Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
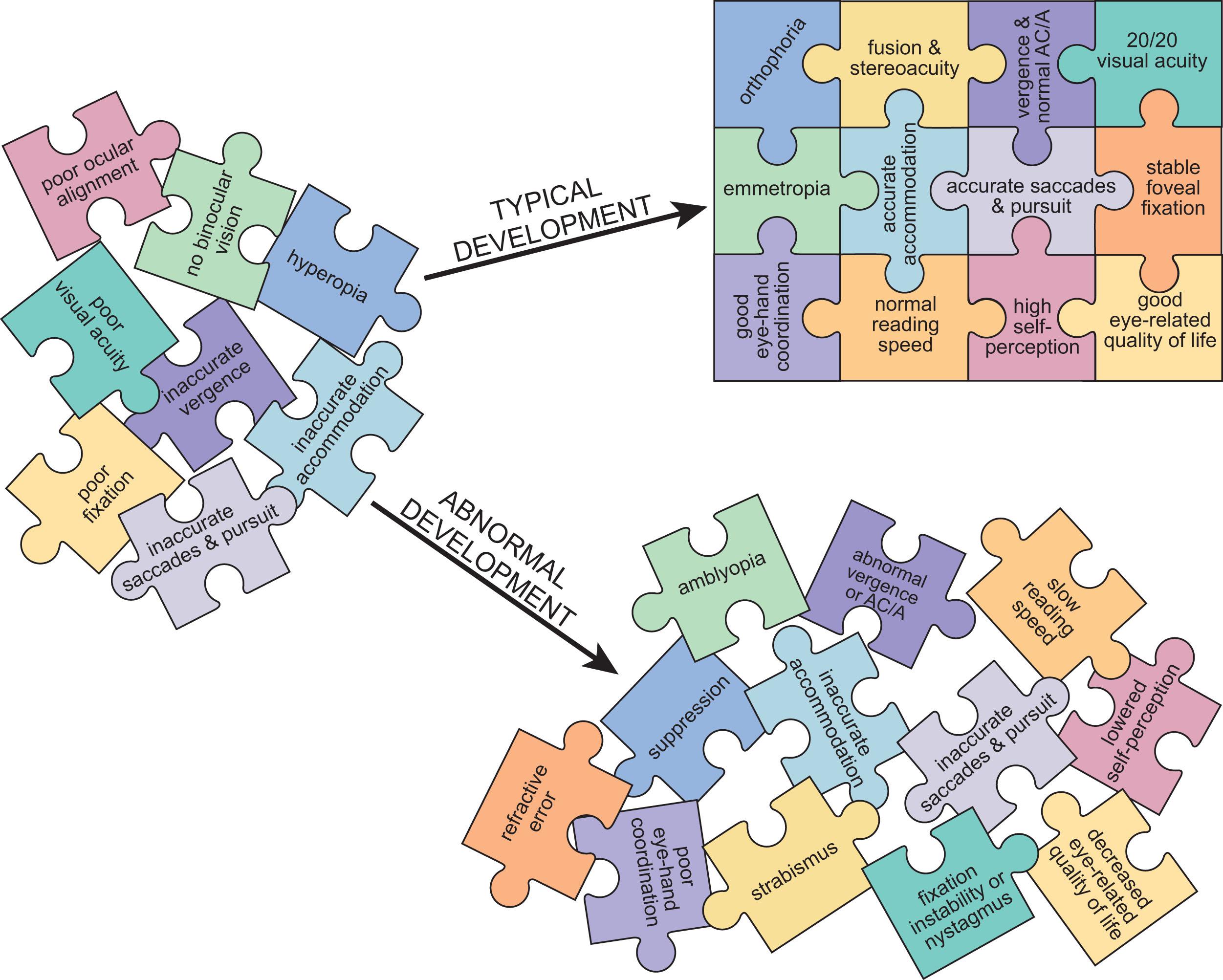
Since the late 1970s, there has been an explosion of new information about the maturation of the human visual system. A growing appreciation of the role of visual experience in shaping the functional organization of the maturing visual system has driven the research effort to define the normal course of maturation and its susceptibility to early abnormal visual experience. There are several critical periods during which visual functions emerge and rapidly develop, and during which they are especially vulnerable to abnormal visual experience. Visual maturation relies on a complex interplay of structural changes in the eye and the brain, dependent on both genetic factors and visual experience. Binocular interactions, including competition and cooperation, are essential in visual development. A disruption in balanced, binocular visual inputs during these critical periods can lead to sensory and ocular motor impairments that persist throughout life if not treated promptly and effectively, suggesting that there also exists a critical period for recovery of visual function. Moreover, disrupted visual development impacts daily life functioning tasks like reading and motor ability, which in turn impact self-perception and quality of life. This chapter will describe typical and atypical visual development, and how aspects of vision are interconnected to complete the puzzle of visual development ( Fig. 3.1 ).
