Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Nutritional and gastrointestinal (GI) symptoms are two of the most common themes encountered in general pediatric medicine. Globally, undernutrition and diarrheal disease cause substantial morbidity and mortality. In the developed world, nutritional assessment is paramount in well-childcare and chronic illness management. GI symptoms are common in acute and chronic illnesses. Since one of the main functions of the GI system is to provide fluid, vitamins/minerals, and the energy requirements to sustain viability and growth, the relationship between gastroenterology and nutrition is readily apparent. However, for the purposes of this chapter, nutrition and gastroenterology are divided into two sections, with a focus on the entities commonly seen by pediatric primary care providers.
The first year is marked by unprecedented growth when compared with any other period of life. Birth weight approximately doubles in the first 6 months and triples by 1 year. Length generally increases by 50% by age 1. As such, the normal caloric and protein requirements for growth are accordingly higher ( Table 11.1 ). Human breast milk or formulas that have been derived to simulate the major components of breast milk fuel this growth. In addition to cost/convenience, there are many reasons why ( Table 11.2 ) there is a general acceptance that breast milk offers advantages that formulas to date cannot emulate. Although generally considered nutritionally replete, breast milk lacks substantial amounts of vitamin D and fluoride, requiring supplementation. Low amounts of vitamin K stores at birth and in breast milk have led to a standard practice of intramuscular vitamin K injection at birth. Breast milk also contains a lower amount of iron, albeit in a bioavailable form, requiring additional supplementation via a multivitamin or solid food as an infant uses the iron stores obtained in utero and reaches a physiologic nadir after the first 6 months of life. Standard infant formulas do contain sufficient iron, vitamin K, and vitamin D and when diluted in fluoridated water are nutritionally replete. There has been rapid growth in the varieties of infant formulas. Many of the “niche” formulas are designed for specific GI illnesses and not