Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute and chronic renal impairment resulting from urinary tract obstruction is common among patients with cancer and not solely limited to patients with pelvic tumors. Unlike benign causes of urinary tract obstruction, urinary obstruction resulting from malignancies poses several unique clinical challenges. The rate of recurrence of the obstruction and complications from measures undertaken to relieve the obstruction, tends to be higher in the cancer population. The recovery of renal function is related to the severity and duration of obstruction. Hence it is important to diagnose and treat urinary obstruction in cancer patients promptly.
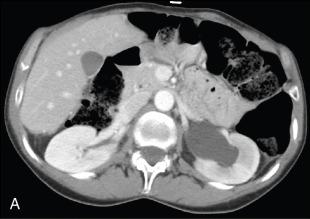
A 57-year-old woman was evaluated in the onconephrology clinic for elevated creatinine and hypertension. She had a history of stage III ovarian carcinoma treated with debulking surgery, followed by chemotherapy with cisplatin, paclitaxel, and bevacizumab 9 years ago. She was diagnosed with disease progression 7 years after the initial diagnosis and was treated with gemcitabine maintenance therapy. After 2 years of being on gemcitabine therapy, she was found to have schistocytes in the peripheral smear, normal serum creatinine, nonnephrotic range proteinuria, and a new diagnosis of hypertension requiring three antihypertensive agents. Imaging of her abdomen showed incidental finding of new left-sided hydronephrosis and dilatation of the ureter to the level of midpelvis ( Fig. 31.1 ). In addition, peritoneal involvement of malignancy was noted to be stable. She underwent retrograde ureteric stent placement with exchanges every 3 to 4 months. She continued to have difficult to control hypertension and eventually developed acute renal dysfunction for which she was referred to the onconephrologist. Repeat imaging was obtained without contrast and this showed stable peritoneal carcinomatosis and nonobstructed kidneys with left-sided ureteric stent in place. She subsequently underwent a biopsy of the right kidney, which showed features of acute and chronic thrombotic microangiopathy. Gemcitabine was discontinued, and chemotherapy was switched to paclitaxel. Hypertension control and kidney function improved, and she survived another 2 years before succumbing to her cancer.
As illustrated in the aforementioned case vignette, obstructive nephropathy in cancer patients often presents insidiously and in conjunction with other causes of renal dysfunction. This is in stark contrast to benign causes of urinary obstruction, such as nephrolithiasis or benign prostatic hyperplasia, where the clinical presentation is quite straightforward. The clinical team should perform a careful review of the patient’s history, current and previous cancer therapies to manage the renal dysfunction, and obstruction to the urinary tract. A high degree of suspicion and vigilance is warranted in managing obstructive nephropathy in the onconephrology world.
Hydronephrosis is defined as dilation of the renal pelvis and calyxes proximal to the point of obstruction. Obstructive uropathy refers to blockage of urine flow because of a functional or structural derangement, anywhere from the tip of the urethra back to the renal pelvis that increases pressure proximal to the site of obstruction. Obstructive uropathy may or may not cause renal parenchymal damage. Such functional or pathologic parenchymal damage is referred to as obstructive nephropathy . Hydronephrosis and obstructive uropathy are not interchangeable terms—dilation of the renal pelvis and calyces can occur without obstruction and urinary tract obstruction may occur in the absence of hydronephrosis.
Urinary obstruction may occur anywhere along the urinary tract, but certain anatomic sites are more prone for obstruction. This is due to the physiologic narrowing of the tract, which increases the risk of obstruction at the uretero pelvic junction, the crossing of the ureter over the common iliac vessels at the pelvic brim and the ureterovesical junction. Ureteric diameter varies along its course: the diameter is 2 to 3 mm at the ureteropelvic junction, it widens to 10 mm until it reaches the pelvic brim and narrows again to 4 to 6 mm, and finally it is narrowest at the ureterovesical junction, about 1 to 5 mm. In women, a fourth area of ureteric narrowing exists at the level of pelvic blood vessels and broad ligament. These are sites of physiologic narrowing of the ureteric diameter and hence prone for obstruction from intrinsic causes, such as calculi. In the setting of malignancy, ureteral obstruction may result from direct tumoral invasion of the ureter or extrinsic compression from lymph nodes or encasement of the ureters in tumoral tissue. Pelvic malignancies, such as prostate, bladder, cervical, uterine, ovarian, and colorectal tumors can cause obstruction by direct metastatic involvement or by external compression.
