Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Breathing oxygen at increased atmospheric pressure achieves very high arterial P o 2 values, but venous P o 2 , and therefore minimum tissue P o 2 , only increases at 3 atm absolute pressure.
Normal metabolic processes, particularly in the mitochondria, produce a range of powerful oxidizing derivatives of oxygen, some of which are referred to as reactive oxygen species.
The harmful effects of reactive oxygen species are countered by a combination of ubiquitous enzymes that inactivate reactive oxygen species and endogenous antioxidant molecules.
The lungs are susceptible to oxygen toxicity, the first measurable signs occurring in healthy subjects after breathing 100% oxygen for approximately 24 hours. The clinical effects of hyperoxia in the perioperative and critical care setting, as well as in acute medical conditions, are becoming better understood.
Hyperbaric oxygen is used to treat a variety of conditions such as tissue infections, carbon monoxide poisoning and sports injuries, but its use remains controversial.
Chapter 23 described the disastrous consequences of lack of oxygen for life forms that depend on it, but for most organisms hypoxia is an infrequent event. However, oxygen itself also has toxic effects at the cellular level, which organisms have had to oppose by the development of complex antioxidant systems. The activity of toxic oxygen derivatives and antioxidant systems is perfectly balanced most of the time. Nevertheless, there is a strengthening opinion that over many years oxidative mechanisms predominate and may be responsible for the generalized deterioration in function associated with ageing. In a variety of diseases, or when exposed to extra oxygen, the balance is radically disturbed, and unwanted physiological changes or direct tissue damage results.
Hyperventilation while breathing air can raise the arterial P o 2 to about 16 kPa (120 mmHg). Higher levels can be obtained only by oxygen enrichment of the inspired gas and/or by elevation of the ambient pressure. Although the arterial P o 2 can be raised to very high levels, the increase in arterial oxygen content is usually relatively small ( Table 25.1 ). The arterial oxygen saturation is normally greater than 95% and, apart from raising saturation to 100%, additional oxygen can be carried only in physical solution. Provided that the arterial/mixed venous oxygen content difference remains constant, it follows that venous oxygen content will rise by the same value as the arterial oxygen content. The consequences in terms of venous P o 2 ( Table 25.1 ) are important, because minimum tissue P o 2 approximates more closely to venous than to arterial P o 2 . The rise in venous P o 2 is trivial when breathing 100% oxygen at normal barometric pressure, and it is necessary to breathe oxygen at 3 atm absolute (ATA) pressure before there is a large increase in venous and therefore tissue P o 2 . This is because most of the body requirement can then be met by dissolved oxygen, and the saturation of capillary and venous blood remains close to 100%.
| At Normal Barometric Pressure | At 2 ATA | At 3 ATA | ||
| Air | Oxygen | Oxygen | Oxygen | |
| Inspired Gas P o 2 (Humidified) | ||||
| (kPa) | 20 | 96 | 190 | 285 |
| (mmHg) | 150 | 713 | 1425 | 2138 |
| Arterial P o 2 a | ||||
| (kPa) | 13 | 80 | 175 | 270 |
| (mmHg) | 98 | 600 | 1313 | 2025 |
| Arterial Oxygen Content b | ||||
| (mL.dL –1 ) | 19.3 | 21.3 | 23.4 | 25.5 |
| Arterial/Venous Oxygen Content Difference | ||||
| (mL.dL –1 ) | 5.0 | 5.0 | 5.0 | 5.0 |
| Venous Oxygen Content | ||||
| (mL.dL –1 ) | 14.3 | 16.3 | 18.4 | 20.5 |
| Venous P o 2 | ||||
| (kPa) | 5.2 | 6.4 | 9.1 | 48.0 |
| (mmHg) | 39 | 48 | 68 | 360 |
a Reasonable values have been assumed for P co 2 and alveolar/arterial P o 2 difference.
