Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter addresses a variety of lung lesions that represent repair reactions, inclusions, deposition disorders, and masses of uncertain etiology. They may present as single or multiple lesions or as infiltrative lesions. They may be secondary to systemic diseases, but unlike those diseases in Chapter 35 , they represent masses or infiltrates that are secondary depositions of material (amyloidosis, light chain deposition disease, calcifications). They are presented in this chapter because they do not fit well into the topics of other chapters; yet they are important to be aware of as one constructs differential diagnoses.
A proliferation of bone and cartilage arising out of the cartilage rings of the trachea and major bronchi, extending beyond the cartilage ring into the airway lumen
More commonly found in males and more common in people over 50 years
Usually asymptomatic but has been known to cause dyspnea, wheezing, changes in voice, and, when severe, obstructive symptoms
Beadlike nodules on the anterior and lateral walls of the trachea and major bronchi, sparing the posterior airway walls
Excellent prognosis
Slow-growing lesion
Laser photovaporization therapy can be effective
Firm, white nodules on the anterior and lateral walls of the trachea and major bronchi
Absence of these lesions on the posterior airway wall is helpful in the differential diagnosis
Bone and/or cartilage replacing the submucosa
Squamous metaplasia and rare case of squamous dysplasia may accompany
Tracheobronchial amyloidosis
Benign and malignant tumors of the airway
Tracheobronchopathia osteochondroplastica (TBO) is a rare pathologic finding in the trachea and major bronchi. It represents proliferations of bone and cartilage arising out of the cartilage rings of the trachea, extending into the airway lumen. TBO is unusual and found in approximately 1 in 200 bronchoscopies, an incidence that will likely increase as pulmonologists do more bronchoscopies for diagnosis and staging. The pathogenesis of TBO is not known. Speculation regarding a role for either bone morphogenetic protein-2 or transforming group factor-beta in this process has been reported.
TBO is an incidental finding at bronchoscopy, more common in men and more common over the age of 50 years. It is unclear if any clinical symptoms are attributed to TBO, but, if present, the most common is chronic cough. Others may include dyspnea, wheezing and perhaps changes in the voice. With unusually large lesions, patients may present with both inspiratory and expiratory airflow limitations.
The imaging studies of TBO show scalloped or beaded lesions involving the anterior and lateral walls of the trachea and sometimes the major bronchi. Chest computed tomography (CT) will highlight the presence of calcium, which is usually not seen on the regular chest x-rays.
The descriptions of TBO have ranged from a “stalactite-like” mucosal surface to cobblestone or “rock garden” appearance in the airways ( Fig. 25.1 ). The lesions are hard, sessile nodules that spare the posterior walls of the trachea and major bronchi. The nodules can be single, diffuse, or occasionally, a confluent aggregate, and the mucosa is usually smooth, without ulceration.
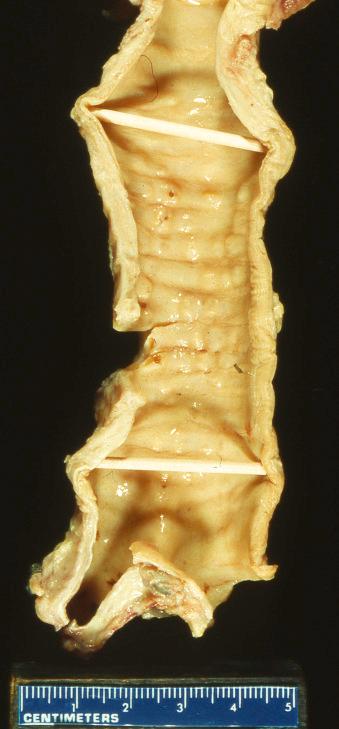
Excisions or biopsies of these lesions usually reveal an intact respiratory mucosa, sometimes with squamous metaplasia and rarely, dysplasia, though there is no definitive evidence that the dysplasia is related to the TBO because other risk factors, including smoking, are commonly present. Bone and/or cartilage are directly beneath the mucosa, many times replacing the submucosa, including the bronchial glands that are present in the trachea ( Fig. 25.2 ). This bone and cartilage can be continuous with or distinctive from the large airway cartilage ring. Calcification and osseous metaplasia, sometimes even including hematopoietic bone marrow, have been reported.
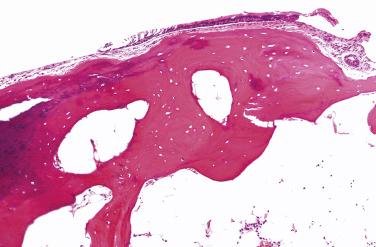
Histochemical studies for amyloid, including a Congo red or a crystal violet stain, should be negative and will help to exclude amyloidosis.
