Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Olfactory System
In most vertebrates, the olfactory system is altogether more important than it is in humans. Although damage to the olfactory pathway on one side is associated with anosmia on that side, olfactory deficits are often found in neurodegenerative disorders.
Limbic System
Cortical and subcortical limbic areas are prominent features of the brain in primitive mammals where they are intimately concerned with mechanisms of attack and defence, procreation, and feeding. The principal effector elements of the limbic system are the hypothalamus and the reticular formation.
The elements, pathways, and transmitters of the human limbic system provide the bedrock on which most of psychiatry and clinical psychology are built.
The olfactory system is remarkable in four respects:
The somas of the primary afferent neurons occupy a surface epithelium.
The axons of the primary afferents enter the cerebral cortex directly; second-order afferents are not interposed.
The primary afferent neurons undergo continuous turnover, being replaced from basal stem cells.
The pathway to the cortical centres in the frontal lobe is entirely ipsilateral.
The olfactory system consists of the olfactory epithelium and olfactory nerves, the olfactory bulb and tract, and several areas of the olfactory cortex.
The olfactory system possesses a dynamic neuroepithelium from which olfactory neurons project to the brain. Due to their location, the olfactory sensory neurons are vulnerable to damage. The olfactory epithelium retains neural stem and progenitor cells in the basal layers, providing the tissue an ability to effect self-repair.
The olfactory epithelium occupies the upper one-fifth of the lateral and septal walls of the nasal cavity. The epithelium contains three cell types ( Fig. 33.1 ):
Olfactory neurons are bipolar neurons, each with a dendrite extending to the epithelial surface and an unmyelinated axon contributing to the olfactory nerve. The dendrites are capped by immotile cilia containing molecular receptor sites. The axons run upward through the cribriform (‘sieve-like’) plate of the ethmoid bone and enter the olfactory bulb. The axons (some 3 million on each side) are grouped into fila (bundles) by investing Schwann cells. The collective fila constitute the olfactory nerve .
