Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hyaline articular cartilage is an avascular and insensate tissue that allows low-friction transmission of physiologic loads in diarthrodial joints. Its functional structure ideally is maintained in homeostasis over the lifetime of an individual but remains incapable of mounting an effective repair response when injured in a skeletally mature adult. The treatment threshold for surgical intervention is not unequivocal, but patients with symptomatic lesions are generally considered candidates for cartilage restoration procedures.
The use of osteochondral grafts of autologous or allogeneic origin is well supported on a basic science level and has a long successful clinical history as a means of biologic resurfacing. Both modalities rely on transplanting mature hyaline cartilage containing viable chondrocytes attached to subchondral bone to restore the architecture and characteristics of native tissue in acquired osteoarticular defects. By transplanting structurally complete osteochondral units with an intact tidemark, the fixation issue is mostly relegated to that of osseous ingrowth. Although both graft sources represent a common cartilage organ transplantation paradigm and are complementary, each has its unique, reciprocal challenges with regard to tissue availability and safety that must be weighed, managed, and communicated whenever the use of osteochondral grafts is being considered.
Autologous osteochondral grafts are best used to address relatively small, yet symptomatic, focal articular lesions of the femoral condyles, especially if these grafts present with associated subchondral abnormalities such as a bone cyst or an intralesional osteophyte. Autologous plugs are also a potential salvage option as in situ fixation for a delaminating osteochondritis dissecans (OCD) lesion (International Cartilage Repair Society [ICRS] grades II-IV; see Chapter 44 ).
Advantages of autologous grafts are immediate availability, relatively low costs, and their nonantigenic and osteogenic behavior that routinely lead to reliable osteointegration. The possibility of arthroscopic delivery of these smaller grafts is appealing, albeit technically challenging.
One obvious disadvantage of autologous graft sources is that the maximum graft surface area is self-limited by the donor area available to be harvested for small and most medium-sized lesions. This is especially true in the previously injured or operated knee, in which suitability regarding tissue quality and overall joint topography has to be critically assessed. In addition, donor site morbidity can significantly add to the disease burden during intraarticular transfer.
Osteochondral allografts are suitable to treat medium to large chondral and osteochondral lesions. In particular, osteochondral allografting may be considered the most appropriate treatment for large and high-grade (ICRS grades III-IV) OCD in the knee. Other specific conditions amenable to allografting include osteonecrosis and posttraumatic defects, such as after periarticular fractures. Further indications for allografting of the knee include treatment of patellofemoral chondrosis or arthrosis and for highly select cases of multifocal or bipolar posttraumatic or degenerative lesions. In a case in which meniscus replacement is also necessary, a composite tibial plateau with attached meniscus can be transplanted. Allografts are also increasingly employed in the salvage of knees that have failed other cartilage resurfacing procedures. Primary treatment may be considered in large chondral defects whose size presents a relative contraindication for other treatments and for which the surgeon believes other procedures may be inadequate.
Osteochondral allografting is the only treatment option that restores mature orthotopic hyaline cartilage and that reproduces the site-appropriate anatomy of the native joint both macroscopically and microscopically, without the risk of inducing donor site morbidity. Osteochondral allografts are versatile when addressing even very large, complex, or multiple lesions in topographically challenging environments, especially if they involve an osseous component.
Obvious drawbacks to the allograft paradigm are the relative scarcity of donor tissue, financial and logistical issues of graft procurement, and residual risk of infection, a discussion that is an essential part of the informed consent process. Although rare allograft-associated bacterial infections have been reported, there are no available published data quantifying this risk or that of viral transmission. Patients are counseled that the risk for disease transmission from a fresh osteochondral allograft is comparable with that associated with banked blood transfusion. In a 35-year experience at our institution using more than 800 fresh allografts, no cases of transmission of disease from donor to recipient have been documented.
Fresh, cold-stored osteochondral allografts have shown to maintain viable chondrocytes and mechanical properties of the matrix many years after transplantation. These findings have generally supported the use of tissue for small osteochondral allografts in the setting of reconstruction of chondral and osteochondral defects. Chondrocyte viability and structural integrity of the matrix are preserved during hypothermal storage in nutritive culture medium containing human serum, with cell density, viability, and metabolic activity remaining essentially unchanged from baseline for as many as 14 days before deteriorating significantly after 28 days, although the hyaline matrix remains relatively intact. The clinical consequences of these storage-induced graft changes have yet to be determined, but 28 days is generally considered the threshold of graft utility in present clinical practice.