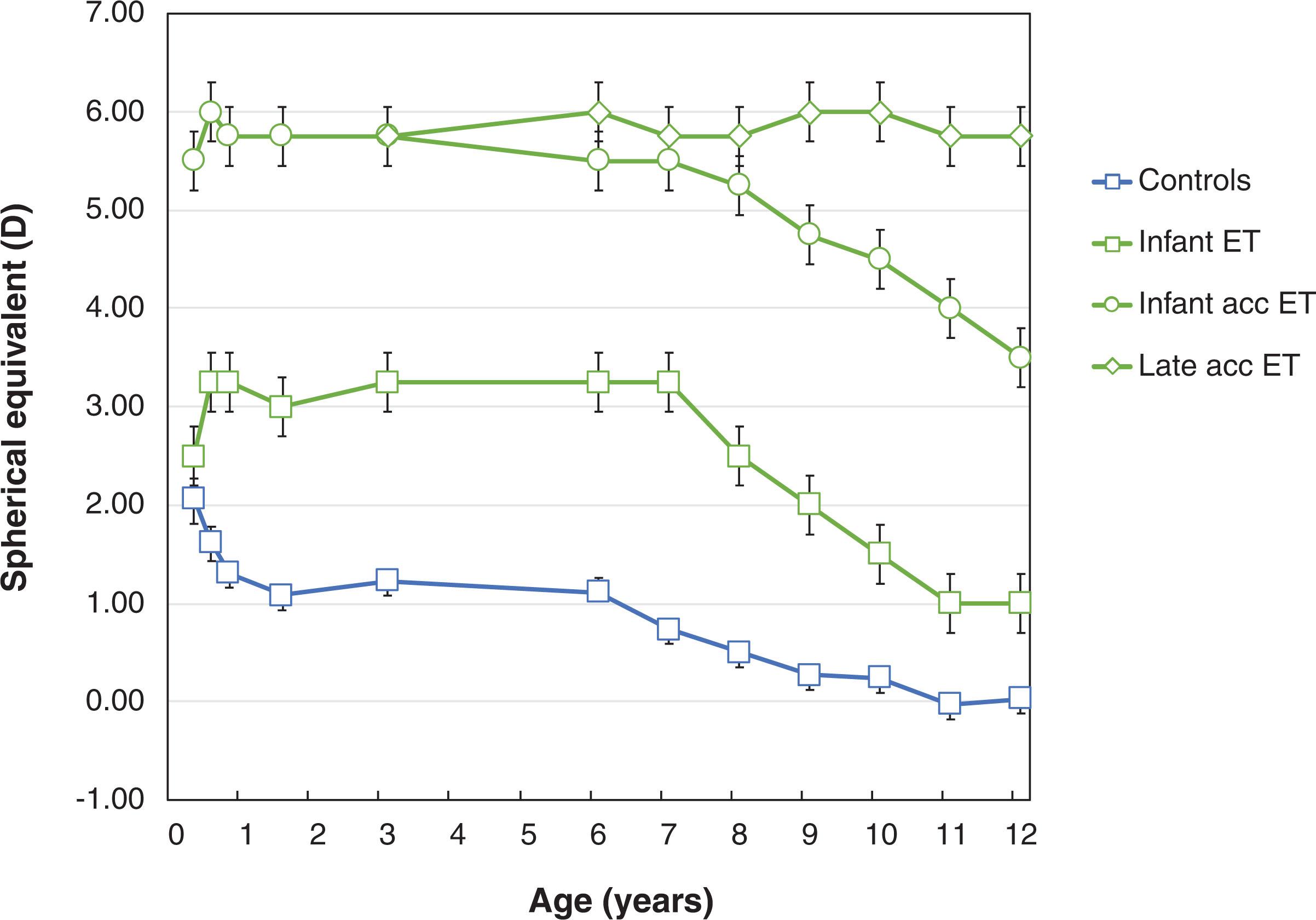
Retinal image quality plays a key role in postnatal visual experience and depends on both the optical properties of the eye and the accuracy of accommodation. Clinically, fundus examination of the infant eye suggests that the optical quality is good and this clinical impression is supported by data on the transmission properties of ocular media and chromatic and higher order aberrations. On the other hand, there is significant growth of the eye and its ocular components postnatally. The axial length of the newborn eye is only 18 mm and typically increases by more than 2 mm during the first 9 months of life. Axial length growth is accompanied by decreasing corneal power, and changes in lens power, radius, and thickness. Infants are born with a wide range of refractive errors, with a median of approximately +2.50 D hyperopia. Growth of the eye and changes in ocular components result in a significant loss of hyperopia, and a significant decrease in the variability among infants in refractive error from +2.09±1.32 D at 3 months of age to +1.31±1.02 D at 9 months of age ( Fig. 3.2 ). Between 3 and 9 months, the dioptric effects of axial growth to reduce hyperopia exceed the effects of losses in corneal and lens power, and an imbalance results in a net loss of hyperopia. After that, axial growth and changes in lens power soon reach a balance point where further increases in axial length are ineffective at decreasing hyperopia; there is little change in refractive error up to at least 3 years of age. Interestingly, more hyperopic eyes grow faster during infancy, so emmetropization generally is effective in decreasing hyperopia. However, the most highly hyperopic infants, those in excess of +5 D, tend to not emmetropize and may be at risk for conditions such as accommodative esotropia, which is associated with high hyperopia. As can be seen in Fig. 3.2 , children with either infantile or late onset accommodative esotropia maintain a mean refractive error of >+5.00 D until age 7 years. Interestingly, children with infantile esotropia have only moderate hyperopia but also fail to emmetropize during the first year of life, maintaining approximately +3.00 D hyperopia throughout their preschool years. After age 7 years, children with either infantile esotropia or infantile accommodative esotropia experience a myopic shift, losing 0.4–0.5 D of hyperopia each year, so that by age 12 years they typically have a significantly reduced amount of hyperopia. In contrast, children with late-onset accommodative esotropia experience little change in refractive error after 7 years of age, whether or not an attempt is made to wean them from their glasses.