The location of these lesions, excluding the posterior walls and major bronchi, is helpful in limiting the pathologic differential diagnosis. Tracheobronchial amyloidosis and proximal airway tumors (both benign and malignant) represent the main differential diagnosis, and both can be seen in both the trachea and major bronchi and do not spare the posterior wall. Nonetheless, because it is difficult to definitively exclude either benign or malignant tumors based on the bronchoscopic gross evaluation, biopsy is usually recommended. Finally, tracheobronchial amyloidosis will be positive for amyloid by Congo red and/or crystal violet, whereas TBO is negative for amyloid stains.
The growth of these nodules is slow, and usually no treatment is necessary. If the lesion does cause severe obstructive symptoms, laser therapy, especially laser photovaporization, can be tried. Radiation has been tried in some symptomatic patients, though no dramatic effect has been reported.
Chronic aspiration of animal, vegetable, or mineral lipid-containing material in the lungs producing a granuloma-like and fibrotic reaction
More commonly found in older adults who may take oil or cathartics
Usually occurs in patients with neuromuscular disorders, esophageal abnormalities such as dysphagia, or anatomic defects of the oral cavity and neoplasms of the head and neck
Dense airspace consolidation or irregular masslike lesions in dependent portion of lungs
Excellent prognosis; may require surgery if cavitation or infection occur
Golden-yellow, firm, masslike area
Cavitation may occur if infection is present
Intraalveolar and interstitial foamy macrophages with lipid vacuoles
Giant cells and cholesterol clefts are common
Lipid (whorled) bodies within macrophages consisting of electron-dense lipid membranes
Large vacuolated macrophages; Sudan black or oil red O stain confirms the lipid material in the vacuoles
Endogenous lipid pneumonia
Storage diseases
Erdheim-Chester disease
Infections: nontuberculous mycobacteria, R. equi, H. capsulatum
Lipoid pneumonia (sometimes referred to as exogenous lipoid pneumonia ) is an uncommon condition that results from chronic aspiration of animal, vegetable, or mineral lipid-containing material into the lungs. The macrophages phagocytize the lipid and cause a granuloma-like or fibrotic reaction within the area of aspiration. This produces either a dense infiltrate or masslike lesion. Endogenous lipoid pneumonia, sometimes referred to as postobstructive pneumonia, commonly contains areas of foamy macrophages. It usually develops when lipids that normally reside in lung tissue, commonly cholesterol and its esters, escape from destroyed alveolar cell walls distal to an obstructing lesion, such as a tumor or from lung parenchyma damaged by a suppurative process. This is an entity distinct from exogenous lipoid pneumonia.
Exogenous lipoid pneumonia usually occurs in patients with neuromuscular disorders, esophageal abnormalities such as dysphagias, and anatomic defects such as cleft palates or head and neck neoplasms that may predispose them to aspiration. It has also been seen in patients without esophageal disorders but who work with mineral oil compounds such as lubricants and cutting fluids in industrial settings. The usual clinical presentation is an insidious onset of cough, which may or may not be productive with the development of dyspnea. Chest pain, weight loss, hemoptysis, and intermittent fevers may also develop, the latter sometimes secondary to the inflammatory reaction to oil or to concurrent infection. Crackles, wheezes, and rhonchi may be heard on auscultative examination.
Chest x-rays usually reveal dense airspace consolidation or irregular masslike lesions, most commonly in the dependent portions of the lung. Chest CT scans reveal consolation with fat attenuation or a “crazy-paving” pattern with septal and centrilobular thickening. Traction bronchiectasis and fibrosis may be seen in severe chronic cases.
The gross appearance of the exogenous lipoid pneumonia varies depending on the stage. Early in the disease process, the involved lung can appear gray to yellow with a moderate firm, spongy texture and exude oil upon sectioning. With progression, the lung appears fibrotic, and the lesions grow firmer; turn gray-white; and can become nodules, masses, and cavitation.
The characteristic microscopic appearance is one of abundant intraalveolar and interstitial foamy macrophages with variably sized vacuoles of lipid ( Fig. 25.3 ). Giant cells and cholesterol clefts are common. Collagenous fibrosis may occur with reactive type 2 pneumocytes. Necrosis is uncommon unless infections are present. Importantly, when necrosis is present, the possibility of superinfections by nontuberculous mycobacterium, including Mycobacterium chelonae, M. fortuitum, colonizers in gut fluid, and M. abscessus is increased. It has been suggested that this occurs, despite the usual weak pathogenicity of these organisms, because oil and lipid enhance the pathogenicity of these mycobacteria.
