Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The most common primary intraocular tumors are choroidal melanoma and retinoblastoma. The most common affecting the ocular adnexa are lymphoma, rhabdomyosarcoma, optic nerve glioma, and epithelial and melanocytic malignancies of the eyelid and conjunctiva.
Many systemic neoplasms can involve the eye and adnexa, especially breast and lung cancer, lymphoma, and leukemia.
Retinoblastoma is the prototypical model of a genetically transmissible tumor via loss of a tumor suppressor gene.
Molecular genetic studies have led to advances in the understanding of the pathways associated with the development of conjunctival and eyelid malignancies.
Key mutations associated with tumor initiation and the development of metastatic potential have been identified for uveal melanoma (UM).
Intraocular tumors can be directly visualized. A combination of funduscopic examination, imaging, and ultrasonography greatly facilitates diagnosis. Biopsies are limited to special circumstances.
Tumors of the conjunctiva and eyelid may be diagnosed through clinical examination, biopsy, and histopathologic examination.
Orbital and adnexal tumors are diagnosed with computed tomography, magnetic resonance imaging, and biopsy.
Eyelid and conjunctival malignancies are often managed with local excision with or without topical chemotherapy, cryotherapy, or radiation.
An understanding of the genetic alterations leading to tumorigenesis in the conjunctiva and eyelid has led to the development of targeted therapies. In some circumstances, targeted therapies are used as primary treatment of conjunctiva and eyelid malignancies. They are also used preoperatively to shrink the primary tumor and adjuvantly to prevent disease recurrence.
Treatment of retinoblastoma is evolving quickly. New modalities for the delivery of chemotherapy have led to significant improvements in globe salvage and visual outcome.
Intraocular tumors are treated with local modalities such as brachytherapy, photocoagulation, and external beam irradiation with or without chemotherapy. If these methods are not sufficient to control the tumor, of if the eye becomes painful with no useful vision, the eye is enucleated.
Orbital malignancies are frequently treated with excision and radiation or chemotherapy. In far advanced cases, exenteration may be necessary.
Metastatic malignancies may be palliated by external beam irradiation and adjuvant chemotherapy.
The most commonly encountered malignancies in the eyes of adults and children—uveal melanoma (UM), metastatic tumors to the globe, leukemia, intraocular lymphoma, and retinoblastoma (RB)—are reviewed in this section.
Conjunctival or skin carcinoma a
Orbital sarcoma
Lacrimal gland carcinoma
Most intraocular tumors arise in the retina or in the uvea, which is composed of the iris, ciliary body, and choroid. The characteristics and prognosis of uveal tumors vary depending on location within the uvea. The optic nerve head can be involved as direct infiltration or secondary to increased intracranial pressure or retrolaminar invasion. Some intraocular tumors affect the vitreous directly with seeding of neoplastic cells, hemorrhage, or secondarily as an inflammatory reaction to the neoplastic cells.
UM is the most common primary malignancy of the adult eye, but it constitutes only about 5% of all melanomas in the body. Up to 85% of ocular melanomas are uveal (primarily choroidal) in origin.
In the United States the mean age-adjusted incidence of UM is 5.1 per million. In Europe the incidence varies with latitude, with more cases in the north (>8 per million) compared with the south (2 per million).
The incidence of UM increases with age, with the median age of diagnosis being 59 to 62 years in the United States and Europe. Iris melanomas are much less common and are usually diagnosed 10 to 20 years earlier than choroidal and ciliary body melanomas.
Risk factors for the development of UM include Caucasian ethnicity, light eye and skin color, inability to tan, dysplastic nevus syndrome, ocular melanocytosis, the presence of germline breast cancer gene (BRCA) 1–associated protein 1 (BAP1) mutation, and exposure to welding ( Table 64.1 ). The evidence linking sunlight exposure with an increased risk of UM is equivocal.
|
UM develops as a result of the proliferation of uveal melanocytes, either de novo or from a nevus or melanocytoma. It has been estimated that 1 in 8845 choroidal nevi transform into melanoma, peaking during the eighth decade of life to 1 in 3238. The risk of malignant transformation and metastatic disease in UM is strongly correlated with its cytogenetic and genetic profile.
Chromosomal abnormalities have been detected in UM and include monosomy 3, trisomy 8, deletions in chromosome 1, and structural or numeric abnormalities of chromosome 6. The most reliable chromosomal predictors of metastasis are loss of chromosome 3 and gain of chromosome 6p. Gain of chromosome 6p occurs in nonmetastasizing tumors. Loss of one copy of chromosome 3 (monosomy 3) occurs most frequently in metastasizing tumors and is strongly associated with a poor outcome. In contrast, tumors with disomy 3 rarely metastasize. Monosomy 3 in UMs tends to be accompanied by chromosomal instability. Chromosomal abnormalities that occur in association with monosomy 3 include gain of chromosome 8q, which is predictive of a poor prognosis, and loss of chromosome 1p, which is involved in the progression of monosomy 3. Loss of chromosome 8p is a late genetic alteration that occurs independently from monosomy 3. However, in the setting of monosomy 3 it forecasts earlier metastasis.
UM has a low mutation burden. The oncogene and tumor suppressor mutations that are common in other cancers are mostly deficient in UM. It is also important to note that the molecular characteristics of UMs are distinct from cutaneous or mucosal melanomas, in which BRAF and NRAS mutations dominate.
The most frequently mutated genes that are considered to be drivers in UM development and progression are BAP1, X-linked eukaryotic translation initiation factor (E1F1AX1), protein Q polypeptide (GNA11), guanine nucleotide binding protein alpha11 (GNAQ), and splicing factor 3B subunit (SF3B1) ( Table 64.2 ).
| Early mutations | GNAQ or GNA11 a | |
| Downstream mutations | ||
| Poor prognosis | BAP1 b | |
| Good prognosis | SF3B1 and EIF1AX c |
a Protein Q polypeptide (GNAQ) or guanine nucleotide binding protein alpha11 (GNA11).
b Breast cancer gene 1–associated protein 1 mutation (BAP1).
c X-linked eukaryotic translation initiation factor (E1F1AX) splicing factor 3B subunit (SF3B1).
Activating mutations in GNAQ and GNA11 are the most common oncogenic driver mutations and occur in 80% to 90% of UMs. These mutations inhibit guanosine-5-triphosphatase (GTPase) activity, thereby inhibiting return to an inactive state, and activate downstream proliferation pathways, such as the RAS/RAF/MEK/ERK pathway. An inactivating somatic mutation in the BAP1 gene has been reported to occur in 84% of metastasizing UM tumors. It has been proposed that BAP1 regulates cell cycle progression, although the underlying mechanism is unknown. SF3B1 mutations are correlated with late-onset metastasis and are rarely coincident with BAP1 mutations. EIF1AX gene mutations are also associated with a good prognosis and have been reported in 24% of UMs. Preferentially expressed antigen in melanoma (PRAME) mRNA expression has been associated with an increased metastatic risk in patients with UM. PRAME expression has been found to be a marker of poor prognosis in several cancers and is thought to promote tumor progression through a variety of mechanisms including inhibition of differentiation, growth arrest, and apoptosis, and stimulation of chromosome instability.
Pathologic samples from UMs may be obtained by incisional biopsy or excision (enucleation or local tumor resection). In those eyes not undergoing incisional biopsy or excision, prognostic fine-needle aspiration biopsy (FNAB) of UM is now routinely recommended at the time of primary treatment.