Sustentacular cells are interspersed among the bipolar neurons.
Basal stem cells lie between the other two cell types. Olfactory bipolar neurons are unique in that they undergo a continuous cycle of growth, degeneration, and replacement. The basal cells transform into fresh bipolar olfactory neurons, which survive for about a month. Replacement declines over time accounting for the general reduction in olfactory sensitivity with age.
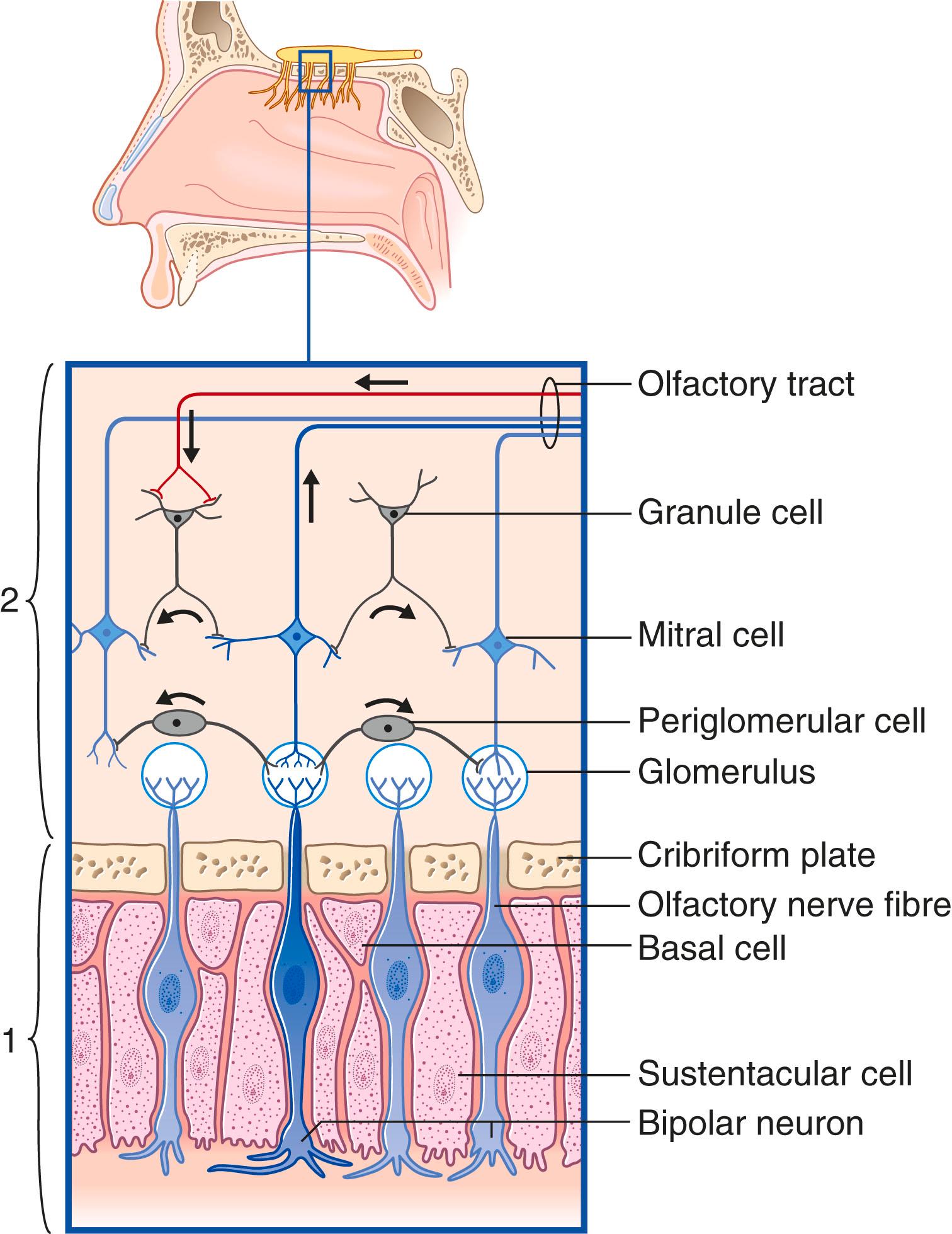
The olfactory bulb is small in comparison to the neocortex, but it is a functionally complex structure that is central to the processing and transmission of olfactory information. Research findings strongly suggest that the human adult olfactory bulb is a highly plastic structure. The exact mechanisms of the plasticity are as yet unclear.
Anatomically, the olfactory bulb consists of a three-layered allocortex surrounding the commencement of the olfactory tract. The chief cortical neurons are some 50,000 mitral (or tufted) cells , which receive the olfactory nerve fibres and give rise to the olfactory tract.
Contact between olfactory fibres and mitral cell dendrites takes place in some 2000 glomeruli , which are sites of innumerable synapses and have a glial investment, but each receives its input from sensory neurons that respond to the same stimulus ( odorant ). Glomeruli that are ‘on-line’ (active) inhibit neighbouring, ‘off-line’ glomeruli through the mediation of γ-aminobutyric acid (GABA)ergic periglomerular cells (cf. the horizontal cells of the retina), representing an initial stage of signal (specific odour) processing. Mitral cell activity is also sharpened at a deeper level by granule cells that are devoid of axons (cf. the amacrine cells of the retina), representing the next stage of signal processing and contrast enhancement between mitral cells. The granule cells receive excitatory dendrodendritic contacts from active mitral cells, and they suppress neighbouring mitral cells through inhibitory (GABA) dendrodendritic contacts.