It is convenient to consider two degrees of hyperoxia. The first applies to the inhalation of oxygen-enriched gas at normal atmospheric pressure, whereas the second involves inhaling oxygen at raised pressure and is termed hyperbaric hyperoxia . Firstly, however, it is necessary to understand the molecular basis by which oxygen causes damage to biological molecules.
The dioxygen molecule ( Fig. 25.1 ) is unusual because it has two unpaired electrons in the outer (2P) shell, but stability is conferred because the orbits of the two unpaired electrons are parallel. The two unpaired electrons also confer the property of paramagnetism, which has been exploited as a method of gas analysis that is almost specific for oxygen (page 158).
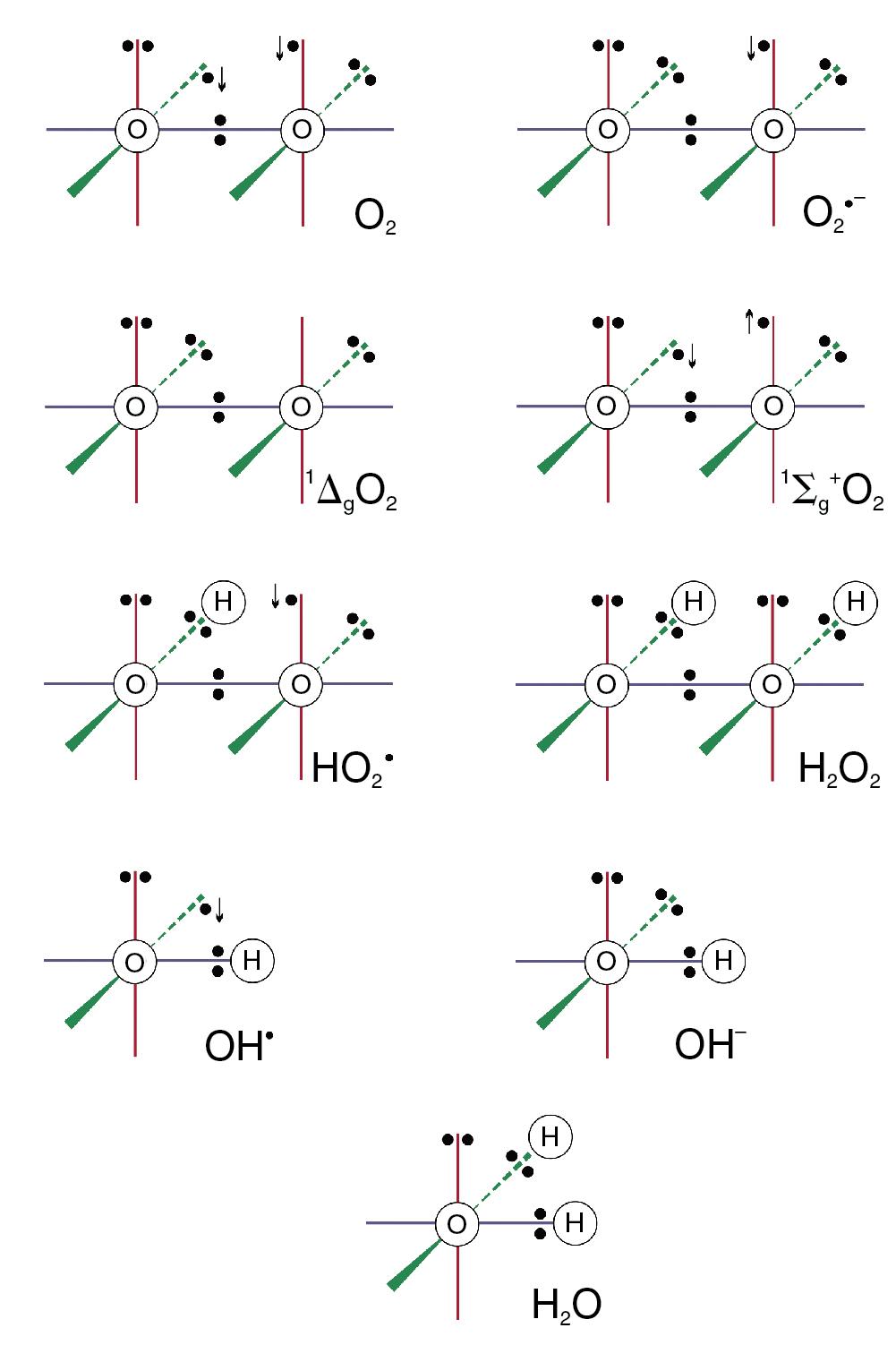
Although ground state oxygen (dioxygen) is a powerful oxidizing agent, the molecule is stable and has an indefinite half-life. However, as discussed below, the oxygen molecule can be transformed into a range of reactive oxygen species (ROS) and other highly toxic substances, most of which are far more reactive than oxygen itself.