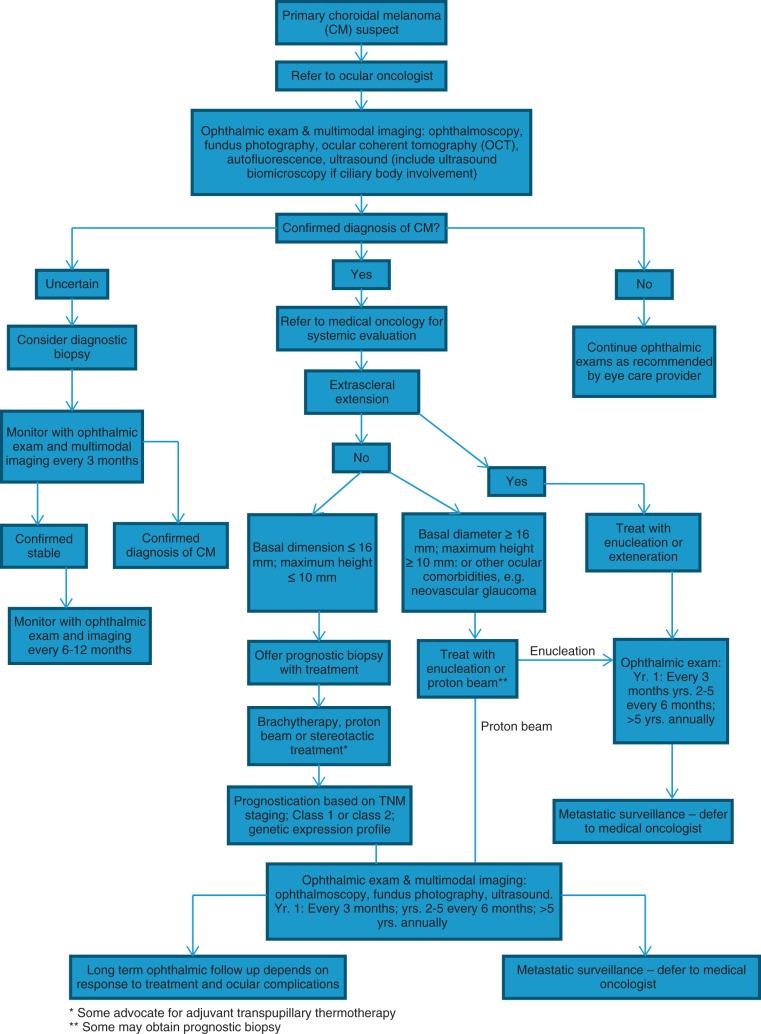
Gross examination of an enucleated eye will reveal the tumor's location, size, and growth pattern, in addition to the presence and extent of extrascleral or anterior chamber invasion. Rarely, choroidal melanoma will grow laterally, resulting in a diffuse pattern of growth. The more common direction of growth is vertical, with displacement or rupture through the Bruch membrane (inner layer of the choroid). A retinal detachment can occur owing to the accumulation of exudate between the retinal pigment epithelium (RPE) and the retina. Choroidal melanoma may on occasion invade the retina but rarely seeds the vitreous cavity. Scleral invasion has been found to be present in up to half of all medium-sized and large tumors. Intraocular melanoma can spread outside the eye through vortex veins, aqueous channels, ciliary arteries, and nerves. Extrascleral extension is rare and estimated to occur in 5% to 15% of cases. Invasion of the optic nerve is even less likely and has been reported to occur in 2% to 7% of UMs ( Fig. 64.2 ).
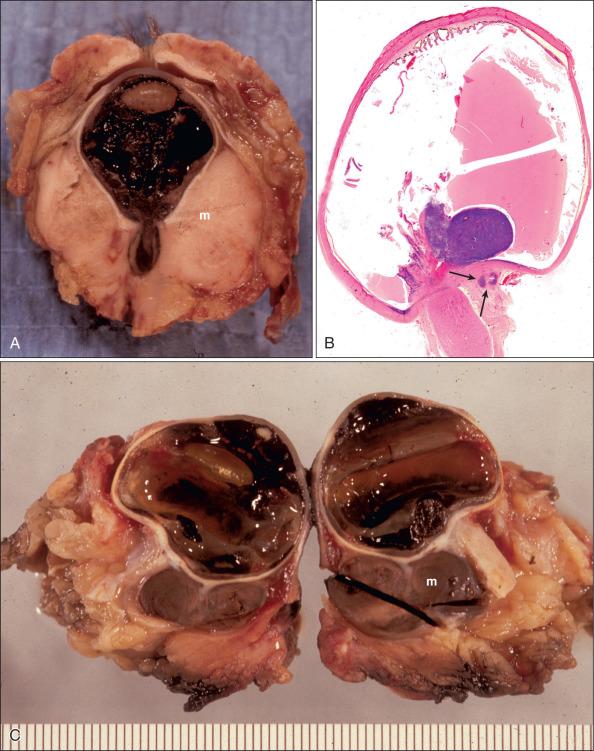
UMs are composed predominantly of epithelioid or spindle cells or a combination of these two cell types. Most iris melanomas are diagnosed as spindle cell melanoma. Large posterior UMs usually have a higher proportion of epithelioid cell than small tumors ( Fig. 64.3C–D ).
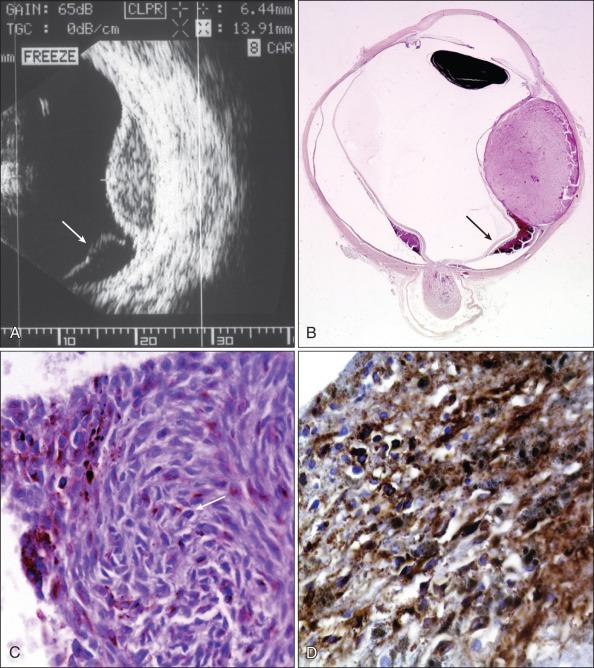
UM spreads via a hematogenous route, and up to 90% of metastases occur in the liver. Liver metastases exhibit histologic features similar to those of primary tumors.
Most patients remain asymptomatic unless the tumor involves the macula or results in a secondary retinal detachment, macular edema, lenticular astigmatism, cataract, or glaucoma. The most common symptoms, when they occur, include blurred vision (38%), photopsia (9%), floaters (7%), visual field loss (6%), visible tumor (3%), pain (2%), and metamorphopsia (2%).
Iris melanomas usually are incidental findings. The two patterns of growth for iris melanomas are circumscribed (90%) and diffuse (10%). Most circumscribed iris melanomas have a flat or rounded contour, and they are usually yellow, tan, or brown. Diffuse iris melanomas usually manifest as a unilateral dark iris (heterochromia) without nodular thickening. Iris melanomas may be associated with secondary glaucoma, angle seeding, hemorrhage (hyphema), and, rarely, extraocular extension. Ring melanoma of the anterior chamber is an uncommon variant of iris melanoma that exhibits a flat circumferential growth limited to the trabecular meshwork and anterior chamber angle. It can manifest as refractory pigmentary glaucoma.
The ciliary body is located behind the iris, in an area of the eye that is challenging to visualize directly. Consequently, ciliary body melanomas tend to be larger at the time of diagnosis, with a mean basal diameter of 11.7 mm and thickness of 6.6 mm. An extremely rare variant of ciliary body melanoma (0.3% of all uveal melanomas) occurs when the tumor extends circumferentially around the entire ciliary body. This configuration is termed a ring melanoma .
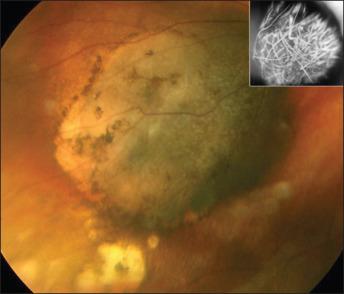
Typically a choroidal melanoma manifests as an elevated, dome-shaped subretinal mass ( Fig. 64.4 ). The mean basal diameter of a choroidal melanoma has been estimated to be 11.3 mm, and the width to be 5.5 mm ( Table 64.3 ). About 20% of the time there is rupture of the Bruch membrane, which usually results in a mushroom-shaped appearance. Approximately 6% of choroidal melanomas occur as a diffuse variant.
| Dimension | T umor S ize | ||
|---|---|---|---|
| Small | Medium | Large | |
| Diameter (mm) | < 10 | 10–15 | >15 |
| Height (mm) | < 2 | 2–5 | >5 |
UMs are usually unilateral and can be pigmented (55%), amelanotic (15%), or of mixed color (30%). They can be associated with a retinal detachment about 70% of the time and intraocular hemorrhage 10% of the time. In contrast to many other tumors, UM is usually diagnosed on the basis of clinical examination and imaging without pathologic assessment. To reduce the risk of local dissemination and vision-threatening complications, pathologic samples are usually only obtained at the time of primary local treatment.
There is compelling evidence that earlier detection and treatment of a small posterior melanoma allow for a better prognosis than with a large melanoma. Many studies have focused on clinically differentiating melanomas from lesions simulating a melanoma. The lesions that frequently mimic posterior UMs, and must be considered in the differential diagnosis, include choroidal nevi, peripheral exudative hemorrhagic chorioretinopathy, congenital hypertrophy of the RPE, hemorrhagic detachment of the retina or RPE, circumscribed choroidal hemangioma, and age-related macular degeneration. Distinguishing a small malignant melanoma from a benign nevus can be particularly challenging. Choroidal nevi and melanomas share many common characteristics. Features that help to differentiate nevi from malignant lesions include retinal pigment epithelial hyperplasia, drusen, and retinal pigment epithelial atrophy. Features that overlap with melanoma include subretinal fluid and overlying orange pigment.
