Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
An osteochondral lesion (OCL) is a defect on the articular surface of a joint with or without subchondral bone involvement. The first report of removal of a loose body from a knee joint, likely an unstable OCL, is attributed to Ambroise Paré in 1558, followed by a description of “cartilage of the joint of the knee separated and ossified” by Alexander Monro in 1738 and descriptions in joints other than the knee by John Hunter in 1759. The term “joint mice” to describe loose OCL was proposed by René Laennec in 1817 due to their tendency to move around the articular space. A nontraumatic etiology of OCL was proposed by Paul Broca in 1854, who suggested “spontaneous necrosis” of the articular surface could lead to a loose body, and also by Sir James Paget in 1870 who described “quiet necrosis” in the knee joint of two patients. The term “osteochondral bodies” was coined by Franz König in 1887 in a treatise on loose bodies in the elbow, which included the most thorough description of the condition at the time.
The term OCL encompasses isolated cartilage lesions with an intact bony base, combined bone and cartilage injuries, and subchondral avascular and cystic changes of the bone with an intact chondral surface. Diffuse cartilage loss, bicameral involvement, and cystic changes associated with inflammatory arthritis or osteoarthritis are typically not classified as OCL, though the distinction between multiple OCL and diffuse cartilage loss is not well defined. Similarly, articular cartilage injury as part of an intraarticular fracture is not labeled an OCL, though OCL is often associated with an acute ankle fracture or sprain. This chapter describes our current understanding of OCL of the foot and ankle including their pathogenesis and incidence, evaluation strategies, treatment options, and results of treatment. While we have learned a great deal about OCL of the foot and ankle in the last decade, it must be stressed that no treatment strategy has achieved clear superiority in all circumstances. Recommendations in this chapter are based on a synthesis of data from the literature combined with the personal experience of the author.
An osteochondral lesion of the talus (OLT) is the most common osteochondral injury treated by the foot and ankle surgeon. Numerous terms have been used to describe OLT including transchondral fracture, osteochondral fracture, osteochondritis dissecans, osteochondral defect (OCD), talar dome fracture, and flake fracture.
An improved understanding of symptom etiology and results of various treatment options have led to an evolving set of tools designed to bring patients lasting relief. Marrow-related cartilage repair techniques are most commonly applied to smaller lesions, while great heterogeneity exists regarding recommended treatment strategy for larger lesions. Management of OLT through arthroscopic or with minimally open approaches can reduce the morbidity associated with an extensive surgical approach, which otherwise may require ankle arthrotomy and malleolar osteotomy.
A thorough understanding of the myriad treatment strategies and rapidly changing surgical options is challenging for surgeons and patients alike. A number of excellent reviews of the basic science, clinical techniques, and outcomes have been published. Systematic reviews and meta-analyses help the clinician to weigh the available evidence when making treatment decisions for their patients, but as of yet have not revealed a superior treatment strategy. Continued prospective comparison studies are needed to identify treatments with the best chances of success for treating OLT.
For the last 100 years, physicians have hypothesized that various events lead to OLTs. These include developmental abnormalities of the bone, traumatic shearing, and spontaneous avascular necrosis. Trauma was initially proposed as the principal etiologic factor, earning names like transchondral fracture of the talus meant to reflect the proposed pathogenesis. Alternatively, idiopathic osteonecrosis has been proposed to be the underlying cause of OLTs, which is supported by the fact that trauma is not documented in many cases.
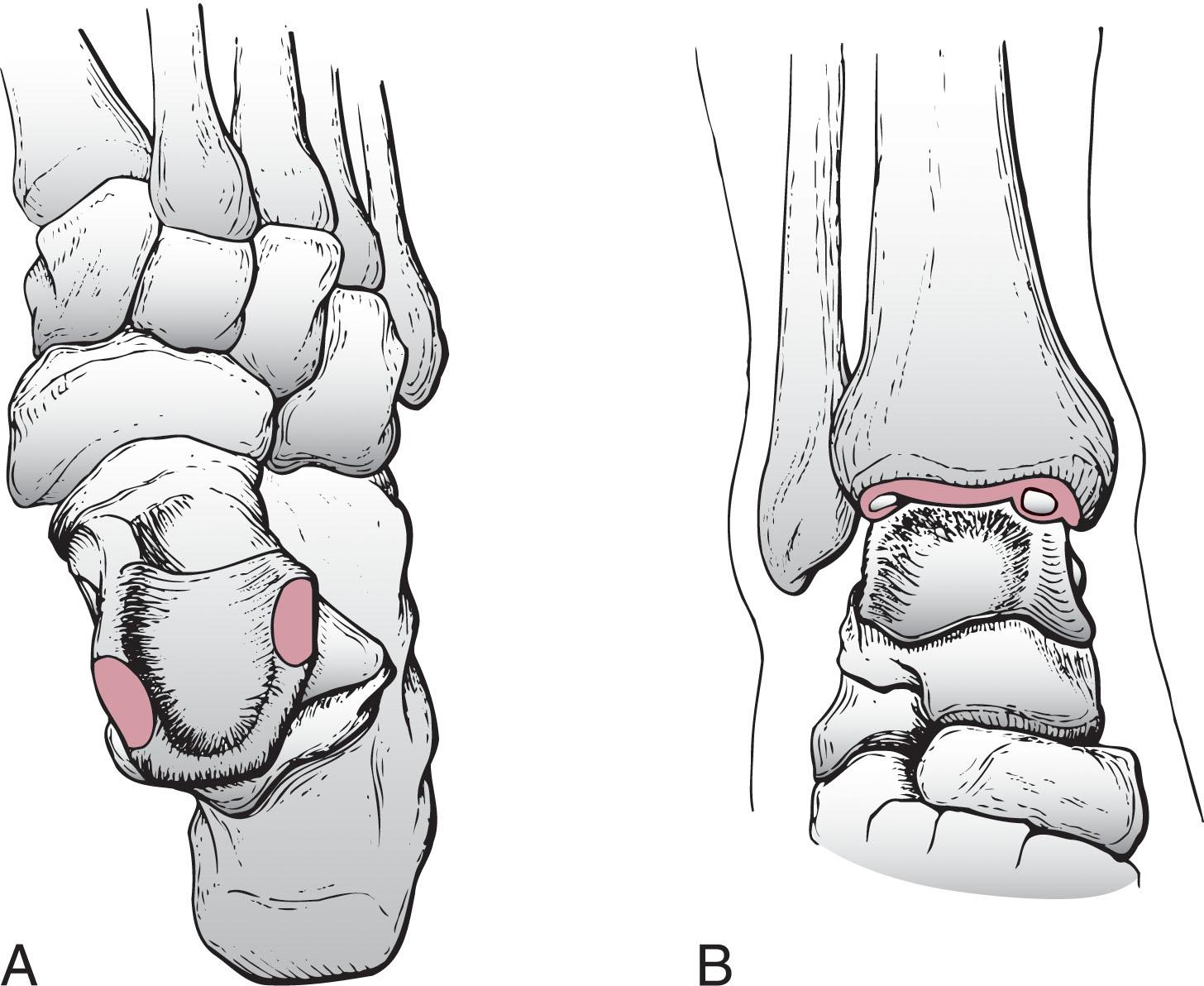
The dissimilar nature of medial and lateral OCL suggests either a different underlying etiology or unique local factors determines their characteristics. Canale and Belding reported that 17 of 17 (100%) lateral talar dome lesions were associated with trauma, whereas only 9 of 14 (64%) medial lesions had prior trauma. Medial lesions tend to be deeper, cup shaped, and more commonly develop into cystic lesions. Contact stress on the surrounding talar bone increases from a depth of between 1 and 3 mm, which may explain cyst formation in the larger medial lesions. On the other hand, lateral lesions are usually shallow, wafer shaped, often displaced, and they are more commonly associated with a traumatic injury.
Patient factors including ankle morphology, ligamentous integrity of the ankle, and bone quality also play a role in development of OLT. A CT study of 19 ankles found patients with medial OLT were more likely to have a medial malleolus that opens distally, and the anterior opening angle of the talus was large. Furthermore, ankle laxity based on radiographic talar tilt is inversely correlated with presence of OLT, suggesting joint restraint is a component of the traumatic etiology of lesions, while joint laxity may be protective. Patients with OLT compared with a cohort of ankle sprain patients without OLT were found to have lower 25(OH) vitamin D levels (31 ± 13 ng/mL vs. 37 ± 14 ng/mL), suggesting bone health may be a factor in patients that develop OLT.
Perhaps both trauma and avascular necrosis play a role in the pathogenesis of OLTs. The lesion may represent the chronic phase of a compressed talar dome fracture resulting from a single episode of trauma or repeated cumulative microtrauma. Similarly, in a person predisposed to talar dome ischemia, an osteonecrotic process could result in subchondral insufficiency fracture and bony collapse. That would explain the presence of subchondral cysts without overlying chondromalacia and osteochondral fragments without a history of trauma.
The incidence of OLT is 2.08 per 100,000 people per year based on a large health care system database review. OLT represented about 60% of all OCLs and were twice as common in men than women. The incidence of bilateral lesions is around 10% in a series of 526 consecutive OLT.
OLTs are frequently associated with trauma to the ankle. A meta-analysis of 2087 OLT found 78% were associated with prior trauma. A systematic review of ankle fracture studies through 2020 including six studies and 446 ankles found the pooled incidence of chondral or OLT by arthroscopy or MRI was 58% (95% CI 48%–71%). The reported rate based on CT scans after ankle fracture is lower (10%–14%) and is not related to the requirement of a relocation maneuver. The number of lesions is associated with the severity of fracture based on the Lauge-Hansen classification grade in some but not all studies. OLT discovered at 2 months after an ankle fracture were found to lead to decreasing functional scores over the subsequent 2 years, whereas fracture patients without OLT improved functional scores over 2 years. Consequently, they were found in 30 of 50 (60%) ankles undergoing arthroscopy for pain after ankle fracture.
Soft tissue trauma to the ankle also results in OLT. Patients undergoing lateral ligament repair were found to have OLT in 17% to 28% of cases. They were found in 21 of 100 (21%) ankles in a group of patients undergoing arthroscopy for chronic ankle pain after sprains, and 46% of OLT had an identified ankle ligament injury in a cohort of 90 OLT. Risk factors for OLT after lateral ankle ligament injury include male sex, older age, and duration of symptoms over 5 years.