Internal rearrangements of the unpaired electrons of dioxygen result in the formation of two highly reactive species, both known as singlet oxygens (1O 2 ). In 1ΔgO 2 one unpaired electron is transferred to the orbit of the other ( Fig. 25.1 ), imparting an energy level of 22.4 kcal.mol −1 above the ground state. With no remaining unpaired electron, 1ΔgO 2 is not a ROS. In 1Σg + the rotation of one unpaired electron is reversed, which imparts an energy level of 37.5 kcal.mol −1 above the ground state, and this molecule is a ROS. 1Σg + is extremely reactive and rapidly decays to the 1ΔgO 2 form, which is particularly relevant in biological systems, and especially in lipid peroxidation.
Under circumstances considered below, the oxygen molecule may be partially reduced by receiving a single electron, which pairs with one of the unpaired electrons, forming the superoxide anion (O 2 •- in Fig. 25.1 ), which is both an anion and a ROS. It is the first and crucial stage in the production of a series of toxic oxygen-derived ROS and other compounds. The superoxide anion is relatively stable in aqueous solution at body pH, but has a rapid biological decay because of the ubiquitous presence of superoxide dismutase (see later). Because it is charged, a superoxide anion does not readily cross cell membranes.
The hydroperoxyl radical is a ROS. A superoxide anion may acquire a hydrogen ion to form the hydroperoxyl radical thus:
The reaction is pH-dependent with a p K of 4.8, so the equilibrium is far to the left in biological systems.
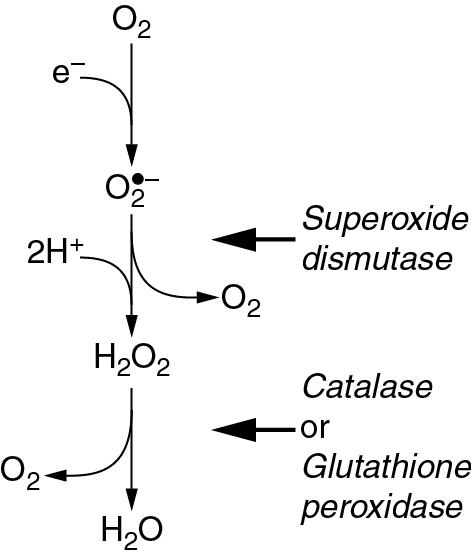
Superoxide dismutase (SOD) catalyses the transfer of an electron from one molecule of the superoxide anion to another. The donor molecule becomes dioxygen, while the recipient rapidly combines with two hydrogen ions to form hydrogen peroxide ( Fig. 25.1 ). Although hydrogen peroxide is not a ROS, it is a powerful and toxic oxidizing agent that plays an important role in oxygen toxicity. The overall reaction is as follows:
Hydrogen peroxide is continuously generated in the body. Two enzymes ensure its rapid removal. Catalase is a highly specific enzyme active against only hydrogen, methyl and ethyl peroxides. Hydrogen peroxide is reduced to water thus:
Glutathione peroxidase acts against a much wider range of peroxides (R–OOH), which react with glutathione (GSH) thus:
Catalase and glutathione peroxidase are discussed further in a later section.