One of the most widely accepted risk factors for malignant potential is documented tumor growth. However, growth is not always a reliable indicator of malignancy, because choroidal nevi can also show a very slow rate of growth and some melanomas can remain clinically stable, without growth, for years.
The Collaborative Ocular Melanoma Study (COMS) group investigated the risk factors for growth and possible malignant transformation. They reported these to be greater initial tumor thickness and diameter, presence of orange pigment, absence of drusen, and absence of areas of retinal pigment epithelial changes adjacent to the tumor. Shields and colleagues explored risk factors, based on routine funduscopic examination, for malignant behavior of small melanocytic choroidal lesions. They proposed the use of the mnemonic “to find small ocular melanoma using helpful hints daily,” which stands for thickness greater than 2 mm; subretinal fluid; presence of symptoms; presence of orange pigment; margin within 3 mm of the disc; ultrasound hollowness (medium internal reflectivity, often compatible with acoustic hollowness on B-scans); halo absent (no circular band of depigmentation surrounding the pigmented choroidal lesion); and drusen absent.
Conventional imaging techniques used for the diagnosis of choroidal melanoma include fundus photography, Optomap (Optos, Marlborough, MA), intravenous angiography with fluorescein (IVFA) and/or indocyanine green (ICG), A- and B-scan ultrasonography, three-dimensional scan ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI). Ultrasonography is an important diagnostic modality in differentiating the melanocytic lesion from a lesion mimicking choroidal melanoma ( Table 64.4 ). Ultrasound is also important to define the tumor shape and measure tumor dimensions. Standardized A-scan ultrasonography can reliably differentiate the low to medium internal tumor reflectivity of melanomas from the medium to high internal tumor reflectivity of metastatic tumors and the high internal reflectivity of nevi, choroidal hemangiomas, and osteomas (see Fig. 64.3 ). Other typical ultrasonographic features of a choroidal or ciliary body melanoma include acoustic hollowing, choroidal excavation, orbital shadowing, and spontaneous vascular movement in highly vascularized tumors. IVFA and ICG have limited value in the diagnosis of choroidal melanoma. Of the two tests, ICG affords better visualization of the intrinsic vasculature in choroidal melanoma, and the pattern of fluorescence helps to differentiate the choroidal melanoma from a choroidal hemangioma. CT and MRI are not routinely used to diagnose UM. On CT scans, posterior melanomas are hyperdense with slight to moderate contrast enhancement. With MRI, the melanomas appear hyperintense on T1- and hypointense on T2-weighted images.
| Acronym | Quality | Quantity |
|---|---|---|
| To | Thickness | >2 mm |
| Find | Subretinal fluid | Any |
| Small | Symptoms | Any |
| Ocular | Orange pigment | Any |
| Melanomas | Margin | Within 3 mm of disc |
| Using Helpful | Ultrasound | Hollowness |
| Hints | Halo | Absent |
| Daily | Drusen | Absent |
Newer imaging modalities (enhanced depth imaging–optical coherence tomography [EDI-OCT] and fundus autofluorescence [FAF]) are facilitating the detection of very early changes in small melanocytic lesions concealing malignancy. When EDI-OCT images of similar-sized melanomas and nevi were compared, the characteristic features suggestive of malignancy included increased tumor thickness, subretinal fluid, subretinal lipofuscin deposits, and retinal irregularities including shaggy photoreceptors. Melanomas show a slight intrinsic hyperautofluorescence on FAF, the brightness of which increases with pigmented tumors, with larger tumors, with associated RPE disruption, and with the presence of orange pigment ( Table 64.5 ).
| Imaging Modality | Finding suggestive of CM |
| Fundus photography | Growth |
| Ultrasonography | Low to medium internal reflectivity Acoustic hollowing Choroidal excavation Spontaneous vascular movement |
| EDI-OCT a | Subretinal fluid Subretinal lipofuscin deposits Shaggy photoreceptors |
| Fundus autofluorescence | Increased autofluorescence |
Approximately 2% of patients have evidence of detectable metastasis at initial presentation. With diagnosis of a UM, the patient will require management from a multidisciplinary team including a medical oncologist. Whether to proceed with staging is individualized for the patient and should not delay the primary management of the melanoma. Staging may consist of whole-body staging with CT scan or positron emission tomography (PET) scan; MRI with diffusion-weighted imaging to stage liver disease; and contrast-enhanced CT to stage extrahepatic disease. Brain imaging and bone scans should be reserved for patients with corresponding symptoms.
There are several prognostic factors for metastatic death from UM. Prognostic information is important to guide clinical care and to facilitate the selection of patients for trials evaluating systemic adjuvant therapy.
The anatomic prognostic indicators for metastasis are topographic location of the tumor, tumor size, and the status of any extrascleral extension. Iris melanoma has the best prognosis, whereas ciliary body melanoma has the worst prognosis. Most studies indicate a direct relationship between the size of a UM and increased risk of metastasis. Most of these studies consider small tumors to be no larger than 10 mm in largest diameter and 2 to 3 mm in height. Extrascleral extension is rare, yet when present it increases the 10-year mortality rate to as high as 75%. The American Joint Committee on Cancer (AJCC) 7th edition TMN staging system is a complex classification of variables based on the tumor size, topographic location of the tumor in the eye, and the status of any extrascleral extension. The staging system also takes into account the presence of secondary glaucoma in iris melanoma.
The pathologic prognostic factors correlating with a poor prognosis include epithelioid cell morphology, a high mitotic index, a high cell proliferation index (Ki-67), vascular invasion, higher microvasculature, and vascular loops. Specific chromosomal abnormalities and the tumor's molecular profile appear to provide a more accurate predictor for metastasis than the tumor's anatomic and histopathologic features. Gain of chromosome 6p in the presence of monosomy 3 is considered to be a low risk predictor; monosomy 3 and gain of chromosome 8q and 8p are indicators of poor prognosis.
Chromosomal abnormalities are detected by karyotyping with fluorescence in situ hybridization (FISH). This method requires a large tissue sample and is labor-intensive. It can also have a high technical failure rate with FNAB. One method of determining the genetic makeup of a tumor with much less tissue and with a much lower technical failure rate (3%) is RNA expression profiling. RNA expression profiling divides UM into two groups that correlate with chromosome 3 status and patient outcome. Class 1 tumors have good prognosis and are usually diploid chromosome 3. Class 2 tumors have poor prognosis with loss of chromosome 3. Class 1 tumors can be further subdivided into class 1A (lowest metastatic risk) and class 1B (moderate long-term metastatic risk). This test is available in the United States as DecisionDx-UM (Castle Biosciences, Friendswood, TX) ( Table 64.6 ). Recently, PRAME mRNA expression has been identified as an independent prognostic biomarker indicating increased metastatic risk in patients with class 1 and class 2 tumors. It may help to predict which patients with class 1 tumors are at increased risk for metastasis.
| Gene Expression Profile | Five-Year Metastatic Risk |
|---|---|
| Class 1 | |
| Class 1A | 2% |
| Class 1B | 21% |
| Class 2 | 72% |
Another UM prognostic test (DNA based) uses copy numbers of chromosomes 1p, 3, 6, and 8 along with microsatellite analysis of chromosome 3 and confirmation of GNAQ and GNA11 gene mutation status. In the United States this test is obtainable from Impact Genetics (Bowmanville, Ontario, Canada). Outside the United States, multiplex ligand-dependent probe amplification, which detects 31 loci across chromosomes 1p, 3, 6, and 8, is a commonly used stand-alone test that predicts high-risk and low-risk tumors using fresh or snap-frozen tissue.
Gene expression profiling (GEP) molecular class has been proposed to be a superior prognostic indicator to the anatomic features used in the TNM staging system. In addition, basal tumor diameter (and perhaps topographic location) may enhance the prognostic impact of GEP molecular classification. An online tool combining clinical, histologic, and genetic data has been developed to estimate the survival probability and approximate timing of metastatic death following treatment for choroidal melanoma (Liverpool UM Prognosticator Online [LUMPO]) and has been validated in the United Kingdom and the United States.
The primary objective of any treatment for UM is to prevent extraocular spread or metastases ( Table 64.7 ). All forms of current therapy have the potential to ultimately lead to vision-impairing complications. The management of UM is highly individualized. The choice among a wide range of therapeutic modalities is based on the patient's age and general health; tumor location and size; the extent of the tumor; the patient's preferences regarding vision and globe preservation; and the risk of metastases.
| OBSERVATION |
|
| RADIATION THERAPY |
|
| ENUCLEATION OR EXENTERATION |
|
| SURGICAL EXCISION (EXORESECTION OR ENDORESECTION) |
|
Currently there are five management options: observation, radiation therapy, enucleation or exenteration, surgical excision, and laser therapy. Close serial observation is considered in patients with indeterminate findings not typical of melanoma, or rarely in patients who happen to be medically unstable or who have vision loss in the fellow eye. The patients are usually monitored for the development of definitive features indicating malignancy as described earlier in this section.
