Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Primate and human infants must normally pass head first through their mother's pelvis to accommodate the limited opening determined by the bony configuration. Therefore the size of the mother's pelvis limits the head and brain size of the infant. Specifically, the brain of an infant ape is 55 percent of its full size, and the brain of a present-day human infant is only 23 percent of the adult size. The result is a human infant who is neurologically immature. Notice that the baby monkey can immediately cling tightly to the fur on its mother's stomach, whereas the human infant has poor muscle strength, has little motor control, and is completely dependent on the mother for survival. While immature, the human infant lives in a restricted and artificial reality, interacting primarily with the mother. The human infant interacts little with the forces of life in the outside world.
It is possible that this early immaturity and restricted world contact are naturally beneficial. The infant's restricted curriculum concentrates on a few priorities necessary for survival. Without words, the infant must be able to announce all his or her needs and encourage a high level of motherly devotion. To communicate with the mother, the infant must be able to read facial expressions and respond with a non-verbal vocabulary. If this speculation is correct, what vision equipment does the infant have to perform these functions?
Larsen noted that the axial length of the neonate's eye was 17 mm and that it increases 25 percent by the time the child reaches adolescence. The size of the normal infant's eye is about three-fourths that of the adult size. Geometric optics teaches us that the retinal image of the normal infant eye is therefore about three-fourths the size of the adult's image. * A smaller image also means that much less fine detail is recorded. The small retinal image may be but one reason why an infant's visual acuity is poorer than that of the adult. In fact, experiments have shown that the neonate's visual appreciation for fine detail at birth is one-thirtieth, or approximately 3 percent, of the development of the adult, * yet the neonate appreciates large objects (e.g. nose, mouth, eyes of close faces) as does the adult.
* Specifically, the size of the retinal image depends on an entity known as the nodal distance, which averages 11.7 mm in the newborn and 16.7 mm in the adult emmetropic schematic eye, giving a ratio of adult to infant retinal images of 1.43.
* The infant's visual acuity is about 20/600 versus the normal visual acuity of an adult, which is 20/20.
Figure 1.1 shows that visual acuity swiftly improves, and by the age of 12 months, the infant's level of visual acuity is 25 percent (20/80) of optimal adult visual acuity. This improvement in acuity seems to parallel eyeball growth. By the age of 5 years, the child usually has 20/20 vision. What other factors beyond eye size account for the young child's lowered visual acuity? As the eye grows, the optical power of the eye lens and cornea must weaken in a tightly coordinated fashion so that the world stays in sharp focus on the retina. Patients with myopia highlight the developmental process of balancing the growth of the eye while maintaining a sharp retinal image. Most cases of myopia have an elongated eye. The stretching and weakening of the sclera seems to depend on two major factors. First, that the intraocular pressure maintains a constant force on the sclera. Second, there is digestion of sclera architecture (collagen I fibers and extracellular matrix) by metalloproteinase enzymes. This idea of strengthening the sclera to prevent myopic expansion is supported experimentally. In one series of experiments, 7-methylxanthine (a caffeine metabolite) was used to enhance concentration and thickness of collagen fibers in the posterior sclera of animals. In humans, scleral collagen fibers were cross-linked using riboflavin activated by ultraviolet light.

However, we do not know what activates the process of scleral weakening and stretching in myopia. Some human studies have shown that use of atropine drops in children partially inhibits the development of myopia. The reason is unclear. Some feel that atropine reduces the pull of the ciliary muscle on the sclera which otherwise allows the sclera to elongate. Another theory suggests that the atropine reduces vitreous pressure, thus reducing a stretching force. However, atropine has the same effect in chickens that have aciliary muscle unresponsive to the effects of atropine. Another school of thought is that retinal receptors somehow activate the process of scleral weakening, e.g. M1 muscarinic receptors present in the neural retina but not in muscle. Animal experiments using specific M1 blockers such as pirenzepine show the same effects as atropine, without blockade of accommodation. The presence of pirenzepine-inhibited receptors within the choroid and retina could also explain why elongation in eyes with transected optic nerves is inhibited by pirenzepine. Human use of topical pirenzepine was reported in two studies which showed a small but statistically significant reduction in myopia and axial length. These data suggest that during childhood the retina can record information concerning the sharpness of the retinal image and then use this information to control the eye's axial length via scleral stretching.
If this tight coordination of growth fails, the infant may become nearsighted or farsighted. Because the coordination of eye length growth and the focusing power of the cornea and eye lens may be imperfect, is there some compensation provided, early in life, guaranteeing that almost every child can process a sharply focused retinal image of the world? Accommodation is the safety valve that can help provide a sharp image, even if all the ocular components are not perfectly matched. In the young child the range of accommodation is greater than 20 diopters. This range, in addition to the farsightedness of almost all infants, means that most young eyes can focus almost any object by using part or all of this enormous focusing capacity.