Some of the contraindications to allograft transplantation are uncorrected ligamentous instability, meniscal deficiency, or axial malalignment of the lower extremity, as well as the presence of inflammatory or crystal-induced arthropathy or any unexplained global synovitis of the knee.
Autologous plugs are not advised in lesions presenting with a lack of containment or substantial subchondral bone loss, which might lead to loss of fixation and graft failure. Whereas “kissing” lesions are also widely considered unsuitable for autologous grafting, bipolar and multicompartmental allografting has been modestly successful in the younger individual. However, advanced multicompartmental arthrosis is a relative contraindication to the allografting procedure, and neither osteochondral grafting technique should be considered an alternative to prosthetic arthroplasty in an individual with symptoms and acceptable age and activity level for prosthetic replacement.
A careful and focused history and a physical examination are essential to determine candidacy for any cartilage restoration procedure. Because articular cartilage itself is insensate, it is important to identify contributory pain generators and to distinguish them from mechanical symptoms. Tools such as the International Cartilage Repair Society (ICRS) Cartilage Injury Evaluation Package, which incorporates several validated outcome measures, can be helpful in systematically documenting the anatomic condition and functional envelope of the knee and in establishing a baseline for therapy.
The clinician should elucidate the location and quality of pain, onset (acute vs. chronic) and duration of symptoms, alleviating and exacerbating events or measures, prior treatment and surgical history (if any), as well as activity at the time of injury and expected future level of occupational and recreational performance. Significant medical factors potentially relating to knee pathology should be sought such as prior trauma, collagen-vascular/inflammatory disorders, or corticosteroid use. It should be noted that the time from the initial cartilage injury and pending disability compensation claims is universally recognized as inversely related to outcome. The onset and character of symptoms are worth noting in an effort to distinguish pain at rest (indicative of an underlying, more advanced degenerative process) from activity-related pain or mechanical symptoms such as catching or locking that suggest meniscal injury, loose bodies, or cartilage flaps associated with acute injury or early degenerative disease.
The physical examination should begin with a visual inspection of the patient's gait and limb alignment. With the patient placed supine, the examiner can commence with a closer inspection of the knee joint and comparison with the uninjured side, focusing on the area of the chief complaint. Range of motion (ROM) and ligamentous stability should be noted using standard maneuvers. Presence of effusion should be noted, and the patellofemoral joint should be assessed for alignment, baja or alta position, mobility, grind, tracking, tilt, and signs of apprehension. It is particularly useful to palpate the femoral condyles and other accessible articular surfaces because focal tenderness at the site of cartilage lesions is an important physical finding. Symptomatic trochlear or patellar lesions can be stressed with a bounce test or by applying prepatellar pressure during the grind test, whereas condylar lesions often become painful when loaded with a valgus or varus stress test or during a McMurray maneuver. Accordingly, joint line pain and other complaints caused by meniscal or synovial symptoms or originating from extraarticular structures such as bursae or the iliotibial band should be distinguished from chondral pathology.
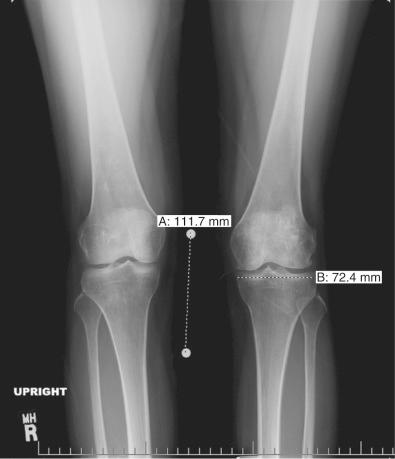
Patients who are considered for an osteochondral grafting procedure should optimally be fully imaged, including a complete radiologic series and magnetic resonance imaging (MRI) studies with cartilage-specific sequencing, if available. Depending on the type and location of cartilage injury being suspected, this should include at least standing anteroposterior (AP) weight-bearing and flexed knee lateral radiographs. Many chondral lesions and their subchondral sequelae are detectable on the AP views ( Fig. 32-1 ), which also give an indication of possible secondary changes such as joint space narrowing and osteophytes. Imaging both knees side by side allows for a built-in comparison view. Of note, OCD presents with bilateral lesions in about a third of the cases that are often easily detected on an x-ray in their typical location on the lateral aspect of the medial femoral condyle toward the intercondylar notch. The lateral flexed view is an important supplementary tool to help assess lesion size, triangulate locations, and identify patient's morphology such as patella alta or baja and trochlear groove topography. Additional views that can be obtained include standing posteroanterior 45-degree flexed knee (Rosenberg) views and supine flexed knee (Merchant) views. The Rosenberg view brings the posterior condyles into view, which helps assess the posterior joint space during loading. Merchant views are standard for the evaluation of the patellofemoral articulation. Long-leg (hip to ankle) standing AP weight-bearing radiographs should be obtained if axial alignment is deemed contributory to the patient's symptomatology and are essential for preoperative planning if a realignment procedure is being considered.