Laser therapy has always been considered to be an attractive potential treatment method for management of choroidal melanoma; however, its applications, even with modern technology, have been limited. At present, the most effective form of laser treatment for UM is transpupillary thermal therapy (TTT). TTT is performed with an 810-nm–wavelength diode laser in an outpatient setting. The advantages of TTT include precise targeting of the laser, immediate tumor necrosis, and less collateral damage compared with plaque radiotherapy . Although vision-threatening complications of TTT can occur, TTT may be particularly advantageous for melanomas located adjacent to the macula and optic nerve, where avoidance of radiotherapy and its side effects are considerations. Unfortunately, there is a relatively high local tumor recurrence rate with TTT. The risk of relapse may be reduced if TTT is performed in combination with plaque radiotherapy on small melanomas with three or fewer risk factors.
Brachytherapy with an apex dose of 80 to 100 Gy is frequently used for UMs less than 12 mm in thickness and less than 18 mm in basal diameter ( Fig. 64.5 ). Iodine-125 ( 125 I) is the most frequently used radioactive source in the United States because of its accessibility, relatively long half-life, amount of tissue penetration, and ease of shielding ( Table 64.8 ). The current treatment delivery design includes use of a lead or gold shield with radioactive seeds set within a silicone holder inside the plaque template (see Fig. 64.5 ).
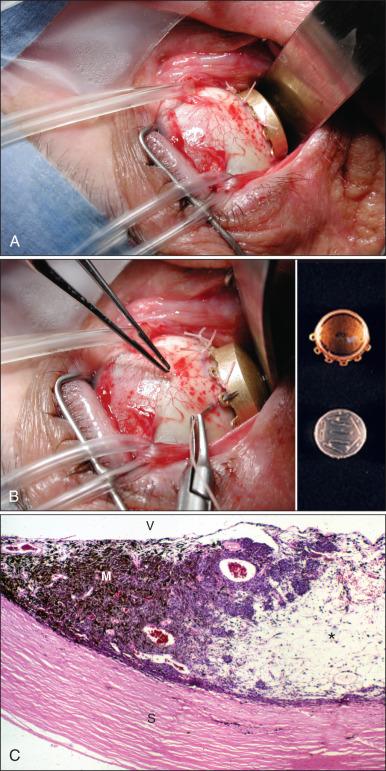
| Radionuclide | Half-Life |
|---|---|
| Cobalt-60 | 5 yr |
| Iodine-125 | 60 days |
| Ruthenium-106 | 366 days |
| Palladium-103 | 17 days |
The COMS trial reported that by the fifth year of follow-up excellent tumor control was achieved in 90% of patients; treatment failure was defined as extrascleral extension, continued growth of the tumor, or recurrence of a tumor that initially responded to radiation. Unfortunately, the visual acuity had deteriorated to 20/200 or worse in 63% of eyes.
Complication rates and final visual acuity after brachytherapy are dependent on the patient's age, systemic medical problems, and initial visual acuity; tumor location and size; presence or absence of subretinal fluid; and the type of isotope used. Visual acuity was best preserved in eyes with small tumors and those with tumors located away from the optic disc and fovea. Complications following 125 I plaque radiotherapy include radiation retinopathy, optic neuropathy, vitreous hemorrhage, cataract, neovascular glaucoma, radiation-induced dry eye, scleral necrosis, and diplopia ( Figs. 64.6 and 64.7 ).
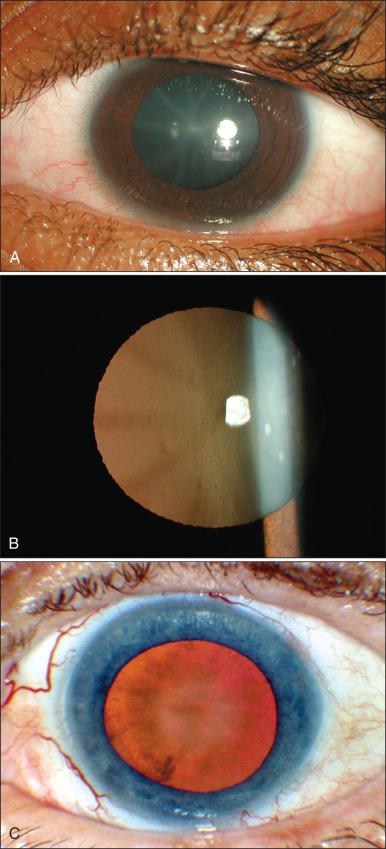
Strategies aiming to minimize vascular complications from the irradiation are being investigated and include combination of radioactive treatment with sector photocoagulation ; intravitreal injections of antivascular endothelial growth factor (anti-VEGF) or triamcinolone ; periocular triamcinolone ; surgical removal of the vitreous; and use of retinal-protective vitreous substitutes, such as 1000-cSt silicone oil.
The COMS trial demonstrated that survival rates in patients with medium-sized melanomas (basal diameter <16 mm and apical height 2.5–10 mm) treated with brachytherapy are not statistically different from those for patients whose eyes were enucleated. The 5-year rates of metastasis were 9% after brachytherapy and 11% after enucleation. Studies of juxtapapillary tumors have demonstrated similar rates of metastatic disease. Small choroidal melanomas had a much lower 5-year melanoma-specific mortality rate of approximately 4% following 125 I plaque radiotherapy.
Proton beam radiotherapy (PBRT) is another radiation delivery system used for posterior UM. Although a theoretical advantage of PBRT is better capability of directing the radiation onto the lesion, the efficacy in terms of tumor control, visual outcome, systemic prognosis, and complications is similar to that of brachytherapy. Long-term preservation of eyes is dependent on tumor size and thickness, as with plaque therapy. Proton therapy may have a substantial role in tumors that are located near the optic disc, where it may be difficult to physically place a plaque but where eye preservation is still a high priority.
Stereotactic photon beam radiation therapy using CyberKnife, Gamma Knife, or a linear accelerator may also play a role. The data regarding local tumor control, visual outcome, and survival after stereotactic photon beam radiation therapy compared with PBRT have, however, been mixed so far.
At initial presentation, approximately 30% of tumors are too large for treatment with current eye salvage techniques. Patients with these tumors are managed with primary enucleation. Enucleation is advised in tumors greater than 18 mm in basal diameter and greater than 12 mm in thickness, eyes with poor visual potential, and disease with moderate extraocular extension. Data from the COMS revealed that preoperative external beam radiation therapy (EBRT) before the enucleation of eyes containing large choroidal melanomas offers no protection against metastatic disease. Exenteration is limited to extreme cases of orbital involvement and for palliative cases.
Local resection involves en bloc tumor removal through a scleral opening (partial lamellar sclerouvectomy [PLSU]) or in a piecemeal fashion during intraocular surgery (vitrectomy). Local resection of choroidal melanoma can be performed as monotherapy and in combination with radiotherapy. These procedures are performed at only a few ocular oncology centers because of the surgical challenges, length of time required for the procedure, and potentially high rates of vision-threatening complications. Currently there is no evidence that patient survival is any longer with local resection of posterior UMs compared with enucleation or radiotherapy. However, PLSU may be an effective treatment option in selected cases of iridociliary and ciliary body tumors.
Despite effective local treatment for UM, half of the patients develop metastasis and 5-year survival rates have not improved in over 30 years. Although there are no available adjuvant systemic therapies, early detection of metastases may improve survival by allowing for identification of disease amenable to local therapies. It would also enable eligible patients' participation in clinical trials. The chapter authors believe that surveillance should be individualized and carried out by a multidisciplinary team that includes a medical oncologist. Risk stratification with anatomic criteria, cytogenetic evidence, and GEP can be used to guide surveillance decisions. There are currently no strict standards for metastatic surveillance of UM patients in the United States. However, lifelong surveillance every 6 months in patients considered to be at high risk has been recommended within the UK UM National Guidelines.
The COMS procedures for surveillance included chest radiography and liver function tests (LFTs). LFTs are no longer considered adequate. Despite high specificity (92%), LFTs have a sensitivity of less than 15% in the diagnosis of metastatic UM. Chest radiographs are no longer routinely recommended for use in surveillance. The sensitivity of chest radiography has been reported to be as poor as 2%, and CT is superior in the detection of intrathoracic metastases. In addition, chest radiographs are low yield, given that metastases predominantly occur in the liver. It has been reported that the majority of oncologists currently perform hepatic imaging as part of systemic surveillance for metastases. Hepatic imaging was also recommended in the UK UM National Guidelines.