Because of the infant's smaller pupil, a second factor that helps the infant achieve a sharper retinal image is an increased depth of focus. Photographers use this device when they use larger F-stops (F32, F64) to keep objects at different distances all in focus.
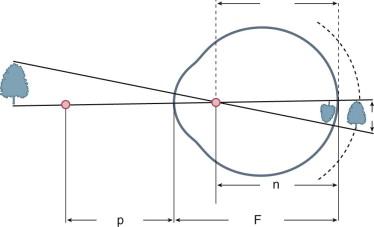
Figure 1.2 shows the importance of the nodal point in determining the size of the retinal image in a typical human eye. To help us appreciate the basic optical principles operating within the human eye and avoid being confused by their many details (e.g. the many different radii of curvature, the different indices of refraction), an all-purpose, simplified eye was developed. Such model eyes have many names (e.g. reduced, schematic, simplified eye) and were developed by some of the true giants of physiologic optics. *
* A partial list of giants of physiologic optics who have created schematic or reduced eyes includes Listing, Helmholtz, Wüllner, Tserning, Matthiessen, Gullstrand, Legrand, Ivanoff, and Emsley.
Figure 1.2 depicts such an eye with its cardinal points (the principal points, focal points, and nodal points). Knowing the location of the cardinal points of a lens system, the optical designer can calculate all of the relationships between an object and an image. For example, to determine the image size of the reduced eye, one simply traces the ray, starting from the top of the object, which goes undeviated through the nodal point and lands on the top of the inverted retinal image. As the distance between the nodal point and the retina increases, the image size increases. The addition of a plus spectacle lens to the eye's optic system moves the nodal point of the new system forward (increasing the nodal point to retina distance), thus magnifying the retinal image. The reverse is true with a negative spectacle lens. Therefore a contact lens or a refractive cornea on which surgery has been performed enlarges the image size for a person with myopia who previously wore spectacles. The change in retinal image size should be taken into account when evaluating the visual acuity results after corneal refractive surgery. From an optical point of view, an 8 diopter hyperope exchanges a larger image produced by their +8 spectacle lens for a smaller image after corneal refractive surgery. Thus, they should theoretically lose a line of visual acuity after refractive surgery, but instead often gain a line. A possible reason is that the aberrations of the high plus spectacle lens cancel the effects of the larger retinal image, but this area requires further study.
The coordination of the power of the cornea, crystalline lens, and axial length to process a sharp retinal image of a distant object is known as emmetropization. In the United States, more than 70 percent of the population is either emmetropic or mildly hyperopic (easily corrected with a small accommodative effort).
With age, the cornea, lens, and axial length undergo coordinated changes. Essentially, the optical components (cornea and lens) must lose refractive power as the axial length increases so that a sharp image remains focused on the retina.
The cornea, which averages 48 diopters of power at birth and has an increased elasticity, loses about 4 diopters by the time the child is 2 years of age. One may assume that the spurt in growth of the sagittal diameter of the globe during this period pulls the cornea into a flatter curvature. The fact that the average corneal diameter is 8.5 mm at 34 weeks of gestation, 9 mm at 36 weeks, 9.5 mm at term, and about 11 mm in the adult eye supports this “pulling, flattening” hypothesis.
On the other hand, other coordinated events also occur, such as the change of lens power and the coordinated increase in eye size (most important, an increase in axial length). The crystalline lens, which averages 45 diopters during infancy, loses about 20 diopters of power by age 6 years. To compensate for this loss of lens power, the axial length increases by 5–6 mm in that same time frame. (In general, 1 mm of change in axial length correlates with a 3-diopter change in refractive power of the eye.)
Now let us examine a possible mechanism that could account for most of the data. As the cross-sectional area of the eye expands, there is an increased pull on the lens zonules and a subsequent flattening of the lens (the anterior lens surface is affected a bit more and the posterior lens surface a bit less), thus decreasing the overall lens power. There also may be a related decrease in the refractive index of the lens, which also contributes to the reduction in lens power. Because the incidence of myopia starts to accelerate significantly around the age of 10, one may question whether there could be a decoupling of the previously described coordinated drop in lens power and increase in axial length. An increased amount of near work (e.g. schoolwork) is associated with a higher incidence of myopia. It is also well known that genetic predisposition influences myopia incidence, as evidenced by the fact that more Asian children than Caucasian children are myopic. Thus one might hypothesize that the long periods of accommodation that accompany schoolwork (ciliary body contraction) may tend to stretch and weaken the linkage between the enlarging scleral shell and the ciliary body. If this were to happen, the lens would flatten less during eye growth. Another way of looking at this phenomenon is to theorize that with the linkage weakened, the restraining effect of the lens-zonule combination on eye growth is also weakened, which results in an increase in axial length in the myopic student. Many studies demonstrating that the myopic eye has a greater axial length than the emmetropic eye support this idea.