Overall, prevalence of anisometropia ≥1.00 D during infancy and preschool years is low (1%–4%) but increases to 6% by the age of 15 years and 9%–15% in adulthood. Although there is clearly a correlation between the interocular differences in refraction and interocular differences in axial length, what triggers different growth rates between the two eyes is unclear. Longitudinal data on anisometropia in children without any other eye condition suggest that anisometropia during infancy is largely transitory, and often resolves with age. On the other hand, severe infantile anisometropia (>3.00 D interocular difference) and lower amounts of anisometropia in the presence of high hyperopia may be sustained over a long period, and increase risk for development of strabismus and amblyopia during the preschool years. Because infants are hyperopic, accommodation is needed to eliminate defocus and improve retinal image quality. Chronic defocus can disrupt cortical development. For 2- to 4-month-old infants, accommodative accuracy is in the order of 0.5 D or better binocularly and 0.75 D monocularly. The finding of more accurate binocular accommodative response amplitude than monocular amplitude is not unique to infants; it agrees with adult data that accommodative lag is greater when one eye is occluded. Infant accommodation is less stable than adults’, either due to elevated accommodative microfluctuations or less sustained visual attention. Studies of school-age children with hyperopia and/or astigmatism have demonstrated that accuracy of accommodation can vary substantially depending on visual task demand. The accuracy of accommodation is reduced in uncorrected hyperopia and in the presence of high astigmatic errors, but improves at near and with increasingly demanding visual tasks. Poor accommodative accuracy is present in some children with amblyopia when their fellow eye is patched, but accommodative accuracy improves as visual acuity of the amblyopic eye improves with patching treatment. In children with Down syndrome, underaccommodation is substantial; spectacles do not improve accommodative accuracy. The accommodation system of children with Down syndrome appears to have the physical capacity to respond, but the neural control of accommodation has an anomalous set point. Children with albinism also underaccommodate, although there is a range of accommodative response and better visual acuity is correlated with better accommodative response.
Visual acuity, stereoacuity, and fusion comprise the most basic aspects of the sensory assessment during an eye examination. Evaluating each of these elements poses unique challenges in infants and young children. Visual acuity depends on both optical and neural factors: the quality of the retinal image (discussed above), and the health and functioning of the retina optic nerve, tract, and visual cortex. As a result, measurement of visual acuity is a simple way to assess the overall health status of the visual pathways.
Routine testing of visual acuity in older children and adults with letter charts evaluates recognition acuity; i.e., the ability to discern letters at a given distance according to a fixed standard. Recognition acuity cannot be tested in infants and preverbal and nonverbal children. The most common visual acuity assessment for these groups is the Teller Acuity Cards, which assess resolution acuity. The Teller Acuity Cards present a square patch of high contrast black and white stripes on one side of a rectangular gray card. The background gray is such that, if the stripes are too fine for the infant to resolve, they will fade into the gray background. Infants innately preferentially fixate the side of the Teller Acuity Card with a more interesting stimulus (stripes) rather than the side with a less interesting stimulus (gray background only), but only if they can see the stripes. Each successive card presents finer stripe patterns, ranging from 1.9 to −0.08 logMAR (20/1600 to 20/16) in approximately 0.15 logMAR (1.5 lines) steps. The smallest stripe size that elicits a consistent preferential looking response indicates the child’s visual acuity. As shown by the blue lines in Fig. 3.3 , at 1 month visual acuity as measured with Teller Acuity Cards is 1.5 logMAR (20/640) but improves rapidly to 0.7 logMAR (20/100) by 6 months, and 0.5 logMAR (20/60) by 1 year. By 3 years, children typically have visual acuity of 0.1–0.3 logMAR (20/25 to 20/40).