Contrast-enhanced MRI is the most sensitive hepatic imaging tool. MRI provides high-resolution images without radiation exposure and is relatively accessible. In one study MRI detected metastases before symptoms in 92% of patients. The downsides to MRI are contraindications for patients with metallic implants and a large body habitus; expense; and the possibility of gadolinium-associated renal toxicity.
Another hepatic test that has increased sensitivity and specificity over LFTs is ultrasonography. Ultrasonography is noninvasive, inexpensive, and widely available. One screening program revealed a 95% detection of metastasis in UM patients with use of semiannual ultrasound examination with LFTs. The drawbacks of ultrasonography are that there can be false-negative findings, the examination is operator dependent, and the imaging can be limited by body habitus.
Triphasic CT has excellent sensitivity. It is faster and less expensive that MRI. However, it has a low positive predictive value because of the high frequency of misleading benign lesions, and there is radiation exposure.
PET-CT has been deemed superior to the conventional methods of screening for metastatic disease but inferior to MRI at detecting small metastases when used alone. The benefits of PET-CT include its facility to capture images of the full body and its usefulness for establishing whole-body staging. The main disadvantages of PET-CT imaging are high cost, suboptimal image resolution, and radiation exposure. It has also been suggested that only 41% of UMs are fluorodeoxyglucose (FDG) avid (in contrast to cutaneous melanomas).
There is no proven standard of care for the treatment of patients who develop metastatic disease. Despite the distinct molecular characteristics of the two cancers, chemotherapeutic treatments used for cutaneous melanoma such as dacarbazine, bleomycin, vincristine, interferon-α (IFN-α), interleukin (IL)-2, and fotemustine have been investigated for UM with disappointing results. There are limited data for the use of ipilimumab in UM. However, some studies have shown a slightly improved overall survival (OS) and progression-free survival. The effects of PD-1 inhibitors have not been established in metastatic UM. However, several clinical trials with nivolumab and pembrolizumab are in progress.
Since the liver is usually the first and only site of metastasis in most patients with UM, efforts have focused on liver-directed treatments. A survival benefit has been established in select patients undergoing surgical resection of liver metastasis. The patients who benefit are those stable enough for surgery with low tumor volume, limited to the liver. Direct targeting of the hepatic artery with fotemustine demonstrated improved progression-free survival but did not improve OS. A trial using a derivative of isolated hepatic infusion, percutaneous hepatic perfusion, and melphalan revealed an improved PFS but also did not improve OS. One study of chemoembolization demonstrated improved OS if there was less than 25% involvement of the liver. Poor responses and major complications were reported with greater than 75% tumor involvement.
More recently, increased emphasis has been placed on designing therapy that is directed toward molecular targets. The oncogenic mutations in GNAQ or GNA11 are the potential drivers of the MAPK-ERK pathway, similar to oncogenic BRAF mutations in cutaneous melanoma. Efforts have targeted the downstream components of the molecular pathways driving tumor growth including MEK (MAPK kinase) and protein kinase C (PKC).
A randomized phase II trial of selumetinib (a highly selective MEK1/2 inhibitor) versus chemotherapy (temozolomide or dacarbazine) produced promising preliminary outcomes for UM. However, further evaluations are required and are ongoing. A limited response was reported for another MEK1/2 inhibitor, trametinib.
Other small phase I and II trials targeting different pathways include gefitinib (an epidermal growth factor inhibitor), thalidomide, lenalidomide, thalidomide and lenalidomide, bevacizumab (a VEGF-blocking antibody) plus IFN-α, bevacizumab plus temozolomide, aflibercept (a “decoy” receptor that binds circulating VEGF), carboplatin-paclitaxel-sorafenib (a multikinase inhibitor), imatinib (a KIT inhibitor), and sunitinib (a multiple receptor tyrosine kinase inhibitor). Unfortunately, none of these agents or combinations have provided encouraging responses in patients with metastatic UM.
Improved understanding of UM has led to earlier detection and treatment, in addition to improved prognostic prediction. Despite this, UM remains a disease with a high mortality. Several targeted approaches are being evaluated in the hope of eventually being able to treat micrometastases before the detection of metastatic disease.
RB, the most common intraocular neoplasm of childhood, has an incidence of approximately 6 in 100,000 live births, accounting for approximately 10% of cancers during the first year of life. Approximately 300 new cases are diagnosed annually in the United States. Approximately one-third of cases are bilateral tumors. This neoplasm, primarily a disease of early childhood, has been detected even in fetal life. It also may rarely occur in children older than 5 years and more rarely in young adults.
RB is the prototypical model of hereditary neoplasms; it develops as a result of mutational inactivation of both alleles of the retinoblastoma gene (RB1). This gene is mapped to chromosomal band 13q14. The RB1 -encoded protein (pRB) is a tumor suppressor that plays a pivotal role in negative control of the cell cycle and in tumor development. Gene inactivation of pRB through chromosomal mutations is one of the principal reasons for RB development. Functional inactivation of pRb by viruses also is documented in many malignancies, including cervical cancer, mesothelioma, and Burkitt lymphoma.
The two-mutation model of Knudson (the “two-hit” hypothesis) dictates that the development of RB is caused by two corresponding chromosomal mutations. In hereditary RB, the initial occurrence, or “hit,” is a germline mutation that, through inheritance, is present in all the child's cells. The second hit occurs during development; if it occurs in a somatic cell such as the primitive photoreceptors, then the tumor develops. Therefore in hereditary RB, all cells in the body are predisposed to neoplastic development, because of the germline. This predisposition helps to explain the high incidence of multiple nonocular tumors, such as sarcomas, lymphomas, and brain tumors, in patients with hereditary RB. On the other hand, in most cases of unilateral, sporadic RB, the two hits occur during development of the retina, and both are somatic mutations. Theoretically, the remainder of the body carries no higher risk for the development of other tumors, because affected persons have a normal chromosomal pattern in cells elsewhere in the body.
It is well known that survivors of hereditary RB have an increased risk for multiple malignant and benign neoplasms, especially soft tissue sarcomas. Some reports indicate a greater than 10-fold increase in overall mortality in patients with RB compared with the general population because of the multiple primary malignancies that develop later in life. For practical purposes, a child with RB has approximately a 5% chance of having another malignancy develop during the first 10 years of follow-up, with a 20% chance during the first 20 years and a 25% chance within 30 years. Survivors of hereditary RB have a statistically significant increase of osteogenic sarcoma, leiomyosarcoma, and other soft tissue sarcomas that persists decades after the initial diagnosis. These patients should undergo lifelong medical monitoring for sarcomas. The 30-year cumulative incidence of nonocular tumor development is approximately 30% for patients with RB treated with EBRT, compared with approximately 10% for patients who did not receive radiation. Among patients with RB treated with radiotherapy, an increased incidence of soft tissue sarcomas, especially osteogenic sarcomas and leiomyosarcomas, is found both within and outside the radiation field.
Morphologically and clinically, hereditary and nonhereditary RBs are generally indistinguishable. The most important differences are that hereditary RB usually occurs at a younger age and is more likely to be bilateral and multicentric. Although approximately one-third of cases of RB are inherited, only about 5% of newly diagnosed patients have a family history. Patients with bilateral tumors and those with a positive family history can be safely assumed to have a germline mutation for the RB1 gene; these patients are at a 50% risk of transmitting the RB gene to their offspring by autosomal dominant transmission. The gene is approximately 80% penetrant, and thus clinical expression will be seen in approximately 40% of these patients' children; some offspring may be merely carriers of the gene, without development of the tumor. Approximately 15% of unilateral tumors develop by germline mutations that, by chance, affect only one eye. The remaining 80% to 85% of unilateral tumors are due to somatic mutations that involve only the retina; in such cases the disease is not passed on to future generations.
Leukocoria, or cat's eye reflex (seen in 55% of cases), and strabismus (seen in 20% of cases) are the most common presenting signs of RB in children in Western countries ( Fig. 64.8 ). RB also may manifest with atypical features such as uveitis, vitreous hemorrhage, and orbital cellulitis, particularly in older children. Any invasive treatment, including biopsy and vitrectomy, should be avoided in these children until the possibility of underlying RB has been excluded.