The cone photoreceptors of the retina are responsible for sharp vision under daylight conditions. The denser the cones are packed, the more acute the vision. To use a photographic analogy, film with the highest resolution has smaller photosensitive grains, packed tightly, whereas a film with large grains of silver halide yields a coarser picture.
The most sensitive part of the retina is the fovea. * Here the cones are even finer and are packed together even tighter. The fovea of the infant eye is packed less than one fourth the density of that of the adult. Furthermore, the synapse density in the neural portion of the retina, as well as the visual brain, is low at birth. The combination of these two anatomic configurations means that fewer fine details of the retinal image are recorded and sent to the brain.
* F. W. Campbell quotes Stuart Ansti's clear analogy of how the fovea functions: “A retina with a fovea surrounded by a lower acuity periphery can be compared to a low magnification finder telescope with a large field of view which will find any interesting target and then steer on to it a high powered main telescope, with a very small field which could examine the target in detail.”
Finally, the nerves that transmit the visual information to the brain, as well as the nerve fibers at the various levels within the brain, are poorly myelinated in the infant. Myelin is the insulating wrap around each nerve fiber. A normally myelinated nerve can transmit nerve impulses swiftly and without static or “cross talk” from adjacent nerves. To use a computer analogy, one might think of the infant brain as being connected with poorly insulated wires. Therefore sparks, short circuits, and static all slow or interfere with perfect transmission, and only the strongest messages get through. Figure 1.3 represents an appropriate analogy. The face of Albert Einstein is shown on a computer screen with larger and larger pixels. The infant's early vision might be comparable to the picture with the biggest block pixels. With maturation of the brain processing elements, the neurologic equivalent of pixel size gets smaller and more details can be registered. Thus the equivalent of the photographic film grain size in the retina and the equivalent of pixel size in the brain processor both get smaller as the child grows. Lakshiminarayanan, who created Figure 1.3 , speculated that the immaturity of the infant's memory capacity may be one reason why its visual images have less detail. In other words, the coarseness of the infant visual system does not overtax the immature memory system.

Experiments with infants reveal that good color vision does not appear for about 3 months. The infant also takes longer to “make sense” of the retinal image. Specifically, the infant must stare for relatively long periods (1–3 minutes), blinking rarely during this period.
The remarkable thing about the infant's eyesight is that the relatively poor level of resolution just described still allows the infant to recognize different faces and different facial expressions. We know this to be true in some newborn infants, who can accurately imitate the expressions of an adult ( Fig. 1.4 ), almost as if the baby uses his or her own face as a canvas to reproduce the facial expression of the onlooker.
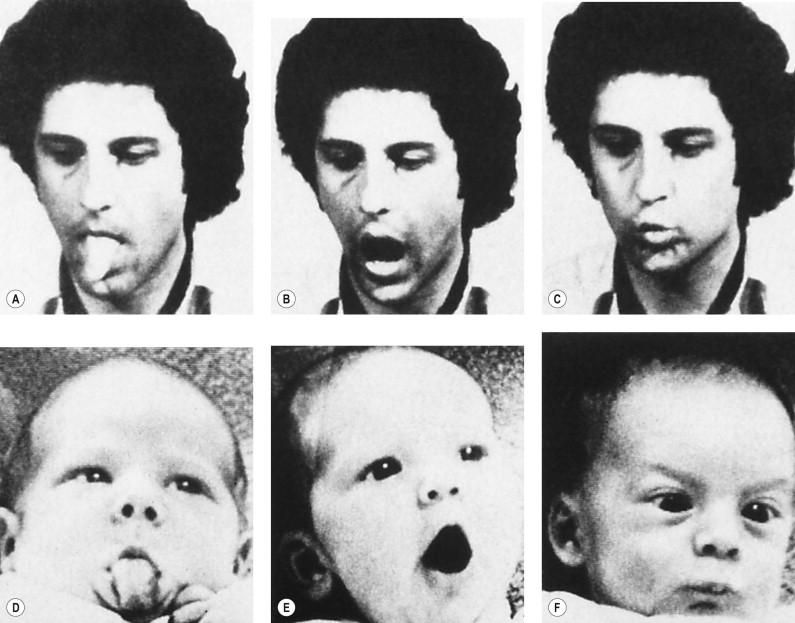
Experiments with infants demonstrate that infants prefer to look at faces or pictures of faces rather than to look at other objects. By the age of 6 weeks, infants can discern specific features of the face. For example, they can lock in on the mother's gaze. By age 6 months, they can also recognize the same face in different poses. In fact, they are experts at recognizing a face, be it upside down or right side up, till the age of 6 years. After age 6, infants actually lose their skill at quickly recognizing upside-down faces.