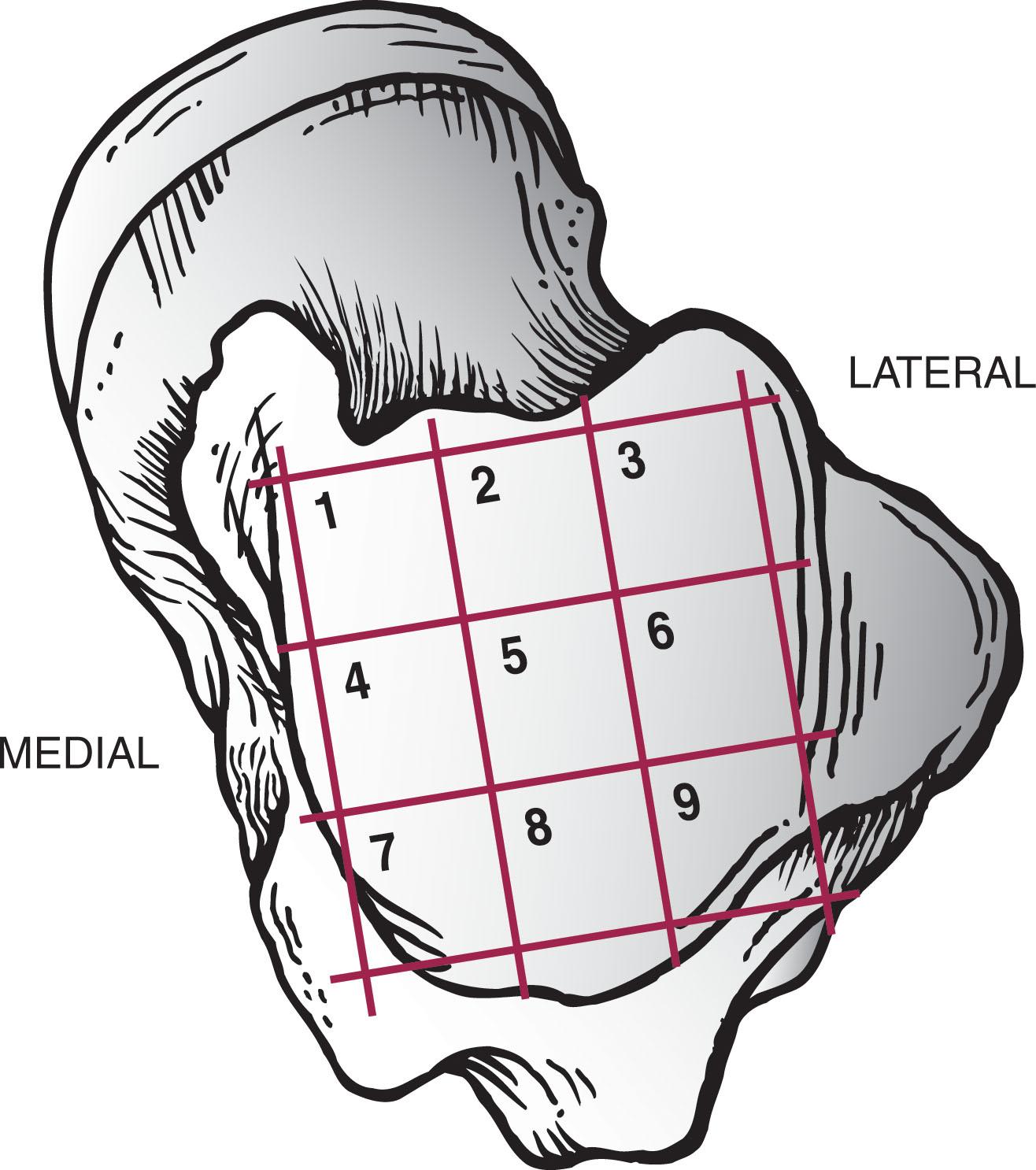
The incidence of OLT by location is best described based on a 3 × 3 grid system overlaid on the talar dome, first described by Elias et al ( Fig. 40-1 ). A meta-analysis of 51 articles and 2087 OLT focused on lesion location found 59% were in the centro-medial (31%) to posteromedial zones (28%) and 19.7% in the combined anterolateral and centro-lateral zones ( Fig. 40-2 ). A review of 428 ankle MRIs of patients with talar OCLs found that the mid-medial zone was the most common location (53%), and these lesions were the largest and deepest. The next most common zones were the mid-lateral zone (26%), posteromedial zone (7%), and posterolateral zone (5%). Other studies confirmed that medial lesions are more common than lateral lesions, overall representing about three fourths of all lesions.
Workup begins with a thorough history from the patient. While the diagnosis of OLT may be made during evaluation of an acute ankle injury, more often the patient presents with chronic ankle pain. They frequently report a remote lateral ankle sprain or other associated injuries, such as lower-extremity fractures or a fall. In certain patients, especially younger patients, no specific mechanism of injury is found. Risk factors for idiopathic osteonecrosis or accumulative microtrauma should be elicited. Symptoms frequently include deep ankle pain with weight bearing, recurrent ankle swelling, stiffness, weakness, giving way, mechanical catching, clicking, or locking. The differential diagnosis should include ankle soft tissue impingement syndrome, degenerative arthrosis, occult fracture, lateral ankle instability, infection, tarsal coalition, peroneal subluxation or tendinitis, subtalar dysfunction, and complex regional pain syndrome. At times, periodic follow-up examination and selective lidocaine injections may be useful in making the correct diagnosis.
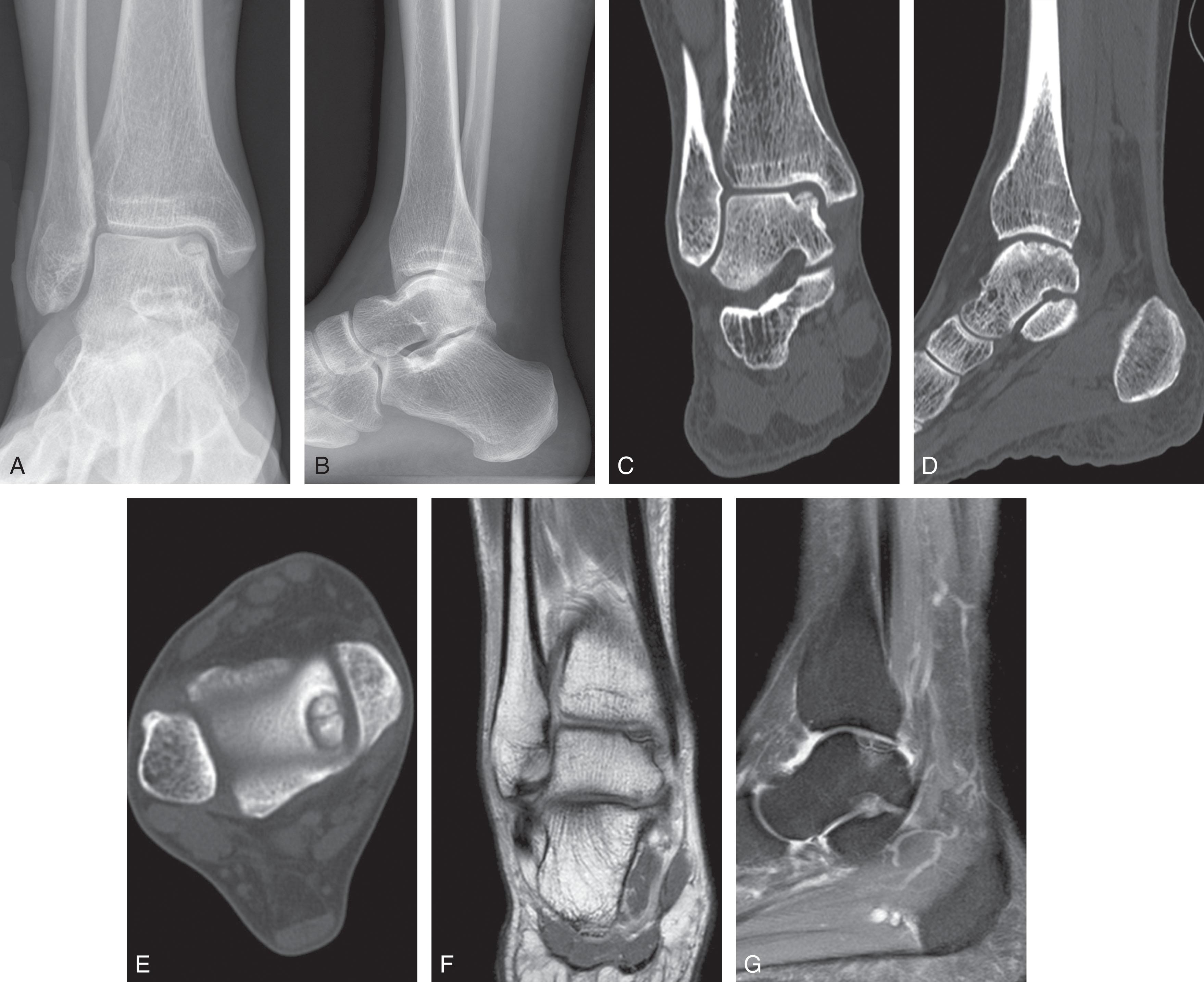
Diagnostic work-up continues with appropriate imaging. While diagnosis of OLT has traditionally been made using plain radiography, CT and MRI have improved our ability to detect lesions due to their improved sensitivity and positive predictive value ( Table 40-1 ). External traction applied at the time of MRI or intraarticular contrast during CT scan improves visibility of the cartilage surface when evaluating OLT.
| Modality | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Plain radiography | 0.59 | 0.91 | 0.70 | 0.86 |
| CT | 0.81 | 0.99 | 0.96 | 0.94 |
| MRI | 0.96 | 0.96 | 0.89 | 0.99 |
| Bone Scan | 0.94 | 0.76 | 0.56 | 0.94 |
| SPECT-CT | 1.0 | 0.57 | 0.66 | 1.0 |
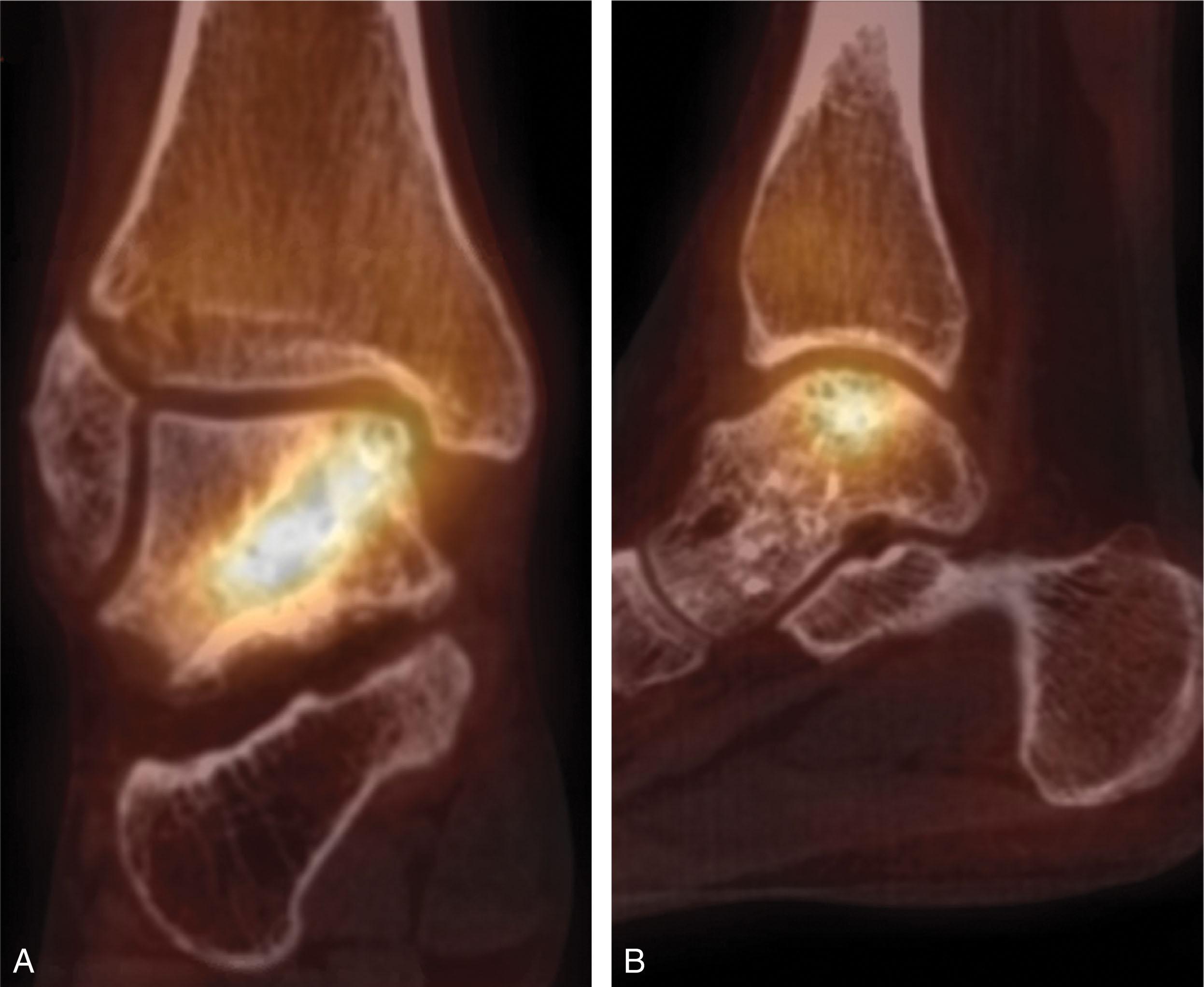
Staging of OLT follows imaging. In 1959, a staging system based on plain radiographs was described by Berndt and Harty based on stability and displacement of the fragment. It was later modified by Loomer and Scranton to include presence of a subchondral bone cysts ( Table 40-2 ). The classification has persisted since pain symptoms correlate with the Berndt and Harty stage, and the stage helps determine treatment. Three-dimensional imaging is typically obtained when OLT is seen on plain radiographs or suspected based on history and physical exam. Both CT and MRI staging systems have high intraobserver and interobserver reliability as well as excellent agreement between the modalities ( Fig. 40-3 ).