Mitral cell axons run centrally in the olfactory tract ( Fig. 33.2 ). The tract divides in front of the anterior perforated substance into medial and lateral olfactory striae .
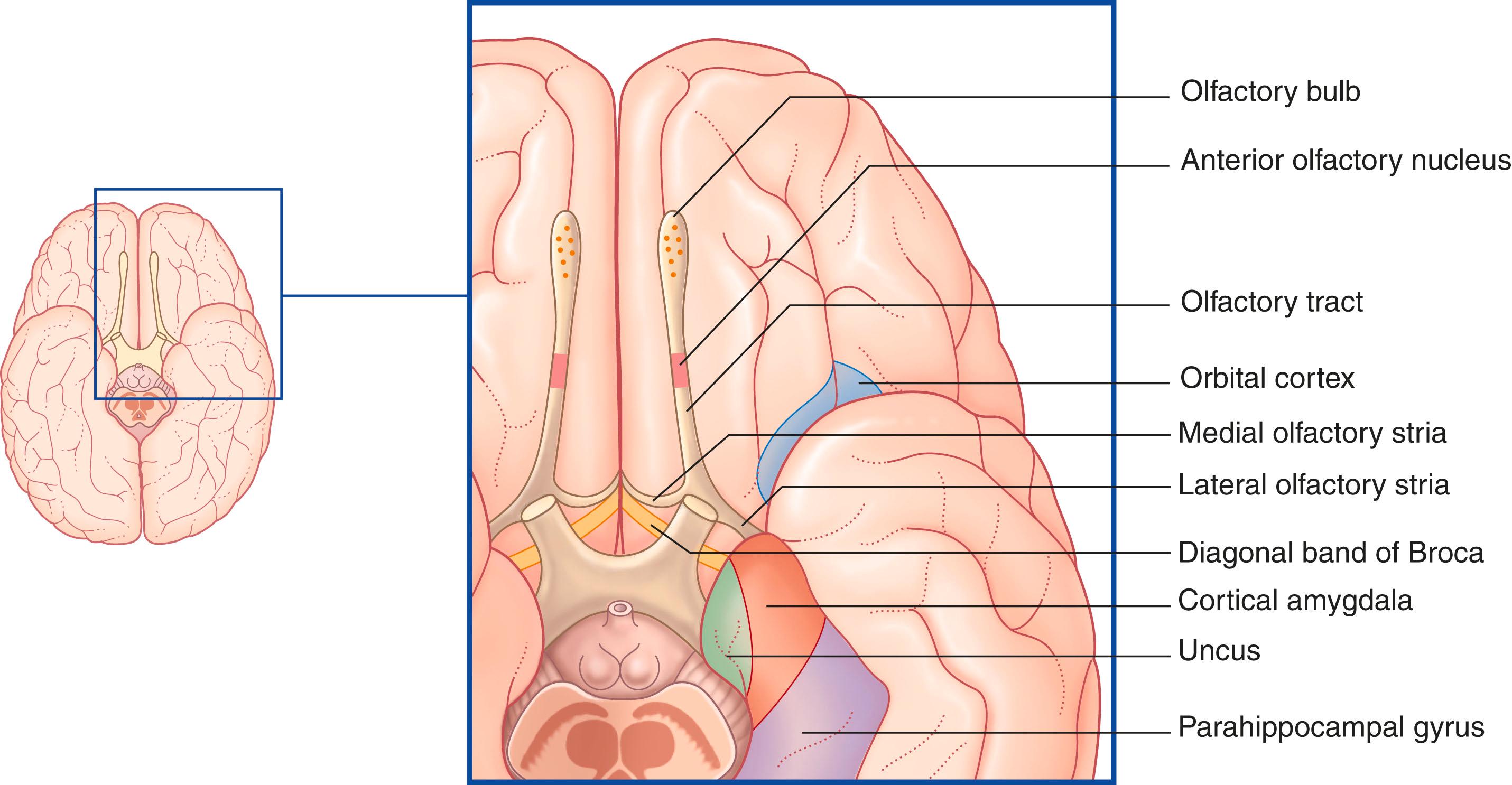
The medial stria contains axons from the anterior olfactory nucleus , which consists of multipolar neurons scattered within the olfactory tract. Some of these axons travel to the septal area via the diagonal band (see later, under Limbic System). Others cross the midline in the anterior commissure and inhibit mitral cell activity in the contralateral bulb (by exciting granule cells there). The result is a relative enhancement of the more active bulb, providing a directional cue to the source of olfactory stimulation.
The lateral olfactory stria terminates in the piriform lobe of the anterior temporal cortex. The human piriform lobe includes the cortical part of the amygdala, the uncus, and the anterior end of the parahippocampal gyrus. The highest centre for olfactory discrimination is the posterior part of the orbitofrontal cortex, which receives connections from the piriform lobe via the dorsomedial nucleus of the thalamus.
The medial forebrain bundle links the olfactory cortical areas with the hypothalamus and brainstem. These linkages trigger autonomic responses such as salivation and gastric contraction and arousal responses through the reticular formation.
Points of clinical interest are mentioned in Clinical Panel 33.1 .
The term ‘limbic system’ is most useful when defined as the limbic cortex (further defined below) and related subcortical nuclei. The term ‘limbic’ (Broca, 1878) originally referred to a limbus or rim of cortex immediately adjacent to the corpus callosum and diencephalon. The limbic cortex is now taken to include the three-layered allocortex of the hippocampal complex and septal area, together with transitional mesocortex in the parahippocampal gyrus, cingulate gyrus, and insula. The principal subcortical component of the limbic system is the amygdala, which merges with the cortex on the medial side of the temporal pole. Closely related subcortical areas are the hypothalamus, the reticular formation, and the nucleus accumbens. Cortical areas closely related to the limbic system are the orbitofrontal cortex and the temporal pole ( Fig. 33.3 ).