A closer examination of the eye at 6 months of age is worthwhile because an unusual change has started to take place in the optics of the eye at this time. Gwiazda et al found that a significant amount of astigmatism develops in 56 percent of the infants studied. This condition remains for only 1–2 years.
The transient astigmatism just described tends to elongate tiny dots of the retinal image into lines. In essence, these create the equivalent of a line drawing of the retinal image. For example, think of a mime (i.e. a painted face with a few dark lines and spots for eyes and nose) as creating different line drawings of the face. What is astonishing is that, although made of only a few dark lines, the mime can recreate most human expressions. It seems reasonable to imagine that the mime presents faces similar to those seen by the young child or found in a child's drawing. The faces have no texture, no shadowing, and no creases – only a line for a mouth, circle for eyes, and occasionally, a dot for a nose. Is it not then possible that infant astigmatism helps represent faces as line drawings to the infant visual system? Line drawings also save memory storage space, which would be an advantage for the small infant brain. * Figure 1.5 illustrates this point in a different way. The face of Albert Einstein is shown with a complete gray scale on the left and is shown as a line drawing (only black and white) on the right. The line drawing requires much less computing power than a face with texture and would be more compatible with the child's immature processing system.

* This idea was suggested by David Marr in “Early Processing of Visual Information,” published in Transactions of the Royal Society of London, Series B 1976; 275:483.
As noted earlier, in many infants the amount of astigmatism can rise to a level of greater than 2 diopters in the first year of life. The orientation of the distortion is usually horizontal (180 degrees) initially. In the course of the next 2 years, the meridian of distortion rotates to the vertical and the amount of the astigmatism diminishes. This slow rotation of the axis of exaggeration can help activate different groups of brain cells, which become sensitive to features in the retinal image with different tilts. In fact, the discovery of these brain cells with orientation selectively leads to a ground-breaking understanding of the functional architecture in the higher brain. In 1958 Torsten Wiesel and David Hubel, working in their laboratory at the Harvard Medical School, implanted electrodes in the visual cortex of an anesthetized cat to record cortical cell responses to patterns of light, which they projected onto a screen in front of the cat. After 4 hours of intense work, the two scientists put the dark spot slide into the projector, where it jammed. As the edge of the glass slide cast an angled shadow on the retina, the implanted cell in the visual cortex fired a burst of action potentials. Torsten Wiesel described that moment as the “door to all secrets.” The pair went on to prove that cells in the cortex responded only to stimuli of a particular orientation. Similar responding cells were all located in the same part of the cortex. This work opened up the area of how and where the brain encodes specific features of the retinal image. Fittingly, Drs. Hubel and Wiesel were awarded the Nobel Prize for Medicine in 1981.
Another function of great survival value to the infant is the ability to follow the eye movements of his or her caretakers. Consider this a near task involving the contrast discrimination of a 12-mm dark iris against a white sclera framed by the palpebral fissure; such a task can be accomplished with a visual acuity of 20/200.
The British psychologist Simon Baron-Cohen, in his book Mindblindness , * suggests that a major evolutionary advance has been the human's ability to understand and then interact with others in a social group (e.g. playing social chess). He further suggests that we accomplish this social intelligence primarily by following the eye movements of others, which begins at a young age. For example, an infant of 2 months begins to concentrate on the eyes of adults. Infants have been shown to spend as much time on the eyes as all other features of an observer's face.
* In his book Mindblindness: An Essay on Autism and Theory of Mind , published by MIT Press in 1995, Baron-Cohen ferrets out the key features of “eye following” in the normal child by comparing them with those of the autistic child.
By 6 months of age, infants look at the face of an adult who is looking at them two to three times longer than an adult who is looking away. We also know that when infants achieve eye contact, a positive emotion is achieved (i.e. infants smile). By age 14 months, infants start to read the direction that an adult is looking. Infants turn in that direction and then continue to look back at the adult to check that both are looking at the same thing. By age 2, infants typically can read fear and joy from eye and facial expression.
Infants are capable of putting up their arms to block a threatening movement. This act tells us that infants appreciate both movement and the implied threat of this particular movement. Admittedly, infants cannot respond if the threat moves too quickly, probably because the immature myelinization of the nerves slows the neural circuits. Nevertheless, a definite appreciation of movement and threat exists.
For movement to be registered accurately, the infant retina probably records an object at point A. That image is then physiologically erased (in the brain and/or retina), and the object is now seen at point B. This physiologic erasure is important because, without it, movement would produce a smeared retinal image. Researchers think that the infant probably sees movement as a smoothed series of sharp images, not smears. This hypothesis is supported by other experiments demonstrating that the infant can appreciate the on/off quality of a rapidly flickering light at an early age. It seems logical that the movement of an image across the retina (with the inherent erasures) is physiologically related to the rapid on/off registration of a flickering light.
