Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A radioactive isotope (radioisotope) is an unstable form of an element that emits radiation from its nucleus as it decays. Eventually, the end product is a stable, nonradioactive isotope of another element.
Radioisotopes can be produced artificially, most frequently by neutron enrichment in a nuclear reactor or in a cyclotron, or may occur naturally. Naturally occurring radioisotopes include uranium and thorium. The vast majority of radioisotopes are produced artificially.
Radiopharmaceuticals are combinations of radioisotopes attached (for the purposes of this chapter) to a pharmaceutical that has binding properties that allow it to concentrate in certain body tissues such as the lungs, thyroid, or bones. Radioisotopes used in clinical nuclear medicine are also referred to as radionuclides, radiotracers, or sometimes simply tracers.
Various body organs have a specific affinity for, or absorption of, different biologically active chemicals. For example, the thyroid takes up iodine , the brain utilizes glucose , bones utilize phosphates, and particles of a certain size can be trapped in the lung capillaries (see Fig. 1.12 ).
After the radiopharmaceutical is carried to a tissue or organ in the body, its radioactive emissions allow it to be measured and imaged using a detection device called a gamma camera.
eTable A.1 outlines various radioisotopes and pharmaceuticals used in nuclear imaging.
| Organ | Radioactive Isotope | Pharmaceutical |
|---|---|---|
| Brain | Technetium-99m (Tc-99m), iodine-123 | Pertechnetate, glucoheptonate, diethylenetriaminepenta-acetic acid (DTPA) |
| Cardiac | Thallium-201, Tc-99m | Pyrophosphate, pertechnetate, sestamibi, teboroxime, labeled red blood cells |
| Lung | Xenon-127, xenon-133, krypton-81m, Tc-99m aerosolized | Macroaggregated albumin |
| Bone | Tc-99m | Phosphates, diphosphonates (e.g., MDP) |
| Kidney | Tc-99m, iodine-131, iodine-123 | Glucoheptonate, mercaptoacetyltriglycine, Hippuran, DTPA |
| Thyroid | Iodine-131, iodine-123, iodine-125, Tc-99m | Pertechnetate with Tc-99m |
| Gallium | Gallium-67 | Citrate |
| White blood cells (infection) | Indium-111, Tc-99m | White blood cells |
Unstable isotopes attempt to reach stability by one or more processes. They may undergo splitting (fission), or they may emit particles (e.g., alpha or beta particles ) and/or energy (e.g., gamma rays ) in the form of radiation.
Fission is a destructive process that occurs primarily in nuclear reactors.
Alpha particles have a relatively high energy, are large, are strongly absorbed by adjacent tissue, and can cause substantial damage to nearby molecules. They are not used diagnostically in medicine.
Beta particles are high-energy, high-speed electrons or positrons (positive electrons) that have a penetrating power between alpha particles and gamma rays. Their main disadvantage in diagnosis is the relatively high radiation dose they deliver to the patient.
Gamma decay involves the emission of energy from an unstable nucleus in the form of electromagnetic radiation. Gamma rays are identical to x-rays except that gamma rays originate from nuclei whereas x-rays emanate from outside the nucleus.
Radioisotopes undergo gamma decay at discrete energies. These energies are usually expressed in the form of the electron volt (eV). Most radioisotopes produce energies in the range of thousands (keV) to millions (MeV) of electron volts.
eTable A.2 describes some of the most commonly used terms in nuclear medicine.
| Term | Description |
|---|---|
| Physical half-life | The time required for the number of radioactive atoms in a sample to decrease by 50%. |
| Biologic half-life | The time needed for half of a radiopharmaceutical to disappear from the biologic system into which it has been introduced. |
| Effective half-life | Time dependent on both the physical half-life and the biologic clearance. |
| Isotopes | Species of atoms of a chemical element with the same atomic number (protons in nucleus) but with different numbers of neutrons and thus atomic masses (total number of protons and neutrons); every element has at least one isotope. |
| Stable isotopes | Do not undergo radioactive decay. |
| Unstable isotopes | Undergo spontaneous disintegration. |
| Atomic number (Z) | Defines an element; all atoms with the same atomic number have nearly the same properties. |
| Mass number (A) | The number of protons and neutrons in the nucleus; different numbers of neutrons is what produces isotopes. |
In order for a radioisotope to be useful for medical diagnosis, it must be capable of emitting gamma rays of sufficient energy to be measurable outside of the body.
