Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intercellular communication is critical to embryonic development, tissue differentiation, and systemic responses to wounds and infections. These complex signaling networks are in large part initiated by growth factors. Such factors can influence cell proliferation in positive or negative ways, as well as inducing a series of differentiated responses in appropriate target cells including survival, apoptosis, and differentiation. The interaction of a growth factor with its receptor by specific binding in turn activates a cascade of intracellular biochemical events ultimately responsible for the biological responses observed. Several classes of receptors are involved in transducing these extracellular signals. These include receptor tyrosine kinases, G-protein coupled receptors, and cytokine receptors. Cytoplasmic molecules that mediate these responses have been termed second messengers. The transmission of these biochemical signals to the nucleus leads to the altered expression of a wide variety of genes involved in mitogenic, survival, and differentiation responses.
As knowledge has accumulated in the area of signal transduction and the complexities increase, it is becoming apparent that overlap exists in cell signaling. This functional redundancy may be seen at several levels. The simplest example would be the fact that several different extracellular signals can lead to the activation of the same pathway. Physiologically, this may serve to allow a cell to respond to a variety of different situations or stresses while conserving some of the downstream machinery. Although some of the components of certain pathways may be common for two different stimuli, the ultimate physiological response may differ greatly because of the activation of a different repertoire of nuclear response elements. In addition, although redundancy may exist in terms of the ability of a stimulus to perturb a specific pathway, it is conceivable that the kinetics and magnitude of activation may differ, leading to distinct outcomes.
There is often redundancy among different isoforms of certain proteins or with members of particular gene families. This is illustrated by the fact that targeted disruptions of some genes fail to produce detectable phenotypes in mice, indicating that other proteins can compensate for their loss. An attractive explanation for this redundancy is that it serves as a fail-safe mechanism to ensure proper functioning in the face of damaging mutations that lead to a loss of function. Indeed, as discussed in more detail later, proteins within a family often have overlapping functions and may in some situations complement one another.
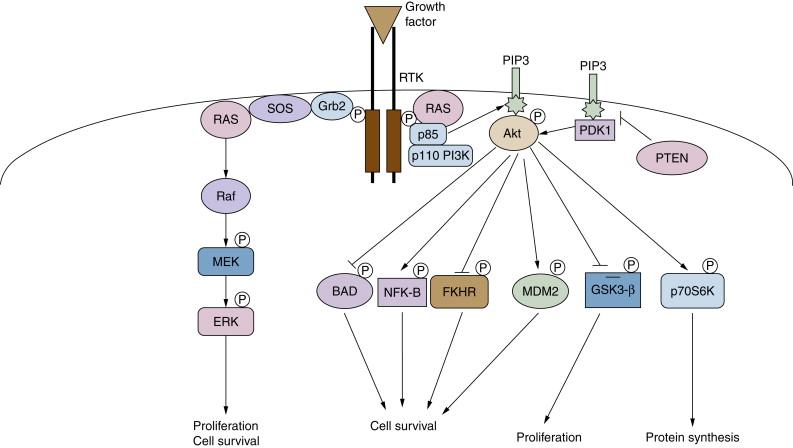
In order to effectively coordinate signaling cascades, nature has created a variety of molecules known as adaptor and scaffolding proteins. These proteins play an integral role in intracellular signaling by recruiting various proteins to specific locations, as well as by assembling networks of proteins particular to a cascade. Adaptor proteins, through protein-protein interactions via specific motifs, provide a link between molecules of a signaling cascade and proteins such as receptor tyrosine kinases (RTKs; Figure 2-1 ). Adaptors can be docking proteins, which provide multiple binding sites on which effector molecules can attach, thereby expanding the magnitude of responses from an activated RTK. Scaffolding proteins also exist in signaling cascades and allow the formation of multienzyme complexes that are involved in a particular cascade. These are important for two reasons. The first is that the activation of a signaling cascade by a growth factor is an extremely rapid process and is not likely to occur as a result of proteins randomly floating in the intracellular milieu until they happen to come in contact with each other. Scaffolding proteins ensure the close proximity of the necessary components. The second reason is that several enzymatic components of a particular signaling cascade may be shared, although the substrates of each may differ. Thus, scaffolding proteins ensure the proper routing of signals by preventing unwanted cross talk between pathways.