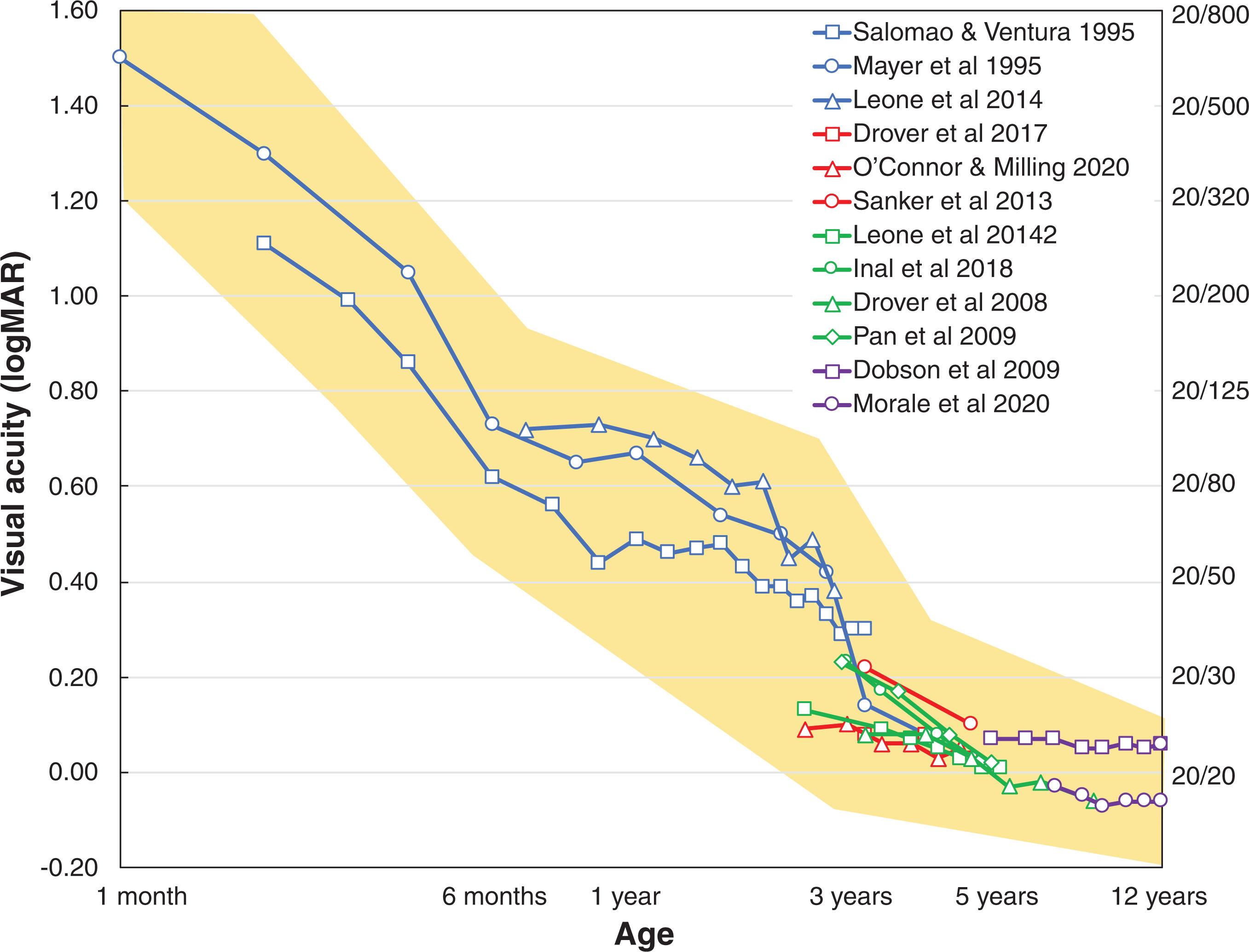
Teller Acuity Card test results may appear to be too good. Resolution acuity is typically better than recognition acuity, especially in conditions like amblyopia where recognition acuity may be reduced by a factor of 1.5 compared with resolution acuity. The discrepancy is likely due to the more complex spatial information in optotypes compared with simple stripe patterns, the presentation of optotypes in crowded or linear format, and the more complex recognition task required for identifying optotypes. The overestimation of optotype visual acuity when using Teller Acuity Cards is greater when amblyopia is severe. Despite the overestimation, most cases of amblyopia are accurately identified using Teller Acuity Cards, with a sensitivity of 80%. Because of the overestimation, children’s Teller Acuity Card test results must be interpreted with some caution, particularly when deciding whether a child meets criteria for vision services or vision-related accommodations. In these cases, Teller Acuity Card test result reports should include expert interpretation.