| Stage | Description |
|---|---|
| 0 | Not detected on radiographs |
| I | Focal compression of subchondral bone |
| II | Partially detached fragment |
| III | Fully detached fragment, nondisplaced |
| IV | Fully detached fragment, displaced |
| V | Radiolucent subchondral cyst |
Generally, when an OLT is seen on radiographs, a CT scan is obtained to gather additional information about its size, depth, and stability (see Fig. 40-3 ). This study is more useful than MRI in delineating the cyst margins from surrounding bone edema, as MRI overestimates OCL size in over 50% of cases. CT arthrography improves diagnosis of partial thickness cartilage defects difficult to detect on MRI. Furthermore, CT scan can help determine the viability of bone associated with an OLT and predict the quality of the overlying cartilage seen during arthroscopy. Ferkel et al as well as Loomer et al updated the Berndt and Harty classification using CT scans to consider the extent of osteonecrosis, subchondral cyst formation, and the separation of fragments that are difficult to see radiographically ( Fig. 40-4 ). Lopez used CT arthrography to classify OLT based on size, depth, and presence of overlying cartilage damage, though it is too new to assess its usefulness ( Table 40-3 ).
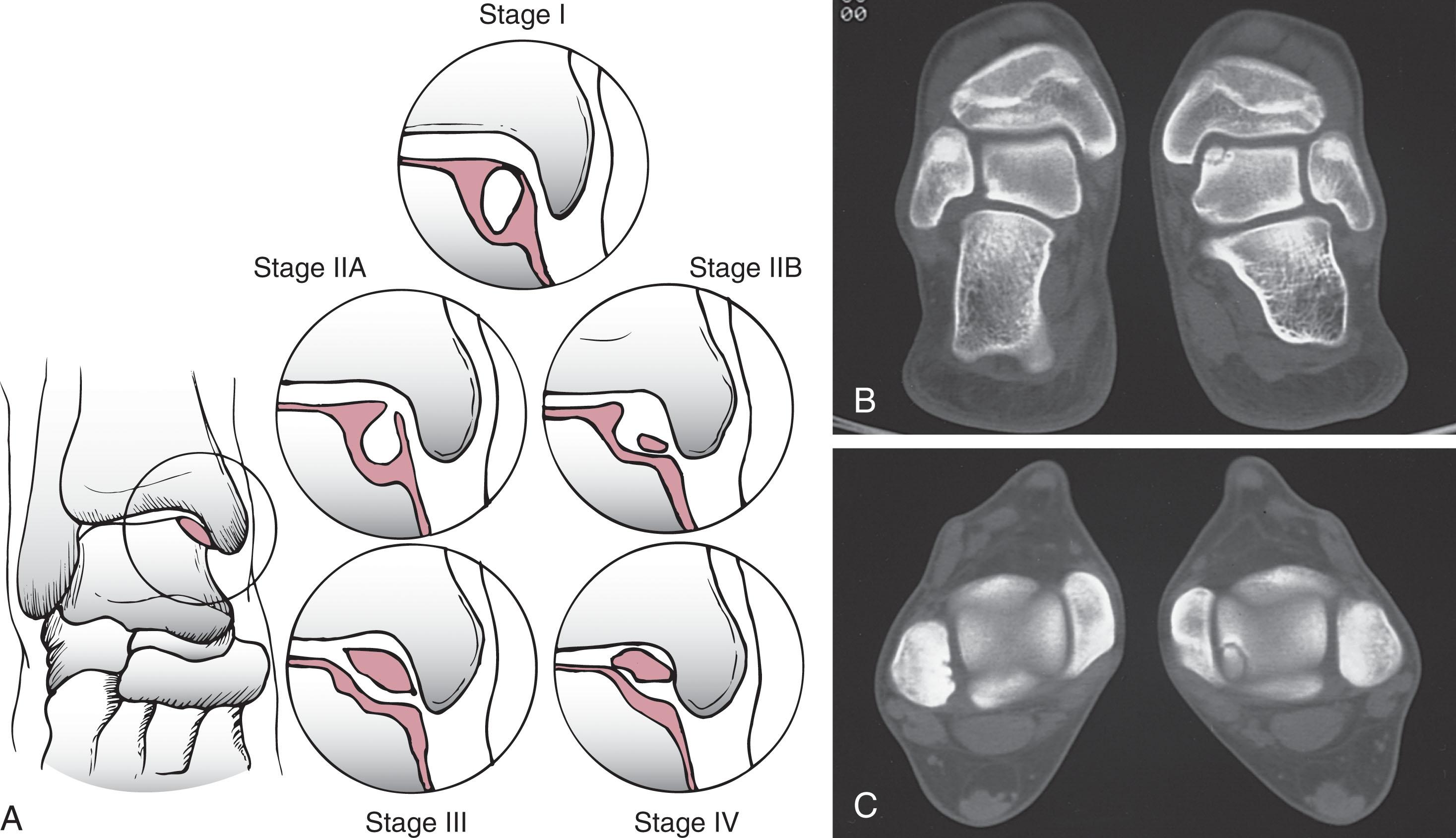
| Stage | Ferkel & Sgaglione | Lopes |
|---|---|---|
| I | Cystic lesion within dome of talus, with intact roof on all views | <10 mm diameter and <5 mm deep |
| II | ≥10 mm diameter and/or ≥5 mm deep, intact cartilage | |
| IIA | Cystic lesion with communication to talar dome surface | |
| IIB | Open articular surface lesion with overlying nondisplaced fragment | |
| III | Nondisplaced lesion with lucency | ≥10 mm diameter and/or ≥5 mm deep, damaged cartilage |
| IV | Displaced fragment |
MRI is also used to stage OLTs ( Table 40-4 , Fig. 40-3 ). MRI is the preferred imaging modality for evaluation of undiagnosed chronic ankle pain and detection of suspected OLT not seen on initial plain radiographs due to its high sensitivity and the additional information gained by imaging the surrounding soft tissue structures (see Table 40-1 ). Quantifiable characteristics of the high signal line under an OCL by T2 MRI can help differentiate unstable lesions, cysts, and bone resorption. Other studies suggest that determining lesion stability by MRI may be inaccurate, particularly in skeletally immature patients who tend to have more stable lesions than adults.
| Stage | Dipaola | Anderson | Hepple |
|---|---|---|---|
| Stage I | Thickening of articular cartilage and low signal change | Subchondral trabecular compression Plain radiograph normal, positive bone scan Marrow edema on MRI | Articular cartilage damage only |
| Stage II | Cartilage breach with low signal rim behind the fragment | Incomplete separation of fragment | |
| Stage IIA | Incomplete separation with formation of subchondral cyst | Cartilage injury with underlying fracture and bone edema | |
| Stage IIB | Cartilage injury with underlying fracture without bone edema | ||
| Stage III | Cartilage breach with high signal rim behind the fragment | Detached, nondisplaced fragment, with synovial fluid around fragment | Detached, nondisplaced fragment |
| Stage IV | Loose body | Displaced fragment | Detached displaced fragment |
| Stage V | Bone cyst |
Nuclear medicine studies also have a high sensitivity for detecting OLT, though their use has largely been supplanted by MRI to diagnose chronic ankle pain with normal radiographs. Sensitivity of bone scan for detecting OLT is as high as 99%. SPECT-CT adds the specificity of CT scan to the sensitivity of bone scan and has a high intraobserver and interobserver reliability when used to diagnose OLT ( Fig. 40-5 ). It may identify lesions not seen on MRI and has potential to help assess subchondral bone involvement.
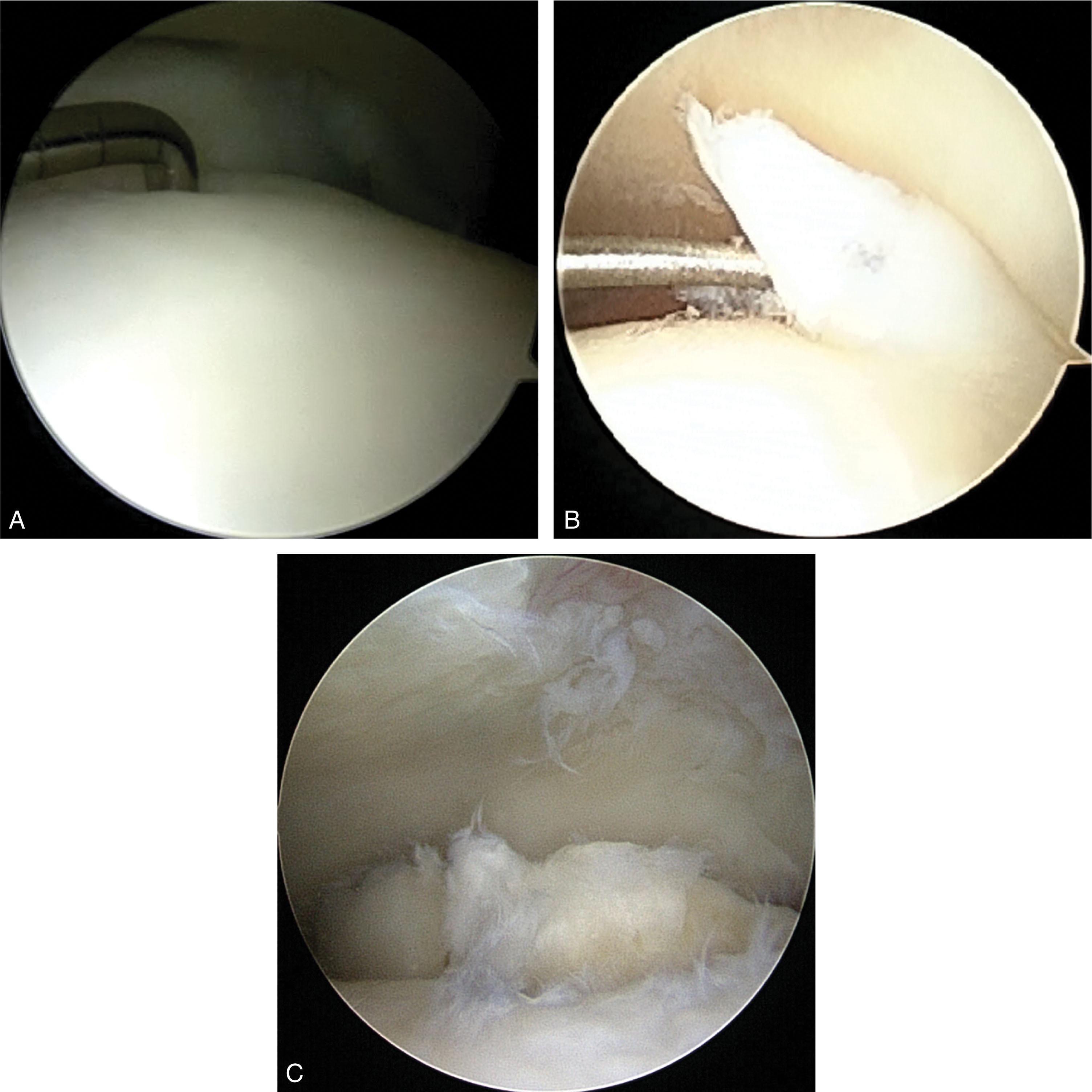
Ankle arthroscopy is the preferred surgical method for the definitive staging and treatment of OLTs since MRI grading only matches arthroscopic findings in approximately 70% of OLT. Visualization and access to difficult-to-reach lesions are now routine with the use of small joint arthroscopic instrumentation, distraction devices, and accessory portals. Cheng et al has validated an arthroscopic classification system based on blinded independent review of 100 arthroscopy videos ( Table 40-5 ). The International Cartilage Repair Society also has an arthroscopic grading system for OCLs ( Fig. 40-6 ).