necessary for standard infant feeding. Table 11.3 categorizes the elements of the most common variants of infant formulas used today. Although whole cow’s milk and other forms of mammalian milk have features like human breast milk, their higher protein concentration and thus higher renal solute load has led the American Academy of Pediatrics to recommend their use be delayed until an infant is 12 months of age.
| Age | Calories (kcal/kg/day) | Protein (g/kg/day) |
|---|---|---|
| 0–6 months | 100–120 | 2.5–3.0 |
| 6–12 months | 90–100 | 2.0–2.5 |
| 1–7 years | 75–90 | 1.5–2.0 |
| 7–12 years | 60–75 | 1.5–2.0 |
| 12+ years | 30–60 | 1.0–1.5 |
|
| Carbohydrate Source |
|
| Protein Source |
|
| Fat Source |
|
| Additives |
|
| Other Considerations |
|
As an infant reaches 1 year, the caloric requirements continue to diminish as the rate of growth decreases (see Table 11.1 ). Solid foods, typically introduced around the sixth month of life, take on the role as a major source of calories and nutrients and formula is typically replaced by whole cow’s milk. As motor, language, and social development progresses, psychological and behavioral aspects of feeding from both the toddler’s and caregiver’s perspective become a common discussion during health care visits. Variations of feeding patterns that do not affect growth but may lead to GI symptoms are often recognized during this time period. Nutritional assessment, including dietary monitoring and simple anthropometric monitoring, continues to be essential in and after this time period to recognize tendencies in undernutrition, overnutrition, and the recognition of both acute and chronic disease.
Growth assessment continues to be commonplace in pediatric practice as a proxy for nutritional adequacy and general health status. Growth charts are used to monitor simple anthropometric data to achieve these goals. Nationally standardized growth charts, in use since 1977, have been updated over time. Use of the World Health Organization (WHO) growth charts, released in 2006, has largely supplanted the Centers for Disease Control and Prevention (CDC) growth charts in children less than 2 years of age. These charts provide growth standards that describe healthy growth under optimal conditions and include longitudinal data from 