A related reflex, the foveal reflex, is triggered by stimulation of the peripheral retina, activating the eye movement system so that the fovea is directed to the visual stimulus.
Although the visual system of the infant is immature, some infants can recognize and respond to adult facial expressions on the first day of life and follow the mother's glances by 6 weeks of age. Clearly, the infant's top priority is to maintain a social relationship with his or her mother or other caring adults. This idea was described succinctly by the “language expert” Pinker: “Most normally developing babies like to schmooze.” As infants grow, they learn to see people and objects in the way that their culture demands and communicate in the expected manner; that is, “We don't see things as they are but as we are.” Is it possible that the immature eye and visual system actually facilitate socially biased seeing? Perhaps the smaller, simpler retinal images, along with the less sophisticated brain processing, prevent the many other details of life from confusing the key message; that is, social interaction takes top priority.
Even in adulthood there is plasticity of the visual system, e.g. after the implantation of multifocal IOLs (intraocular lenses). These IOLs superimpose blurred near images on a sharply focused distant image. Thus, the patient's brain must filter and suppress the third, fourth and so on blurred images from the scene. This is a time-dependent phenomenon that may take days to months to occur.
The image quality of the human adult eye is far superior to that of the human infant, although probably inferior to certain predator birds. Its wide focusing range is smaller than that of certain diving birds, and its fine sensitivity to low light levels is weaker than spiders or animals with a tapetum lucidum. Its ability to repair itself if probably not as efficient as some animals (e.g. newts, which can form a new lens if the original is damaged). Finally, the human eye has the ability to transmit emotional information (e.g. excitement by means of pupil dilation, sadness by means of weeping), but with less forcefulness than some fish, who uncover a pigmented bar next to the eye when they are about to attack, or the horned lizard, which squirts a jet of blood from its eye when it is threatened. Thus, in reading this chapter, one must appreciate the eye's level of performance in light of its large spectrum of functions.
When our retinas receive an image of a spotted puppy in a room, what is really happening in terms of information transfer? Light waves from the ceiling light are cast onto the puppy. The puppy's body reflects and scatters the light waves into our eyes. In a sense, information about the puppy has been encoded into visible light waves. The optical elements of the eye focus the encoded light waves onto our retina as a map of bright and dim colored dots, known as the retinal image. Nerve signals report the retinal image to the brain. In the brain the nerve signals are recreated into the impression that a real puppy is in the room.
One might liken the function of the eye to that of a radio, which receives radio waves carrying a Beethoven symphony. The specific station broadcasting the symphony beams it out on a specific radio wavelength. The radio then receives the radio waves, and the speaker reconverts the waves to musical sounds. If the eye is to receive and process visible light, it must be constructed with the ability to be tuned to the wavelengths of visible light. The physicist would substitute the term resonate for tune. Literally, resonate means to resound another time. A simple example of resonance is an opera singer who can make a wine glass hum when the frequency (or wavelength) of the note is the same as the natural frequency of the glass. What is the natural frequency of anything? The answer has to do with composition and size. Organ pipes of different lengths resonate at different frequencies and wavelengths. Changing the length of the antenna on a car radio allows the frequencies of different stations to be received. The best receiver for a specific wavelength (frequency is the reciprocal of wavelengths) is physically the same size as the wavelength, a precise number of wavelengths, or a precise fraction (one-fourth, one-half) of the size of the wavelength. Therefore optical theory demands that the size of the key components of the eye be the size of a wavelength of visible light or some number ( n ) times the size of, or a fraction of the size of, the wavelengths of visible light; in addition, the key components must be made of a resonating material.
The human cornea is a unique tissue. First, it is the most powerful focusing element of the eye, roughly twice as powerful as the lens within the eye. It is mechanically strong and transparent. Its strength comes from its collagen fiber layers. Some 200 fiber layers crisscross the cornea in different directions. These fibers are set in a thick, watery jelly called glycosaminoglycan. The jelly gives the cornea pliability. For a long time, no one could explain convincingly the transparency of the cornea. No one could understand how nature combined tough, transparent collagen fibers (with their unique index of refraction) with the transparent glycosaminoglycan matrix, which had a different index of refraction, and still maintain clarity. Perhaps an everyday example of this phenomenon will help. When a glass is filled from the hot water tap, the solution looks cloudy. Looking closely, one can see many fine, clear expanded air bubbles (which have a unique index of refraction) within the water (which has different refractive properties). Conversely, cold water appears clear because its air bubbles are tiny. The normal corneal structure might be considered optically similar to the structure of the cold water (i.e. tiny components with different indices of refraction).
Miller & Benedek were ultimately able to prove that if the spaces consisting of glycosaminoglycan and the size of the collagen fibers were smaller than one-half a wavelength of visible light, the cornea is clear, even if the fibers were arranged randomly. An orderly arrangement of the fibers also helps maintain corneal transparency.