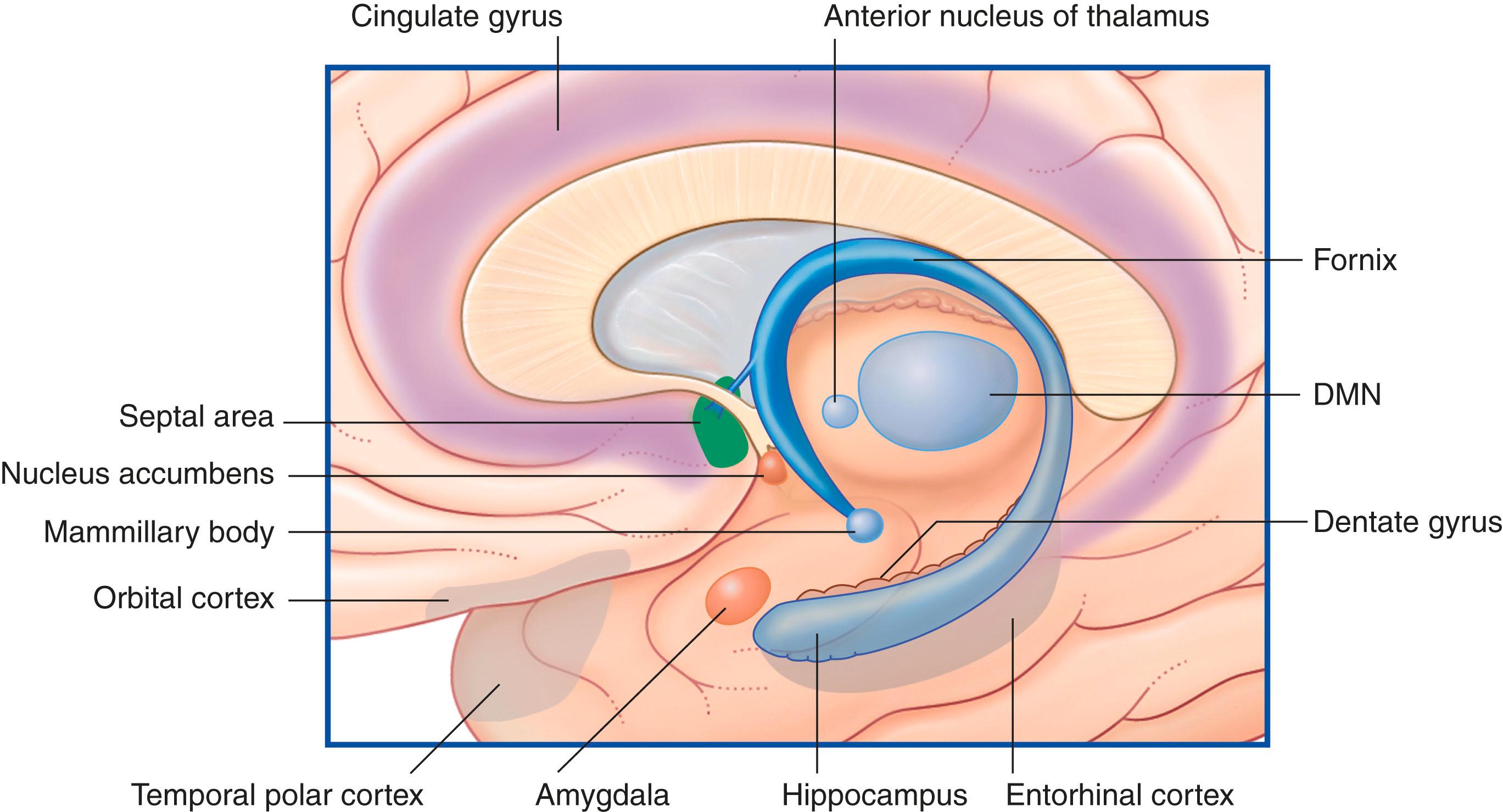
Fig. 33.4 is a graphic reconstruction of mainly subcortical limbic areas.