| Grade | Ferkel & Cheng | Grade | ICRS |
|---|---|---|---|
| A | Smooth, intact, but soft or ballotable | OCD I | Stable, continuity: Softened area covered by intact cartilage |
| B | Rough surface | ||
| C | Fibrillations/fissures | ||
| D | Flap present or bone exposed | OCD II | Partial discontinuity, stable on probing |
| E | Loose, nondisplaced fragment | OCD III | Complete discontinuity, not dislocated |
| F | Displaced fragments | OCD IV | Dislocated fragment, loose within the bed or empty defect <10 mm in depth |
| OCD IVB | Dislocated fragment, loose within the bed or empty defect >10 mm in depth |
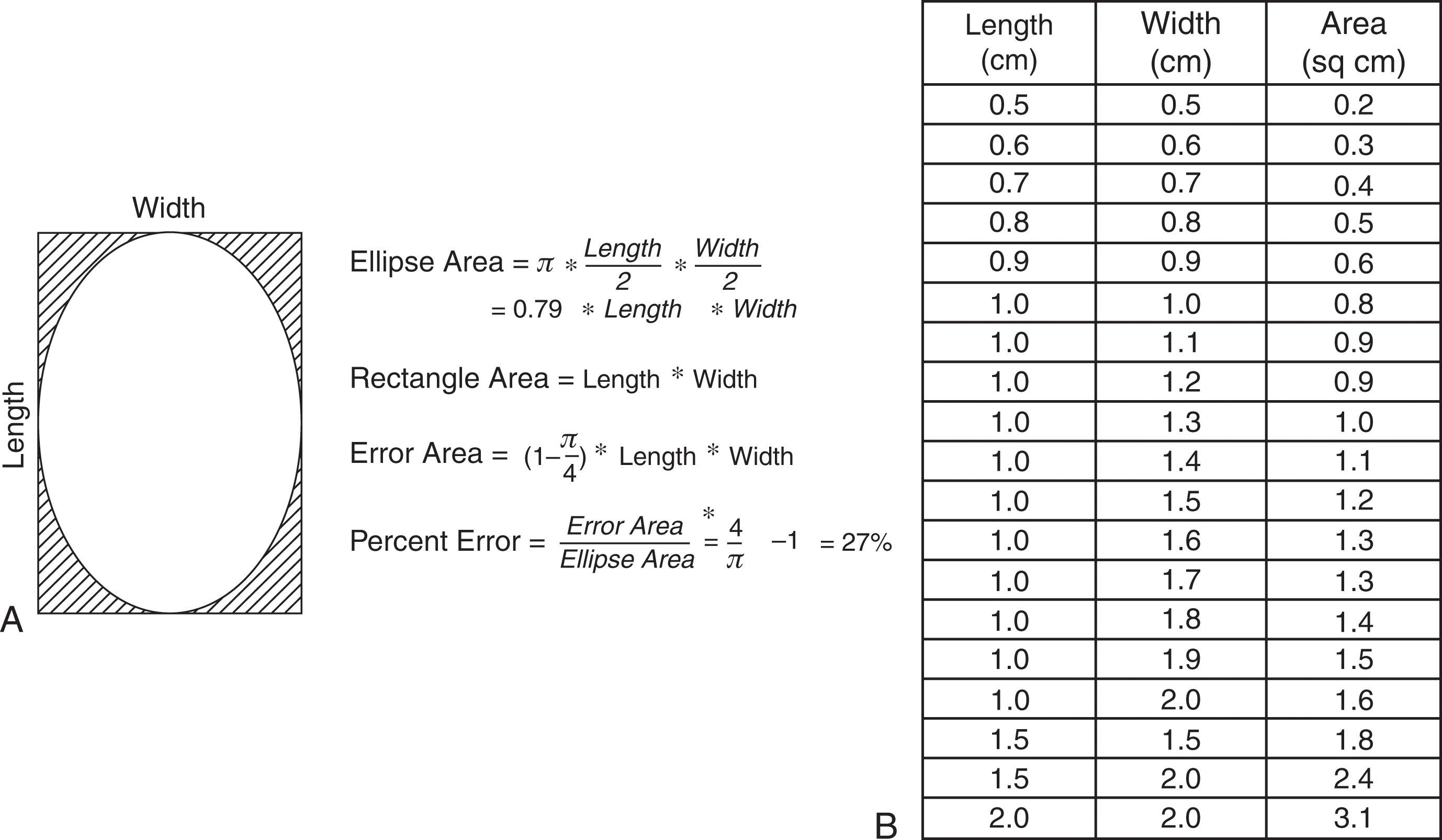
Describing the clinical size of a lesion is most accurate when largest width, length, and depth are included. Studies that describe lesions size by area should include the method of calculation. Since most lesions are elliptical rather than rectangular in cross section, we prefer calculating area by π × maximum width × maximum length / 4. Using the simpler formula, maximum width × maximum length, will overestimate cross sectional area by 27% ( Fig. 40-7 ).
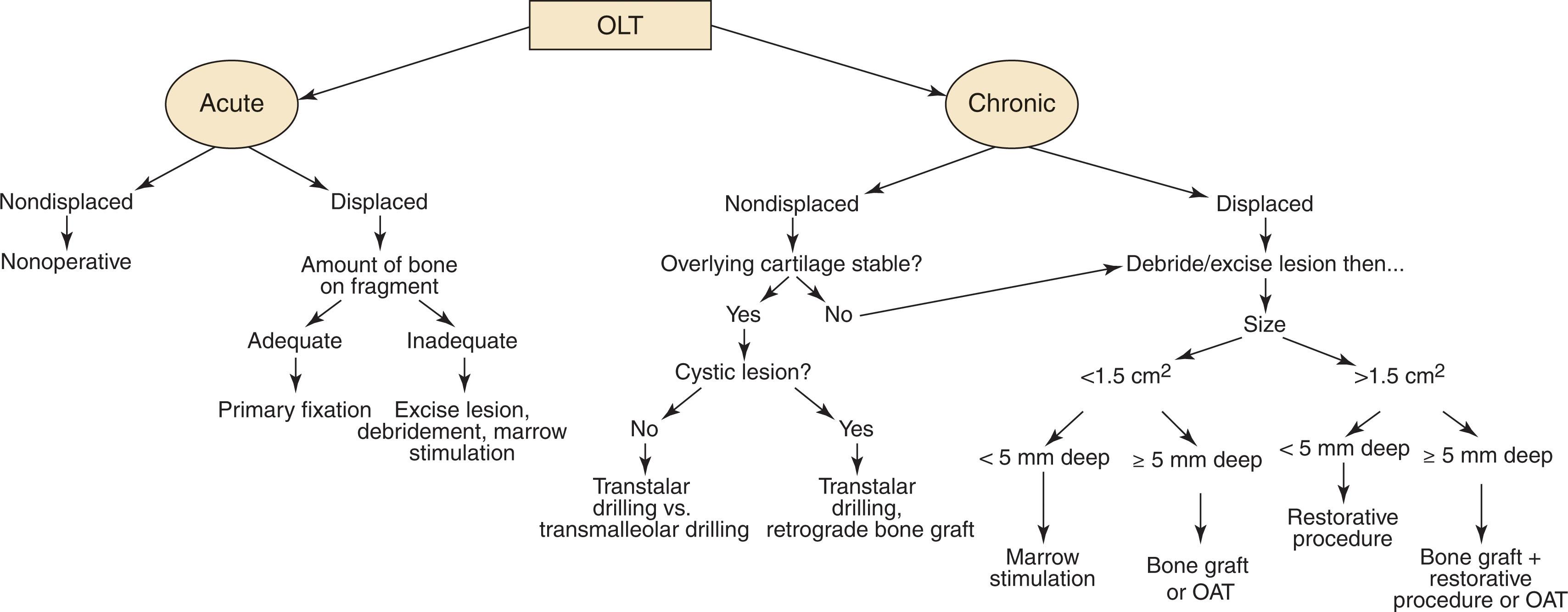
There are several important variables to consider when selecting an appropriate treatment for an OCL. Decision making regarding treatment of OLT depends on the severity and duration of symptoms, response to conservative care, and the size and stability of the lesion ( Fig. 40-8 ). Treatment selection for chronic lesions can be challenging because there are few high-level or comparison studies to guide decision making, and the variety of factors taken into account makes it difficult to compare most clinical series. Patients with high-grade bicameral “kissing” lesions do not do as well with treatments strategies for OLT and should be considered for ankle osteoarthritis treatment protocols.
Nonoperative management of OLT typically is reserved for nondisplaced or minimally symptomatic lesions. It can be challenging to determine if a nondisplaced lesion is an acute injury or incidental finding of a stable chronic lesion. No clear consensus on the ideal nonoperative regimen has been reached. For acute injuries, a period of non–weight bearing in a boot to allow range of motion is recommended, though some recommend a longer period of casting for unstable fragments. Most authors recommend a trial period ranging from 3 to 6 months.
Surgery is recommended for acute, displaced OLT, as well as for those whose symptoms are not resolved by conservative treatment. Although MRI or CT may provide clues about a given lesion, arthroscopic evaluation allowing probing of the lesion to determine its stability and the quality of the underlying bone provides the most accurate information. Therefore a detailed and thorough arthroscopic assessment of the lesion must be performed to select the ideal treatment, and surgical plans need to be flexible if intraoperative findings are not what was expected based on preoperative imaging.
Surgical treatment options for OLT include debridement, reparative techniques, restorative techniques, and techniques that replace the damaged OCL. Palliative measures include debridement and lavage. Primary repair is recommended for an acute traumatic OLT with adequate bone attached to the osteochondral fragment, typically over 3 mm thick. Secondary repair techniques involving elevating an osteochondral flap, drilling and autografting the underlying bony bed, and fixation with a bioabsorbable implant (Lift Debride Fill & Fix) have also been described.
Other techniques seek to restore cartilage in the damaged area through local cellular infiltration. Traditional techniques stimulate cartilage repair by creating bleeding from the underlying bone marrow, which fills the defect, and promotes creation of fibrocartilage. These treatments include abrasion arthroplasty, microfracture, and drilling techniques. Microfracture has been augmented with membranes to protect the fragile clot, a procedure termed autologous matrix induced chondrogenesis (AMIC). The membranes are made from a variety of biocompatible materials including hyaluronic acid (HA), chitosan, and collagen. Application of extracellular matrix cartilage allograft (EMCA) to the defect after microfracture is used with hopes of providing a scaffold for improved cartilage growth.
Since the turn of the twenty-first century, there has been a surge in interest in restorative techniques for OLT using cells derived from beyond the local bone marrow. These include cells derived from cultured autologous chondrocytes, concentrated bone marrow aspirate (cBMA), adipose derived mesenchymal stem cells, and juvenile particulated cartilage allograft. The cells may be secured with a periosteal autograft (ACI), imbedded in a matrix or membrane (MACI), imbedded in a fibrin gel, covered with collagen, or a variety of other options.