Another way to explain it is to say that the cornea is basically transparent to visible light because its internal structures are tuned to the size of a fraction of the wavelengths of visible light. Figure 1.6 is an electron micrograph showing the fibers of the human cornea. The black dots are cross sections of collagen fibers imbedded in the glycosaminoglycan matrix. In this specimen ( Fig. 1.6A ), the fibers are spaced closer than half a wavelength of visible light apart, and the fibers in each of the major layers are arranged in an orderly manner. In an edematous, hazy cornea there are large spaces between collagen fibers ( Fig. 1.6B ).
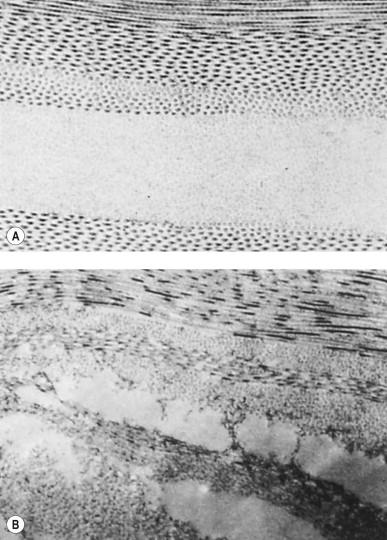
This arrangement of corneal fibers serves a number of important functions. First, the arrangement offers maximal strength and resistance to injury from any direction. Second, the arrangement produces a transparent, stable optical element. Third, the spaces between the major layers act as potential highways for white blood cell migration if any injury or infection occurs. Horizontal arrangement of corneal lamellae, which can slide over each other during eye rubbing, facilitates the clinical development of pathological conditions such as keratoconus. The lack of interlamellar adhesion and corneal thinning facilitate a bulging under IOP and gravity. Enzymatic digestion seems to be the causative agent in the thinning, which eventually deforms the anterior surface of the cornea. This conical cornea is irregularly astigmatic and will produce multiple blurred images in the retina. Collagen cross-linking helps connect the adjacent lamellae, inducing greater corneal strength by resisting interlamellae sliding, and thus reduces the progression of keratoconus ( Box 1.1 ).
An example of immunofluorescent staining for confocal microscopy (40×) of a bovine cornea soaked with a riboflavin (0.01 percent) solution. Prior to staining the specimen was submitted to 30 minutes exposure to UV light (365
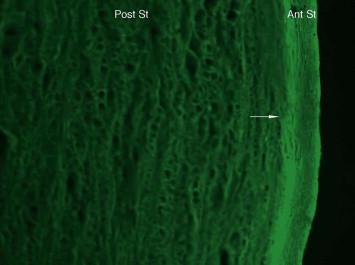
nm). Note the connection between lamellae in the anterior stroma (ant st) compared to the laxity of the posterior stroma (post s). (Courtesy Bottos, Schor, Chamon, Regatieri, Dreyfuss and Nader.)
Is it easier to see underwater with goggles? * Without goggles, the water practically cancels the focusing power of the cornea, † leaving objects blurred. The goggles ensure an envelope of air in front of the cornea, restoring its optical power. If water cancels optical power, how can we explain the focusing ability of the crystalline lens, which lies inside the eye and is surrounded by a fluid known as aqueous humor ? The answer is that the focusing power resides in the unusually high protein content of the lens. The protein concentration may reach 50 percent or more in certain parts of the lens. * Such a high concentration increases the refractive index above that of water and allows the focusing of light. Now we are ready to appreciate the real secret of the lens.
* Because the index of refraction of water is greater than air, objects underwater appear about one-third closer and thus one-third larger than they would in air (i.e. magnification = 1.33×).
† The cornea is a focusing element for two reasons. First, it has a convex surface. Second, it has a refractive index greater than air. Actually, its refractive index is close to that of water. Thus, when one is underwater, the surrounding water on the outside and the aqueous humor inside the eye combine to neutralize the cornea's focusing power.
* The chemical composition of a focusing element such as the crystalline lens determines the refractive index. Water has a refractive index of 1.33. As the protein concentration of the lens rises, the index of refraction approaches 1.42.
Normally, a 50 percent protein solution is cloudy, with precipitates floating about like curdled lumps of milk in a cup of coffee. However, the protein molecules of the normal lens do not precipitate. In a manner not fully understood, the large protein molecules known as crystallins (large protein molecules ranging in size from 45 to 2000 kD) seem to repel each other, or at least prevent aggregation, to maintain tiny spaces between each other. The protein size and the spaces between them are equivalent to a small fraction of a light wavelength. Spaced as they are, one might say that they are tuned to visible light and allow the rays to pass through unimpeded. On the other hand, if some pathologic process occurs, the protein molecules clump together and the lens loses its clarity. When this happens, light is scattered about as it passes through the lens. The result is a cataract.