0 to 24 months of age. The WHO growth charts are based on the breastfed infants. The updated version of the WHO growth charts uses weight for age, length/height for age, and occipital-frontal circumference for age. The CDC growth charts, used in children from 2 to 20 years old, include the body mass index (weight [kg]/height [m] 2 ) in response to the growing epidemic of overnutrition as a measure of obesity. The CDC growth charts are generally not used in children younger than 2 years of age. As a distribution based on percentages, it should always be recognized that there will always be a population that is over the greatest isopleth (>97th percentile) and under the lowest isopleth (<3rd percentile) in all of these growth charts. Despite this, the characteristics of growth of a child over time can be an invaluable comparative measure of what would be considered normal growth to actual growth. Growth charts are also available for specialized populations such as infants with genetic conditions (e.g., Trisomy 21) and premature infants. Care must be taken when using these growth charts, as the numbers of patients in the sample section are less than those in the standard growth charts.
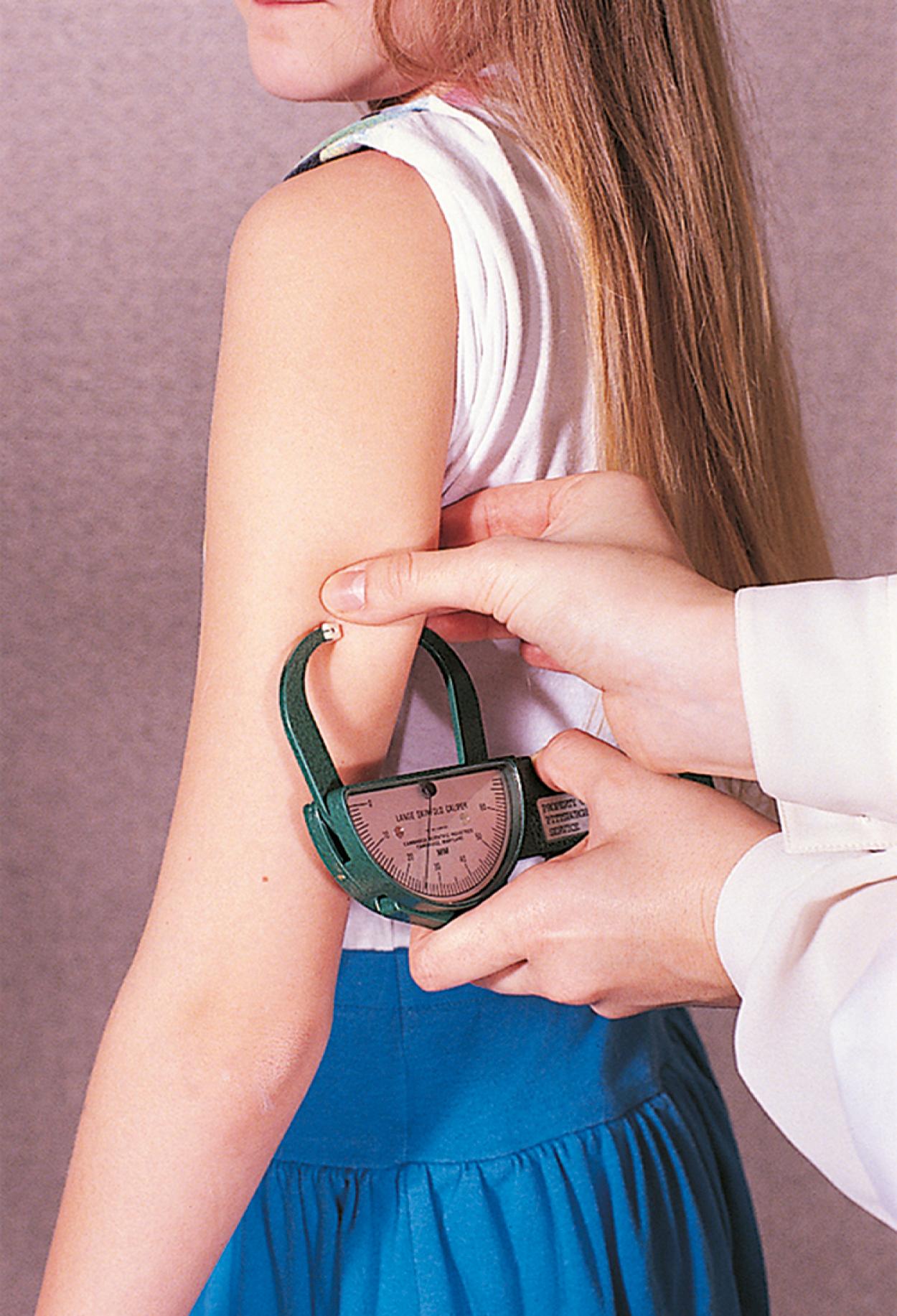
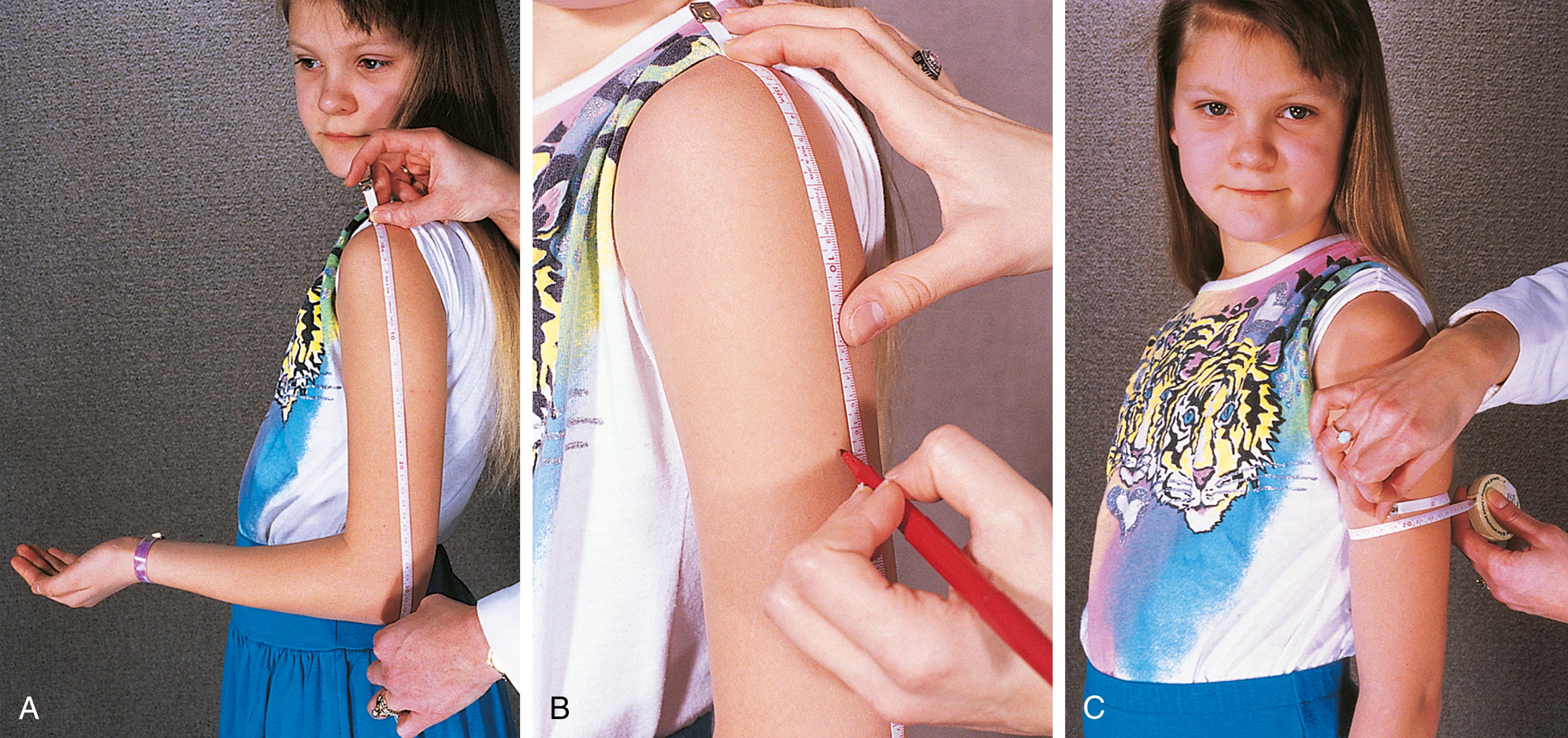
Other measurements, such as triceps skinfold thickness ( Fig. 11.1 ) and midarm circumference ( Fig. 11.2 ), when done by a well-trained examiner can be useful when growth charts are insufficient. Example populations of where these measurements would have utility would include infants with end-stage liver disease and marked ascites or vascular malformations/hypertrophy of an organ or appendage, which falsely elevates true body weight. For the great majority of infants and children, growth charts are usually adequate to assess overall growth.
Malnutrition can be defined as both undernutrition and overnutrition, although it is most often associated with an undernourished state. The state of malnutrition results from an imbalance between the intake and/or absorption of calories and the consumption of calories required for growth. Obesity is due to overnutrition and has grown to epidemic proportions in the United States and other developed nations. Undernutrition results from inadequate intake, absorption, or