Another treatment strategy for OLT involves replacing the damaged osteochondral unit entirely, either with bone or bone and cartilage. Bone grafting techniques, osteochondral autologous transfer (OAT; mosaicplasty), and fresh osteochondral allograft have all been used successfully. Novel synthetic and xenograft strategies have also been proposed.
Adjuvant treatments to these techniques have also been recommended. Injections of HA or platelet-rich plasma (PRP) have been proposed to stimulate healing and cartilage restoration. Externally applied pulsed electromagnetic field application in the postoperative period has been studied as well, showing improvement in patient outcome scores in randomized nonblinded studies but not in randomized blinded studies.
An algorithm for helping make treatment decision is shown in Fig. 40-8 . If the lesion is acute and reparable, a primary repair is performed. If the cartilage surface is intact, retrograde treatment is used. If the overlying chondral lesion is found to be unstable, debride the unstable cartilage back to stable vertical margins and determine the true lesion size. For chronic lesions less than 1.5 cm 2 in size (approximately 1 × 2 cm) after debridement with intact or minimal bone loss, marrow stimulation techniques produce favorable results.
For chronic lesions greater than 1.5 cm in size, treatment selection is controversial. If the lesion is less than 5 to 7 mm deep, an initial marrow stimulation procedure may be considered; however, these have a higher percentage of unfavorable results than smaller lesions based on low level-of-evidence studies. Alternatively, a cartilage restoration procedure can be considered, such as microfracture plus application of morselized cartilage allograft, EMCA, or autologous chondrocyte implantation (ACI). For large, cystic lesions deeper than 5 to 7 mm, a procedure that restores bone and cartilage is recommended, such as, autologous bone grafting plus morselized cartilage allograft application or osteochondral transfer. Some surgeons prefer OAT because it provides type II hyaline cartilage with attached bone to fill the cystic defect and aid in graft incorporation. In the revision setting, a cartilage restoration procedure with or without bone grafting, depending on the depth of the lesion, is used.
Comfort with a variety of surgical approaches allows the surgeon adequate exposure to the pathology while minimizing tissue dissection. Extensile ankle arthrotomy or malleolar osteotomy require significant tissue trauma and may be associated with nonunion or malunion of the malleoli, postoperative joint stiffness, prolonged rehabilitation time, suboptimal cosmetic appearance, and inadequate visualization of the posterior talar dome lesion. They have largely given way to ankle arthroscopy for both evaluation and treatment of OLTs due to superior visualization of the talar dome, improved access to the lesion, and reduced morbidity. Mini-open approaches and occasional use of modest debridement of the tibial plafond overlying the lesion are required for certain cartilage restoration techniques. For larger autograft or allograft transfers, formal malleolar or tibial plafond osteotomy may still be required. Prone Achilles splitting posterior arthrotomy has been proposed for posterior lesions. Retrograde techniques combine an ankle arthroscopy with an open approach to the sinus tarsi. For some patients, a closed percutaneous technique using fluoroscopy may be considered.
Ankle instability and malalignment should be addressed at the time of treating OLT. Results if instability is addressed with ankle stabilization should be expected to match results with isolated OLT treatment without ankle instability, though a higher proportion of AOFAS scores in the fair to poor range (<80) has been reported in chronically unstable ankles after microfracture and ligament stabilization. Distal tibial malalignment, if felt to lead to overload of the OCL, may be addressed with supramalleolar osteotomy.
Small (<0.50 cm ) chronic, symptomatic lesions and partial thickness cartilage defects may benefit from arthroscopic lavage and debridement, which makes no attempt to repair or replace damaged articular cartilage. However, with more aggressive early weight-bearing protocols and improved outcomes after marrow-stimulating cartilage regeneration techniques, debridement alone should be reserved for partial thickness lesions or older and lower demand patients. Lavage alone has yielded variable short-term results and no demonstrable long-term benefits.
Arthroscopic debridement removes loose bodies, rough and irregular cartilage surfaces, and catabolic cytokines from the ankle, creating a more hospitable environment. Using standard arthroscopic portals and small joint tools such as biters, graspers, and shavers, loose cartilage or osteochondral flaps are removed from the joint until a smooth, stable cartilage periphery is achieved ( Fig. 40-9 ). The patient may weight bear in a walking boot immediately and is allowed to rehabilitate rapidly once the arthroscopic portals heal, typically after 1 week.
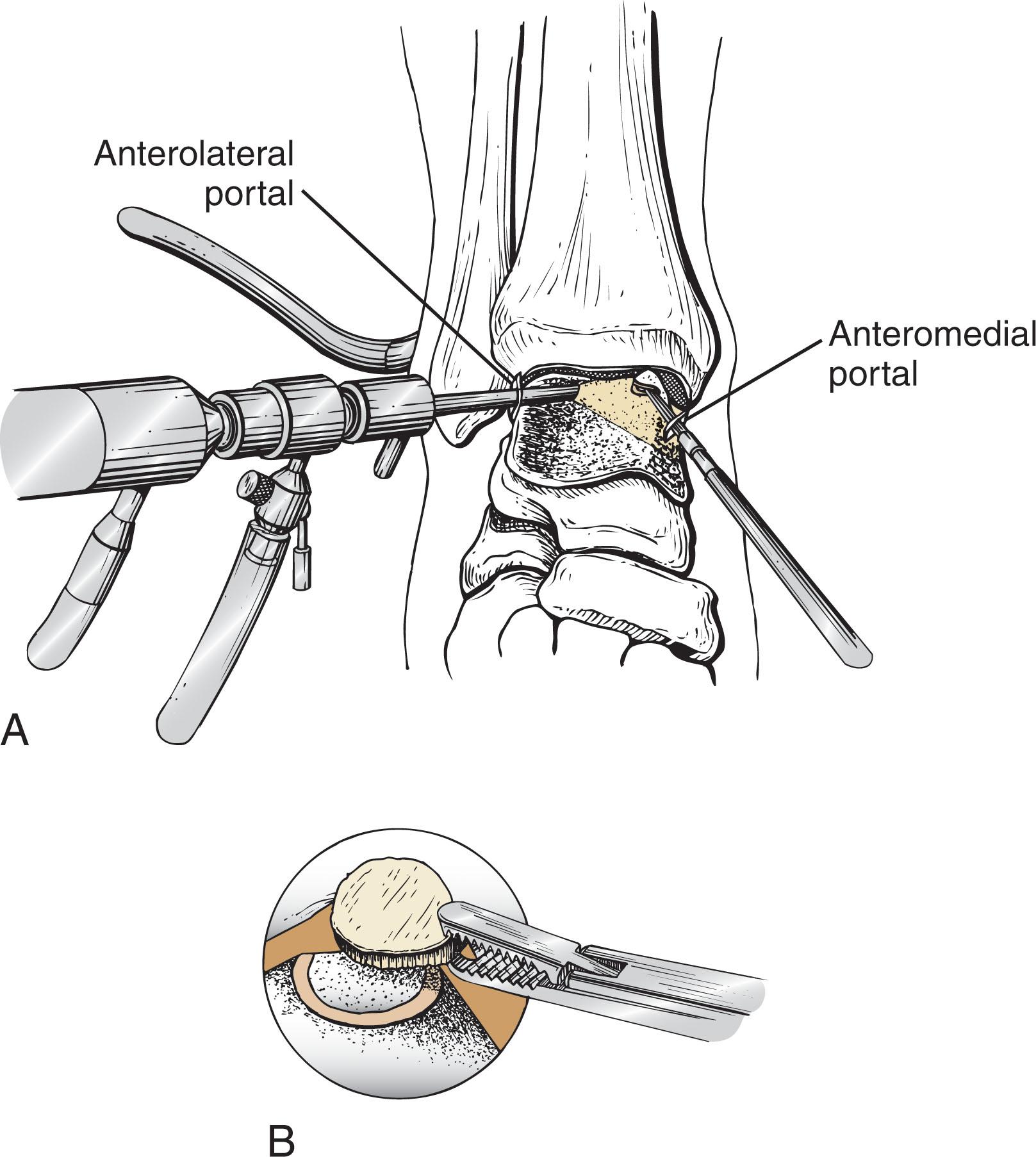
If the overlying chondral surface of an OLT is intact and stable, but a subchondral depression or cyst exists, the subchondral bone should be approached in a retrograde manner, as opposed to drilling through the healthy overlying cartilage. Because the subchondral bone cannot be directly visualized, the surgeon needs to rely on preoperative imaging (CT scan) to determine the extent of the lesion ( Fig. 40-10 ). Most of these lesions are medial, allowing a small lateral sinus tarsi approach to access the nonarticular inferolateral talus. Lesions should be reamed and curetted using a combined arthroscopic/fluoroscopic trans-talar technique, and bone graft should be inserted to restore a stable subchondral plate.
The technique is as follows.
After arthroscopic examination, a small incision is made inferior to the lateral malleolus for a medial lesion, or inferior to the medial malleolus for lateral lesions. The inferior lateral (or medial) aspect of the talus is identified away from the articular cartilage of the posterior facet subtalar joint.