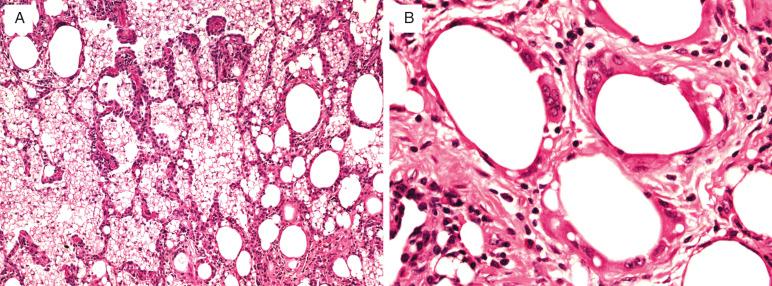
Transmission electron microscopy reveals typical whorled bodies of lipid within the foamy macrophages. These consist of lamellated, concentric, electron-dense lipid membranes.
Large vacuolated macrophages with lipid material are found in fine-needle aspirations of lipid pneumonia. Lipid histochemical stains such as Sudan black or oil red O will confirm the lipid nature of the cytoplasmic vacuoles.
Endogenous lipoid pneumonia has a similar appearance to exogenous lipoid pneumonia, except that the vacuoles of lipid are smaller and more uniform in size. Spaces representing lipid droplets that were leached out during processing are found in exogenous, but not endogenous, lipoid pneumonia. Other foamy macrophage-rich lesions may also be considered, including storage diseases such as Niemann-Pick, Erdheim-Chester disease, and infections (nontuberculous mycobacteria, Rhodococcus equi, and certain disseminated fungal diseases such as Histoplasma capsulatum ). Clinical information and special stains for organisms are helpful for distinguishing these processes from lipoid pneumonia.
Therapy for exogenous lipoid pneumonia usually involves treatment of the underlying disorder that predisposes the patient to aspiration and treatment of any superimposed infections (bacterial, mycobacterial). The prognosis without infection is excellent. When infections occur, especially from nontuberculous mycobacteria, cavitary lung disease may occur, requiring surgical resection.
A spectrum of disease in which nonbranching linear fibrils of protein are deposited as insoluble fibrils in the extracellular spaces
Amyloid localized to the lung is rare and is usually tracheobronchial or nodular
Systemic amyloid involves the lung in 30% to 90% of patients and is usually the diffuse interstitial form
Excellent survival with tracheobronchial and nodular forms, but the median survival is only 18 to 24 months with the diffuse parenchymal form
No gender or racial predilection
Most patients are more than 40 years of age, and the senile form usually occurs at over 80 years of age
Four clinical syndromes of pulmonary amyloidosis:
Systemic (generalized)
Localized
Diffuse alveolar septal
Pleural
Systemic amyloidosis consists of four types:
AL: associated with light chain production
AA: associated with longstanding inflammatory disease (eg, rheumatoid arthritis, chronic osteomyelitis, etc.)
Wild type AATR: associated with normal transthyretin and old age
Hereditary ATTR: associated with a transthyretin mutation
Nodular: single or multiple well-circumscribed nodules
Diffuse: interstitial nodular or linear densities
Tracheobronchial: airway thickening or collapse; postobstructive pneumonia
Cysts have been reported on imaging studies; some appear calcified
Nodular and tracheobronchial amyloidosis are treated with conservative excision (surgical or laser); excellent survival
Diffuse amyloidosis has no effective treatment; poor prognosis
Three forms:
Tracheobronchial: polypoid masses in major bronchi
Nodular: firm, waxy gray to white, well-demarcated nodules
Diffuse: uniform rubber-sponge–like lung parenchyma
Amorphous, eosinophilic substance involving airways, vessels, and interstitium
Tracheobronchial and nodular forms commonly have giant cells “ingesting” amyloid at edges
Plasma cells, calcium, and bone are often present
FNAB of nodular amyloid shows homogenous flocculent material
Amyloid stains with Congo red stains and demonstrates apple-green birefringence when viewed under polarized light
Amyloid is blue-gray with Mallory’s trichrome stain, in contrast to the more definite deep blue color of collagen
Amyloid stains metachromatically with crystal or methyl violet
Tangled or feltlike mass of long, tubular, nonbranching, hollow fibrils 7.5 to 10 nm in diameter, 800 nm long
10% of components consist of P component, a glycoprotein
Antibodies to AA, Aκ, Aλ, ATTR, and Aβ 2 -microglobulin are available