Oncogenes encode proteins that possess the ability to cause cellular transformation. These genes act in a dominant fashion, through either overexpression or activating mutations. There are several criteria that define cellular transformation. These include morphological changes, loss of contact inhibition, anchorage-independent growth, and the ability to form tumors when transplanted into nude mice. For example, under normal physiological situations, a growth factor binding to a receptor produces a very transient activation of a certain signaling cascade allowing tightly regulated responses such as proliferation to occur. When downstream components of these cascades are mutated in a way that causes them to be constitutively active, the signal is no longer transient and regulated but is aberrantly turned on in a continuous fashion. In addition to activating mutations, these genes can be activated by overexpression at levels much higher than in normal cells. Proto-oncogenes are commonly involved in cellular signaling, and specific examples are discussed later in the context of their roles in signal transduction.
Initially, it was believed that cellular transformation was caused solely by unregulated cell proliferation induced by activation of oncogenes. It is now known that although deregulated proliferation is most likely a necessary component for transformation, it is probably not sufficient, and other changes, such as modulation of cell survival functions, are critical as well. In fact, as discussed later, certain oncogenes function to modulate cell survival.
In the early 1980s, approaches aimed at identifying the functions of retroviral oncogenes converged with efforts to investigate normal mitogenic signaling by growth factors. Analysis of the predicted sequences of a number of retroviral oncogene products uncovered several with similarities to the prototype v-src product, whose enzymatic function as a protein kinase had been identified. Unlike many protein kinases, which phosphorylated serine and/or threonine residues, the v-src product was a protein kinase capable of specifically phosphorylating tyrosine residues. Later efforts to identify oncogenes led to the discovery of the small GTPase Ras, which was unmasked as a transforming gene by transfection of tumor cell genomic DNA.
Independent efforts to purify and sequence growth factors led to the discovery that the sequence of the platelet-derived growth factor (PDGF) B chain matched the predicted product of the transforming gene of simian sarcoma virus, designated v -sis . The v-erbB gene of avian erythroblastosis virus, which predicted a v-src–related protein tyrosine kinase, was then found to represent a truncated form of the epidermal growth factor receptor (EGFR). Independent evidence demonstrated that EGF triggering of its receptor resulted in tyrosine autophosphorylation. Thus, a direct link between growth factors, receptors with tyrosine kinase activity, and oncogenes was firmly established. The proliferation, differentiation, functional activity, and survival of cells can be affected by a wide array of other cytokines that signal through transmembrane receptors that lack protein tyrosine kinase activity. Because these signaling systems have also been implicated in malignant transformation, they are described in this chapter as well.
Membrane-spanning RTKs contain several discrete domains, including their extracellular ligand binding, transmembrane, juxtamembrane, protein tyrosine kinase, and carboxy-terminal tail domains. Interaction of a growth factor with its receptor at the cell surface leads to a tight association, so that growth factors are capable of mediating their activities at very low concentrations. In the general model of RTK activation, ligand binding induces receptor dimer or oligomer formation associated with activation of the tyrosine kinase domain. However, the dimerization mechanism can be very different among the 20 families of RTKs, and the contribution of the ligands varies. For example, nerve growth factor (NGF) dimers directly mediate the coupling of two TrkA receptors, whereas EGF molecules do not contribute to the EGFR dimerization interface, but instead induce a conformational switch, which unmasks a dimerization domain in the EGFR extracellular region. Also, it should be noted that certain RTKs, such as the insulin and insulin-like growth factor receptors, form inactive dimers that are triggered in the presence of their ligands through conformational changes.
Among the different RTKs, the tyrosine kinase is the most conserved domain, and its integrity is absolutely required for receptor signaling. For example, mutation of a single lysine in the ATP binding site, which blocks the ability of the receptor to phosphorylate tyrosine residues, completely inactivates receptor biological function. In the presence of their ligands, the conformational changes in the activated receptors overcome cis -autoinhibitory interactions in the kinase, the juxtamembrane, and the carboxy-terminal domains, which allow trans -phosphorylation on tyrosine residues that serve as docking sites for different adaptor and second messenger proteins.
The juxtamembrane sequence that separates the transmembrane and cytoplasmic domains is not well conserved among different families of receptors. However, juxtamembrane sequences are highly similar among members of the same family, and studies indicate that this stretch plays a role in the modulation of receptor function. For example, addition of PDGF to many types of cells causes a rapid decrease in high-affinity binding of EGF to its receptor, which is mediated by the phosphorylation of the EGFR juxtamembrane domain induced by PDGF-triggered protein kinase C (PKC), a process termed receptor transmodulation. The carboxy-terminal domain of the receptor is thought to play an important role in the regulation of kinase activity. This region typically contains several tyrosine residues, which are autophosphorylated by the activated kinase. In fact, the receptor itself is often the major tyrosine phosphorylated species observed following ligand stimulation. Tyrosine phosphorylation of the carboxy-terminal domain can modulate kinase catalytic activity and/or the ability of the kinase to interact with substrates. Thus, mutations that alter individual tyrosine sites or deletions of this domain have the effect of attenuating kinase function.