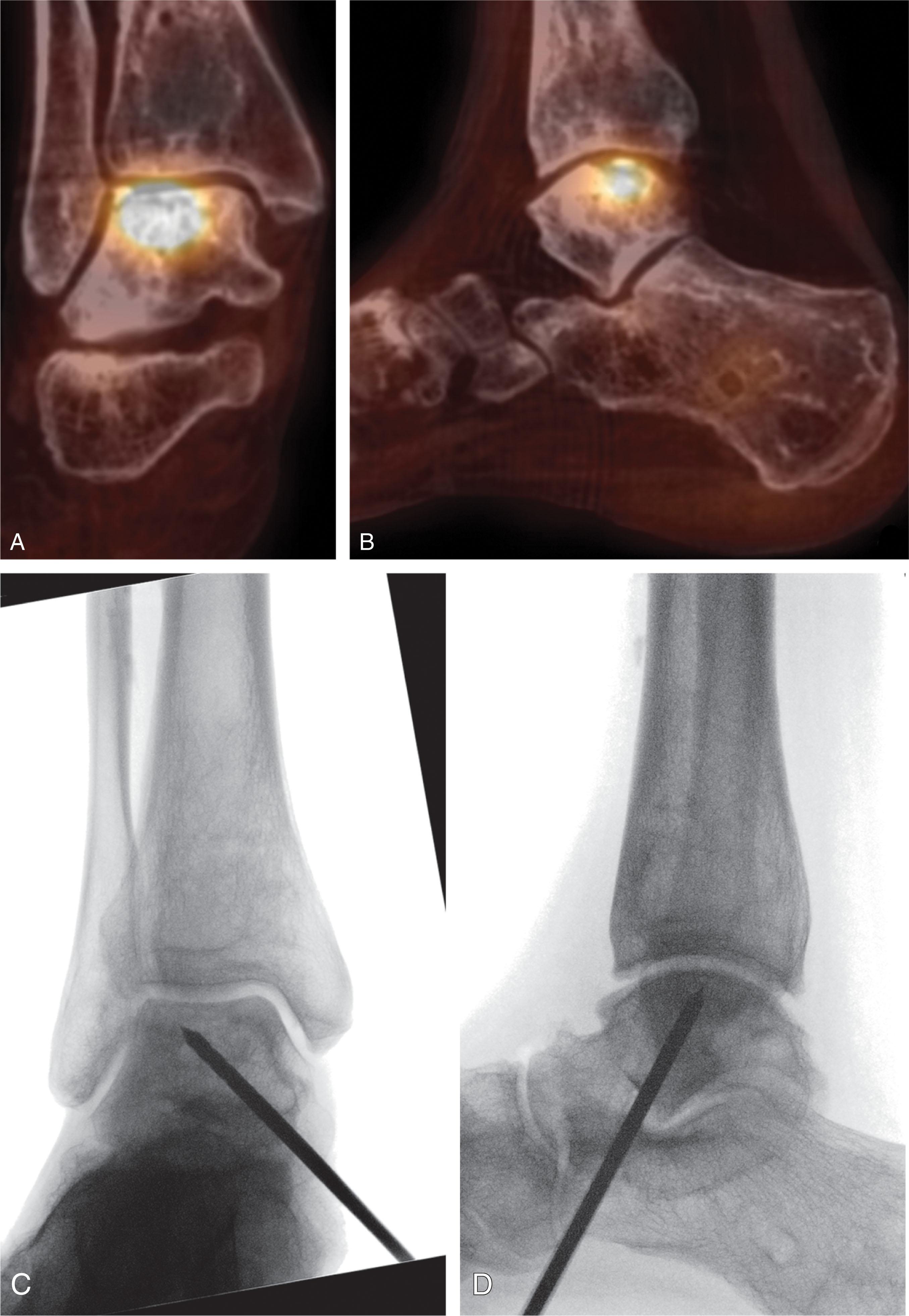
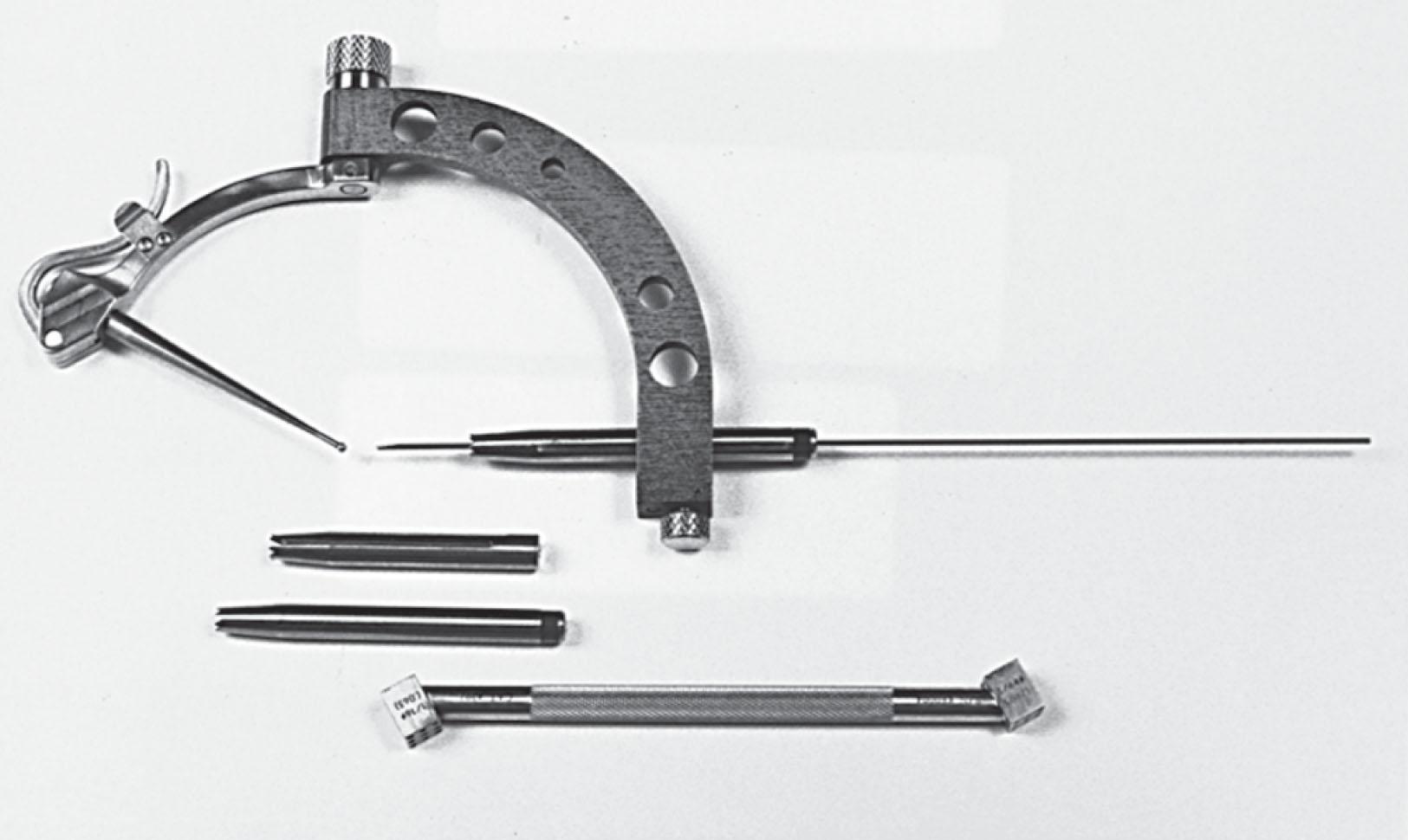
Using a hinged small joint pin guide ( Fig. 40-11 ), a guide wire is passed from the inferior talus, across the body of the talus, to a few millimeters below the cartilage surface within the OLT. Fluoroscopy is used to double check pin placement.
A cannulated drill is used to create a trans-talar hole to the bone inferior to the OCL, large enough to allow a small curette to reach the lesion.
The lesion is curetted from underneath, keeping the overlying healthy bone and cartilage intact.
Trans-talar bone grafting is applied from deep to superficial. Bone graft is obtained from the calcaneus or distal tibia through a small incision and bone graft harvesting trephines. Alternatively, an injectable tricalcium phosphate graft may be used.
Patients are allowed to weight bear in a protective boot for 6 weeks.
Acute osteochondral fractures of the talus may be arthroscopically reduced and stabilized if there is adequate bone on the undersurface of the lesion to allow fixation ( Fig. 40-12 ). While most acute nondisplaced lesions can be treated conservatively, displaced lesions require surgical reduction and fixation to restore articular cartilage congruency. The osteochondral fragment should be carefully inspected to determine the adequacy of the connected bone for reattachment. If sufficient bone (≥3 mm) remains attached to the fragment, all attempts should be made to anatomically reduce the fragment, particularly in younger patients. However, if the fragment is primarily chondral in nature, excision is recommended, with subsequent debridement followed by marrow stimulation. The lateral inverted (upside-down) osteochondral fractures of the talus (LIFT lesions) in particular should be repaired if recognized early ( Fig. 40-13 ).
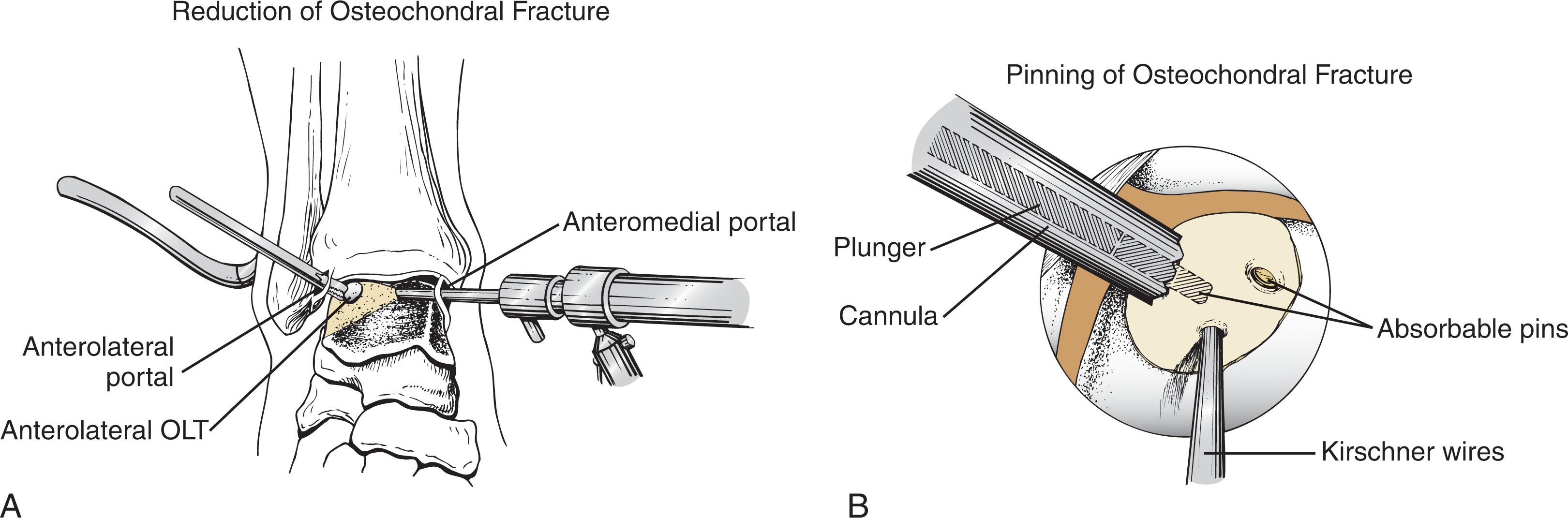
The lift, drill, fill, and fix (LDFF) method of primary repair may allow more chronic lesions to be repaired successfully. With this technique, lesions greater than 1.0 cm in diameter and 3 mm in depth are elevated, the underlying bone is debrided, small drill holes are created into the surrounding metaphyseal bone, a small amount of autologous bone graft is packed into the defect, and the elevated fragment is reduced and fixed in a manner similar to an acute lesion.
Fixation devices include permanent or bioabsorbable low-profile pins, bioabsorbable screws, and headless metal screws. Kirschner wires (K-wires) may be used for temporary fixation. Small bone autograft pegs can be fashioned from the distal tibia to be used for fixation. A small headed screw can be used but will likely require a second procedure for removal.
The technique is as follows.
Acute lesions should be arthroscopically inspected and palpated with a small-joint probe to assess the extent of the overlying cartilaginous softening and blistering and the quality of the underlying bone. Acute OLT with adequate overlying cartilage and subchondral bone should be repaired.
Nondisplaced lesions are repaired in situ. A probe or grasper is used to reduce displaced lesions. If this cannot be achieved arthroscopically, the closest portal is extended to create a mini open window to the lesion.
Temporary fixation with (K-wires) may be required.
Definitive fixation is achieved with absorbable pins or screws to allow accurate reduction and stability, permitting early motion, and avoiding additional surgery for pin removal.
After debridement, a knife blade, biter, or grasper may be used to excise loose chondral fragments and areas of surface blistering. Caution must be exercised with this technique not to damage viable cartilage or displace the repair. The debridement should be limited to significant chondral flaps, maintaining softened but intact cartilage. A grasper is then used to remove any loose chondral pieces. Thoroughly lavage the joint to avoid leaving loose bodies.
The patient remains non–weight bearing in a boot for 6 weeks followed by progressive weight bearing for another 4 to 6 weeks. Early motion is allowed once the incisions heal if the repair is stable. Any hardware that is not buried beneath the chondral surface should be removed prior to weight bearing.
Abrasion, drilling, and microfracture techniques rely on the reparative properties of marrow chondroprogenitor cells released from the underlying cancellous bone ( Fig. 40-14 ). These stem cells populate the fibrin clot that fills the talar defect after surgery and initially develop into a repair tissue composed of type II collagen. This transforms to predominantly type I and III collagen as proteoglycans are depleted and chondrocytes perish, raising concerns about long-term durability. Marrow-stimulating restorative techniques become less effective as lesion size increases and are not recommended in large cartilage defects (>1.5 cm 2 ) and lesions with large or deep cysts of 5 to 7 mm or greater.