Figure 25.2 summarizes the three-stage reduction of oxygen to water, which is the fully reduced and stable state. This contrasts with the more familiar single-stage reduction of oxygen to water that occurs in the terminal cytochrome (page 153). Unlike the single-stage reduction of oxygen, the three-stage reaction shown in Figure 25.2 is not inhibited by cyanide.
Although both the superoxide anion and hydrogen peroxide have direct toxic effects, they interact to produce even more dangerous species. On the right side of Figure 25.3 is the Fenton or Haber–Weiss reaction , which results in the formation of the harmless hydroxyl ion together with two extremely reactive species, the hydroxyl free radical (OH • ) and singlet oxygen (1O 2 )
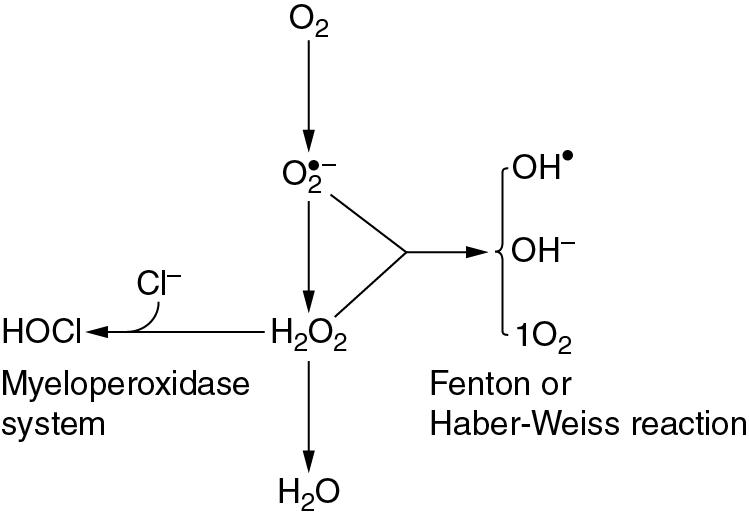
The hydroxyl free radical is the most dangerous ROS derived from oxygen.
On the left side of Figure 25.3 is the reaction of hydrogen peroxide with a chloride ion to form hypochlorous acid. This occurs in the phagocytic vesicle of the neutrophil and plays a role in killing bacteria, facilitated by the enzyme myeloperoxidase. The myeloperoxidase reaction also occurs immediately after fertilization of the ovum, and hypochlorous acid thus formed causes polymerization of proteins to form the membrane that prevents further entry of spermatozoa.
The changes previously described have many features in common with those caused by ionizing radiation; the hydroxyl radical (OH • ) is the most dangerous product in both cases. It is not, therefore, surprising that the effect of radiation is increased by high partial pressures of oxygen. As tissue P o 2 is reduced below about 2 kPa (15 mmHg), there is progressively increased resistance to radiation damage until, at zero P o 2 , resistance is increased threefold. This unfortunate effect promotes resistance to radiotherapy of malignant cells in hypoxic areas of tumours (page 360).
Nitric oxide may behave as a ROS by reacting with the superoxide anion to produce peroxynitrite (ONOO − ). This molecule can either rearrange itself into relatively harmless nitrite or nitrate (page 148) or give rise to derivatives with similar biological activity to the hydroxyl radical. Conversely, nitric oxide may act as an antioxidant, binding to ferrous iron molecules and preventing them from contributing to the formation of a superoxide anion (see next) or the Fenton reaction. The in vivo role of nitric oxide as a ROS or antioxidant therefore remains unclear.