MRI remains as an import tool for assessing the status of the articular cartilage and associated structures of the knee ( Fig. 32-2 ). The most common MRI sequences for cartilage assessment of cartilage repair require cartilage that are fat-suppressed 3-dimensional gradient echo (3D-GRE) and proton-density (PD) and T2-weighted (dual) fast spin echo (FSE) techniques with or without fat suppression. In general, 3D-GRE sequences with fat suppression allow analysis of the thickness and surface of cartilage, whereas dual FSE sequences outline the internal structure of hyaline cartilage. Although not widely used on clinical scanners, recent advancements in specific sequences of MRI such as delayed gadolinium enhanced MRI of cartilage (dGEMRIC), T1 rho, T2 mapping and diffusion weighted imaging can assess biochemical details of cartilage tissue. Additionally, with the widespread deployment and subsequent validation of novel qualitative and quantitative MRI sequences, imaging may play a larger role in the postoperative evaluation of cartilage repair techniques. This includes ultrashort echo time sequences that allow qualitative and quantitative evaluation of the calcified layer of cartilage, which play an important role in the overall function of the osteochondral unit. In an exploratory subgroup analysis, preserving the calcified cartilage layer was particularly useful in predicting biomechanic properties of osteochondral allografts.
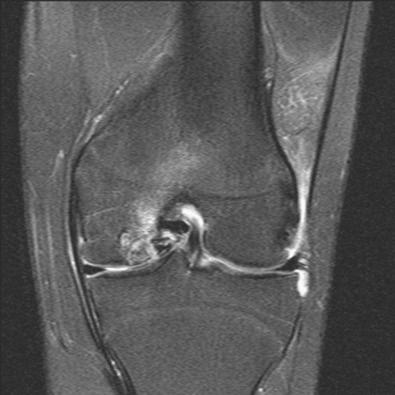
Early degenerative ICRS grade I or II changes (softening, fibrillation) and associated surface irregularities often present as subtle alterations in contour morphology and thickness on MRI. Decreases in thickness can indicate cartilage volume loss, although increases in thickness can signal intrasubstance collagen degeneration and free water accumulation. Advanced degenerative grade III or IV lesions are usually more overt on MRI, manifesting as poorly marginated substance defects often associated with corresponding signal-intensive subchondral edema or cysts, apposing joint surface changes, localized synovitis, or general joint effusion. In contrast, acute, traumatic defects routinely present as focal chondral or osteochondral lesions with distinct margins, often with underlying bone signal changes.
If the patient has had prior surgery, the corresponding operative reports and arthroscopic photographs (if available) are usually helpful not only in assessing the index lesion but also in gauging the overall disease burden and suitability for autologous graft harvest, if applicable. Obviously, results of any modalities and examinations described previously will also factor into the decision and any surgical planning.
Common to all fresh allografting procedures is matching the donor with the recipient. It should be noted that in current practice, small-fragment fresh osteochondral allografts are not human leukocyte antigen (HLA) or blood type matched between donor and recipient and that no immunosuppression is used. The allografts are matched to recipients on size alone. In the knee, an AP radiograph with a magnification marker is used (see Fig. 32-1 ), and a measurement of the mediolateral dimension of the tibia, just below the joint surface, is made and corrected for magnification of the radiograph. This corrected measurement is used, and the tissue bank makes a direct measurement on the donor tibial plateau. Alternatively, a measurement of the affected condyle can be performed. A match is considered acceptable at ±2 mm; however, it should be noted that there is a significant variability in anatomy, which is not reflected in size measurements. In particular, in treating OCD, the pathologic condyle is typically larger, wider, and flatter; therefore a larger donor generally should be used.
Lastly, when considering realigning osteotomy on the same articulating side of an osteochondral graft, staging the procedure is advised to not jeopardize the microvascularity of the recipient bone bed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here