digestion of nutrients, or insufficient intake for increased metabolic needs. Under prolonged conditions, undernutrition and overnutrition will lead to malnutrition. Careful plotting of height, weight, and body mass index as a function of age on an appropriate growth chart greatly aids in the diagnosis of malnutrition. The Waterlow criteria assess undernutrition by plotting weight for height, as a measure of current nutritional status, and height for age, which reflects the chronicity of the malnutrition.
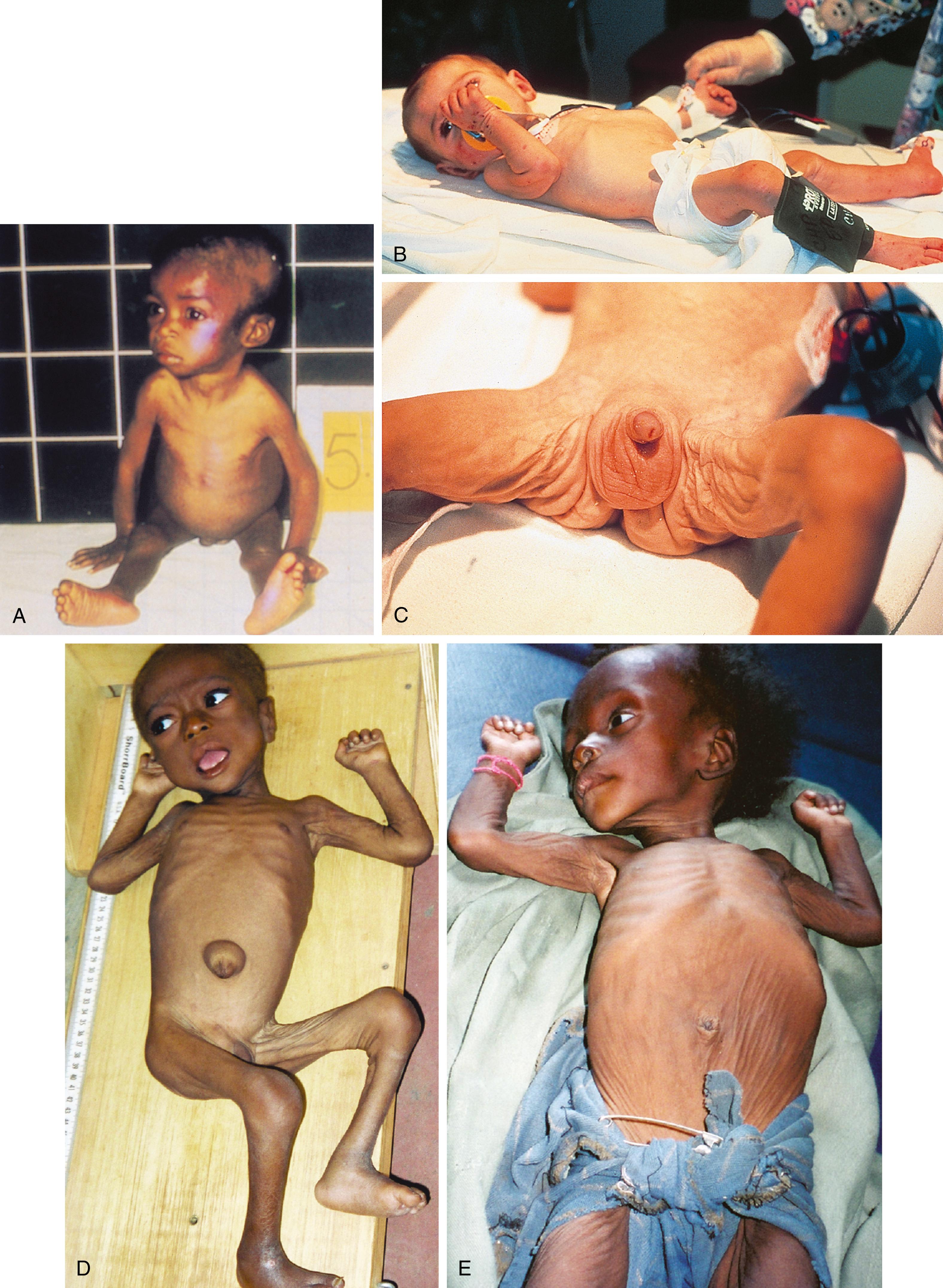
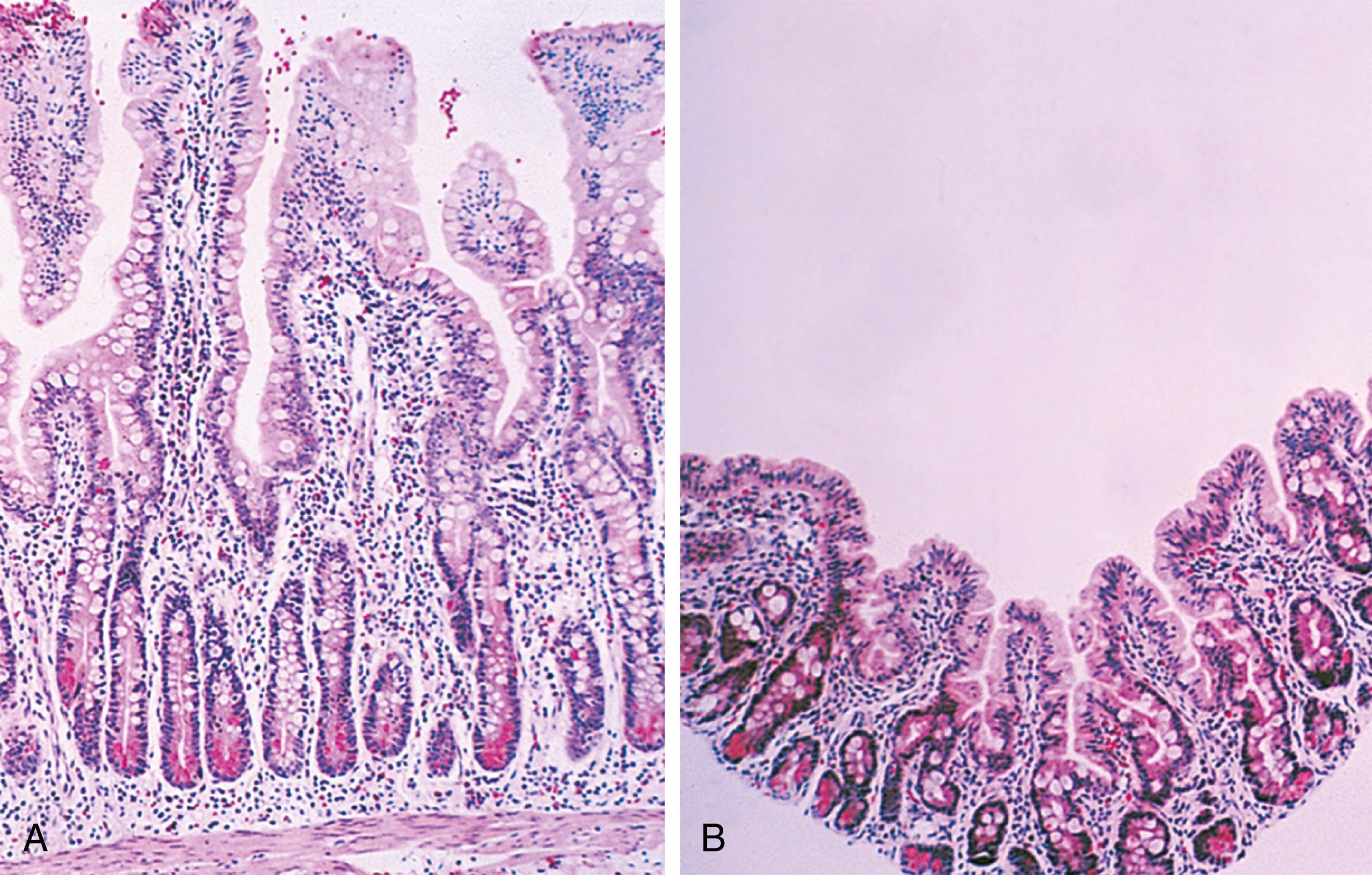
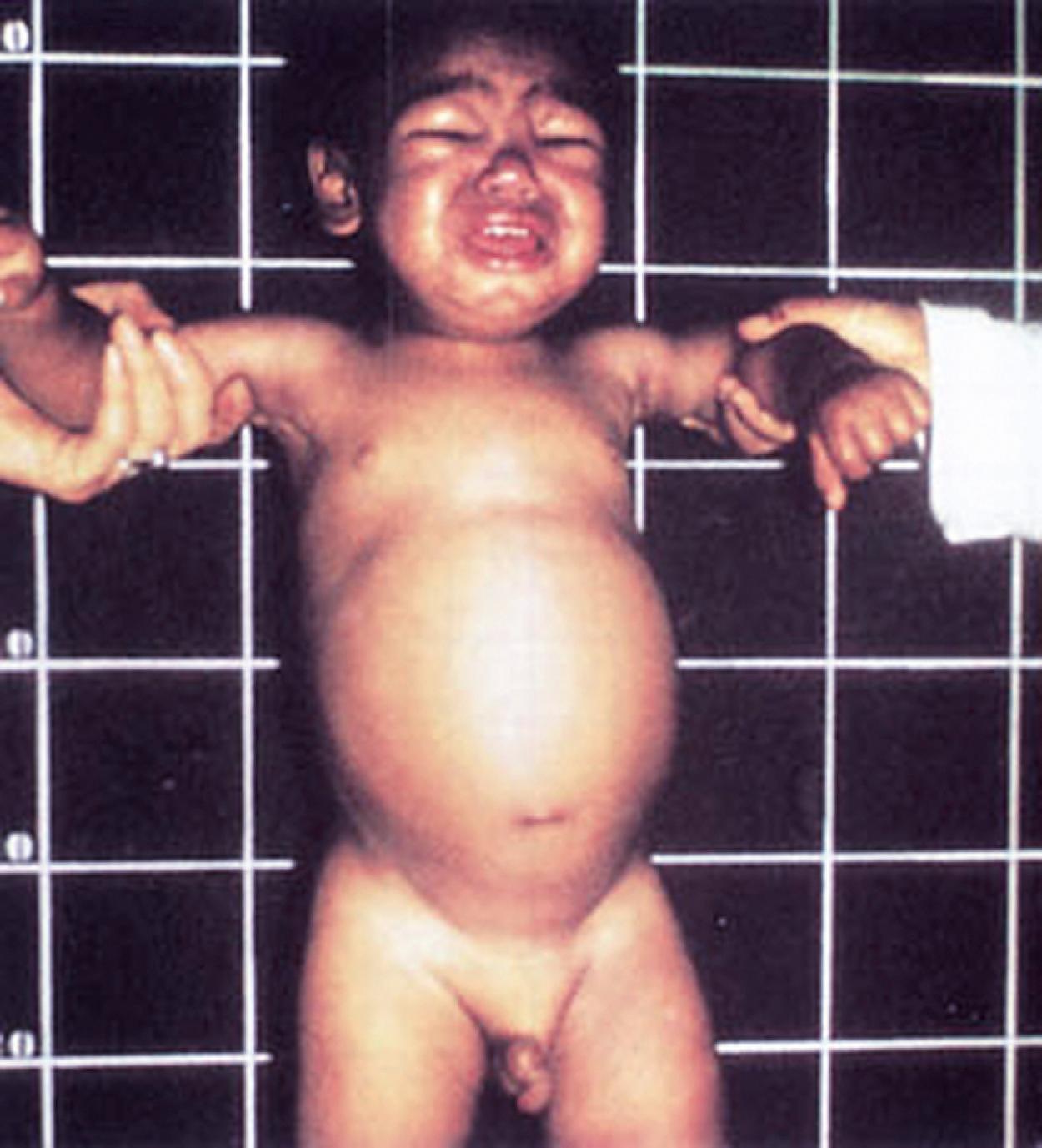
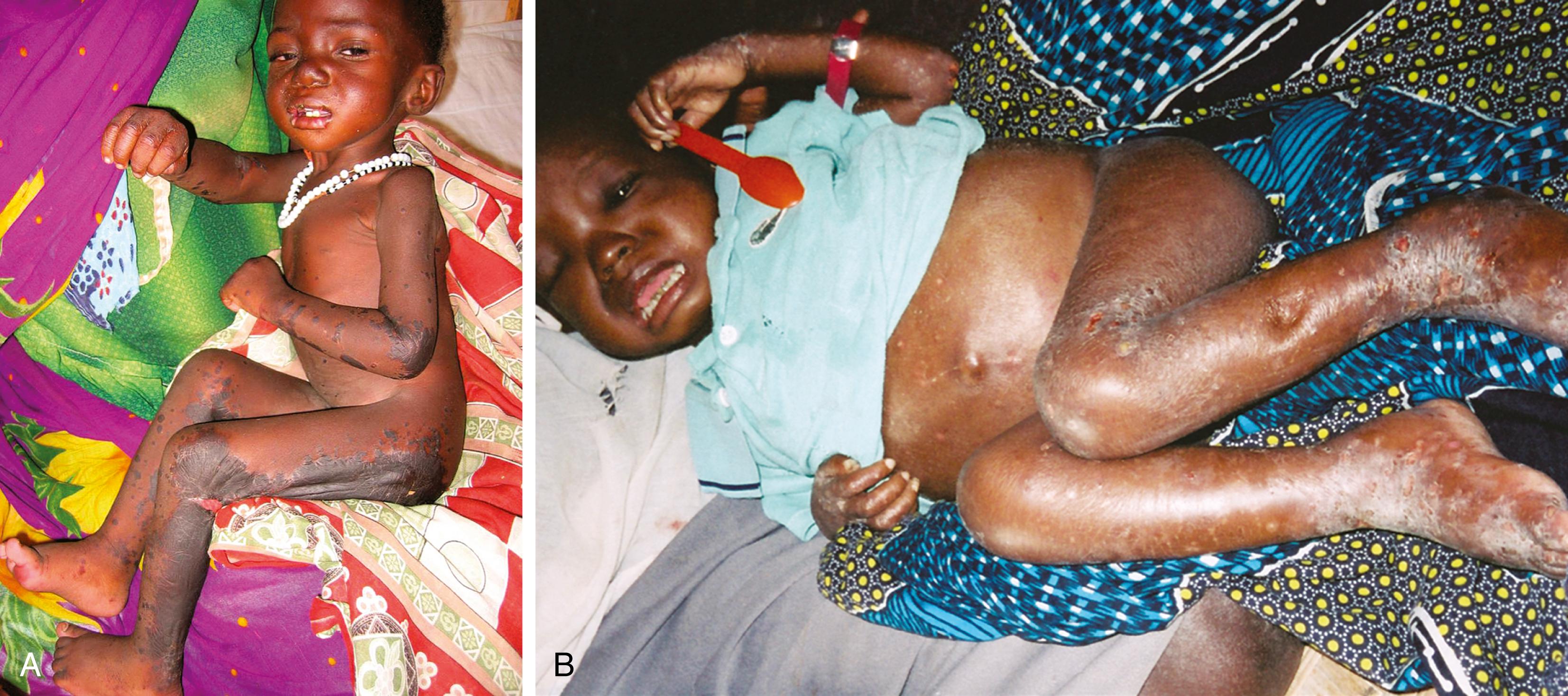
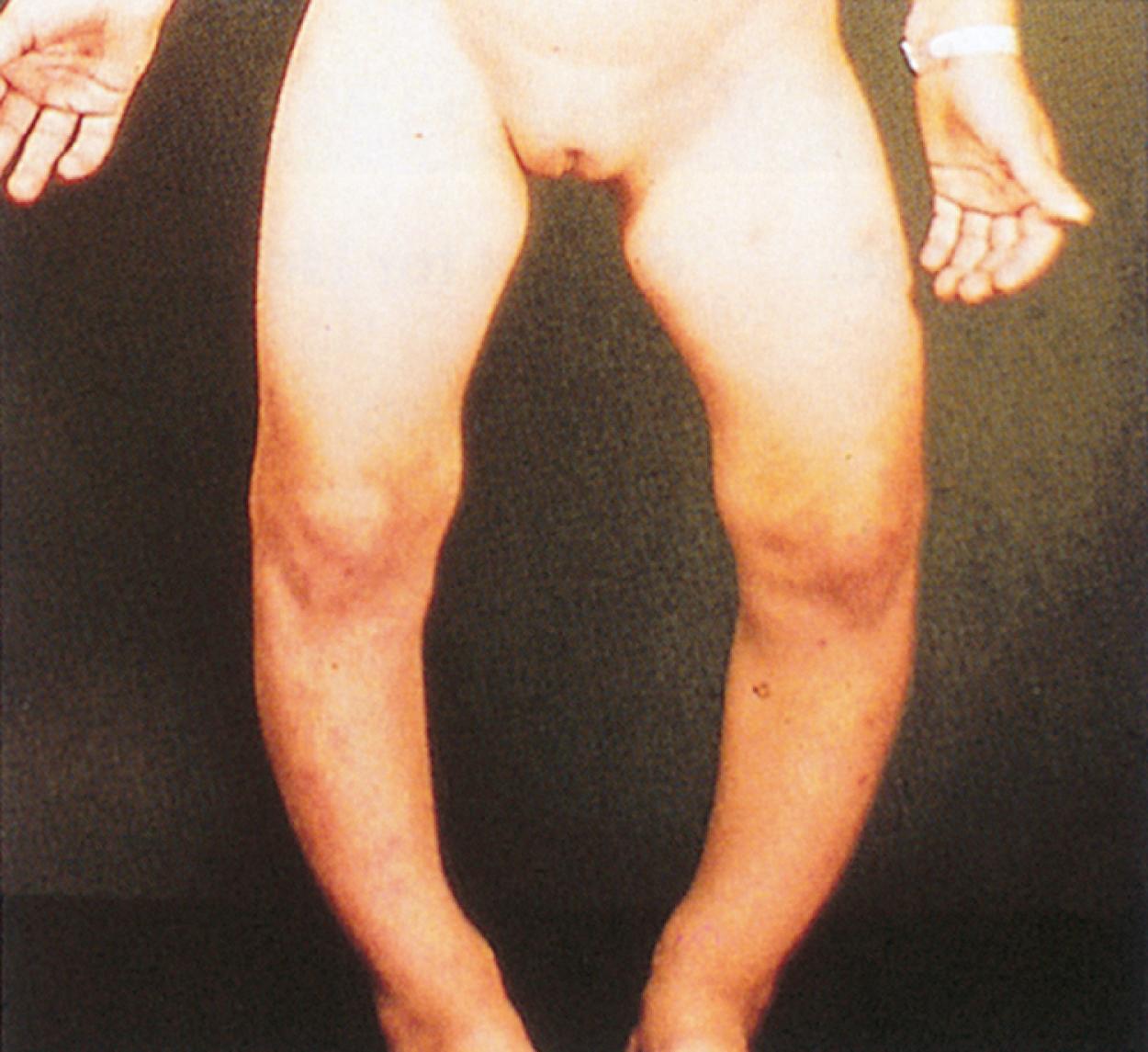
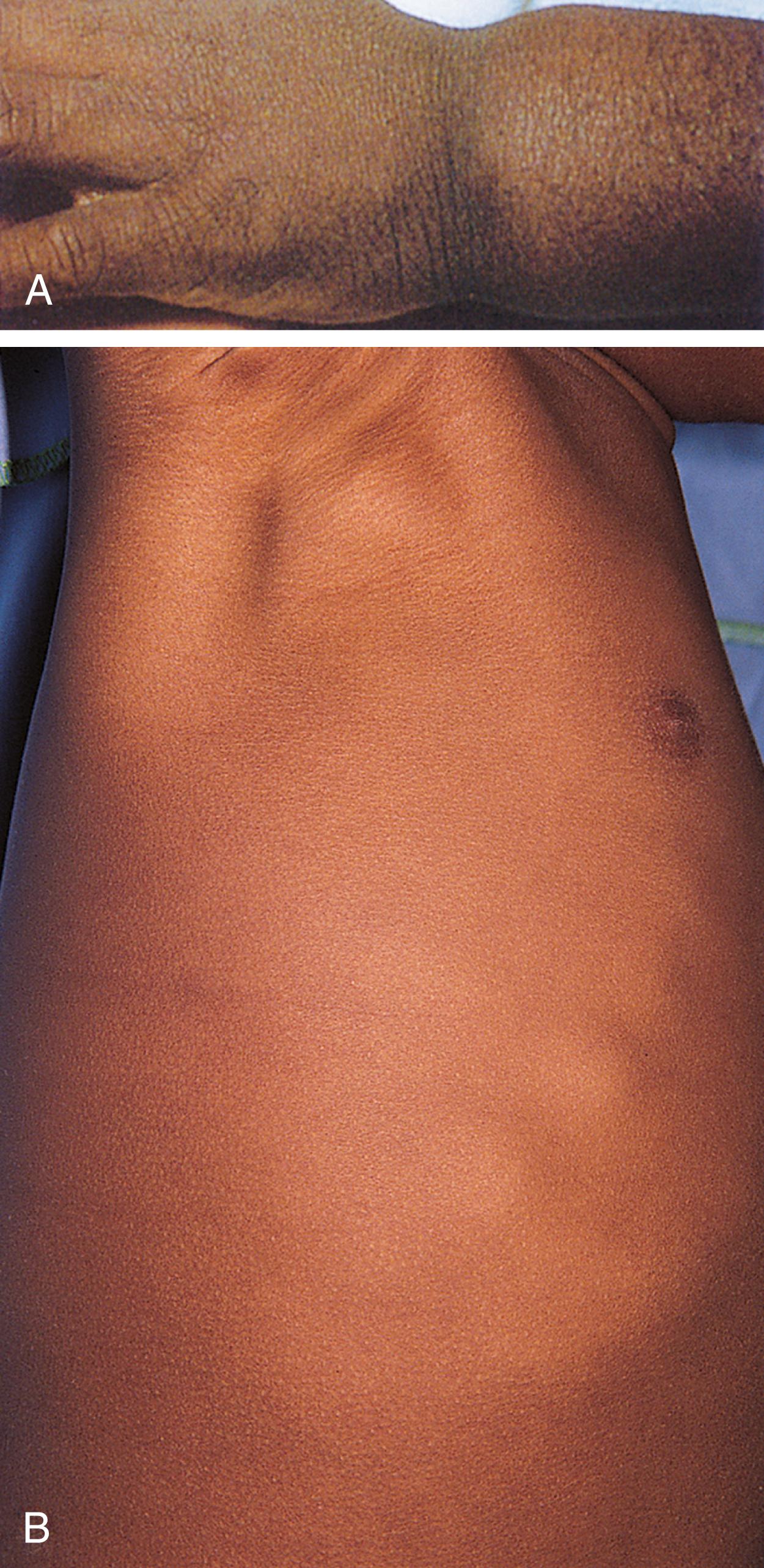
A traditional classification of extreme protein energy malnutrition includes marasmus, kwashiorkor, and marasmic kwashiorkor. Marasmus is defined as predominantly caloric/energy deficiency and is characterized by marked emaciation, loss of subcutaneous fat and muscle, brittle, sparse hair, and poor nail growth ( Fig. 11.3 ). It is the consequence of poor caloric, protein, vitamin, and mineral intake. Diarrhea is common as intestinal cell turnover slows, resulting in intestinal villous blunting and a secondary malabsorptive state ( Fig. 11.4 ). Secondary effects of marasmus, such as hypothermia and bradycardia, occur late in the clinical course. Marasmus is usually seen within the first year of life at the time of weaning from breastmilk/formula, during famine, or at any time when there is a consistent and extreme deprivation of calories. Kwashiorkor, on the other hand, results from a diet lacking protein even though it may be rich in calories. The initial “moon face” of kwashiorkor is often mistaken for proper nutrition. The child is often edematous, which becomes strikingly apparent after nutritional repletion ( Fig. 11.5 ). Hepatomegaly and mental status changes are common. Skin changes in patients with kwashiorkor include hyper- or hypopigmentation with a scaly, weeping dermatitis that may ulcerate and desquamate ( Figs. 11.6 and 11.7 ). In marasmic kwashiorkor, both patterns of malnutrition may develop together, resulting in a combination of clinical features.
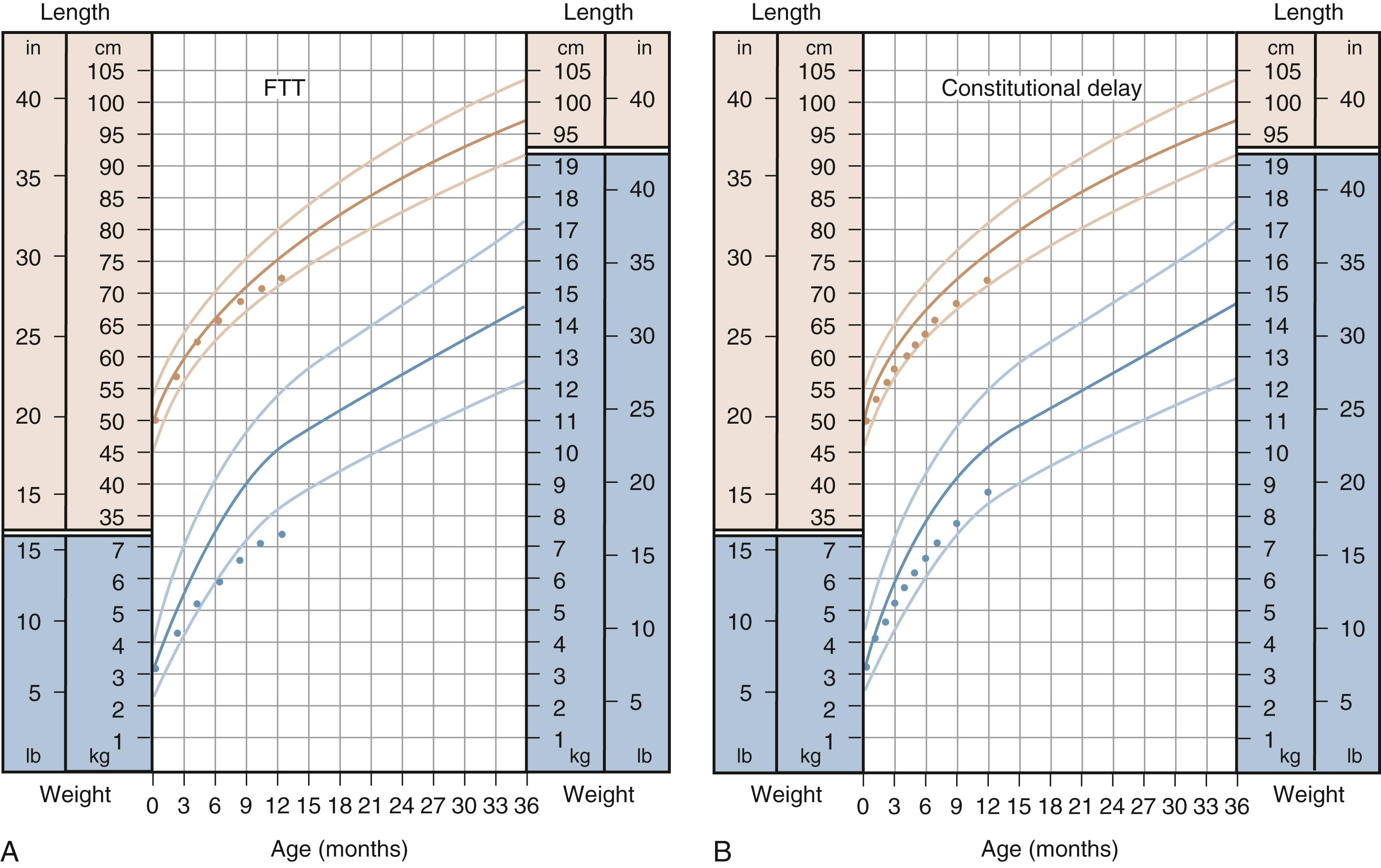