The constitutive expression of a growth factor and its specific receptor by the same cell may be sufficient to establish a so-called autocrine loop that contributes to tumor progression. Autocrine transforming interactions have been identified in a number of human malignancies. At least one PDGF chain and one of its receptors have been detected in a high fraction of sarcomas and in glial-derived neoplasms. Growth factors also contribute to tumor progression by a paracrine mode. For example, continuous stimulation by growth factors in paracrine as well as autocrine modes during chronic tissue damage and repair associated with cirrhosis and inflammatory bowel disease may predispose to tumors. Some tumor cells produce angiogenic growth factors such as the vascular endothelial growth factors (VEGFs). Such growth factors cause paracrine stimulation of endothelial cells, inducing neoangiogenesis and lymphangiogenesis, which contribute to tumor progression.
RTKs are frequently targets of oncogenic alterations, which create a constitutively activated receptor, independent of the presence of ligand. This was initially demonstrated with retroviral oncogenes, v-erbB and v-fms, encoding activated forms of the EGF and CSF-1 receptor, respectively. Alterations affecting a large number of RTKs have been implicated in human malignancies. One mechanism involves the amplification or overexpression of a normal receptor. Examples include EGFR, ERBB-2, and MET (see reviews ). In some human tumors, deletions within the external domain of the EGFR receptor or mutations in its tyrosine kinase domain are associated with its constitutive activation. The RET gene is activated by rearrangement, as a somatic event, in about one third of papillary thyroid carcinomas. Germline mutations affecting the cysteine residues in the extracellular region, resulting in constitutive dimerization of these receptors caused by the formation of intermolecular cysteine bridges, are responsible for multiple endocrine neoplasia (MEN) 2A and for the familial medullary thyroid carcinoma syndrome. In contrast, point mutations in RET kinase domain, such as those involving codons V804, Y806, A883, and M918, are responsible for MEN 2B. These mutations have been shown to upregulate RET catalytic function, resulting in its genetic transmission as an oncogene. MET is overexpressed and/or mutationally activated in a variety of human tumors, including hepatocellular, gastric, and colorectal carcinomas, and activating mutations of MET have been associated with the metastatic progression of head and neck cancers. A direct role of MET in hereditary papillary renal carcinoma has also been established. This hereditary disease is characterized by multiple, bilateral renal papillary tumors, in which mutations activate constitutive kinase activity and transforming properties. Somatic mutations in MET have also been detected in some sporadic renal papillary tumors. Several other receptors, including PDGF-β, TrkA, TrkC, and anaplastic lymphoma kinase (ALK) have been shown to be oncogenically activated in human malignancies by gene rearrangements that lead to fusion products containing the activated TK domain.
Knowledge of the cascade of biochemical events triggered by ligand stimulation of tyrosine kinase receptors has increased rapidly in recent years and provides further evidence of the importance of these signaling pathways in cancer. The PDGF system has served as the prototype for identification of the components of these systems. Certain molecules become physically associated and/or phosphorylated by the activated PDGF receptor kinase. Those identified to date include phospholipase C (PLC)-γ, phosphatidylinositol-3’-kinase (PI-3K) regulatory subunit (p85), Nck, the phosphatase SHP-2, Grb2, Crk, ras p21 guanosine triphosphatase (GTPase)-activating protein (GAP), and src and src-like tyrosine kinases. PLC-γ is one of several PLC isoforms and is involved in the generation of two important second messengers, inositol triphosphate (IP 3 ) and diacylglycerol (DAG), which cause release of stored intracellular calcium and activation of PKC, respectively. PKC participates in cell proliferation, survival, and migration. Although different isoforms can play distinct and sometimes opposite roles in such processes, evidence for the involvement of PKC in tumorigenesis and cancer progression is substantial.
PI-3K phosphorylates the inositol ring in PI in the 3’ position and becomes physically associated with a number of activated tyrosine kinases. This protein contains an 85-kDA regulatory subunit, which is tyrosine phosphorylated, and a 110-kDa catalytic subunit. PI3-K appears to play a major role in cell survival signaling, as discussed later (see Figure 2-1 ).