If the emmetropic eye is in sharp focus for the distant world, it must refocus (accommodate) to see closer objects. † For example, the child's range of accommodation is large, as noted earlier. This allows the child to continue to keep objects in sharp focus from an infinite distance away to objects brought to the tip of the nose. The act of accommodation is fast, taking only about one-third of a second. Our range of accommodation decreases with each passing year, so by the age of 45, most of us are left with about 20 percent of the amplitude of accommodation we started with.
† The question “How does accommodation ‘know’ it has achieved the sharpest focus?” seems to be best answered by a sensing system in the brain. However, some have suggested that the system takes advantage of the naturally occurring chromatic aberration of the primate eye to fine-tune focusing.
With age, the lens enlarges and becomes denser and more rigid. In so doing, it progressively loses the ability to accommodate. Parenthetically, the cornea of many birds, from pigeons to hawks, can change shape to accommodate. The avian cornea does not change flexibility with age; therefore these birds do not become presbyopic. However, there is no “free lunch” in nature. The human lens, sitting within the eye surrounded by protective fluid is far less vulnerable to injury than the cornea.
After light passes through the cornea, the aqueous humor, the lens, and the vitreous humor, it is focused onto the retinal photoreceptors. The light must pass through a number of retinal layers of nerve fibers, nerve cells, and blood vessels before striking the receptors. These retinal layers (aside from blood vessels) are transparent because of the small size of the elements and the tight packing arrangement.
The bird retina does not have blood vessels. The human retina has retinal blood vessels that cover some of the retinal receptors and produce fine angioscotomas. A bird's retina obtains much of its oxygen and nutritive supply from a tangle of blood vessels (the pectin), which is covered with black pigment and sits in the vitreous in front of the retina and above the macula (so as to function as a visor). The negative aspect of such a vascular system is vulnerability to a direct blunt or penetrating injury that can lead to a vitreous hemorrhage and sudden blindness. Obviously, this is less probable in the bird because of its lifestyle.
The rods and cones are made up of a biologic molecule that absorbs visible light and then traduces that event into an electrical nerve signal. The rhodopsin molecule is an example of Einstein's photoelectric effect. * In fact, only one quanta (the smallest possible amount of light) of visible light † is needed to trigger the molecule, that is, snap the molecule into a new shape. ‡
* Some wavelengths of light are powerful enough to knock electrons of certain molecules out of their orbits, thereby producing an electric current. Einstein was awarded the Nobel Prize for explaining the “photoelectric effect.”
† In 1942, Selig Hecht and his co-workers in New York first proved that only one quanta of visible light could trigger rhodopsin to start a cascade of biochemical events eventuating in the sensing of light.
‡ Photoactivation of one molecule of rhodopsin starts an impressive example of biologic amplification, in which hundreds of molecules of the protein transducer each activate a like number of phosphodiesterase molecules, which in turn, hydrolyze a similar number of cyclic guanine monophosphate (cGMP) molecules, which then trigger a neural signal to the brain.
The internal structure of the molecule allows the wavelengths of visible light to resonate within its electron cloud and within 20 million millionths of a second, inducing the change in the molecule that starts the reaction.
Probably the earliest chemical relative of rhodopsin is to be found in a primitive purple-colored bacteria called Holobacterium halobium. Koji Nakanishi, a biochemist at Columbia University, in an article titled “Why 11-cis-Retinal?” (a type of rhodopsin) notes that this bacteria has been on the planet for the last 1.3 billion years. Its preference for low oxygen and a salty environment places its origin at a time on earth where there was little or no oxygen in the atmosphere and a high salt concentration in the sea. Although found in primeval bacteria, bacteriorhodopsin is a rather complicated molecule, containing 248 amino acids. It is thought that this bacteria probably used rhodopsin for photosynthesis, rather than light sensing. Time-resolved spectroscopic measurements have determined that this molecule changes shape within one trillionth of a second after light stimulation. This early form of rhodopsin absorbs light most efficiently in the blue-green part of the spectrum, although it does respond to all colors.
To function as the transducer for vision, the photopigment must capture light and then signal the organism's brain that the light has registered. As noted earlier, one molecule needs only one quanta to start the reaction. Even more amazing is the molecule's stability. Although only one quanta of visible light is necessary to trigger it, the molecule will not trigger accidentally. In fact, it has been estimated that spontaneous isomerization of retinal (the light-sensing chromophore portion of rhodopsin) occurs once in a thousand years. If this were not so, we would see light flashes every time there is a rise in body temperature (a fever). To better understand the rhodopsin mechanism, one may picture a hair trigger on a pistol that takes only the slightest vibration (but only a special type of vibration) to be activated. As noted, the activating quanta must be of the proper energy level to “kick in” the reaction. That is, the quanta of light must be made of wavelengths of visible light.