May be less sensitive than Congo red staining with examination under polarized light
Mass spectrometric analysis is more accurate for assessment of fibril composition
Four major types: AL, AA, AATR, and beta 2 -microglobulin
Light chain disease
Pulmonary hyalinizing granuloma
Hyalinizing fibrosis
Amyloidosis is a spectrum of diseases in which nonbranching linear fibrils of protein are deposited as insoluble fibrillar material in the extracellular spaces. This deposition can be systemic or localized. Overall, amyloid is classified both by the biochemical composition of the fibrils and by its clinical syndromes. The four major types of fibrils include serum protein A (SAA), amyloid light chain (AL), amyloid-associated transthyretin (AATR), and beta 2 -microglobulin. In the lungs, four general clinical syndromes are recognized: systemic (generalized), localized, diffuse alveolar septal, and pleural. Systemic amyloidosis involves the lung 30% to 90% of the time, depending on the series, and consists of AL (associated with light chain–producing plasma cell dyscrasia), AA (associated with longstanding inflammation), wild-type ATTR (associated with normal transthyretin and old age), and hereditary ATTR (associated with a transthyretin mutation). Localized amyloidosis to the lungs is rare but, if present, usually takes the form of tracheobronchial amyloidosis (see later). Diffuse alveolar septal amyloidosis and pleural amyloidosis consist of type AL and are forms of amyloidosis limited to the lungs.
Pulmonary amyloidosis can develop at any time during adult life. The clinical presentation of amyloidosis depends on the form the disease takes. Nodular amyloidosis usually presents as an asymptomatic nodule. When multiple nodules occur, pleuritic chest pain, dyspnea, or hemoptysis may be noted. Diffuse parenchymal amyloidosis usually presents with dyspnea or cough. Tracheobronchial amyloidosis can present with wheezing, atelectasis, or recurrent pneumonia.
Nodular amyloidosis appears as single or multiple well-circumscribed nodules on imaging studies. Cavitation is rare. Cysts have been reported, usually associated with calcified nodules. Diffuse parenchymal amyloidosis usually manifests as interstitial lung disease with linear or nodular densities. Tracheobronchial amyloidosis may present as airway thickening or collapse, as postobstructive pneumonia, or as atelectasis.
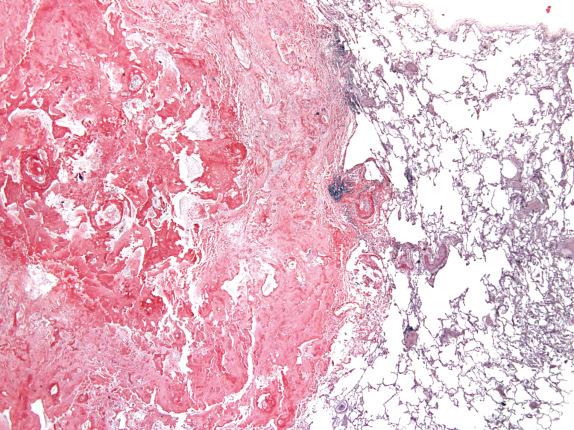
Pulmonary amyloidosis takes three major forms: tracheobronchial, nodular, and diffuse parenchymal (interstitial, septal, and vascular). Tracheobronchial amyloid is usually a polypoid mass or a raised submucosal plaque in a major bronchus ( Fig. 25.4A ). Nodular amyloidosis takes the form of firm, waxy, gray or white nodules with well-demarcated borders present within the lung parenchyma. These range from a few millimeters to 15 cm in diameter. The gross appearance of diffuse parenchymal amyloidosis has been referred to as a “uniform rubber-sponge-like appearance,” which may become brittle or sandy if calcium is present.
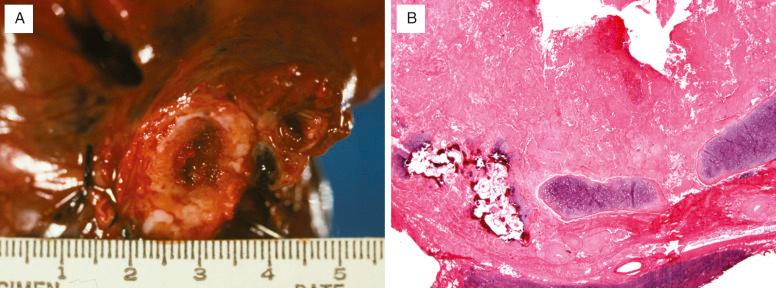
Tracheobronchial amyloidosis presents as amyloid deposits in the submucosa. Multinucleated cells, calcification, chondrification, and ossification are commonly seen ( Fig. 25.4B ). The nodules in nodular amyloidosis often have sharp borders and are present within the pulmonary parenchyma as well-circumscribed masses ( Fig. 25.5 ). Multinucleated foreign-body–type giant cells are frequently seen “ingesting” amyloid around the edges ( Fig. 25.6 ), and plasma cells are usually present in moderate amounts. Calcification and ossification of these nodules is common. Diffuse parenchymal amyloidosis has amyloid deposition in the interstitium and within the vessels ( Fig. 25.7 ). Calcium may accompany the amyloid, and an infiltrate of lymphocytes or plasma cells is common. Giant cells are usually not seen in this form of amyloidosis.