The term failure to thrive is a descriptive term used to depict inadequate weight gain over time, often used when weight for age crosses two percentile isopleths or when weight for age falls below the 3rd percentile for age. As previously stated, caution must be used when weight gain consistently tracks below the 3rd percentile, as this may reflect a normal growth pattern based on standard weight distribution patterns ( Fig. 11.8 ). Most failure to thrive, by Waterlow criteria, is marked by decreased weight for height, with initial sparing of height for age and head circumference for age, with gradual loss in height and head circumference for age as the undernourished state continues. A more accurate description of this abnormal pattern of growth can be classified as inadequate intake, malabsorption, ineffective use of calories, or increased metabolic demand. Table 11.4 lists some of the more common diagnoses associated with failure to thrive. At times, the etiology can be mixed as an underlying diagnosis may be compounded by inadequate intake secondary to its presenting symptoms.
| Inadequate Intake | Inadequate Absorption | Increased Caloric Needs |
|---|---|---|
| Caloric deprivation | Celiac disease | Congenital heart disease |
| Behavioral feeding disorders | Intestinal inflammation | Chronic pulmonary disease |
| Disorders of swallowing | Fat malabsorption (cystic fibrosis) | MalignancyRenal disease |
| Vomiting/reflux | Chronic diarrhea | Genetic disease |
| Anorexia/poor appetite |
A detailed history is often helpful in narrowing the differential diagnosis for children with failure to thrive. This starts with a dietary assessment including the type and amount of oral intake and specific behaviors surrounding eating. In infants, it is important to assess the preparation of formula, as incorrect mixing (e.g., dilution) can lead to decreased caloric intake and the potential for electrolyte abnormalities such as hyponatremia. Three-day diet diaries can be helpful in reviewing caloric intake but are subject to bias after a deficiency of intake is considered. A detailed gastroenterological review of systems including assessment of appetite, vomiting, and stool output is necessary as generally reviewing for symptoms that may point to a chronic medical illness.