Ras small GTP-binding proteins are a major point of convergence in receptor tyrosine kinase signaling and are an important component of the cellular machinery necessary to transduce extracellular signals (see review ). These membrane-bound intracellular signaling molecules mediate a wide variety of cellular functions, including proliferation, differentiation, and survival. The Ras family of GTPases contains 39 proteins encoded by 36 genes in the human genome; it includes H-, N-, and K-Ras, R-Ras, Rap1 (A and B), TC21, and Rheb1/2. Ras proteins are synthesized in the cytosol and become associated with the inner leaflet of the plasma membrane via posttranslational modifications, including a form of fatty acid lipidation, isoprenylation, on Cys-186. The carboxy-terminal CAAX box (Cys, two aliphatic amino acids, followed by any residue) is an essential motif required for Ras function, as it targets the unprocessed protein for this essential modification.
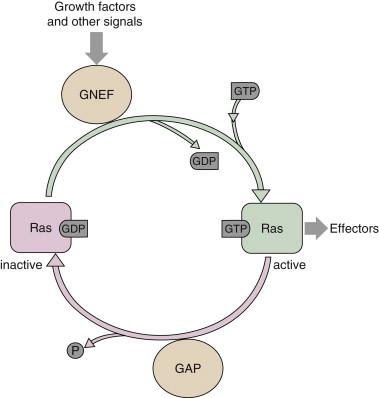
Ras proteins act as molecular switches alternating from an inactive guanosine diphosphate (GDP)-bound state to an active GTP-bound state. The paradigm for Ras activation involves the recruitment of a guanine nucleotide exchange factor (GNEF) to the membrane in response to growth factor binding and subsequent activation of a receptor tyrosine kinase. GNEFs promote the release of GDP from the Ras catalytic pocket, and the relative abundance of intracellular GTP as compared to GDP ensures preferential binding of GTP ( Figure 2-2 ). The best example of a Ras GNEF is SOS (son of sevenless), which is brought to the membrane by its stable association with the adaptor protein Grb2 (see Figure 2-1 ). Grb2 contains an src-homology 2 domain (SH2), which binds to a specific motif containing phosphorylated tyrosine residues on several RTKs, including the PDGFR and the EGFR. Grb2 also has two SH3 domains that mediate its binding to SOS via a carboxy-terminal proline-rich region. Alternatively, another adaptor protein, Shc, can bind to the cytoplasmic tail of the receptor through its SH2 domain, resulting in its phosphorylation on tyrosine and subsequently binding Grb2. The exact sequence of binding of adaptors depends on the receptor and cell type. Once SOS is translocated to the membrane, it can promote the release of GDP from Ras, allowing GTP, which is present in excess in the intracellular environment, to bind and ultimately lead to Ras activation. Although Ras is a GTPase, its intrinsic GTPase activity is actually quite inefficient and requires additional proteins known as GTPase activating proteins (GAPs) to promote GTP hydrolysis (see Figure 2-2 ). GAPs can accelerate GTP hydrolysis by several orders of magnitude and are, thus, negative regulators of Ras functions. The mechanism by which GAP accelerates the GTPase reaction is complex and not completely understood. Currently, several GAPs for Ras have been identified, including p120 GAP and NF1-GAP/neurofibromin, as well as GAPs with preferential activity on related proteins such as Rap. Of particular interest is NF1, as it is found to be frequently inactivated by mutation in patients with the familial tumor syndrome neurofibromatosis type 1.
Ras appears to have a multitude of functions that differ depending on factors such as cell type and extracellular environment. It is paradoxical that a single gene can cause cell cycle entry and DNA synthesis in one type of cell, such as fibroblasts, and terminal differentiation in others, such as PC12. In other cell types such as myoblasts, activated Ras seems to oppose cell cycle withdrawal and differentiation into myotubes and downregulates expression of muscle-specific mRNA transcripts. In addition, Ras has been demonstrated to promote cell survival in some cell types such as those of hematopoietic lineages on cytokine withdrawal, and PC12 cells and primary sympathetic neurons on removal of NGF or other trophic factors. Although Ras mediates such important cellular processes as proliferation, survival, and differentiation, the exact contribution of H-, N-, and K- isoforms is not clear: targeted knockouts to H- and N舐Ras genes resulted in mice that did not exhibit an abnormal phenotype, whereas a K舐Ras knockout is an embryonic lethal and exhibits liver and hematopoietic defects. Of note, H舐Ras knockin into the K舐Ras locus does not perturb mouse embryonic development, implying that the phenotype of K舐Ras −/− mice is probably due to the different expression pattern of the other Ras isoforms in the embryo, rather than their incapacity to compensate for K舐Ras function.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here