% of the pain experienced by outpatients was caused by tumor. Direct tumor involvement of bone, hollow viscera, or nerves are more common causes of pain in adult patients with cancer than in children. Such tumor involvement commonly results in somatic, visceral, and neuropathic pain, respectively. Somatic pain is typically well-localized and is frequently described as aching or gnawing. Examples of somatic pain include pain associated with primary or metastatic bone disease or postsurgical incision pain. Visceral pain results from the infiltration, compression, distension, or stretching of thoracic and abdominal viscera by primary or metastatic tumor. This pain is poorly localized, often described as deep squeezing and pressure and may be associated with nausea, vomiting, and diaphoresis, particularly when acute. An example of visceral pain includes pain associated with tumor of the liver, either primary such as hepatoblastoma, or metastatic, such as neuroblastoma. Neuropathic pain most commonly results from tumor compression or infiltration of peripheral nerves or the spinal cord. Chemical- or radiation-induced injury also may result in this sort of pain. The clinical features of pain resulting from neural injury include:
Abnormal or unfamiliar unpleasant sensations, such as dysesthesias frequently having a burning or electrical quality,
A paroxysmal brief shooting or stabbing component,
Allodynia, or pain that is induced by stimuli that are not normally painful such as light touch of the skin,
Or pain that may be felt in the region of a sensory deficit.
The pattern of symptoms, based on the self-reports of children aged 10 to 18 treated for cancer, was studied. Children were surveyed across the spectrum of illness and included newly diagnosed patients, those receiving a bone marrow transplant, and those receiving palliative care. It showed that children with cancer are very symptomatic and are often highly distressed by their symptoms. A prevalence rate greater than 35% was noted for the symptoms of pain, drowsiness, nausea, cough, anorexia, lack of energy, and psychological symptoms. In-patients reported being more symptomatic than their out-patient cohorts. Children with solid tumors were more symptomatic than children with other malignancies. Pain, nausea, and anorexia were clustered as being highly distressing symptoms. Children 7 to 12 years of age, also treated for cancer, similarly self-reported their symptoms. The most prevalent symptoms were pain, difficulty sleeping, itch, nausea, fatigue, and anorexia.
A retrospective chart review at a tertiary-care hospital summarized the end-of-life care of patients more than 5 years of age and dying from cystic fibrosis in the United States. Increasing pain for this patient population may signal advanced, progressive disease. Twenty-five percent of these patients had been receiving opioids for the treatment of chronic headache and/or chest pain for more than their last 3 months of life. When opioids were used for the treatment of breathlessness and/or chest pain, the proportion increased to 86%. Chest, head, extremity, abdomen, and back pain were the most common pain locations during end-of-life care.
Pain, breathlessness, and oral symptoms such as secretions were highlighted as the most common symptoms by caregivers proxy reports for children in the last month of life at an in-patient hospice. Half of the children were non-communicative. Neurodegenerative illness was the major diagnostic category in this in-patient hospice population. Common sources of pain in children with cognitive and physical impairment include muscle spasticity and problems of the musculoskeletal system, such as hip dislocation or kyphoscoliosis.
HIV/AIDS is known to cause pain and other symptoms for multiple reasons, including primary treatments, associated infections, and other complications. Possible causes of pain include bowel dysfunction, cachexia, pancreatitis and sequelae of infection. In a U.S,-based study, 59% of HIV-infected children reported that their pain impacted negatively on their lives.
Nociception, or the sensation of tissue injury or inflammation, is an important biologic function that alerts an individual to potential or ongoing injury and prompts the avoidance or limitation of further injury. Lack of protective sensation can lead to a variety of medical complications, including compartment syndromes or decubitus ulcers. Conversely, there is often no protective significance to other types of pain, such as that of metastatic cancer or migraine.
Nociceptors in deep and superficial tissues can be activated by chemical, thermal, or mechanical stimuli to send afferent impulses through thinly myelinated A-d fibers or unmyelinated C fibers. Primary afferents synapse in the dorsal horn of the spinal cord. Secondary fibers then convey impulses rostrally through a number of tracts, especially the anterolateral spinothalamic tracts. Descending control systems modulate and inhibit pain perception. Endogenous opioids, serotonin, and norepinephrine all appear to be involved in these descending pain-inhibiting systems. In neonates there is a relative excess of excitatory mechanisms for pain transmission with immature inhibitory mechanisms and large receptive fields. All of these factors contribute to a more generalized response to lower intensity pain stimulation in the neonate.