Given the anatomy of the urinary tract, differences exist in how urinary obstruction affects male and female patients. The commonest malignancies associated with urinary obstruction are cervical cancer in women and prostate cancer in men. Prostate, cervical, and bladder tumors comprise about three-fourths of the tumors causing urinary tract obstruction. The rest are breast cancer, gastrointestinal malignancies, and lymphomas causing urinary obstruction. About 10% of patients with prostate cancer present with or develop symptomatic urinary obstruction during the course of their illness. A significant proportion of patients with colorectal cancer also develop hydronephrosis with renal dysfunction. In fact, any widely metastatic malignancy causing extensive retroperitoneal lymphadenopathy can cause urinary obstruction by extrinsic compression or by causing peritoneal carcinomatosis. In addition, obstruction may also result from radiation therapy and pelvic lymphoceles.
In terms of tumors causing bilateral ureteral obstruction, cervical cancers are the commonest cause followed by stomach cancer and urologic malignancies. Primary tumors of the ureter and urethra are quite uncommon. Ureteric tumors represent only about 2.5% to 5% of transitional cell carcinomas of the urinary tract. Urethral tumors are 4 times more common in women compared with men but are generally uncommon in onconephrology practice.
Acquired obstructive nephropathy in humans results from partial urinary obstruction in most cases and tends to be prolonged in its clinical course. But most physiologic studies of renal function in obstruction are based on models of acute complete obstruction for 24 hours. In the case of cancer patients, the physiology can be altered by numerous other factors, including changes in patients’ body weight, nutritional status, and vascular tone. Regardless, the animal models of acute urinary obstruction illustrate several key elements in the underlying pathophysiology.
Urinary obstruction significantly alters renal blood flow, glomerular filtration rate (GFR), and tubular function even before anatomic changes occur in the kidney. , Within the first 2 to 3 hours of obstruction, there is an early vasodilator response; termed the ‘ hyperemic phase. ’ The rise in hydrostatic pressure in the proximal tubule initially results in reduced resistance of the afferent arteriole and increased glomerular hydrostatic pressure to counteract the proximal tubular pressure. Therefore there is afferent arteriolar dilatation. This effect is demonstrably inhibited by nonsteroidal antiinflammatory drugs (NSAIDs) and is also seen in denervated kidneys, indicating that this is an intrarenal autoregulatory response to obstruction. The reduced distal tubular flow contributes to the initial rise in single nephron GFR as part of tubuloglomerular feedback. Thus in the initial phase of obstruction, single nephron GFR is maintained at approximately 80% of the preobstruction values, despite the marked increase in proximal tubular pressure. As obstruction persists in the next 12 to 24 hours, there is a late vasoconstrictor phase, which is characterized by a drop in renal blood flow to about 40% of normal and poor renal perfusion.
Two major vasoconstrictors, angiotensin II and thromboxane A2, play an important role in the markedly reduced renal blood flow and reduction in single nephron GFR in obstruction. After the obstruction is relieved, there is further vasoconstrictor response in the kidney caused by the release of angiotensin II, as the macula densa senses the change in tubular flow. In animal experiments, simultaneous inhibition of thromboxane A2 and angiotensin production normalized GFR in the postobstructed kidney. The administration of atrial natriuretic peptide after release of obstruction in rats also resulted in an increase of GFR, urine flow, and sodium excretion, suggesting a role of atrial natriuretic peptide in the hemodynamic changes of the postobstructed kidney.
Obstruction affects tubular function by reducing the ability of renal tubules to transport sodium (Na+), potassium (K+), and hydrogen (H+), and reduces the ability to concentrate and dilute the urine. , This contributes to postobstructive diuresis. For example, the apical Na-K-2 chloride (Cl) cotransporter and the basolateral Na+K+ adenosine triphosphatase in fresh suspensions of medullary thick ascending loop cells of obstructed kidneys, show reduced transporter activity. Severe downregulation of aquaporin 2 expression contributes to the impaired urinary concentrating ability. The local increase in prostaglandin E2 synthesis in postobstructed kidney is thought to play a role in aquaporin 2 downregulation. Significant downregulation of apical membrane expression of the distal convoluted tubule Na+ Cl- cotransporter also occurs from obstruction. , The defect in H+ and K+ secretion in the distal nephron in obstructive uropathy has been shown to be independent of aldosterone.