Figure 25.3 shows the superoxide anion as the starting point for the production of many other ROS. The first stage reduction of dioxygen to the superoxide anion is therefore critically important in oxygen toxicity.
Complex 1 (nicotinamide adenine dinucleotide + hydrogen oxidoreductase [NADH oxidoreductase]) and a variety of other mitochondrial enzymes may ‘leak’ electrons to molecular oxygen, producing superoxide anions during normal oxidative respiration. Animal studies indicate that this may account for almost 2% of total mitochondrial oxygen consumption, indicating the importance of the highly efficient mitochondrial form of SOD (see later). The concentration of mitochondrial ROS molecules must be carefully controlled, a task undertaken by the mitochondrial permeability transition pore (mPTP) channels in the mitochondrial membrane. In response to an unfavourable redox state within the mitochondrion, the mPTP channels open very briefly to allow the ROS out into the cell for removal by cytoplasmic antioxidant systems. Excess ROS in the mitochondrion leads to more prolonged opening of mPTP channels, which can damage the mitochondrion and cell. Thus ROS are acting as a signalling pathway during normal circumstances to control their own levels, but can easily become a pathological cause of cell damage.
The NADPH oxidase system is the major electron donor in neutrophils and macrophages. The electron is donated from NADPH by the enzyme NADPH oxidase, which is located within the membrane of the phagocytic vesicle. This mechanism is activated during phagocytosis, and is accompanied by a transient increase in the oxygen consumption of the cells, a process known to be cyanide resistant. This is the so-called respiratory burst, and occurs in all phagocytic cells in response to a wide range of stimuli including bacterial endotoxin, immunoglobulins and interleukins. Superoxide anion is released into the phagocytic vesicle, where it is reduced to hydrogen peroxide, which then reacts with chloride ions to form hypochlorous acid in the myeloperoxidase reaction ( Fig. 25.3 ). For many years the release of ROS into the phagocyte was believed to be the main way in which bacteria were killed by phagocytes. Recent work on pulmonary neutrophils in mice with pneumococcal infection has refuted this claim, finding no evidence of bacterial killing by neutrophil-generated ROS, although ROS were involved in neutrophil regulation. Powerful protease enzymes released into the phagosome by the neutrophil may be the most important bactericidal mechanism.
