Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
An effective testing process requires integration of preanalytic, analytic, and postanalytic steps.
An understanding of workflow is a fundamental prerequisite to any performance optimization strategy.
A variety of techniques should be used to collect workflow data. These include sample and test mapping, tube analysis, workstation analysis, staff interviews, and task (process) mapping.
Though technology is a critical component of every laboratory, it is only a tool to reach a goal. Technology alone does not improve performance and workflow; its success or failure depends on how it is implemented and whether it was truly needed.
Consolidation, standardization, and integration are key strategies that can optimize workflow. Managing test utilization may also change overall operational needs and workflow patterns.
The clinical laboratory is a complex operation that must smoothly integrate all three phases of the testing process: preanalysis, analysis, and postanalysis. Preanalysis refers to all the activities that take place before testing, such as test ordering and sample collection. The analysis stage consists of the laboratory activities that actually produce a result, such as running a sample on an automated analyzer. Postanalysis comprises patient reporting and result interpretation. Collectively, all of the interrelated laboratory steps in the testing process describe its workflow. This, in turn, occurs within the overall design of a laboratory operation as described in its policies and procedures.
The steps in the testing process can be generally categorized according to testing phase, role (responsibility), or laboratory technology ( Fig. 2.1 ). Note that the testing process and the grouping of steps vary somewhat from one facility to another. Depending on the laboratory service model and technology used, some steps may fall into one category or another. For example, centrifugation may be performed in a physician’s office (preanalysis) or in the laboratory as part of a total automation workcell (analysis). Depending on the technology selected, a laboratory may automate some or many of the steps identified in Figure 2.1 . Information technology is the essential “glue” that binds these steps. A more detailed discussion of each testing phase is presented in Chapter 3, Chapter 4, Chapter 5, Chapter 6, Chapter 7, Chapter 8 . This chapter explores the interrelationship of laboratory workflow, technology, and performance.
To fully understand a laboratory’s workflow, one must audit all phases of the testing process. Only then can one determine how to optimize performance and to what degree technologic or nontechnologic solutions are needed. Table 2.1 provides some of the issues to consider.
| Test ordering | Where are orders placed—in the laboratory, patient unit, or office? Are inpatient orders handled differently from outpatient ones? Is there a paper or electronic requisition? |
|---|---|
| Sample collection | Who collects the samples—laboratory or physician? When are they collected—all hours or just in the am ? Are samples bar coded at the site of collection or in the laboratory? How are the labels generated? Is there a positive patient ID system? Does the label contain all the information needed to process the sample? |
| Transportation | How are samples delivered—by messenger, automatic carrier transport, or a combination? Do all laboratories participate? Are all patient care areas served? How are stats handled? What is their impact? Is there a separate system for emergency department and intensive care units? |
| Sample receipt | Is there a central receiving area? How are samples distributed to each laboratory? Does physical layout promote efficient sample flow? How are stat samples distinguished from routine ones? How are problem samples handled? Are samples sorted by workstation or department? |
| Sample processing | Are samples centrifuged centrally or in distributed locations? Are stats handled differently? Are samples aliquoted? If so, where? Is a separate sample drawn for each workstation? |
| Testing | How many workstations are used? How does capacity relate to need? How are samples stored and retrieved? How long are samples kept? When and why are samples repeated? Are repeat criteria appropriate? |
| Reporting | How are results reported? Electronically? By remote printer? How are stat and critical values reported, and are criteria appropriate? How many calls for reports does the laboratory receive and why? How are point-of-care tests reported? |
Data are of paramount importance in any workflow analysis. Although laboratory data are rather easy to produce because they are readily available from automated analyzers and information systems, they may not be complete, valid, or in the format required. Because laboratory data play a central role in laboratory decision making (e.g., determining which analyzer to acquire), they have to be accurate. Otherwise, one may make wrong downstream decisions that can have a negative impact on operations. One must understand how data are collected by each of these systems and whether they are valid. For instance, do the test statistics pulled from an analyzer provide information on how many patient reportable tests are done, or do they count how many total tests are done (with quality control, repeats, etc.)? Are panel constituents counted individually, is only the panel counted, or are both counted? Are the “collect” times accurate on turnaround time reports that measure “collect to result”? Or are samples indicated as “collected” on a patient floor before they are actually collected, thereby making the turnaround time appear longer than it really is? Ultimately, there is no substitute for carefully reviewing data to determine whether they make sense. Sometimes, this requires manually verifying data collected electronically or directly observing a work area. For example, it may be necessary to observe when samples arrive in the laboratory to determine how long a delay exists before staff assign a receipt time in the computer. By doing so, one can determine the accuracy of the sample receipt time.