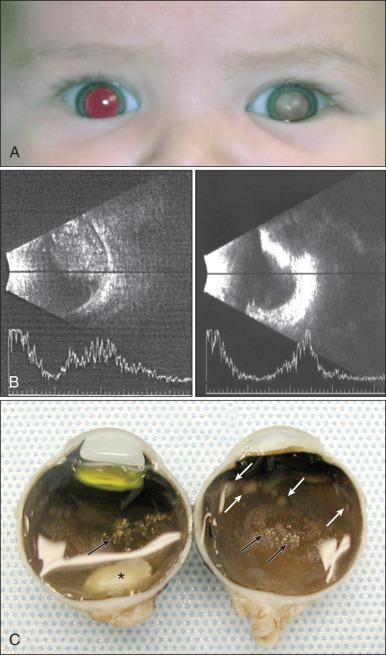
The most dependable way of diagnosing RB is by means of dilated indirect ophthalmoscopic examination performed with administration of a general anesthetic agent; a tumor, if present, is recognized as a characteristic whitish-gray mass within the eye ( Fig. 64.9 ). Dilated fundus examination also permits identification of multifocal tumor or vitreous seeding. Currently this examination is made even more rewarding with the digital image capture and storage capability of the wide-angle fundus visualization systems. The risk of general anesthesia is balanced by the benefit of improved survival of the patient and preservation or maximization of vision. The currently recommended schedule for surveillance among persons in whom a nongermline mutation is proved by genetic screening may perhaps be relaxed in the future, but in today's clinical practice, multiple examinations after administration of a general anesthetic agent within the first 5 years of life is the standard of care. Additional tests, although not always necessary, may be performed to confirm the diagnosis, but ocular biopsy in persons suspected of having RB is not performed for histopathologic confirmation because of the risk of carrying tumor cells to extraocular tissues.
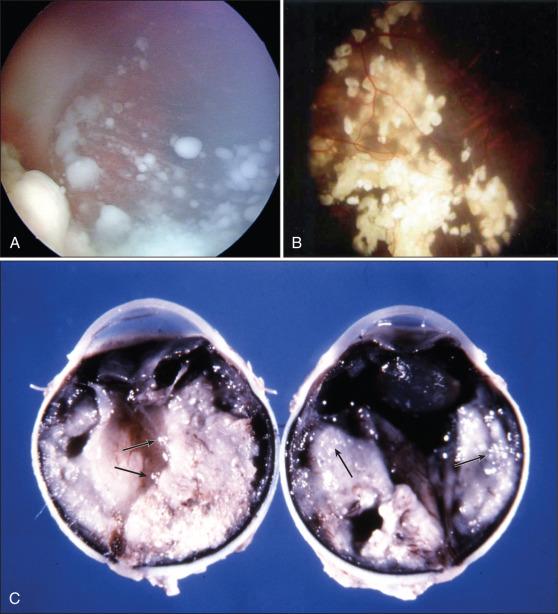
Spontaneous regression of RB is exceptionally rare. If the tumor is not treated, it will rapidly enlarge to occupy the entire globe and extend into adjacent orbital, periorbital, and intracranial tissues ( Fig. 64.10 ). The most common routes of extension are by direct infiltration of the optic nerve or sclera and spread via the choroid. The risk of neural spread depends on the level of the invasion of tumor cells into the optic nerve. Mortality rates of up to 85% and 70% have been reported if tumor cells have reached the surgical transection margin of the optic nerve and posterior to the lamina cribrosa, respectively. Additional routes of spread include dispersion of the tumor cells through the subarachnoid space into the central nervous system (CNS), lymphatic dissemination of the tumor anteriorly into the conjunctiva and eyelids, and hematogenous metastases to distant organs such as the bone, liver, and brain.
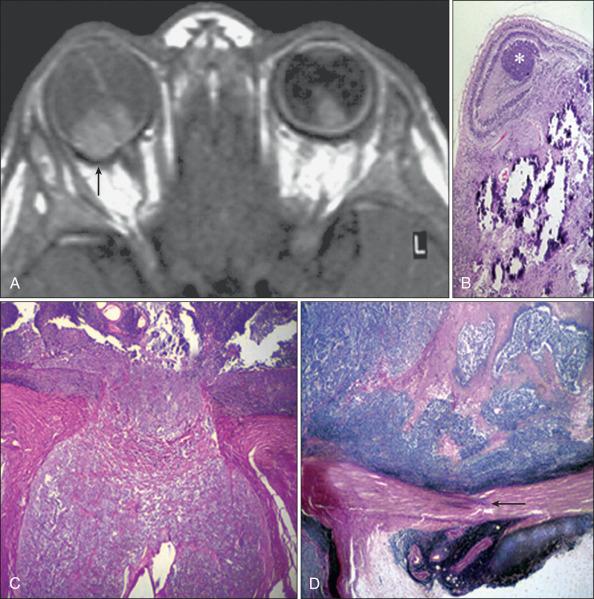
Although diagnosis of RB is usually made by the ophthalmologist on the basis of indirect ophthalmoscopy and ultrasonography, high-resolution MRI with gadolinium is an important adjunct imaging modality. MRI is mostly helpful for detecting tumor extension into the optic nerve, sclera, and orbital soft tissues; for detecting associated brain pathology; and for uncovering midline neuroectodermal tumors (trilateral RB). Implementation of a standardized MRI protocol for RB is valuable for clinical practice, particularly in cases of hereditary RB, because CT increases the exposure to ionizing radiation (see Fig. 64.10 ).
If imaging provides evidence of tumor outside the eye, a metastatic workup should be pursued. Metastatic disease is rarely suspected at the time of initial presentation, and the usual tumor staging studies are typically not performed. In the usual case of endophytic RB in which the optic nerve can be seen, the morbidity associated with metastatic screening tests outweighs their likely value. Symptoms and signs of metastatic disease may include weight loss, vomiting, headache, neurologic impairment, orbital mass, or enlarged neck nodes. A preoperative bone scan is not justified in patients with intraocular disease even when it is advanced, and such scans should be performed only in patients with documented extraocular metastatic disease.
The Reese-Ellsworth classification for RB, which has been used for more than half a century, is based on intraocular tumor staging and globe salvage prediction after EBRT; chemoreduction issues and survival are not taken into account in this categorization. Newer systems that are more suitable to current management scenarios have been proposed. The International Intraocular Retinoblastoma Classification (the “ABC classification”) is used by most practitioners. This classification stages intraocular tumors according to their prognosis after chemoreduction and adjuvant focal therapy. It consists of five groups—A, B, C, D, and E—in descending order of favorable prognosis ( Table 64.9 ).
| Group | Quick Reference | Specific Features |
|---|---|---|
| A | Small tumor | Retinoblastoma <3 mm a |
| B | Larger tumor | Retinoblastoma >3 mm or |
| Macula | Macular retinoblastoma location (<3 mm to foveola) | |
| Juxtapupillary | Juxtapupillary retinoblastoma location (<1.5 mm to disc) | |
| Subretinal fluid | Additional subretinal fluid (<3 mm from margin) | |
| C | Focal seeds | Retinoblastoma with |
| Subretinal seeds <3 mm from retinoblastoma | ||
| Vitreous seeds <3 mm from retinoblastoma | ||
| Both subretinal and vitreous seeds <3 mm from retinoblastoma | ||
| D | Diffuse seeds | Retinoblastoma with |
| Subretinal seeds <3 mm from retinoblastoma | ||
| Vitreous seeds <3 mm from retinoblastoma | ||
| Both subretinal and vitreous seeds <3 mm from retinoblastoma | ||
| E | Extensive retinoblastoma | Extensive retinoblastoma occupying >50% globe or |
| Neovascular glaucoma | ||
| Opaque media from hemorrhage in anterior chamber, vitreous, or subretinal space | ||
| Invasion of postlaminar optic nerve, choroid (>2 mm), sclera, orbit, anterior, chamber |
Other causes of leukocoria such as Coats disease (exudative retinitis or retinal telangiectasis), persistent hyperplastic primary vitreous, retinopathy of prematurity, Toxocara endophthalmitis, large retinal detachments, and, rarely, unilateral congenital cataracts may be confused with RB. Most of these conditions can be differentiated rather easily from RB with today's diagnostic technologies. Coats disease, however, may still be a problem when it occurs in young children. MRI or IVFA is helpful, particularly when Coats disease with retinal detachment is in the differential diagnosis.
RB is now considered a “curable” tumor if it is diagnosed early. Early treatment of the disease is effective at a reasonable cost, saving both life and vision. Undertaken at a late stage, however, treatment is very costly, with poor outcome, particularly for vision. This situation underlines the call for early diagnosis and the importance of public and professional awareness. The hereditary form is associated with increased risk of second nonocular primary malignancies that are even more lethal than the RB itself. Management of RB should be tailored to the individual patient.
All parameters should be taken into account, including the size, location, and laterality of the tumors; threat of metastases; risks for second malignancies; and projected visual prognosis. Today's treatment methods include chemotherapy; TTT; cryotherapy; laser photocoagulation; plaque brachytherapy; EBRT; and enucleation or exenteration.