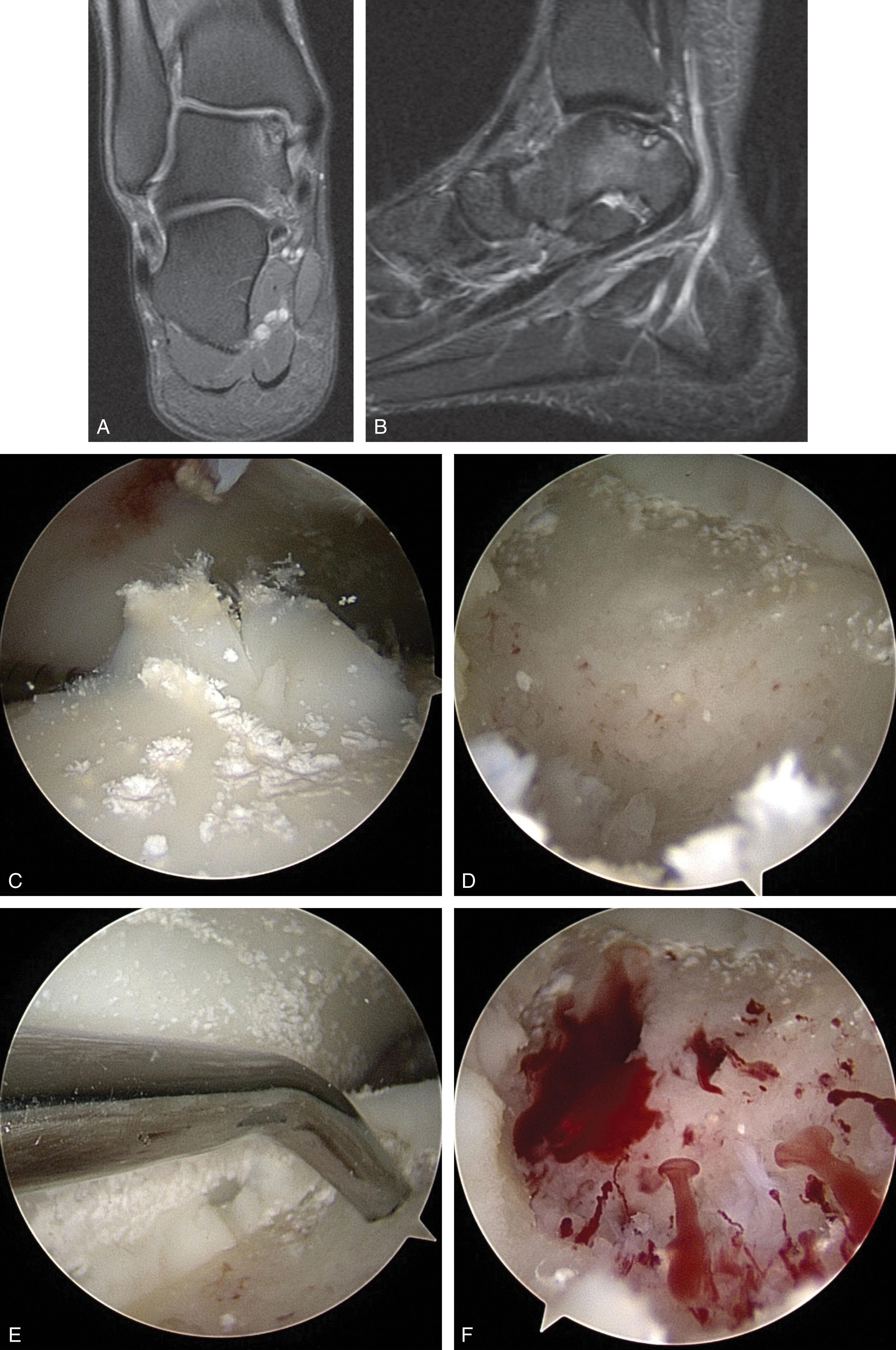
The technique is as follows.
Lesions should be arthroscopically inspected and palpated with a small-joint probe to assess the extent of the overlying cartilaginous damage and the quality of the underlying bone. Most lesions can be reached using the standard anteromedial and anterolateral portals. Far posterior, posterior-central, and posterior lesions in tight ankles may require an accessory posterolateral and/or posteromedial portal.
Any nonadherent cartilage, or cartilage with loss of >50% of its thickness, should be debrided. Flaps are gently elevated to avoid delaminating otherwise healthy cartilage. The edge of a small, sharp curette or ring curette can be used to create vertical walls at the interface between damaged and healthy cartilage ( Fig. 40-15 ).
The bone at the base of the lesion is probed to assess its integrity. If there is well-corticated subchondral bone, a curette is used to remove the calcified cartilage layer. Cystic lesions are debrided of nonviable or very soft bone until a base of healthy bleeding bone is created.
The length and width of the lesion are measured using a probe of known length. The depth is measured with a probe or angled curette ( Fig. 40-16 ).
Small joint microfracture awls of various angles are used to create numerous punctate holes 3 to 4 mm apart through the subchondral bone into bleeding bone marrow ( Fig. 40-17 ). At times, a small fat bubble is released, suggesting the marrow layer has been reached.
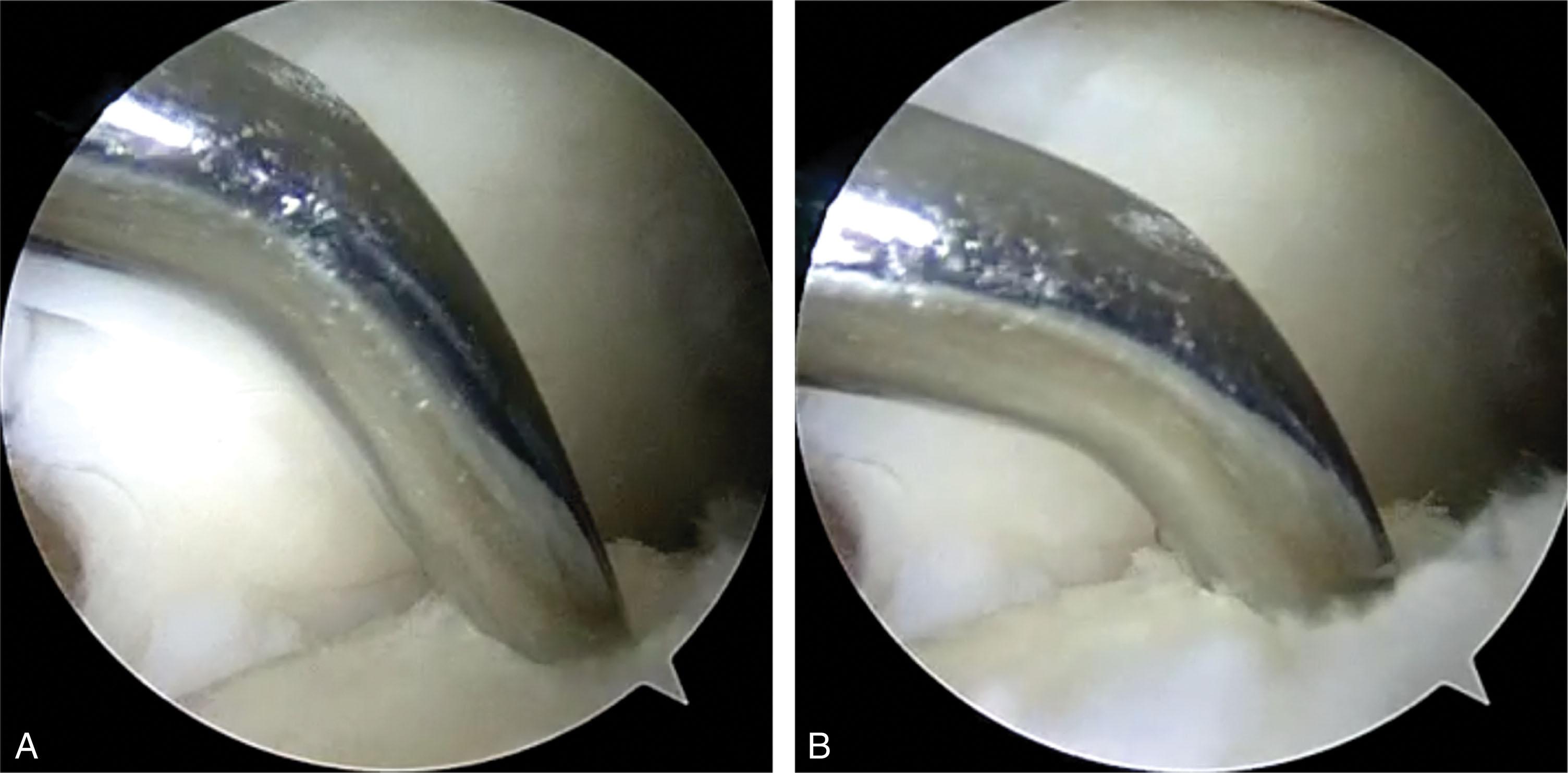
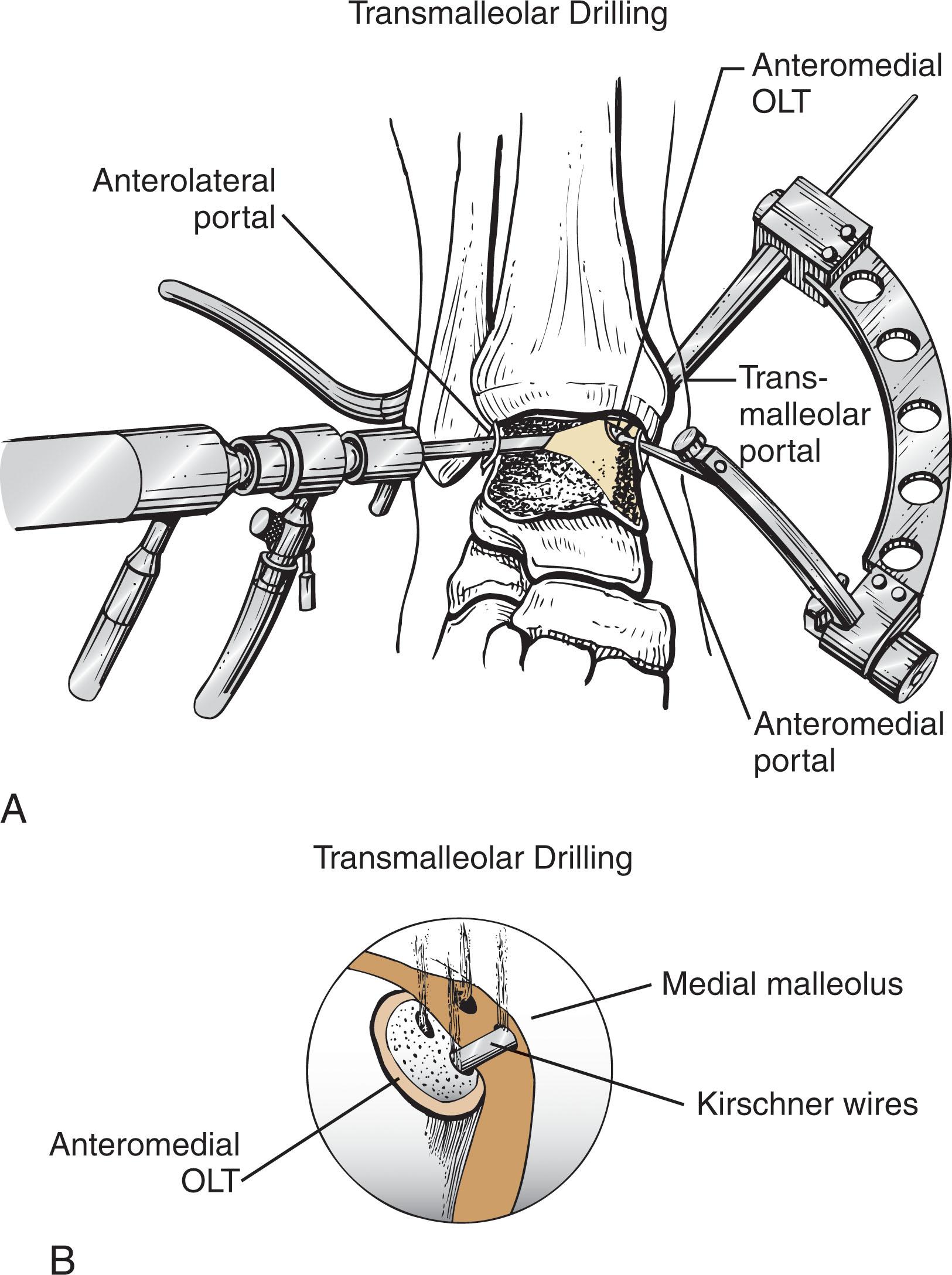
Drilling may be considered for lesions that are difficult to access with microfracture awls. A small joint drill guide is used to triangulate wire placement, and a 1.1- to 1.6-mm K-wires is drilled through the medial malleolus or placed percutaneously ( Fig. 40-18 ). Numerous channels are drilled to a depth of 10 mm 3 to 4 mm apart, facilitated by dorsiflexion and plantarflexing the ankle.