By age 3 years, most children can participate in recognition acuity testing, either giving verbal responses or by using a matching card. Lea symbols and Kay pictures, both designed using modern optotype principles and available in logMAR steps, can be useful for children too young to test with letter optotypes. Standardization of visual acuity measurement is helpful for accurate monitoring of the response to treatment in individual children and in clinical trials. Electronic versions of the HOTV and ETDRS visual acuity tests are available and have been used in many clinical trials. Each uses crowded, isolated letters, have high test–retest reliability, have good agreement with standard charts, and are available on multiple commercially available platforms. The red lines in Fig. 3.3 illustrate normative data for the Lea symbols test, green lines for the electronic ATS HOTV test developed by the Pediatric Eye Disease Investigator Group (PEDIG), and purple lines for the ETDRS test conducted with charts or the electronic ETDRS. At 3 years, average recognition visual acuity is 0.2 logMAR (20/30), improves to 0.0 logMAR (20/20) by 6 years and, by some reports, shows further improvement through age 8 years.
Development of normal vision acuity depends on both the health of the visual pathways and normal, balanced postnatal visual experience. During infancy and early childhood, visual deprivation from a cataract or discordant visual experience due to strabismus or anisometropia can result in a permanent visual acuity impairment of the affected eye if not treated early in life. Amblyopia (visual acuity deficit that results from abnormal visual experience during the early critical period of visual development) is thought to result from prolonged suppression of the affected eye’s input to the visual cortex. The visual acuity deficit is only one component of a collection of sensory and ocular motor deficits caused by abnormal visual experience. Discordant visual experience during the first years of life due to strabismus, for example, typically results in lifelong suppression, subnormal or absent stereoacuity, and a constellation of associated visual and visuomotor sequelae.
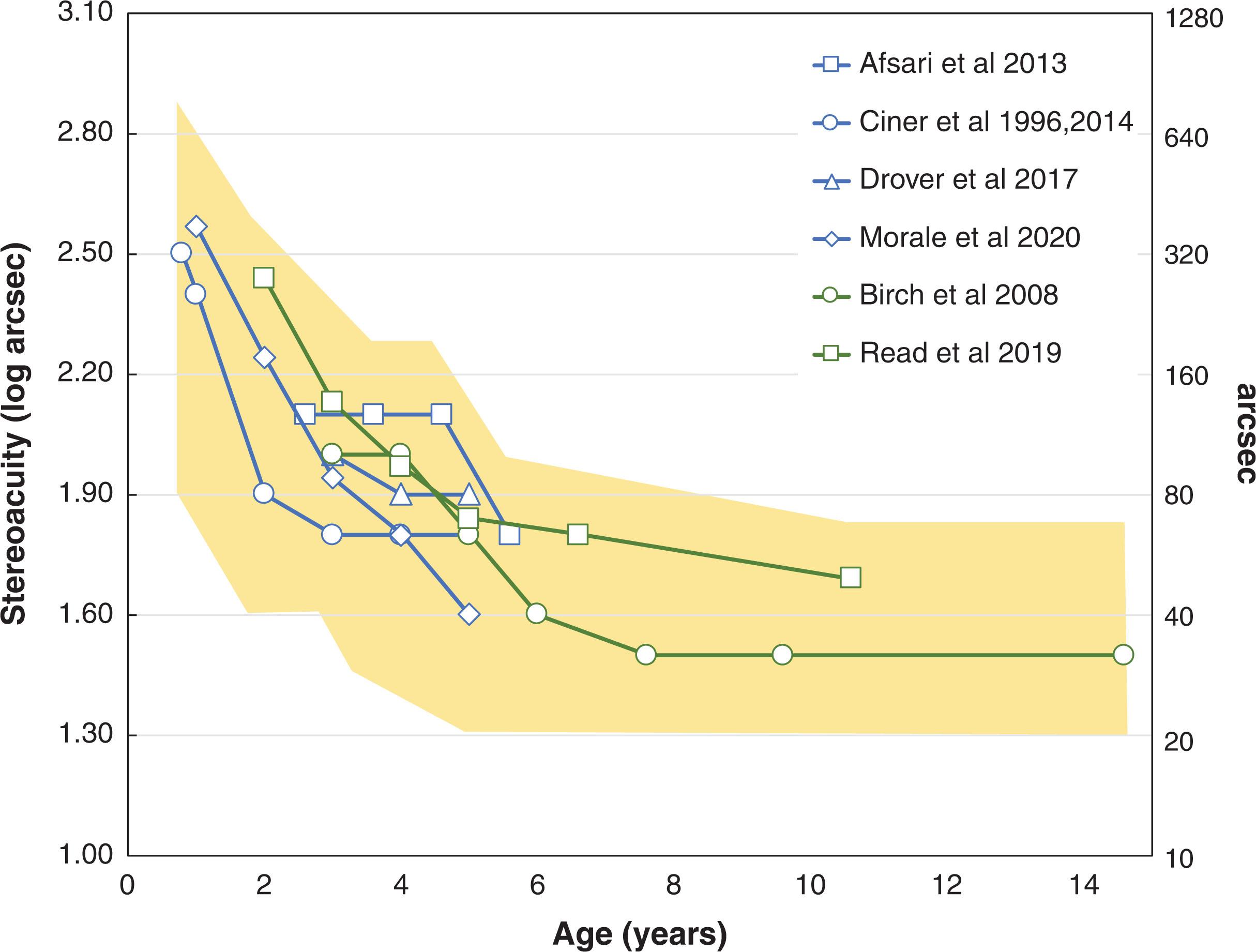