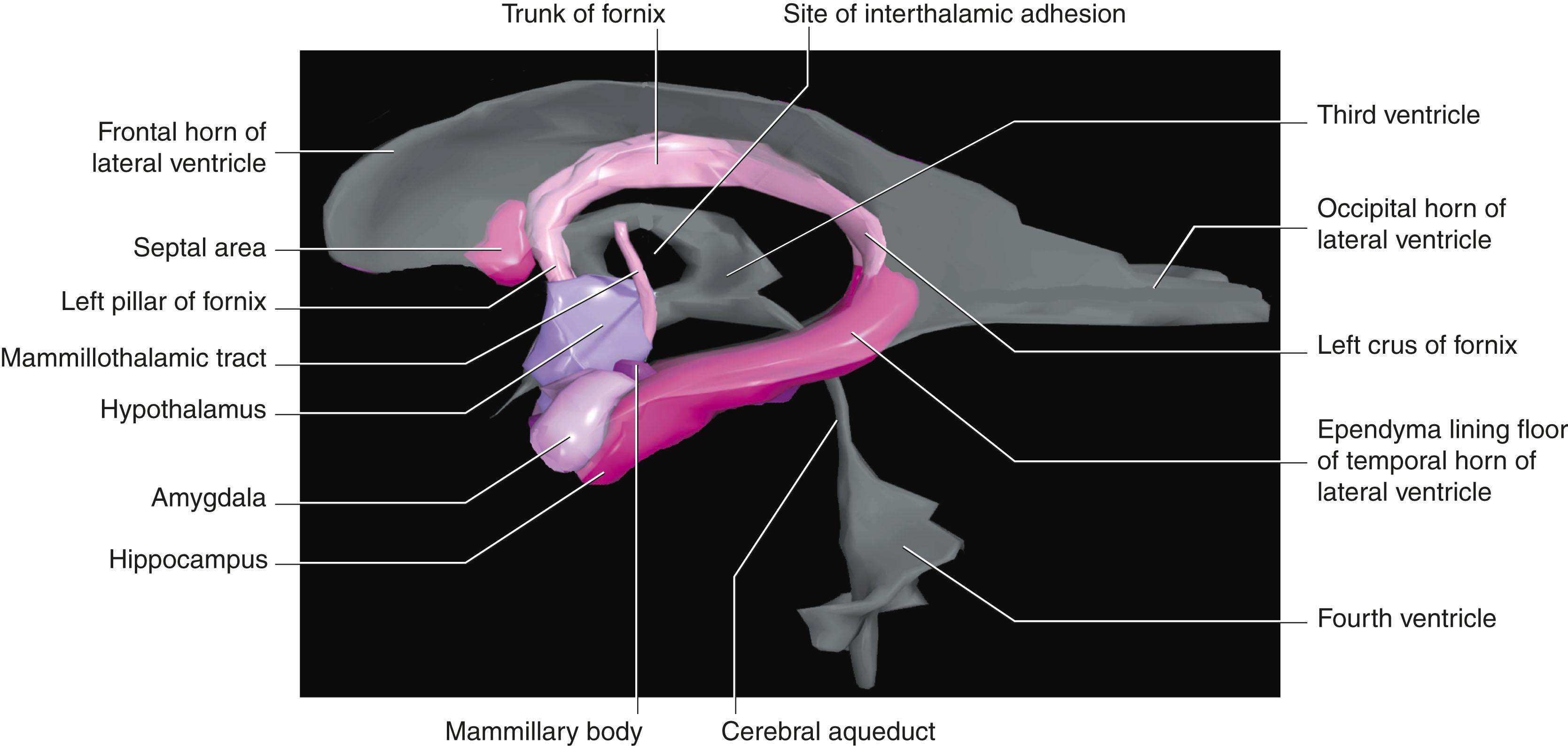
The parahippocampal gyrus is a major junctional region between the cerebral neocortex and the allocortex of the hippocampal complex. Its anterior part is the entorhinal cortex (area 28 of Brodmann) , which is six layered but has certain peculiar features. The entorhinal cortex can be said to face in two directions. Its neocortical face exchanges massive numbers of afferent and efferent connections with all four association areas of the neocortex. Its allocortical face exchanges abundant connections with the hippocampal complex. In the broadest terms, the entorhinal cortex receives a constant stream of cognitive and sensory information from the association areas of the neocortex, transmits it to the hippocampal complex for consolidation (see later), retrieves it in consolidated form, and returns it to the association areas where it is encoded in the form of memory traces. The fornix and its connections form a second, circuitous pathway from the hippocampus to neocortex.
The hippocampal complex (or hippocampal formation) consists of the subiculum , hippocampus proper , and dentate gyrus ( Fig. 33.5 ). All three are composed of temporal lobe allocortex, which has tucked itself into an S-shaped scroll along the floor of the lateral ventricle. The fornix originates from the subiculum and hippocampus as a band-like structure called the fimbria. The early neuroanatomists called the hippocampus ‘Ammon’s horn’ (or cornu ammonis), because it looked to them like a ram’s horn. They further divided the hippocampus into four regions known as cornu ammonis (CA) 1 to 4 ( Fig. 33.6A ).