Systemic chemotherapy is often used as the initial treatment for bilateral RB to treat the intraocular tumor, prevent metastasis, and decrease the likelihood of development of pineoblastoma. Chemotherapy may reduce tumor volume by more than 50%, making the tumor more amenable to local treatments such as laser or cryotherapy ( Fig. 64.11 ). Chemotherapy is also used to treat patients with a high risk of metastasis because of optic nerve invasion and/or massive choroidal involvement. The choice of agent, dosage, and the duration of treatment vary among centers; however, most use vincristine, carboplatin, and an epipodophyllotoxin (etoposide or teniposide). The tumor-related disadvantage of chemotherapy is the recurrence of vitreous or subretinal seeding or the appearance of new crops of RBs elsewhere (in approximately one-fourth of cases) in the eye after the discontinuation of therapy. A permanent response is almost never achieved with chemotherapy alone, and thus the response to treatment should be monitored closely and other focal therapies should be implemented before the recurrent tumor reaches a large size. In addition, intravenous chemotherapy is not without its systemic adverse effects, such as bone marrow suppression, loss of hair, and possibly more serious long-term complications such as the development of second malignancies.
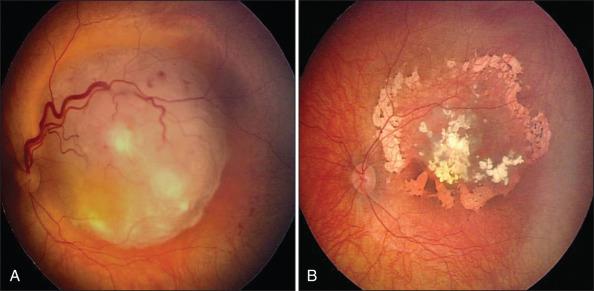
In recent years, the use of intraarterial chemotherapy (IAC) has gained momentum in the treatment of RB. With this treatment modality, anticancer drugs, usually melphalan with or without topotecan, are delivered to the tumor by selective catheterization of the ophthalmic artery. Dosage is determined by age, the size of the tumor, and vascular anatomy. Advantages of IAC include improved visual results, globe salvage, and the avoidance of systemic chemotherapy. In general, IAC is used to treat nongermline RB and recurrent tumors. Some groups have shown that IAC is effective in the management of advanced intraocular RB. Other groups underline the serious vascular complications of this technique.
Treatment of vitreous seeds is difficult with chemotherapy because the vitreous lacks vasculature. In these cases, chemotherapy agents may be injected directly into the vitreous to treat recurrent vitreous seeds or vitreous seeds unresponsive to standard therapy. One study showed a success rate of 83% in the treatment of recurrent vitreous seeds. Melphalan is the drug most commonly used. The needle and injection site are frozen with a cryoprobe as the needle is withdrawn from the eye, in order to prevent tumor seeding.
In addition, chemotherapy may be injected into the subconjunctival or sub-Tenon space. Initially this form of therapy showed promising results. However, when used alone, periocular injection of chemotherapy agents is associated with a significant treatment failure rate. In addition, there is a high incidence of complications including orbital and eyelid edema, orbital fat atrophy, optic atrophy, and fibrosis of the extraocular muscles leading to motility disturbances. Because of the significant side effects, periocular chemotherapy is rarely used anymore.
Plaque radiotherapy is a form of brachytherapy in which a radioactive seed carrier (i.e., plaque) is surgically sutured over the wall of the eye at the base of a tumor focus for transscleral irradiation (see Fig. 64.5 ). Placement of radioactive plaques (e.g., 125 I and ruthenium-106 [ 106 Ru]) is limited to tumors measuring less than 15 mm in cord diameter and 8 mm in thickness and can be used for the primary treatment of medium-sized tumors. When an RB tumor reaches these sizes, however, it usually breaks down, with seeding into the vitreous and subretinal spaces. Therefore the plaques more often are used as a secondary measure for these large tumors.
Currently, the most widely used plaque is 125 I, which delivers low-energy gamma rays that can be easily shielded. When plaque radiotherapy is given in cases with extensive subretinal or vitreous seeding, it has a high failure rate. On the other hand, when this method is used as a primary treatment of RB, it provides long-term tumor control in almost 90% of cases. It also is very effective in eyes in which a focus of RB recurs after chemoreduction. Of note, although plaque radiotherapy is much safer than EBRT in terms of local radiation toxicity, it may lead to localized radiation effects such as radiation vasculopathy, maculopathy, or papillopathy, depending on the location of the lesion. EBRT may be used to treat tumors unresponsive to other treatments. However it is rarely used anymore because of its important long-term adverse effects including midface hypoplasia and the increased risk of development of second tumors such as osteosarcomas, soft-tissue sarcomas, and brain tumors.
The other form of treatment to be taken into account for RB is enucleation. Although enucleation has become less common, it remains the treatment of choice for advanced disease in eyes with no visual potential or with high risk of metastasis. The great majority of group E cases are managed with enucleation or more extensive surgical procedures such as exenteration.
Ocular lymphomas account for 5% to 10% of extranodal lymphomas. Intraocular lymphoma can be classified by location (retinal or uveal) and whether the tumor is primary or secondary to systemic disease. Primary vitreoretinal lymphoma (PVRL) is a subset of primary central nervous system lymphoma (PCNSL). Metastasis of systemic lymphoma to the retina is exceedingly rare. Metastatic intraocular lymphoma secondary to metastasis is usually confined to the choroid. Primary uveal lymphomas may arise in the choroid, iris, and ciliary body.
PVRL usually manifests in the third to eighth decades, with a median age at diagnosis of 63 years. The true incidence of PVRL is unknown because no central database exists. One retrospective study estimated the incidence in British Columbia to be 0.017 to 0.048 per 100,000 people between 1990 and 2010. CNS involvement is reported to be seen in 16% to 34% of PVRL cases at presentation and to develop in 35% to 90% during the course of the disease. A small proportion (15%–25%) of patients with PCNSL develop PVRL. As with PCNSL, extracerebral dissemination is rare.
PVRL is considered to be a subset of PCNSL and can be classified as diffuse large B-cell lymphoma (DLBCL). Owing to distinctive clinical and biologic qualities, PVRL (PCNSL) is recognized as a specific subtype of lymphoma in the WHO classification. Over 80% of PVRL and PCNSL cases belong to the activated B-cell type of DLBCL. PVRL is characterized by the expression of B-cell markers CD79a+, CD20, PAX5, BCL-2, MUM1/IRF4, and BCL-6, and lack of CD10 and plasma cell markers. PVRL typically expresses monotypic immunoglobulin light chains and IgM or IgM and IgD heavy chains. The proliferation and apoptotic rate is high, and there is a high rate of somatic mutation of the rearranged immunoglobulin genes.
Most of the published data regarding the genetics of PVRL come from studies of PCNSL. There is contradictory information regarding chromosomal translocations. Copy number gains on chromosomes 1q, 18q, and 19q and losses on 6q have been demonstrated in PVRL. In addition, mutations in the MYD88 gene have been reported in several studies.
The pathophysiology of PVRL is not fully understood. Hypotheses include an infectious antigen-driven B-cell expansion, and chemokine influenced B-cell survival and attraction to the RPE. The lack of immune surveillance in the eye and brain might also result in development of molecular characteristics that could prevent the seeding of extracerebral sites.
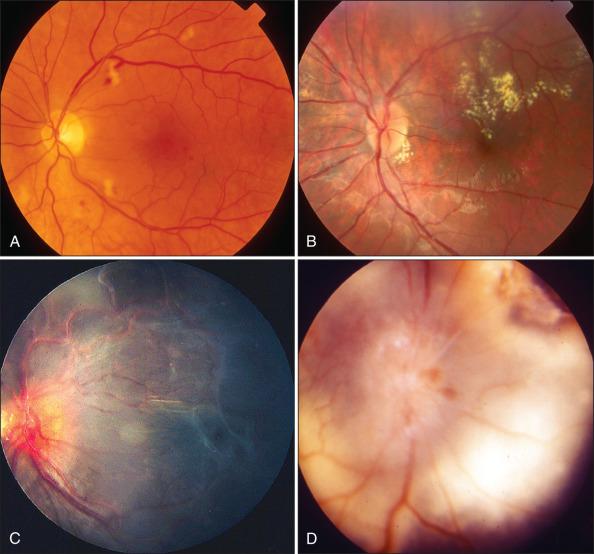
Bilateral ocular involvement is common (60%–90%), but PVRL may occur as a unilateral disease. In most cases of PVRL, at presentation the patient has uveitis-like symptoms including pain, blurred vision, and floaters. The pathognomonic features of PVRL include vitritis consisting of large vitreous cells and solid detachments of the RPE with irregular creamy-yellow deposits ( Fig. 64.12A–B ). The anterior segment is not often involved, although cells can sometimes accumulate on the posterior surface of an intraocular lens.