Two types of stereoacuity tests are widely available: contour tests and random dot tests. Contour tests have the disadvantage of providing monocular or nonstereoscopic binocular cues that enable some children with no stereoacuity to pass the initial 2–4 levels. Random dot tests contain no monocular or non-stereoscopic binocular cues; depth can only be appreciated by global binocular evaluation of corresponding points and disparate points. It is important to optically correct the child and it is best to perform stereoacuity testing early in the examination, before the child's eyes are dissociated by examination procedures. For infants and preverbal children, stereoacuity can be evaluated with the preferential looking test called the Preschool Assessment of Stereopsis with a Smile (PASS) Stereotest. For children age 3 years and older, stereoacuity can be tested with the matching, pointing, or naming tasks available in the PASS, Randot Preschool, Toegepast Natuurwetenschappelijk Onderzoek (TNO), and Frisby Stereoacuity tests. Coarse stereoacuity can be assessed using the Randot Butterfly (2000 arcsec) or with the Stereo Fly test (3000 arcsec). Unfortunately, the Stereo Fly test often yields false “pass” outcomes. A modified testing protocol that utilizes a second pair of glasses with polarizers orientated in the same direction for both eyes can be used to verify that the tentative “pass” is based on binocular disparity cues, and not simply a result of the child’s expectation that wings are elevated, of their repeated exposure to the test with only two possible responses, or from alternation of fixation with observation of image jump. The onset of stereopsis occurs at approximately 4 months of age, with rapid maturation of stereoacuity during infancy, and further slow improvement of stereoacuity continuing during childhood ( Fig. 3.4 ). Stereoacuity depends on good vision in both eyes, accurate and stable alignment of the visual axes, and intact binocular cortical mechanisms. Abnormal binocular experience early in life associated with anisometropia or strabismus may result in maldevelopment of binocular vision, suppression, and reduced or nil stereoacuity. Moreover, there is evidence that discordant binocular experience that is early and prolonged (>3 months duration) is associated with a constellation of profound visual and ocular motor abnormalities. For esotropic children, rehabilitation of even subnormal binocular function is associated with long-term stability of postoperative alignment, reduced severity of amblyopia, and reduced risk for recurrent amblyopia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here