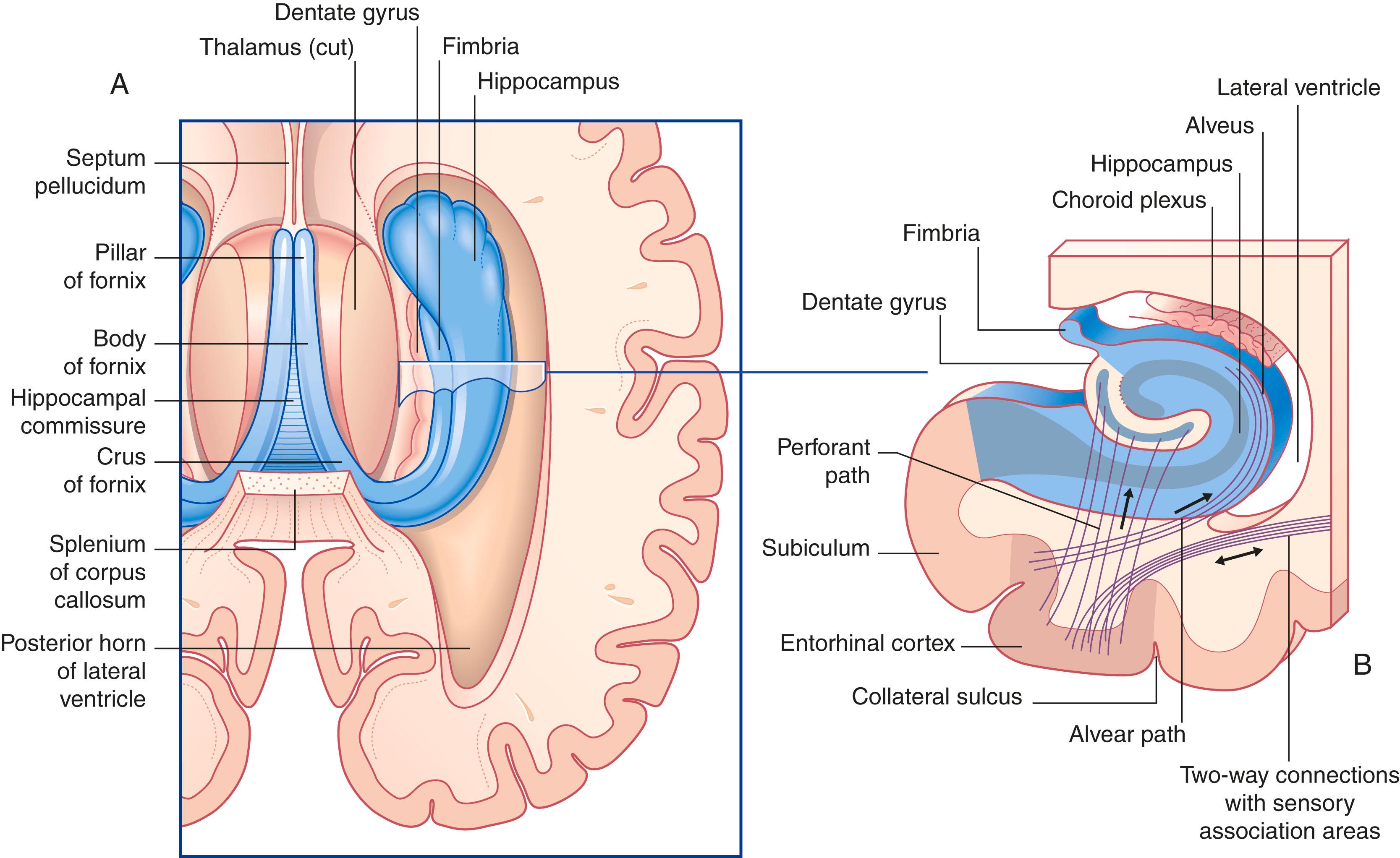
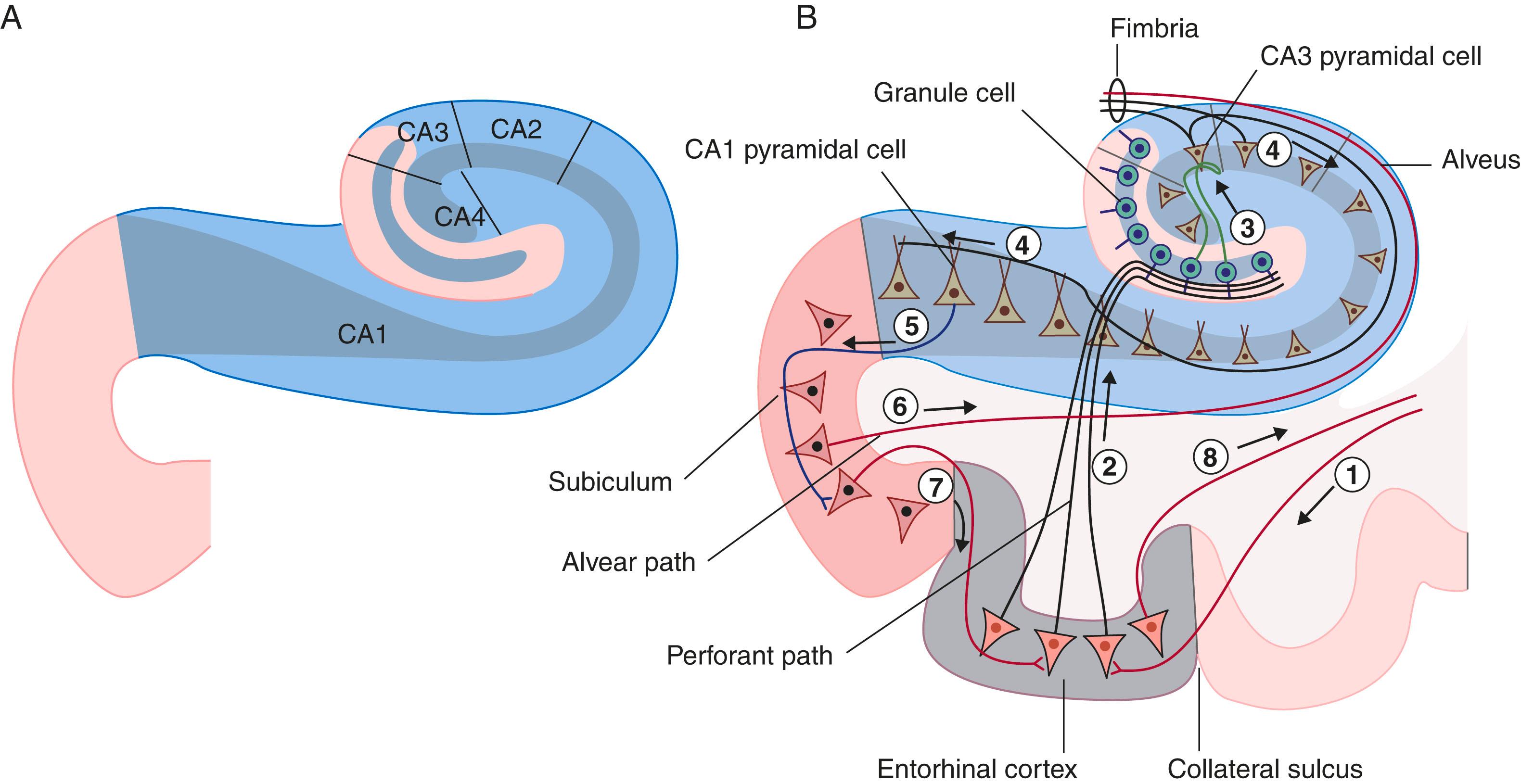
The principal cells of the subiculum and hippocampus are pyramidal cells ; those of the dentate gyrus are granule cells . The dendrites of both granule and pyramidal cells are studded with dendritic spines. The hippocampal complex is also rich in inhibitory (GABA) interneurons.
The largest source of afferents to the hippocampal complex is the perforant path , which projects from the entorhinal cortex onto the dendrites of dentate granule cells ( Fig. 33.6B ). The subiculum gives rise to a second afferent path, the alvear path , which contributes to a sheet of fibres on the ventricular surface of the hippocampus, the alveus .
The axons of the granule cells are called mossy fibres ; they synapse upon pyramidal cells in the CA3 sector. The axons of the CA3 pyramidal cells project into the fimbria; before doing so they give off Schaffer collaterals , which run a recurrent course from CA3 to CA1. CA1 projects into the entorhinal cortex.
Auditory information enters the hippocampus from the association cortex of the superior and middle temporal gyri. The supramarginal gyrus (area 40) transmits coded information about personal space (the body schema described in Chapter 32 ) and extrapersonal (visual) space. From the occipitotemporal region on the inferior surface, information concerning object shape and colour, and facial recognition, is projected to the cortex called perirhinal or transrhinal , immediately lateral to the entorhinal cortex. From here it enters the hippocampus. A return projection from the entorhinal to perirhinal cortex is linked to the temporal polar and prefrontal cortex.