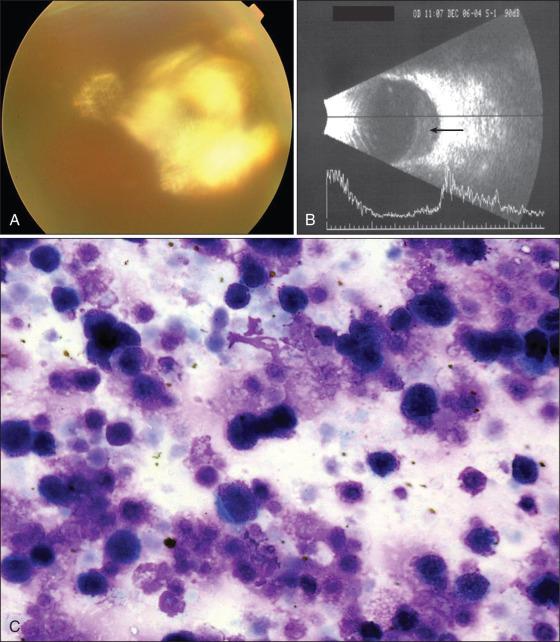
Imaging can be useful in diagnosis. A granular pattern of bright hyperfluorescent spots (corresponding to subretinal infiltrates) and hypofluorescence (secondary to the RPE atrophy) on FAF is suggestive of PVRL. Optical coherence tomography (OCT) may reveal nodular hyperreflective lesions in the RPE. IVFA can reveal a variety of patterns including RPE staining and stippling.
Although a high degree of suspicion for PVRL can be derived from clinical examination, a definitive diagnosis requires confirmation via a vitreous, aqueous, or retinal biopsy. Because the PVRL cells are fragile outside of the eye, processing within 1 hour or placement of the sample in culture medium or a mild fixative that preserves cytologic detail, immunoreactivity, and nucleic acids is recommended.
Cytologic examination of PVRL reveals the presence of large atypical lymphoid cells with increased nuclear to cytoplasmic ratio, and irregular nuclei with one to several nucleoli (see Fig. 64.12C ). Although cytologic confirmation of a diagnosis is desirable, it is challenging to obtain a diagnosis of PVRL based solely on morphologic features. Cytologic assessment confirms a diagnosis of PVRL in only 45% to 60% of cases, and false-positive results are rare. Often the diagnosis is masked by inflammation, poor preservation of the morphologic detail due to degradation of the cells, prior steroid therapy, or limited cellularity of the sample. Accordingly, several adjunctive diagnostic tests have been developed to supplement cytologic evaluation. These include immunohistochemistry, flow cytometry, molecular detection of clonal gene rearrangements, and 191 cytokine profiling of intraocular fluid.
In order to demonstrate the monoclonality of the PVRL cells, immunohistochemistry or flow cytometry is required. Immunohistochemistry involves staining the cytologic slides for CD20, kappa (κ) and lambda (λ) light chains. Flow cytometry enables a more comprehensive profiling of the intraocular cell surface markers. The use of a greater variety of cell surface markers helps to differentiate the PVRL intraocular cells from other disorders such as marginal zone lymphomas and uveitis. The downsides to flow cytometry are that it is usually more accurate with cell numbers greater than can be obtained in a typical vitreous biopsy and that it requires an expert operator.
Molecular analysis of PVRL cells can detect gene rearrangements in either IgH of B-cell lymphoma or T-cell receptor in T-cell lymphoma. In order to improve the diagnostic yield of vitreous samples, adjunct markers such as MYD88 mutations (observed with high frequency in DLBCL) and quantification of ocular microRNA are being investigated.
Cytokine profiling of the aqueous humor has been shown to be a good screening test for patients with suspected PVRL. Immunosuppressive cytokines such as IL-10 are more likely to be elevated in PVRL than proinflammatory cytokines such as IL-6. An IL-10/IL-6 ratio of greater than 1 is strongly associated with PVRL. In larger series the sensitivity of IL-10 measurements and the IL-10/IL-6 ratio in PVRL is 80% to 90%, with virtually no false-positive results reported.
The purpose of treatment for PVRL is to cure and retain vision by controlling the intraocular disease while preventing spread to the CNS. Randomized, controlled clinical trials have not been performed for PVRL, and the optimal management of isolated PVRL is uncertain. Available evidence suggests that some patients with PVRL can be effectively treated with local treatment alone, whereas other patients should be treated with systemic chemotherapy.
The controversy extends to recommendations published in the literature by two different groups. The International PCNSL Collaborative Group guidelines specified systemic treatment only in instances of CNS involvement at the time of diagnosis or bilateral ocular disease. The British Neuro-Oncology Society/National Cancer Action Team (NCAT) rare tumor guidelines (available at http://bnos.org.uk ) recommend systemic chemotherapy incorporating high-dose methotrexate-based chemotherapy with whole-globe irradiation in patients with ocular-only disease. No study has demonstrated superiority of systemic treatment over local treatment in terms of OS or progression free survival.
Local treatment may include intraocular chemotherapy with methotrexate or rituximab, or EBRT. These local treatments are associated with good local control and negligible systemic toxicity. The possible risks of intraocular injections include endophthalmitis, hemorrhage, and cataract. Intraocular methotrexate can also cause several reversible complications such as keratopathy, hypotony, and macular edema. Intraocular rituximab appears to be better tolerated, whereas ocular radiation is associated with a variety of side effects including dry eye syndrome, cataract, radiation retinopathy, and optic neuropathy.
Salvage therapy is controversial. One study showed improved survival with the use of thiotepa, busulfan, and cyclophosphamide, combined with hematopoietic stem cell rescue for relapsed or refractory PCNSL and PVRL. There is no consensus regarding the use of local or systemic therapy for isolated ocular relapse.
The prognosis of PVRL is poor because of CNS involvement. There are very few data regarding pathologic biomarkers that predict the prognosis for PVRL alone. Available prognosis models were developed for PCNSL and can be used only if there is coexistent cerebral involvement.
Although there are a few trials addressing new therapies in patients with concomitant PVRL and PCNSL in progress, there are no ongoing prospective trials exclusively for PVRL. Therapeutic advances in PVRL will require dedicated international and multicenter collaboration.
Uveal lymphomas can occur in the iris, ciliary body, or choroid. The majority of primary and secondary uveal lymphomas are restricted to the choroid. Primary iridal lymphomas are extremely rare. Compared with primary lymphomas, secondary uveal lymphomas have a higher rate of iris (20% versus 4%) and ciliary body (30% versus 8%) lymphoma.
Primary choroidal lymphomas tend to be low grade and have an indolent clinical course. The lymphoid cells of secondary disease are usually of a morphologically higher grade than in eyes with primary choroidal lymphoma. The most common systemic lymphoma subtype is DLBCL. Other systemic subtypes include chronic lymphocytic leukemia or small lymphocytic lymphoma, multiple myeloma, Waldenström macroglobulinemia, extranodal marginal zone lymphoma, and plasmablastic lymphoma.
Secondary intraocular lymphoma has also been reported to arise from orbital Burkett lymphoma and intravascular lymphoma.
Primary choroidal lymphoma is usually unilateral. It is more common in men than in women. The symptoms include recurrent, painless episodes of decreased vision and metamorphopsia. Patients with secondary choroidal lymphoma are more likely to have bilateral disease, eye pain, and severe visual loss at presentation.
Clinical manifestations include multifocal, yellow-pink choroidal swellings, with thickening of the uveal tract and possible serous retinal detachment. Uveal lymphoid infiltration has a tendency to extend through the sclera. Extension manifests as multifocal “salmon patches.” Bulky posterior extension can lead to proptosis and diplopia. Anterior segment venous engorgement, inflammation, and hyphema are more common in patients with secondary uveal lymphoma. Vitritis is also more common in secondary disease.
Primary choroidal lymphoma is distinguished from scleritis by the absence of both pain and scleral thickening on ultrasonography. Echography of the tumor usually reveals a smooth surface and low internal acoustic reflectivity. MRI depicts a smooth mass with enhancement by gadolinium. EDI-OCT can depict the choroid in high resolution (4 microns). Choroidal lymphoma is visualized with EDI-OCT as a calm, flat infiltration of the choroid if thin and an undulating “seasick” appearance if the tumor is thick. IVFA tends not to be diagnostically useful.
Although the diagnosis of uveal lymphoma can be established with a high degree of certainty based exclusively on clinical findings and imaging, definitive diagnosis requires histopathologic confirmation. Tissue for histopathologic, immunohistochemical, and flow cytometry evaluation is procured through biopsy of the epibulbar extensions or aqueous or choroidal infiltrates.
Patients with no known systemic lymphoma should undergo a comprehensive systemic evaluation to exclude the possibility of undiagnosed lymphoma. Regular evaluations are recommended in patients with high-grade histopathologic features or those with high-risk clinical features suggestive of secondary choroidal lymphomas. Without treatment, choroidal lymphoma can result in glaucoma and retinal detachment, resulting in a blind and painful eye.
There currently is no standard treatment for choroidal lymphoma. Treatment options include EBRT, immunotherapy, and chemotherapy. The prognosis for survival after primary choroidal lymphoma is favorable. The prognosis of secondary choroidal lymphoma depends on the aggressiveness of the systemic lymphoma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here