Although the NADPH oxidase system has extremely important biological functions, there seems little doubt that its inappropriate activation in marginated neutrophils can damage the endothelium of the lung, and it may well play a part in the production of acute lung injury ( Chapter 31 ).
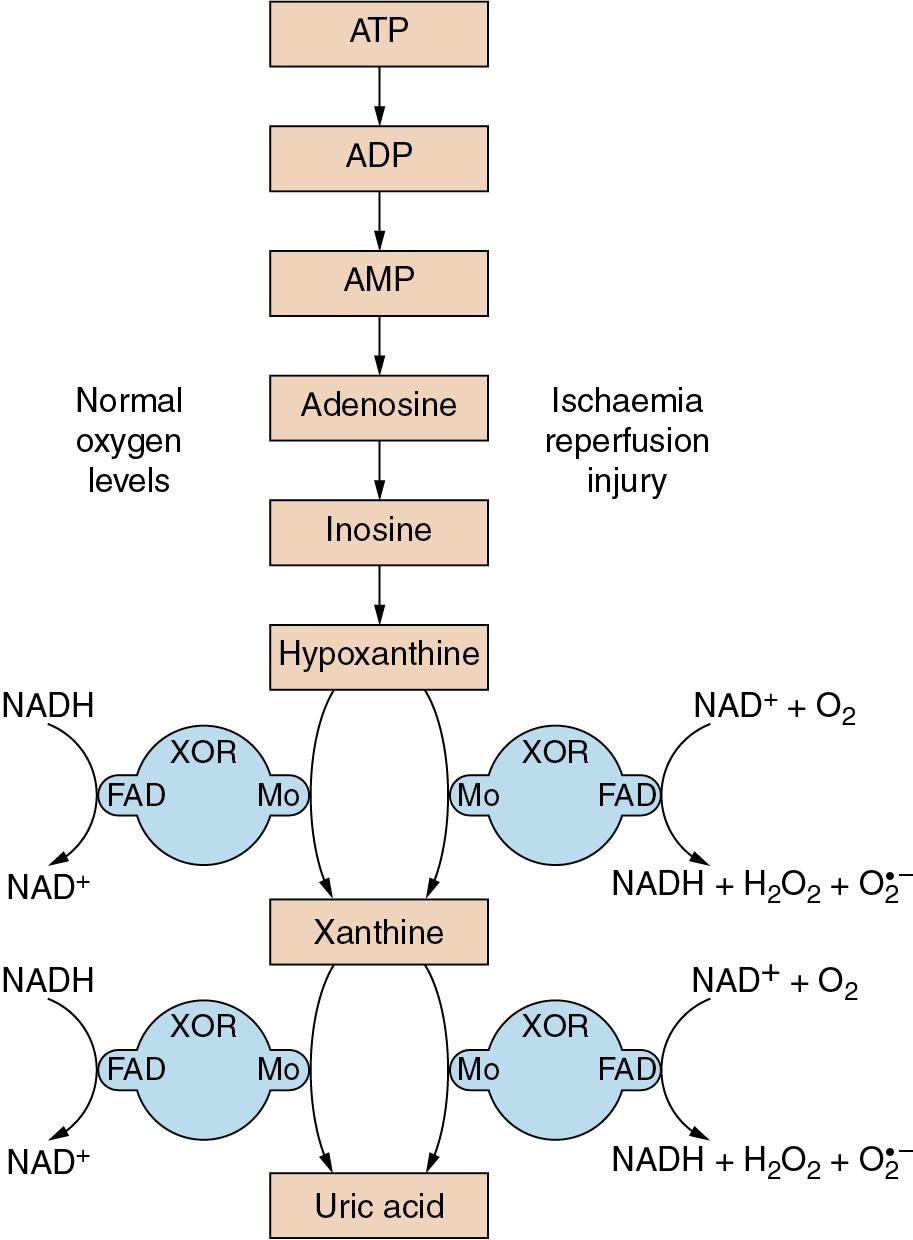
The enzyme xanthine oxidoreductase (XOR) is responsible for the conversion of hypoxanthine and xanthine to uric acid ( Fig. 25.4 ). XOR is a large (300 kDa) protein involving two separate substrate binding sites, one including flavine adenine dinucleotide cofactor and the other a molybdenum molecule. In vivo, XOR exists in two interchangeable forms, with about 80% existing as xanthine dehydrogenase and the remainder as xanthine oxidase. In both forms XOR catalyses the conversion of both hypoxanthine to xanthine and of xanthine to uric acid, and under normal conditions uses NADH as a cofactor. In ischaemic or hypoxic tissue large quantities of hypoxanthine accumulate (page 273), the availability of NADH declines, and the ratio of the oxidase and dehydrogenase forms of XOR may be reversed. As a result of these changes, when oxygen is restored to the cell, the XOR catalysis of xanthine and hypoxanthine is altered, with NAD + and dioxygen now used as cofactors, resulting in the production of hydrogen peroxide and superoxide anions ( Fig. 25.4 ). Thus during reperfusion there may be extensive production of ROS.
Ferrous iron (Fe 2+ ) loses an electron during conversion to the ferric (Fe 3+ ) state. This is an important component of the toxicity of ferrous iron. A similar reaction also occurs during the spontaneous oxidation of haemoglobin to methaemoglobin (page 149). It is for this reason that large quantities of SOD, catalase and other protective agents are present in the young red blood cell. Their depletion may well determine the life span of the cell. Apart from ferrous iron acting as an electron donor, it is a catalyst in the Fenton reaction (see previous discussion).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here