Nociceptive pain refers to pain associated with intact neurons detecting and transmitting impulses associated with tissue injury or inflammation. Neuropathic pain refers to pain associated with abnormal excitability in peripheral or central neurons. Neuropathic pain may persist even after tissue injury or inflammation has subsided. Neuropathic pain often is described as burning, shooting, or stabbing, and it is often associated with paresthesias. Allodynia refers to a condition in which pain can be elicited by normally nonpainful stimuli, such as light stroking of the skin. In the absence of acute inflammation of the skin, allodynia generally implies the existence of an underlying neuropathic condition.
Tumor commonly causes pain from involvement of bone, viscera, and nerves. Visceral stretch, compression, or ischemia activates nociceptive endings that evoke pain. Visceral pain is typically more poorly localized than somatic pain. Animal studies have demonstrated primary visceral afferents that discharge maximally only to apparent noxious stimuli and have small myelinated or unmyelinated axons. Possible mechanisms that may cause pain from bone metastases include stimulation of nerve endings in the endosteum by chemical agents released from the destroyed bone tissue, such as prostaglandins, bradykinin, substance P, or histamine; stretching of the periosteum; fractures; and tumor growth into surrounding nerves and tissues. The periosteum is more sensitive than bone marrow and cortex.
Mechanisms for persistent neuropathic pain after damage to peripheral tissues include:
Deafferentation-induced hyperactivity of dorsal horn pain transmission cells,
Loss of central inhibition by loss of myelinated primary afferents,
Ectopic impulse generation in damaged nociceptive primary afferents,
Sympathetic efferent activation of damaged or intact primary afferents,
Changes in autonomic reflexes.
The pain assessment of the child receiving palliative care may be a complex process. Regular pain assessment of the child receiving post-operative pain management is standard practice at most children's healthcare facilities. It is a less-established practice that the child with progressive illness receives a regular pain assessment. Pain may still not be thought of or asked about when the child's condition is rare, poorly understood, and/or impairs cognition; and clinicians may erroneously believe that if patients do not volunteer information about pain, then it is not a relevant clinical issue. In addition, children and their caregivers may not volunteer information about pain due to a fear that it may indicate progressive disease. Continually assessing a child's pain is an essential component of competent pain management in pediatric palliative care.
Assessment necessitates finding out what language is used by the child to describe pain and a pain history including: location, radiation, duration, quality, exacerbating or relieving factors, and impact on the usual activities of play and mobility. A physical examination should also be performed where appropriate. History and physical examination are of critical importance in determining a diagnosis. It is possible that therapies directed at the primary cause of pain, such as antibiotics for infection as a cause of pain, will be ultimately more effective than the administration of analgesics. Assessment is sometimes made indirectly by quantifying the type, frequency, and dosing of medications. The report from someone other than the child is frequently relied upon for a proxy measure of pain in children who are cognitively impaired.
Measuring pain is one component of the assessment of pain in children. Measurement relies on a metric applied to a specific aspect of the pain experience, usually pain severity.
Self-report measures of pain in children have largely focused on the assessment of acute pain severity. Generally the data support the use of visual analogue scales (VAS) or faces scales for children over the age of 5. VAS have been used in the assessment of pediatric cancer pain; frequently they have anchors of no pain and the worst pain possible. To use such scales, children must understand proportionality, to be able to conceptualize their pain experience along a continuum and be able to translate that understanding to the visual representations on the line and the anchors ( Fig. 31-1 ).