Laboratory testing can serve as (1) a diagnostic tool for defining an underlying condition, (2) a marker for the degree of malnutrition, and (3) a screening tool for specific nutrient or micronutrient deficiencies. A complete blood count can suggest an underlying inflammatory condition, identify iron deficiency or other type of anemia, and overall serve as a basic immunologic assessment. Serum electrolytes may reflect abnormal losses due to diarrhea or excessive vomiting and identify acidosis as an underlying metabolic disease. A 72-hour fecal fat level or fecal pancreatic elastase may depict fat malabsorption. Fecal α 1 -antitrypsin and urinalysis can aid in the diagnosis of abnormal protein losses. Analysis of stool for reducing substances may reflect carbohydrate malabsorption. Analysis of amino or organic acids in specific cases can point to metabolic disorders. Diagnosis-specific screens such as a sweat test for cystic fibrosis or tissue transglutaminase IgA antibody for celiac disease can also be useful in the patient with abnormal growth patterns. Decreases in specific proteins can be helpful as a marker of catabolism. Albumin ( t ½ 20 to 24 days) is commonly used, although not particularly specific, to depict the current nutritional status. Transferrin ( t ½ 9 days), prealbumin ( t ½ 2 days), and retinol-binding protein ( t ½ 12 hours) may be more sensitive indicators, although most of these proteins have limited use because they can act as acute-phase proteins. Lean body mass may also be assessed by measuring 24-hour urinary creatinine compared with the standard values for height (creatinine height index). Although laboratory tests can be helpful, they are not mandatory and the decision for testing should be derived from the history and physical examination.
Therapy for malnutrition is twofold—treat the underlying cause and rehabilitate. All attempts should be made to treat the underlying symptom cause. In parallel, nutritional rehabilitation via providing sufficient calories for growth should be implemented once malnutrition is recognized. Care must be undertaken when providing nutritional rehabilitation, as one consequence of aggressive therapy is refeeding syndrome ( Table 11.5 ).
| Physiologic |
|
| Electrolyte Abnormalities |
|
| Monitoring |
|
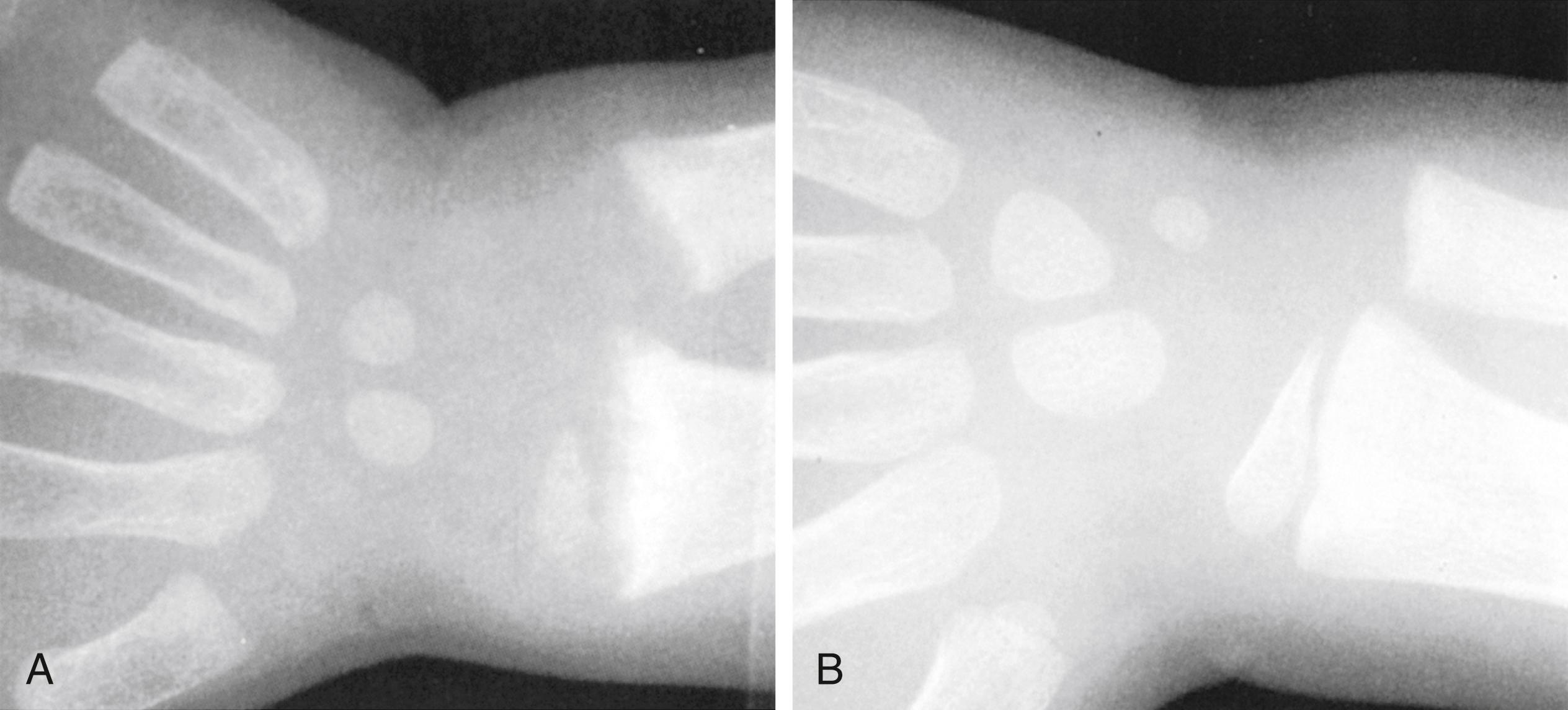
In addition to more general malnutrition states, excess or deficiencies of specific nutrients, vitamins, and minerals can be seen alone or as complicating factors of chronic disease ( Table 11.6 ). For example, fat-soluble vitamin deficiency (vitamins A, D, E, and K) can be found in isolation due to inadequate intake or with fat malabsorption secondary to chronic diseases (e.g., cystic fibrosis, celiac disease). Vitamin D is essential for normal calcium, phosphorus, and magnesium homeostasis. Vitamin D deficiency may lead to rickets, the inadequate mineralization of growing bones, osteomalacia, osteoporosis, heart disease, various cancers, type 1 diabetes, and multiple sclerosis later in life. There is growing evidence that it also plays a role in various physiologic functions including immune function. Classic vitamin D deficiency presents at weight-bearing age and results in poor growth, curvature of weight-bearing bones ( Fig. 11.9 ), widening of epiphyses, and costochondral “rachitic rosary” beading ( Fig. 11.10 ). Craniotabes, or softening of the skull, may be seen in infants. With appropriate vitamin and mineral supplementation, resolution occurs followed by bone remodeling ( Fig. 11.11 ). Common laboratory findings are summarized in Table 9.5 . Vitamin D deficiency is especially prevalent in breastfed infants, infants with darker skin, and infants and children with decreased sun exposure. Systemic conditions that can lead to poor vitamin absorption include children with cholestasis, Crohn disease, cystic fibrosis, and celiac disease and abnormal hydroxylation in end-stage renal disease. In fact, the high prevalence of vitamin D deficiency without rickets places otherwise healthy children and adolescents at risk if they are not consuming the recommended daily allowance in dairy and other vitamin D–fortified foods. The American Academy of Pediatrics has recommended an intake of 400 IU/day to prevent complications of vitamin D deficiency. Other fat-soluble vitamin deficiencies are seen in patients with fat malabsorption such as cholestatic liver disease, cystic fibrosis, and celiac disease. Vitamin K deficiency namely results in prolongation of the prothrombin time and international normalized ratio (INR) and requires supplementation of vitamin K either orally, or, in some cases, intramuscularly. Vitamin A deficiency can lead to night blindness, xerophthalmia, follicular hyperkeratosis, or Bitot spots. Vitamin E deficiency is marked by absence of deep tendon reflexes and subsequent ataxia. It has also been linked to hemolysis and possible retinopathy of prematurity. Isolated deficiencies of vitamins A and E are rare and are generally found in the context of fat-soluble vitamin malabsorption. Water-soluble vitamin deficiencies are also uncommon unless unintentionally excluded in the diet. For example, an infant fed goat’s milk, instead of breast milk or a cow’s milk–based formula, is at risk for folate deficiency. Signs of specific vitamin deficiencies can be manifested on physical examination. Angular cheilitis/stomatitis is a common finding of several vitamin deficiencies (B vitamins) as well as iron deficiency ( Fig 11.12 ). The presence of stomatitis should cause at least consideration of a deficiency state. Vitamin B12 is of specific interest as it requires active uptake in the terminal ileum. Patients with terminal ileum disease or resection are at particular risk of vitamin B 12 deficiency, which results in a macrocytic anemia (see Fig. 11.9 ), hypersegmented neutrophils (see Fig. 11.10 ), and/or neurologic findings due to poor myelination of nerve fibers. Neurologic findings can be reversible when detected early. Supplementation is either intramuscular or (more recently) through intranasal mucosal absorption.