Retinal receptor factors that influence the optical limits of visual acuity occur in the foveal macular area. The fovea itself subtends an arc of about 0.3 degree. It is an elliptical area with a horizontal diameter of 100 µm. The area contains more than 2000 tightly packed cones. The distance between the centers of these tightly packed cones is about 2 µm. The cone diameters themselves measure about 1.5 µm (a dimension comparable to three wavelengths of green light) and are separated by about 0.5 µm. Therefore the fine details of the retinal image occupy an elliptical area only about 0.1 mm in maximum width.
A discussion of the diffraction limits of resolution, in a theoretical emmetropic human eye, must involve the anatomic size of the photoreceptors and the pupil. A point or an object is focused on the retina as an Airy disc because of diffraction. The angular size of the Airy disc is determined as follows:
Let the wavelength be 0.00056 mm (560 nm; yellow green/light). Then
For a pupil of 2.4 mm (optimal balance between diffraction and spherical aberration in the human eye):
* One minute of arc is the spacing between the bars of a 20/20 symbol. Interestingly, the sizing of the symbol was originally determined empirically.
Given this angle of 1 minute, the actual size of the Airy disc can be calculated if the distance from the nodal point to the retina is known. The optimal distance depends on the diameter of the photoreceptors. Because these act as light guides, the theoretical limit is 1–2 µm. To obtain the maximum visual information available, Kirschfield calculated that more than five receptors are required to scan the Airy disc. Assume that each foveal cone is 1.5 µm in diameter and that there is an optimal space of 0.5 µm between receptors. The following equation describes the situation for three cones and two spaces (5.5 µm).
Substituting 0.0003 from equation (2) into equation (3) gives equation (4):
From equation (4) the distance from the nodal point to the retina can be rounded off to 18.00 mm, which is close to the distance between the secondary nodal point and the retina in the schematic human eye.
How closely does optical theory agree with reality? The average visual acuity for healthy eyes in the age group younger than 50 was better than 20/16. In the distribution within the group younger than 40, the top 5 percent had an acuity of close to 20/10.
Another related factor must be kept in mind. The fixating eye is in constant motion, as opposed to a camera on a tripod. Presumably, these movements prevent bleaching or fading within individual photoreceptors. These small movements, called either tremors , drifts , or microsaccades , range in amplitude from seconds to minutes of angular arc. Such movements tend to smear rather than enhance our traditional concept of visual resolution. It can only be presumed that to maintain high resolution within the context of this physiologic nystagmus, the visual system takes quick, short samples of the retinal image during these potentially smearing movements and then recreates an image of higher resolution.
The unique essence of the vertebrate retina is that the structure of the transparent optical components, the rhodopsin molecule, and the size of the foveal cones are all tuned to interact optimally with wavelengths of visible light. It is earth's unique atmosphere and its unique relationship to the sun that have allowed primarily visible light, a tiny band from the enormous electromagnetic spectrum of the sun, to rain down upon us at safe energy levels. Our eyes are a product of an evolutionary process that has tuned to these unique wavelengths at these levels of intensity.
With this basic science background, we can discuss how functions such as visual acuity and contrast sensitivity are monitored in a clinical setting.
The idea that the minimum separation between two point sources of light was a measure of vision dates back to Hooke in 1679, when he noted “tis hardly possible for any animal eye well to distinguish an angle much smaller than that of a minute: and where two objects are not farther distant than a minute, if they are bright objects, they coalesce and appear as one.” In the early nineteenth century, Purkinje and Young used letters of various sizes for judging the extent of the power of distinguishing objects. Finally, in 1863, Professor Hermann Snellen of Utrecht developed his classic test letters. He quantitated the lines by comparison of the visual acuity of a patient with that of his assistant, who had good vision. Thus 20/200 (6/60) vision meant that the patient could see at 20 feet (6 m) what Snellen's assistant could see at 200 feet (60 m).
The essence of correct identification of the letters on the Snellen chart is to see the clear spaces between the black elements of the letter. The spacing between the bars of the “E” should be 1 minute for the 20/20 (6/6) letter. The entire letter is 5 minutes high. To calculate the height of “ x ” (i.e. a 20/20 or 6/6 letter), use the following equation:
* The 20/200 (6/6) letter is 10 times larger, or 3.6 inches (9.14 cm).
In clinical visual acuity testing, the chart luminance should (1) represent typical real work photopic conditions and (2) be set at a level where variation produced by dust accumulation in the projector system, bulb decline, or normal variation in electrical current levels minimally affect visual performance. Thus chart luminance between 80 and 320 cd/m 2 meet such criteria (160 cd/m 2 is a favorite level of illumination).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here