The tourniquet is deflated and fluid pressure reduced until bleeding from the holes is verified ( Fig. 40-14F ).
After surgery, patients remain non–weight bearing in a boot for 7 to 10 days until the incisions are healed. They then complete 6 weeks in the boot allowing full weight-bearing and non–weight-bearing ankle range of motion exercises.
The ideal treatment for large (>1.5 cm 2 ) OLTs, lesions with bone loss (depth >5–7 mm), and failed OLTs continues to evolve. Innovative methods of bringing cells to OLT continue to evolve, with the goal of restoring organized, durable hyaline cartilage with viable chondrocytes. Cartilage cell–based restorative techniques have the potential to restore the joint congruity with a hyaline-like cartilage surface but lacking normal cartilage extracellular architecture. Options include ACI, matrix associated chondrocyte implantation (MACI) and its different variations, concentrated bone marrow aspiration (cBMA) application, and particulated cartilage allograft application. The ideal patient is younger, has no malalignment, degenerative joint disease, or instability of the joint. The procedures are not recommended for bipolar (kissing) lesions involving both the tibia and talus.
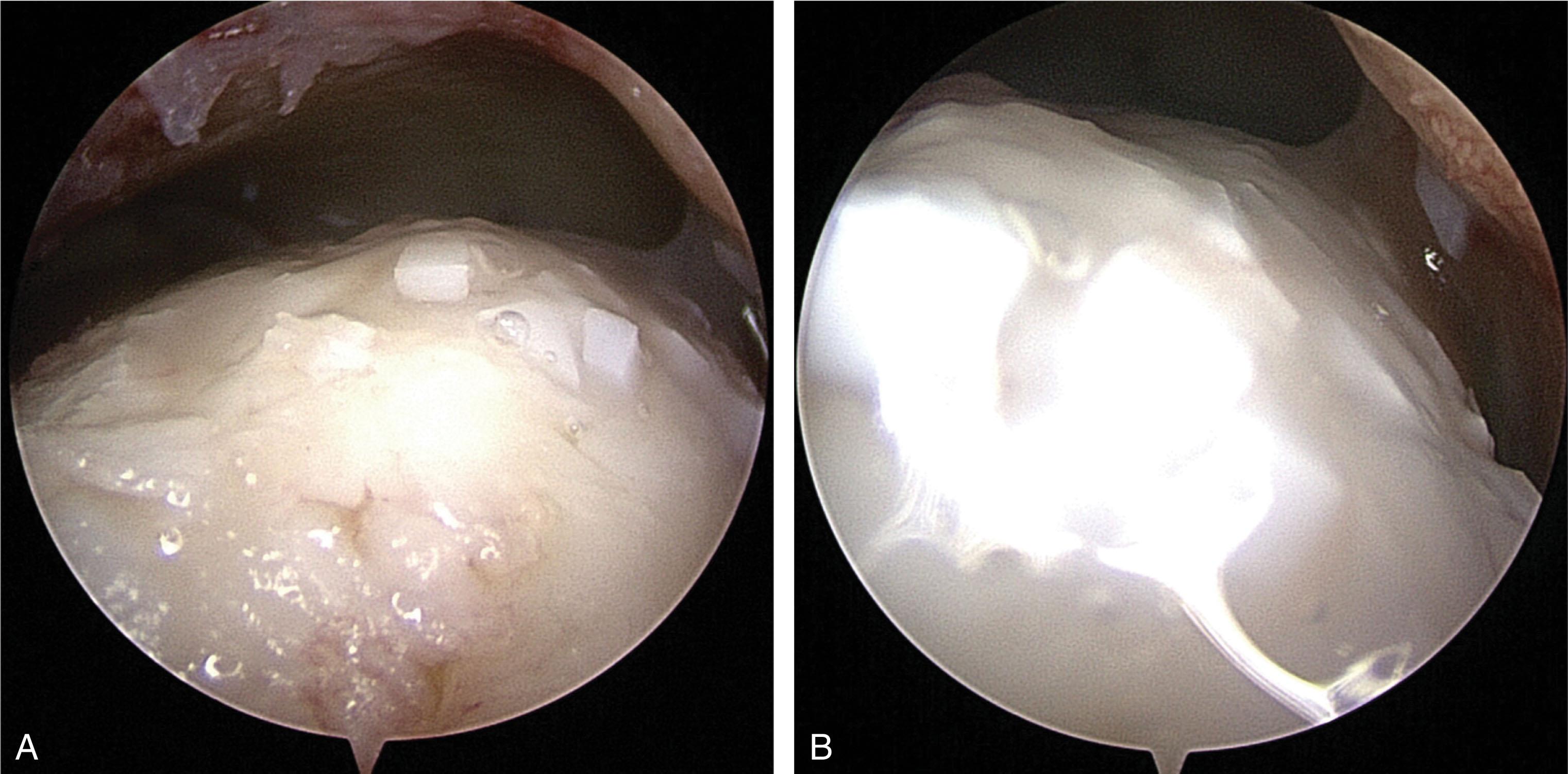
Particulated allograft cartilage transplantation holds the promise to regenerate hyaline cartilage in an OCL with a single surgery. Some techniques allow storage of the graft, so it is available when an unexpected need for its use arises. Available allograft types include preserved juvenile cartilage ( Fig. 40-19 ) and granularized or powdered allograft cartilage matrix mixed with autologous PRP or cBMA ( Fig. 40-20 ). This technique works well for contained defects and is more challenging with uncontained (shoulder) defects due to the need to keep the graft in the lesion while fibrin glue is applied. The technique may be used with bone grafting to fill larger defects (see below).
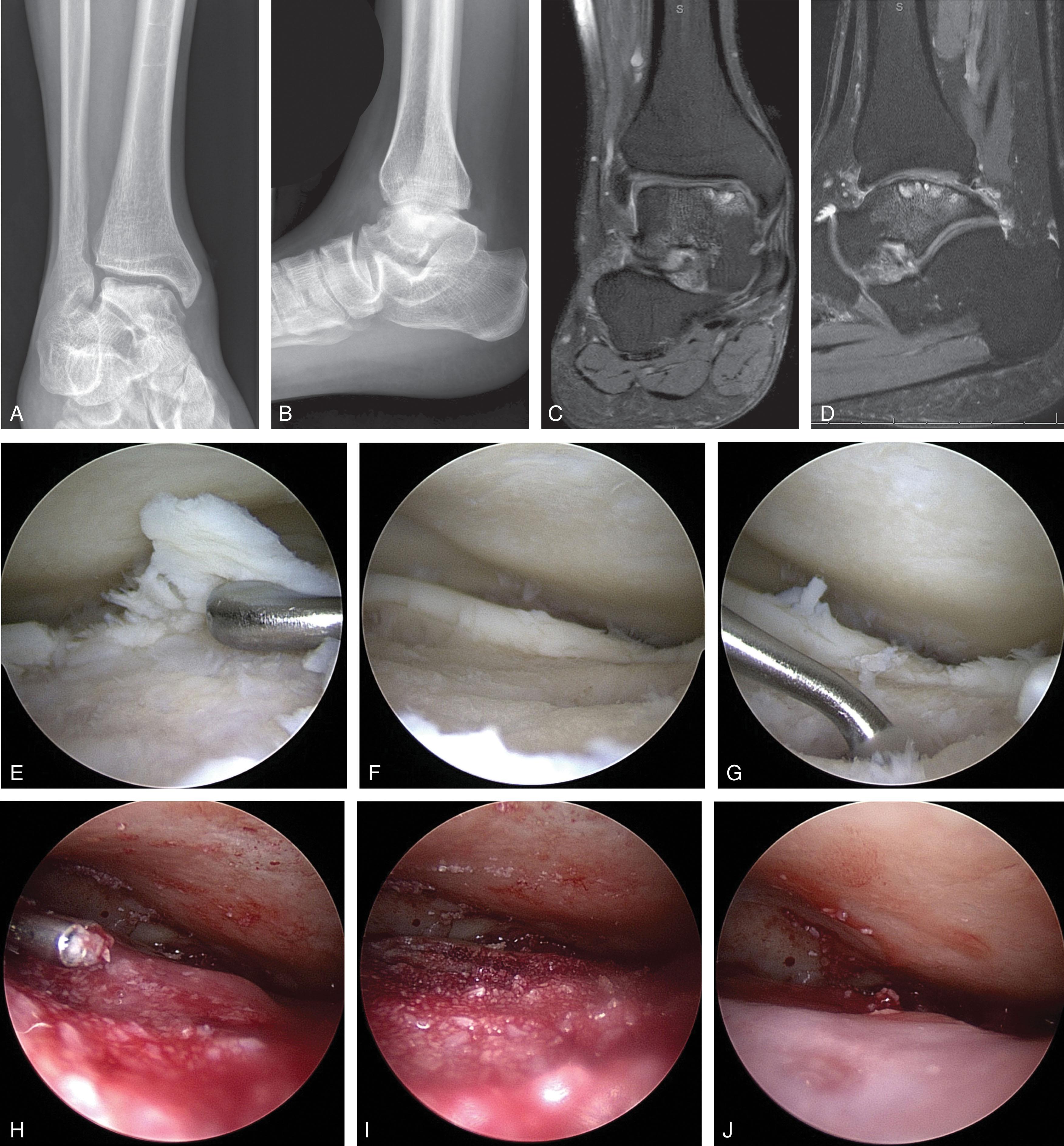
ACI creates hyaline-like cartilage in the defect and can be used in conjunction with bone grafting to fill larger defects. ACI requires a graft-harvesting procedure to obtain 200 to 300 mg of autologous chondrocytes from the superior medial or lateral femoral condyle, the intercondylar ridge of the knee, or from the talus cartilage lesion during diagnostic arthroscopy. The cells are grown in vitro for 2 to 5 weeks before reimplantation through a mini-arthrotomy.
After the lesion is debrided, through a mini-open arthrotomy, an autologous periosteal flap is harvested and sewn over the implanted cells and sealed with fibrin glue ( Fig. 40-21 ). Alternatively, a xenograft collagen patch may be used. In cases of concomitant cystic bone loss, bone graft is applied and covered with a periosteal patch, with its cambrium side facing the cartilage. To create a space for the cells, a second periosteal patch is sewn over the first patch, with its cambrium side facing the bone (“sandwich procedure”).
ACI is indicated in large, well-contained defects, large lesions with extensive subchondral cystic changes, and failed previous surgery. Because the technique requires procurement of cells from a donor site, there is the associated risk of donor-site morbidity and the need for a second surgical procedure. Also, the procedure is expensive and perhaps is best reserved as a salvage procedure for lesions that have failed other treatments.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here