| Vitamin/Mineral | Sign/Symptom |
|---|---|
| Calcium, phosphorus, vitamin D | Rickets/osteomalacia |
| Vitamin A | Night blindness, xerophthalmia, Bitot spots, follicular hyperkeratosis |
| Vitamin C | Scurvy: bone lesions, bleeding |
| Vitamin E | Hemolytic anemia, peripheral neuropathy |
| Vitamin K | Petechiae, ecchymoses |
| Thiamine (vitamin B 1 ) | Beriberi: heart failure, increased intracranial pressure |
| Niacin | Pellagra: dermatitis (sun-exposed areas) |
| Riboflavin (vitamin B 2 ) | Angular stomatitis, cheilosis |
| Vitamin B 6 | Anemia, dermatitis, neuropathy |
| Vitamin B 12 | Anemia, neuropathy |
| Folate | Anemia |
| Iron | Anemia, koilonychia |
| Biotin | Rash, hair loss |
| Essential fatty acids | Rash, coagulopathy |
| Zinc | Rash (acrodermatitis), growth failure, delayed sexual development, ageusia |
| Copper | Bone changes, hypopigmentation, anemia, neutropenia |
| Selenium | Cardiomyopathy (Keshan disease) |
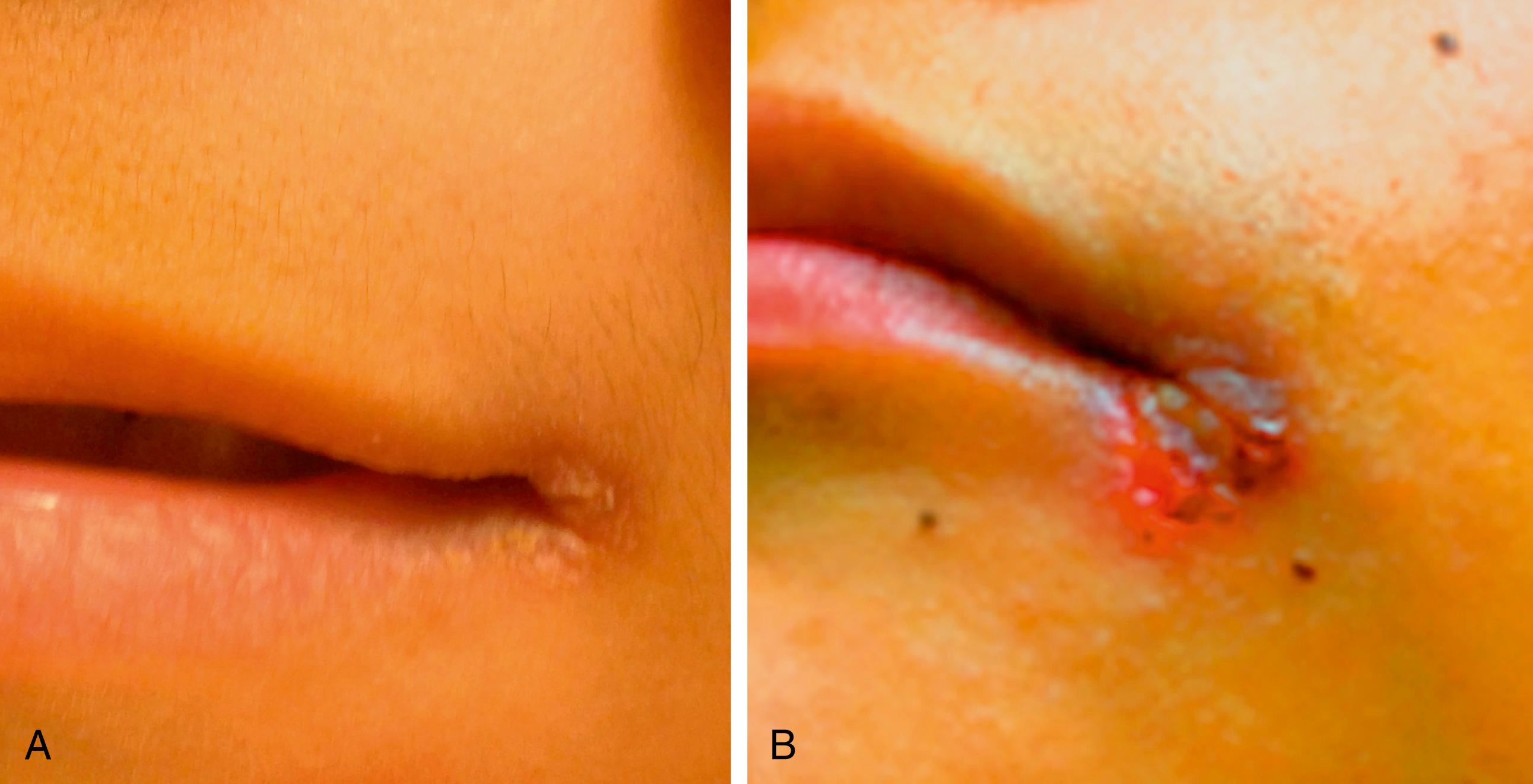
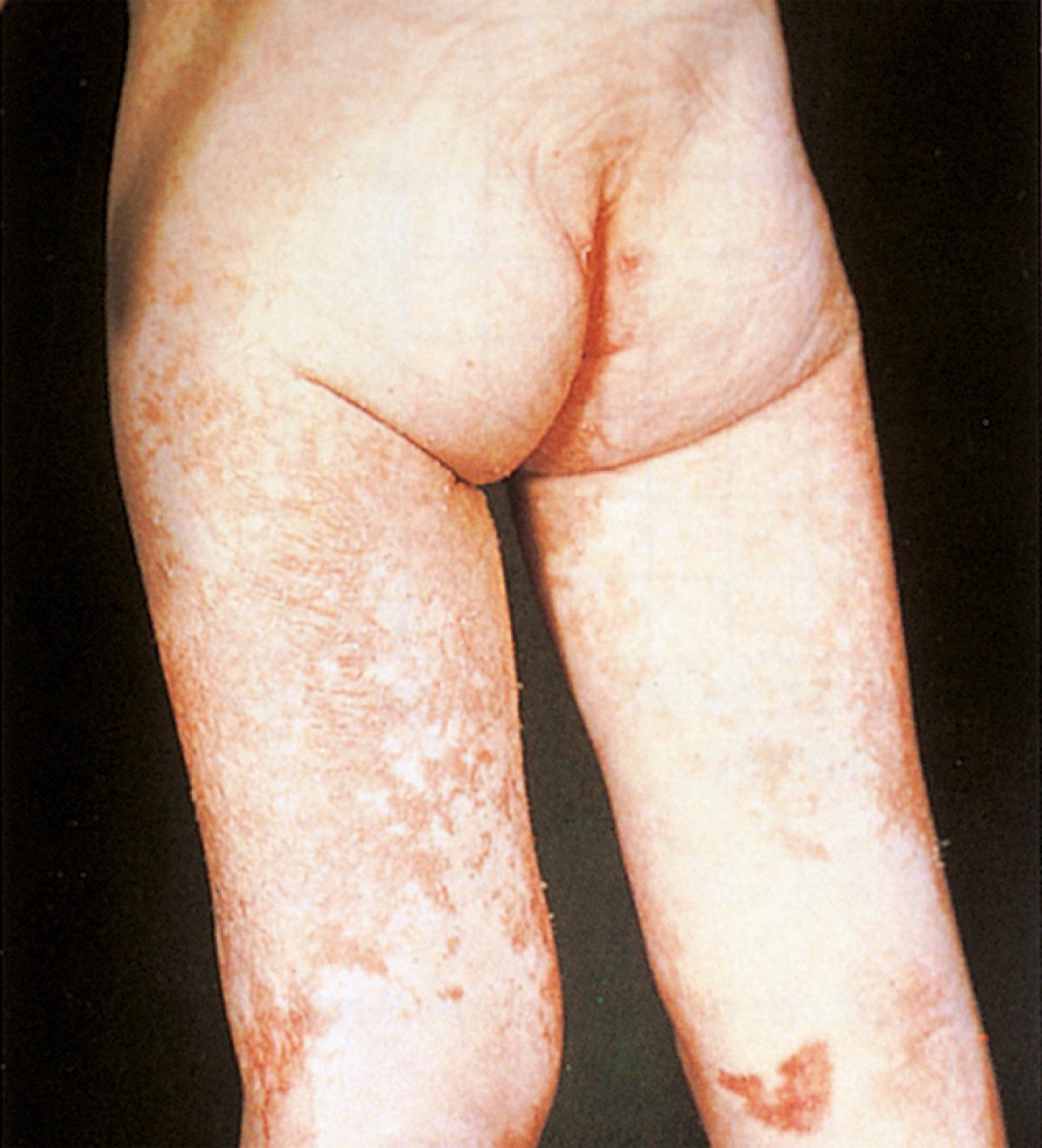
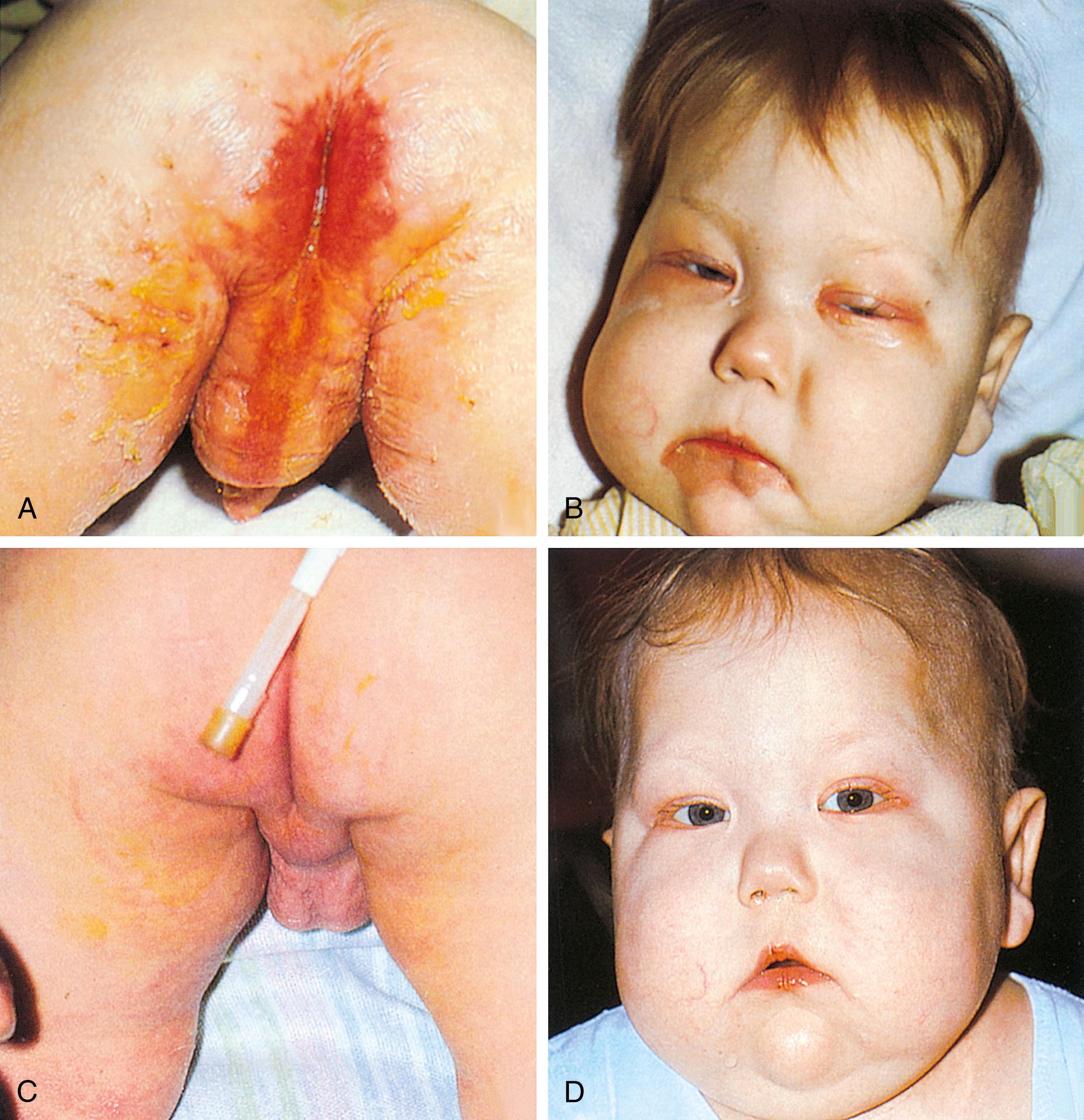
Other specific nutritional deficiencies can sporadically occur in the pediatric population, especially when receiving nontraditional diets. Patients on extremely low-fat diets or who are receiving exclusive parenteral nutrition, in which standard fat emulsions have been limited/omitted because of concern about cholestasis, can present with essential fatty acid deficiency. α-Linolenic acid (ω-3) and linoleic acid (ω-6) are the essential fatty acids, whereas several others may be considered conditionally essential. Symptoms include a scaly dermatitis and dull, brittle hair and nails. Diagnosis can be suggested based on the triene:tetraene ratios. Zinc deficiency encountered in diarrheal diseases and in burns can result in alopecia, diarrhea, and acrodermatitis. A deficiency in copper, which is often reduced in the diets of patients with chronic liver disease, can present as irritability, decreased hair pigmentation, anemia, neutropenia, and bone abnormalities. Copper deficiency can be diagnosed by serum copper levels and depressed ceruloplasmin. When biotin, a conditionally essential amino acid, is deficient, a weeping dermatitis in the perioral, perianal, and eyelid area along with lethargy, malaise, and thinning of hair can occur ( Fig. 11.13 ). Table 11.6 details further signs and symptoms of specific nutrient deficiencies.