In the first few days after onset of obstruction, there is interstitial edema and an influx of leukocytes, predominantly macrophages, into the kidneys. If the obstruction is persistent and not relieved, glomerular size decreases, tubular cells lose apical microvilli and basolateral interdigitations and have fewer mitochondria. Nephrons atrophy from reduced renal blood flow and inflammatory responses. If obstruction is maintained for a longer period of time, hydronephrosis eventually develops and there is tissue loss with tubular atrophy, interstitial fibrosis, and interstitial inflammation.
In murine experimental models, interstitial fibrosis has been shown to develop within days in the obstructed kidney with increased renal synthesis of extracellular matrix proteins and transforming growth factor-β. The interstitial fibrosis is also mediated by angiotensin II. Proliferation of interstitial fibroblasts with myofibroblast transformation leads to extracellular matrix deposition. Phenotypic transition of renal tubular cells, endothelial cells, and pericytes has been implicated in this process. The compression of medullary and cortical tissue from the renal calyceal distension results in widespread apoptosis and tubular atrophy as early as 3 days postobstruction. The interstitial response to urinary tract obstruction further intensifies the injury, resulting in renal dysfunction. In patients with malignant urinary obstruction, these changes happen over time and may not manifest as clinically evident renal failure for several days to weeks.
In general, patients with benign acute unilateral ureteric obstruction, from a kidney stone for example, present with acute pain typical of a renal colic. Pain from acute ureteric obstruction is caused by pressure or stretch in the lumen. The ureter is supplied by two distinct types of neurons with different activation thresholds. U1 units are low threshold units activated by peristalsis in the absence of mechanical stimulus and U2 units are activated in response to mechanical stimuli, such as increased pressure. U2 units can activate nociceptive afferent input in the nervous system, even in the absence of inflammation. ,
In the setting of malignant obstruction, the clinical presentation depends on the duration and location of the obstruction. The process is more chronic, with extrinsic obstruction developing over a period of days to weeks, unlike in the setting of acute renal colic. This type of insidious urinary obstruction may be unilateral or bilateral. Most patients with malignant urinary obstruction present with vague symptoms of nonspecific lethargy, flank discomfort, or feeling of fullness or being bloated. These symptoms may be accompanied by varying degrees of nausea or anorexia. It has been postulated that distension of the renal pelvis and ureter from obstruction may cause a reflex change in pyloric sphincter pressure contributing to the nausea and vomiting experienced by patients—the so-called renogastric reflex . Urinary tract infection (UTI) may also be a heralding symptom of urinary obstruction. Often, the patient is asymptomatic and the obstruction is identified incidentally on imaging.
Patients with severe renal impairment present with symptoms of renal failure—nausea, vomiting, anorexia, weight loss, edema, and change in mental status. It is important to note that patients with unilateral and even bilateral ureteric obstruction are not necessarily oliguric or anuric. Patients with partial obstruction may continue to have a normal urine output. In some cases polyuria may be noted because of the concentration defect in distal nephron. It is only in cases of complete urinary obstruction that anuria is seen.
Patients with lower urinary obstruction may present with varying degrees of lower urinary tract symptoms including urinary frequency, hesitancy, and sensation of inadequate emptying of the bladder. On physical examination, palpable kidney may be seen only in patients with significant hydronephrosis. Otherwise, examination will show findings of the patients’ primary malignancy, such as the presence of pelvic mass, or ascites from peritoneal carcinomatosis.
Though many patients may have a known diagnosis of cancer before presentation, a careful history and physical examination should be performed. In patients with advanced malignancy, more than one cause for renal dysfunction may be present—prerenal injury in the setting of poor oral intake and postrenal failure from obstruction. Most patients with advanced malignancy have poor oral intake and loss of muscle mass may lead to underestimation of the severity of their renal dysfunction. The postrenal component may only reveal itself after the prerenal component is corrected with volume resuscitation. In some patients, urinary obstruction may be the first presentation of malignancy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here