In addition to the discrete afferent connections mentioned above, the hippocampus is diffusely innervated from several sources, mainly by way of the fornix:
A dense cholinergic innervation, of particular significance in relation to memory, is received from the septal nucleus.
A noradrenergic innervation is received from the locus coeruleus.
A serotonergic innervation enters from the raphe nuclei of the midbrain. The linkage between serotonin depletion and major depression is mentioned in Chapter 34 .
A dopaminergic innervation enters from the ventral tegmental area of the midbrain. The linkage between dopamine and schizophrenia is discussed in Clinical Panel 33.2 .
The largest efferent connection from the hippocampal complex is a massive projection via the entorhinal cortex to the association areas of the neocortex. A second projection is the fornix ( Fig. 33.5A ). The fornix is a direct continuation of the fimbria , which receives axons from the subiculum and hippocampus proper. The crus of the fornix arches up beneath the corpus callosum, where it joins its fellow to form the trunk and links with its opposite number through a small hippocampal commissure . Anteriorly, the trunk divides into two pillars . Each pillar splits around the anterior commissure sending precommissural fibres to the septal area and postcommissural fibres to the anterior hypothalamus, mammillary body, and medial forebrain bundle. The mammillary body projects into the anterior nucleus of the thalamus, which projects in turn to the cingulate cortex, completing the Papez circuit from the cingulate cortex to the hippocampus with return to the cingulate cortex via the fornix, mammillary body, and anterior thalamic nucleus ( Fig. 33.7 ).