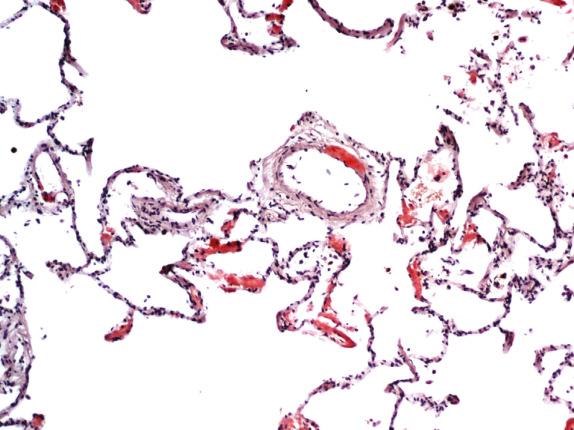
Fine-needle aspiration biopsy (FNAB) may be helpful in the diagnosis of nodular or tracheobronchial amyloidosis. The presence of homogeneous flocculent eosinophilic material in the aspirated sample should raise the possibility of amyloid, which must be confirmed by Congo red staining. Plasma cells, lymphocytes, and giant cells may be present, as well as deposits of calcium (also see Chapter 36 ).
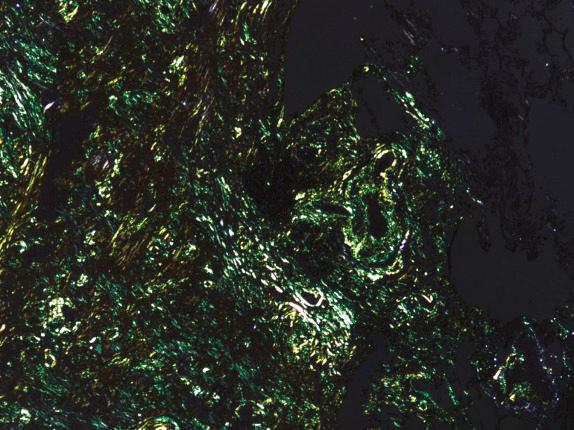
Amyloid stains best with Congo red and has a characteristic apple-green birefringence with polarization ( Fig. 25.8 ). Amyloid presents a light to medium blue-gray color with Mallory’s trichrome stain, in contrast to the more definite deep blue color of collagen. Amyloid also stains metachromatically with crystal or methyl violet, which may be useful as an initial stain for screening purposes.
Amyloid can be classified by the biochemical composition of its fibrils. Protein AL is derived from plasma cells and of immunoglobulin origin, usually representing the variable N-terminal end of light chains. Protein AA is a unique N-terminal sequence nonimmunoglobulin synthesized in the liver and commonly found in primary amyloidosis. Protein ATTR is a prealbumin molecule that binds and transports thyroxine and retinol, deposited in familial amyloid polyneuropathies and senile systemic amyloidosis. Beta 2 -microglobulin is a component of human leukocyte antigen class I molecules and a normal serum protein deposited in patients on long-term hemodialysis.
Immunohistochemistry using antibodies to these proteins confirms the type of amyloid fibril and is essential to guide therapy. Antibodies for AA amyloid and in most cases of ATTR amyloid are widely available and sensitive. For AL amyloid these antibodies are less helpful. Because of this, the proteomic method of mass spectrometric analysis is the new gold standard for all fibril typing. This technique involves microdissection and capture of Congo red deposits from a formalin-fixed, paraffin-embedded section followed by matching of peptides to a protein reference database.
Electron microscopy shows a tangled or feltlike mass of very long, tubular, nonbranching hollow fibrils measuring 7.5 to 10 nm (75–100 Å) in diameter, perhaps some 800 nm (8000 Å) long. About 10% of the components consist of a pentagonal substance (P component) that is glycoprotein and accounts for any periodic acid–Schiff (PAS) staining.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here