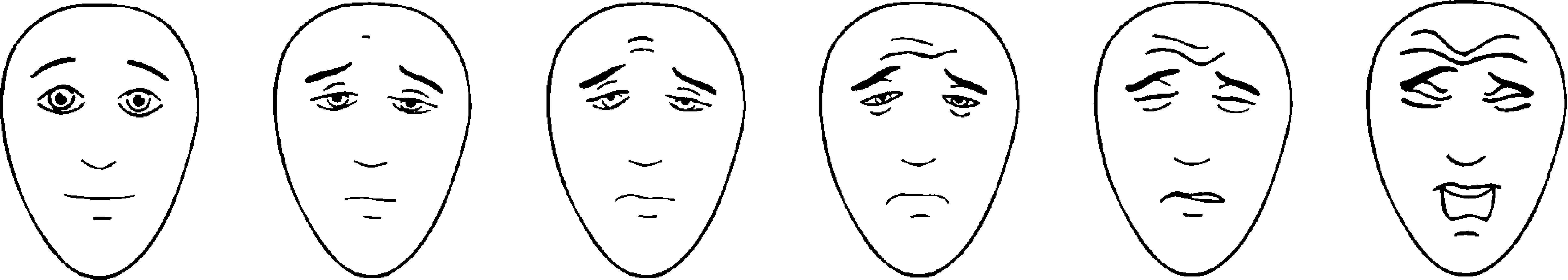
Similar strategies, such as Likert scales with anchor points of 1, no pain, and 5, extreme pain, have been used to assess pain in children with cancer. However, research on the use of verbal rating scales with children 9 years and older has not clearly established the utility of this approach over visual analog scales. Other investigators have used visual cues, such as different pictures of a child's face that are graded from neutral or happy expressions for no pain to sad or distressed expressions for extreme pain.
A child in pain may exhibit some of the following behaviors: irritability and uncooperativeness, lethargy, excess activity, being unusually quiet, loss of appetite, disturbed sleep, anger, flat effect, withdrawn, and paucity of movement. Actions, especially in babies and/or toddlers that are less common than those above but nevertheless can demonstrate pain, include: pulling at ears, banging the head, lying with knees flexed, holding themselves rigid, and clenching fists.
The subjective distress of acute pain, particularly after traumatic medical procedures, often manifests itself in certain facial expressions, verbal, and motor responses. Behavioral methods for assessing pain in children require independent raters recording the physical behaviors of children in pain, as well as the frequency of the occurrence. Behavioral measures of pain in children consist of observation checklists in which a trained observer records the occurrence of certain behaviors. The frequency and duration of the behaviors that occur during the medical procedures are scored to produce a numerical value that represents the child's overall distress. This value is an integrated index of a child's anxiety, fear, distress, and pain, but children's behavioral scores have been interpreted as their global pain scores.
The Gauvain-Piquard rating scale is an observation scale designed to assess chronic pain in pediatric oncology patients aged 2 to 6 years. The lack of operational definitions and the low kappa coefficients question the utility of this scale. The scale consists of 17 items:
7 are related to pain assessment: antalgic rest position, spontaneous protection of painful areas, somatic complaints, the child points out painful areas, antalgic behavior during movement, control exerted by child when moved, and emotional reactions to medical examination of painful regions.
6 are related to depression: child retires into his shell, lack of expressiveness, lack of interest in surroundings, slowness and rarity of movements, signs of regression, social withdrawal.
4 assess anxiety: nervousness and/or anxiety, ability to protest, moodiness and/or irritability, tendency to cry.
A substantial number of children who die have an illness that results in cognitive impairment. Many neurodegenerative diseases impact profoundly on the child's ability to verbally communicate. The physical aspects of certain illnesses, such as grimacing or hypertonia, can mimic features or behaviors commonly attributed to pain. In one post-operative pain study, 24 children aged 3 to 19 years with cognitive impairment were rated by their caregivers and researchers as to their perceived intensity of the child's pain pre- and post-surgery. Familiarity with an individual child was not necessary for observers to have congruent pain measurements. Pain cues reported by 29 caregivers of non-communicative children aged 2 to 12 years with life-threatening conditions were compared against a checklist of 203 items. This study yielded a common core set of six pain cues. These are screaming and/or yelling, crying, distressed facial expression, tense body, difficult to comfort, and flinching when touched.
The Memorial Symptom Assessment Scale 10-18, modified from an adult version, was developed for children aged 10 to 18 years with cancer. In a mean of 11 minutes, the majority of children were able to answer questions about how severe, frequent, and distressing they found their symptoms. For the younger child with cancer, the scale was modified and trialed in 7 to 12 year olds. On this scale, pain is one of many symptoms assessed in 3 dimensions: severity, frequency, and distress.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here