GI symptoms are common in pediatric practice. This section emphasizes the major complaints that bring patients with GI disorders to medical attention, with particular coverage of entities that may be diagnosed by examination.
Gastroesophageal reflux (GER) is defined as the passage of gastric contents into the esophagus, when intragastric pressure exceeds the forces that protect the esophagus. This process differs from physiologic vomiting in that GER is not centrally mediated or associated with reverse peristalsis. GER episodes are considered effortless and the refluxate is most often in the form of undigested food, and in infants, curdled milk. GER is a common and usually self-limited condition beginning in early infancy and resolving by 1 year of age. Symptoms generally begin immediately after feeding but may continue for several hours afterward. GER that does not resolve by 2 years of age or that presents in later childhood more often follows the pattern of adult-onset GER and persists as a chronic condition.
GER associated with one or more concurrent morbidities is often termed gastroesophageal reflux disease (GERD). The symptoms often associated with GERD include irritability/fussiness, heartburn, poor weight gain, upper respiratory symptoms, and wheezing. These symptoms have been recently discussed in a thorough review (Rosen et al. J Pediatr Gastroenterology & Nutrition 2018). Apnea and bradycardia have been associated with reflux in the preterm infant, although these episodes usually resolve as an infant reaches term. Sandifer syndrome is described as abnormal posturing and back arching in response to GER and is an uncommon manifestation. Rumination, which is different from reflux, is the voluntary contraction of abdominal musculature that leads to episodes that manifest similar to reflux. It is generally regarded as a self-stimulating disorder and is more common in adolescence and in various behavioral disorders.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here