Many types of data can be used to assess workflow. Although some of the fundamental data analysis techniques are described in this chapter, they may have to be supplemented with additional data collection to analyze unique characteristics of a laboratory’s operation. It is always useful (some would say imperative) to check that the data collected reflect actual laboratory experience rather than anomalies created by unusual workflow patterns or laboratory information system (LIS) programs or definitions.
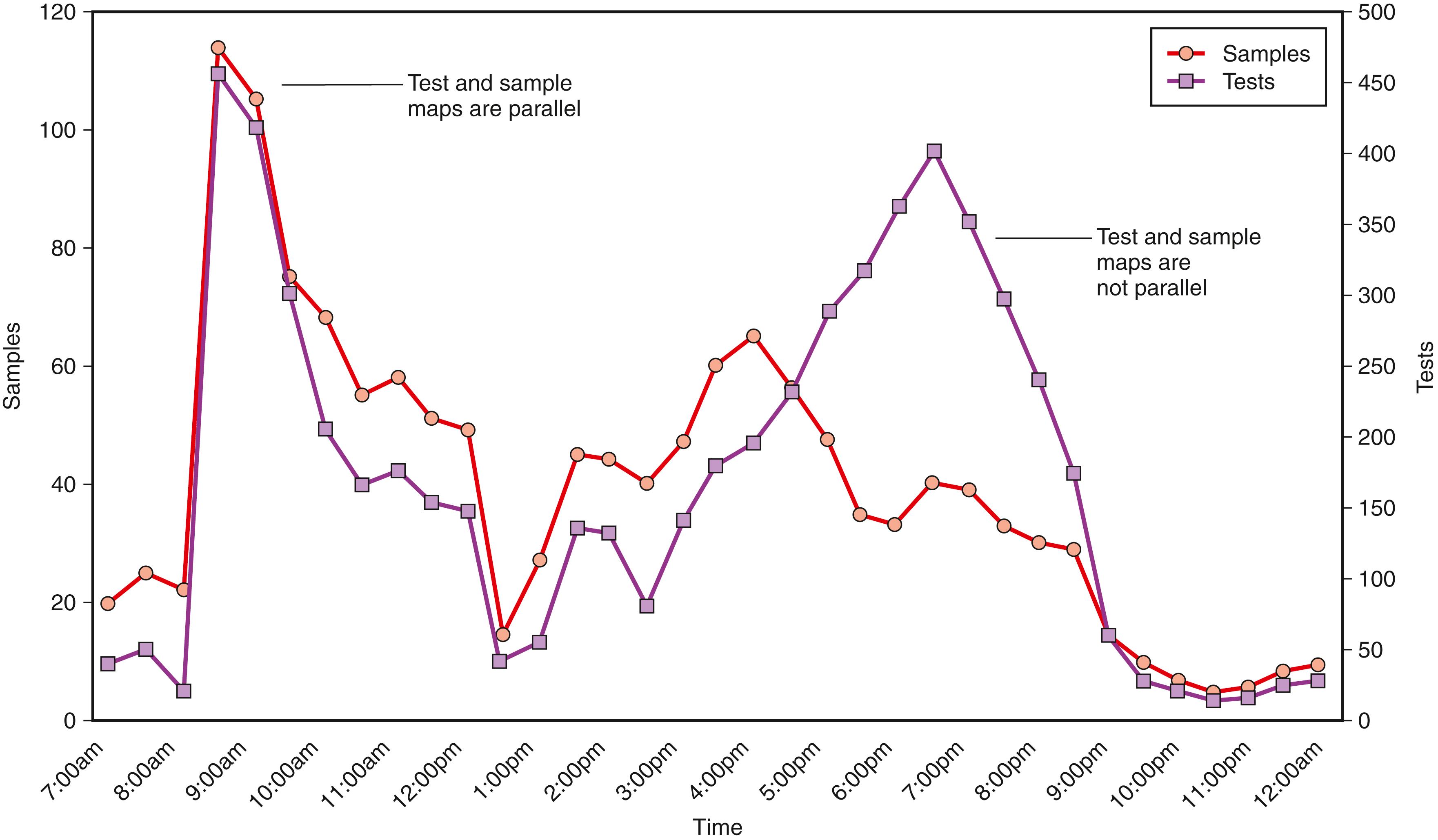
One fundamental data collection technique is to analyze the distribution of samples and tests over time ( Fig. 2.2 ). Depending on what is mapped, the time interval can be a day (e.g., hour increments for frequently ordered tests, such as those in general chemistry) or a week (e.g., daily increments for tests batched several times a week). The goal is to identify overall workload patterns to assess whether resources are appropriately matched to needs and whether turnaround time or other performance indicators can be improved. It is important that the workload measured reflects actual experience. For example, if phlebotomists remotely mark specimens “received” or the laboratory actually orders tests in the LIS, the measured workload distribution may not accurately reflect the underlying processes. As part of the exercise, it is also important to map routine samples versus stat ones and to map locations that may have special needs, such as the emergency department. In addition to sample mapping, one should map key tests and the number or “density” of tests per sample. This is of special interest in the chemistry section. Outpatient samples typically have greater test density than inpatient ones; thus, an equal number of inpatient and outpatient samples may be associated with different inpatient and outpatient workloads. In automated chemistry, sample mapping more closely reflects staffing needs in that much of the labor is associated with handling and processing tubes rather than actually performing the assays. In contrast, test mapping more closely reflects instrument needs (i.e., the test throughput it needs to complete its workload in a timely manner). By mapping samples and tests and relating them to turnaround time and staffing, a laboratory can identify production bottlenecks and alter workflow to achieve better outcomes. Very frequently, laboratories discover that delays are less the result of instrument issues per se and more the result of workflow patterns that are not matched to instrument capabilities.