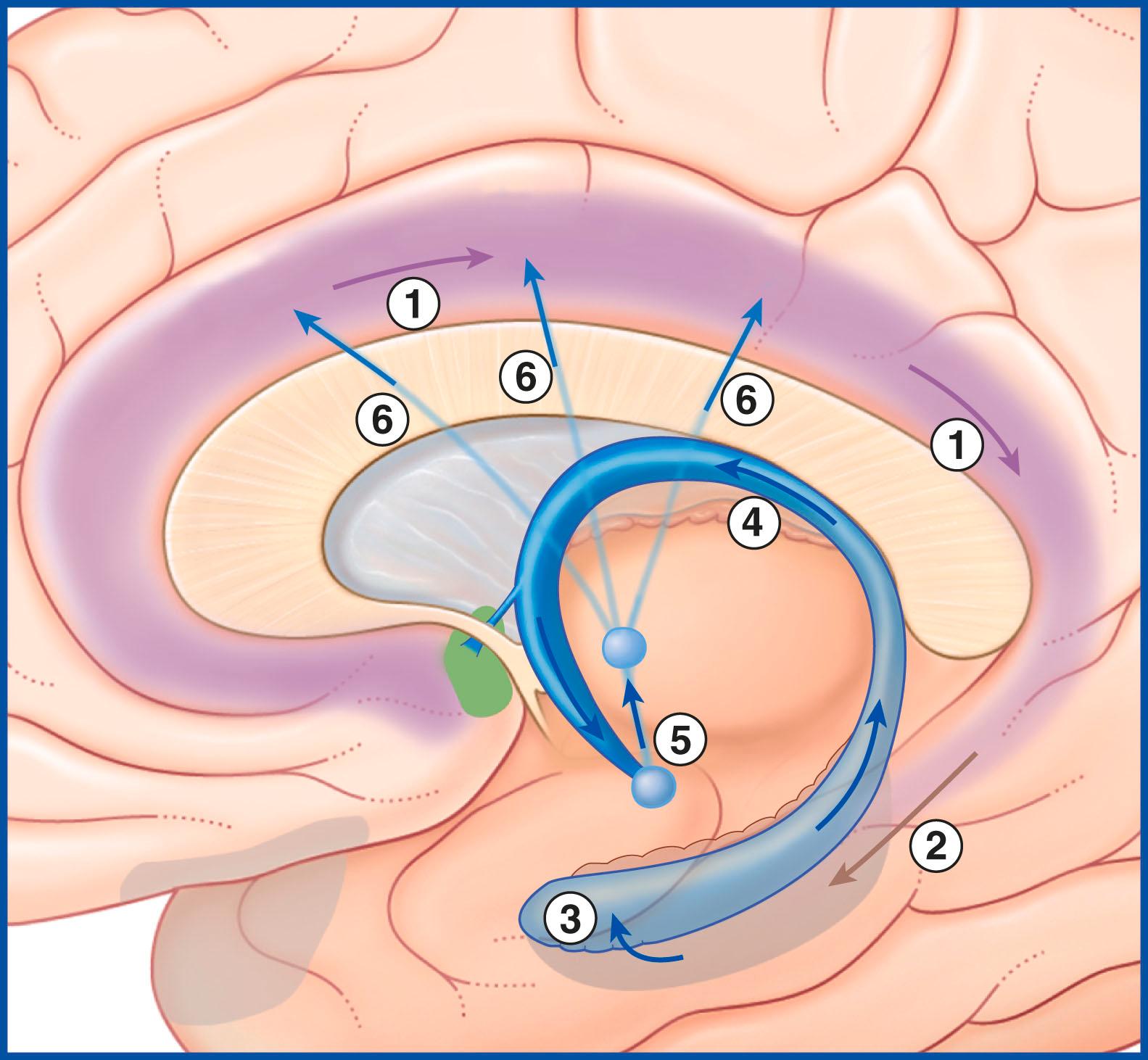
The term medial temporal lobe is clinically inclusive of the hippocampal complex, parahippocampal gyrus, and amygdala. The term is most often used in relation to seizures ( Clinical Panel 33.3 ).
The evidence for a mnemonic (memory-related) function in the hippocampal complex is discussed at considerable length in psychology texts. Some insights are given below.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here