It also must have a half-life that is long enough for it to still be radioactive after shipping and preparation, but sufficiently short so as to decay soon after it is used for imaging.
The physical half-life of a radioisotope is the time required for the number of radioactive atoms in a sample to decrease by 50%. Physical half-life is a property inherent to the radioisotope. Most radioisotopes for medical use must have half-lives of hours or days.
eTable A.3 outlines the physical half-lives of some of the most commonly used radioisotopes.
| Radioisotope | Physical half-life |
|---|---|
| Technetium-99m | 6 hours |
| Iodine-131 | 8 days |
| Iodine-123 | 13.2 hours |
| Gallium-67 | 3.3 days |
| Indium-111 | 2.8 days |
| Thallium-201 | 73 hours |
Biologic half-life accounts for the biologic clearance of a radiopharmaceutical from an organ or tissue. If a radiopharmaceutical is cleared from the body via the kidneys, but kidney function is impaired, the radiopharmaceutical will have a longer biologic half-life than if kidney function had been normal.
The effective half-life is dependent on both the physical half-life and the biologic clearance.
By far, the most widely used radioisotope is technitium-99m (abbreviated Tc-99m, the m standing for metastable ). It has a half-life of 6 hours, meaning that it loses roughly half of its radioactivity in that time. It decays by emitting low-energy gamma rays rather than higher energy beta emission and is easily combined with a wide variety of biologically active substances.
Radioisotope doses in nuclear scanning are typically minute in amount, i.e., at the microcurie or millicurie level.
Geiger counters
Geiger counters are used mostly to detect contaminations (e.g., inadvertent or unknown spills) and are especially good at detecting low levels of radioactivity. Their portability and sensitivity allow them to survey relatively large areas for the presence or absence of radiation.
Scintillation detectors
Scintillation is the process by which a material called a scintillator (the most common compound being sodium iodide mixed with thallium) luminesces when excited by ionizing radiation. The luminescence is in the form of a minuscule flash of light. A scintillation detector receives the emitted light , intensifies its signal in a device called a photomultiplier, and converts that signal into an electrical pulse for further analysis by computer.
Scintillation detectors have the capacity to convert ionizing radiation into electrical energy in an amount proportional to the energy deposited in the crystal, which is key to their ability to produce diagnostic images.
Gamma cameras
A gamma camera uses one or more scintillation detectors made of crystals that scintillate in response to gamma rays emitted from the patient. A computer reconstructs an image based on the distribution and concentration of the radioisotope deposited in the target organ.
Images can be acquired either as static , whole-body, dynamic images (i.e., change in activity in the same location measured over a period of time) or single-photon emission computed tomography (SPECT) images.
SPECT imaging is a nuclear medicine study that is performed by using a gamma camera to acquire multiple two-dimensional (2D) images from multiple angles, which are then reconstructed by computer into a three-dimensional (3D) dataset that can be further manipulated to display thin slices in any projection.
To acquire SPECT images, the gamma camera rotates around the patient.
SPECT scans use the same radiopharmaceuticals as 2D (planar) images.
Any type of nuclear medicine study can be performed using SPECT. SPECT is especially used in myocardial perfusion imaging, bone imaging, and functional brain imaging.
Most nuclear medicine scans have about 1 cm of resolution, meaning that they cannot accurately detect lesions smaller than that.