Part of the laboratory’s daily work is related to processing collection tubes or containers. “Tube labor” includes sorting and centrifuging; aliquoting; racking, unracking, loading, and unloading samples on analyzers; retrieving tubes for add-on tests; performing manual dilutions or reruns (depending on instrument); and storing tubes. Although the time needed to perform a tube task may seem insignificant, it has to be repeated many times per day, which can add up to a substantial amount of time. For example, at an average of 10 seconds per tube, it will take a laboratory 3.3 hours to sort 1200 tubes per day. Automation can often reduce this labor, but redesigning the workflow may be a less expensive and more efficient alternative. To the extent that a laboratory reduces the number of tubes and/or the number of tasks associated with each tube, it can reduce tube labor and positively influence workflow and staffing needs. Reducing tube labor is one of the main goals of consolidating chemistry and immunodiagnostic tests into a single analyzer or workcell. Sample mapping provides information about how many containers are received within a specified interval; tube analysis helps to analyze how many additional “tube-related” tasks have to be done. Tube analysis includes the number of containers other than tubes (e.g., fingerstick collections that may require special processing or aliquoting) and the number of reruns (i.e., repeats) needed as the result of instrument flags and/or laboratory policies ( Table 2.2 ).
| Analyzer A | Analyzer B | |
|---|---|---|
| Total tubes run | 500 | 500 |
| Mechanical error | 13 | 15 |
| Dilution | 7 | 20 |
| Clot/low volume | 20 | 30 |
| Total instrument-related reruns | 40 (32% of total reruns) | 65 (65% of total reruns) |
| Delta check | 62 | 21 |
| Panic value | 23 | 14 |
| Total laboratory criteria–related reruns | 85 (68% of total reruns) | 35 (35% of total reruns) |
| Total reruns | 125 | 100 |
| % reruns | 25% | 20% |
A typical laboratory is divided into stations for allocating work and scheduling staff. Some workstations consist of a variety of tasks or tests that are grouped together for the purpose of organizing work for one or more staff. For example, all manual or semiautomated chemistry tests may be grouped into a workstation, even though testing might actually be performed at different sites or using different equipment around the laboratory. More typically, a workstation is one physical location (e.g., a fully automated analyzer or group of analyzers, such as hematology cell counters, a chemistry workcell, or an automated track that includes preanalytic processing, analytic modules, and postanalytic storage). Regardless of how a laboratory is organized, it is important to understand where, when, and how the work is performed. This is the goal of a workstation analysis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here