Radiopharmaceuticals are prescription drugs that require dispensing by a physician.
Each dose must be assayed for its radioactivity before being administered to the patient. Dose calibration is essential in assuring that a safe and effective amount of radiopharmaceutical is given. This is usually done by inserting a syringe containing the radiopharmaceutical into an ionization chamber that converts the ionization of a sample into a measurable dose , depending on the radioisotope used.
The performance of the dose calibrator itself must be evaluated at set intervals utilizing a series of tests to ensure that the calibrator is accurate and reliable.
A locked and controlled area is needed for the storage and preparation of radiopharmaceuticals. Techniques need to be in place to assure the material being injected is sterile and free of pyrogens.
Spills of liquid radiopharmaceuticals sometimes occur accidentally and there are prescribed methods for containing and cleaning the spill as well as disposing of the material used for the cleanup. The area in which the spill has occurred may be monitored by using Geiger counters.
While there is no absolute contraindication to the use of radiopharmaceuticals during pregnancy, some radioisotopes, for example, radioactive iodine, can cross the placenta and be concentrated in the fetal thyroid. Similarly, women who are breastfeeding may have to suspend breastfeeding for a period of time following administration of some radiopharmaceuticals because the pharmaceutical may pass through breast milk to the child. Renal excretion of some radioisotopes means they collect and concentrate in the urinary bladder of the mother and can pose a potential risk by their proximity to the developing fetus.
Adverse reactions to the radiopharmaceutical itself are extremely rare and are related to the pharmaceutical, such as those composed of human serum albumin, rather than the radioisotope.
Some types of radiotherapy, utilizing radiopharmaceuticals administered at much higher doses than for diagnostic studies, may require the patient to be hospitalized in order to assure radiation safety. Patients may be assigned to private rooms without outside visitors for 24 hours. The Nuclear Regulatory Commission no longer requires hospitalization for iodine-131 treatment of the thyroid.
Patients treated with radioiodine (again in doses much larger than for diagnostic purposes) may be warned to carry certification of their treatment because they may trigger radiation security alarms at airports and elsewhere for up to 4 months after treatment.
Patients undergoing treatment with radiopharmaceuticals must follow instructions so that exposure to other individuals can be maintained as low as is reasonably possible.
Commonly used nuclear medicine studies include:
Bone scans
Ventilation/perfusion scans
Cardiac scans
Thyroid scans
HIDA scans
GI bleeding (blood loss) scans
Bone scans are the screening method of choice for the detection of osseous metastatic disease and for diagnosing fractures before they become visible by conventional radiography.
Bone scans offer the advantage of being widely available, inexpensive, and of being capable of imaging the entire skeleton at the same time. Although MRI scans may be more sensitive in detecting osseous metastases, they are less widely available and usually more expensive. The disadvantages of bone scanning are poorer spatial and contrast resolution.
Technetium-99m (Tc99m) methylene diphosphonate (MDP) is the radiopharmaceutical most frequently used for bone scanning. It combines a radioisotope, technetium-99m, with a pharmaceutical (MDP) that directs the isotope to bone. Diphosphonates are rapidly removed from the circulation and produce little background noise from uptake in soft tissues.
After the intravenous injection of the radiopharmaceutical, most of the dose is quickly extracted by the bone. The remaining radiopharmaceutical is excreted by the kidneys and subsequently collects in the urinary bladder. Less than 5% of the injected dose remains in the blood 3 hours after injection.
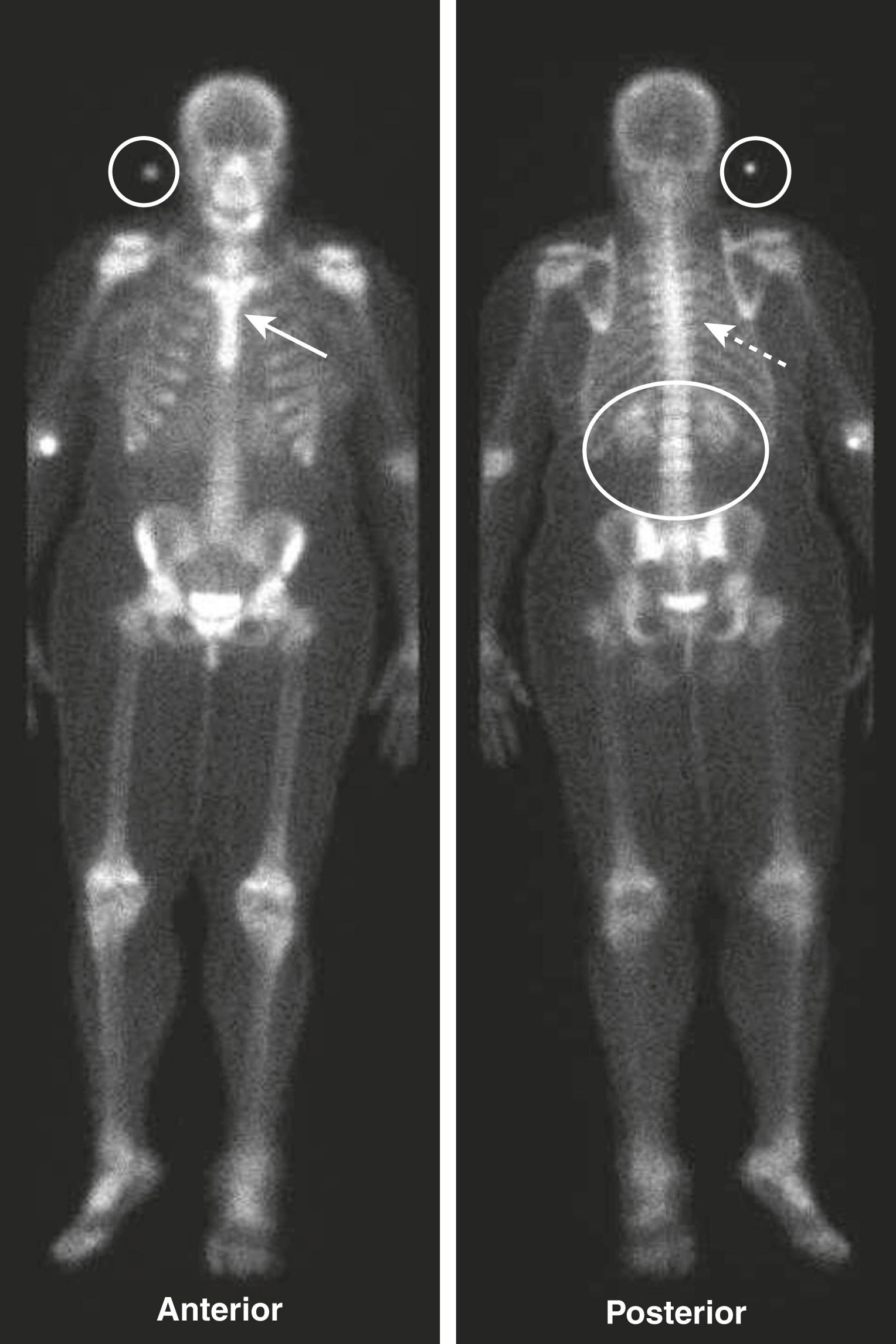
In most instances, the entire body is imaged about 2 to 4 hours after injection, either by producing one image of the whole body, multiple spot images of particular body parts, or both. Anterior and posterior views are frequently obtained, because each view brings different structures closer to the gamma camera for optimum imaging, for example, the sternum on the anterior view and the spine on the posterior view.
Unlike the convention used for viewing other studies in radiology, the patient’s right side is not always on your left in nuclear scans. This can be confusing, so make sure you look for the labels on the scan ( eFig. A.1 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here