Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As is the case for development of the prosencephalic structures discussed in Chapter 2 , the most critical time for development of the form and cellular structure of the brainstem and cerebellum is in the second and third months of gestation. The widespread implementation of prenatal screening protocols that include fetal anatomical ultrasound (US) surveys has made suspected posterior fossa anomalies in the fetus one of the more common indications for prenatal neurological counseling. Some of these posterior fossa anomalies are only identified or require diagnostic confirmation or refinement in the postnatal period. As with other developmental anomalies, a brief discussion of normal development of the major posterior fossa structures is followed by a review of the more common malformations.
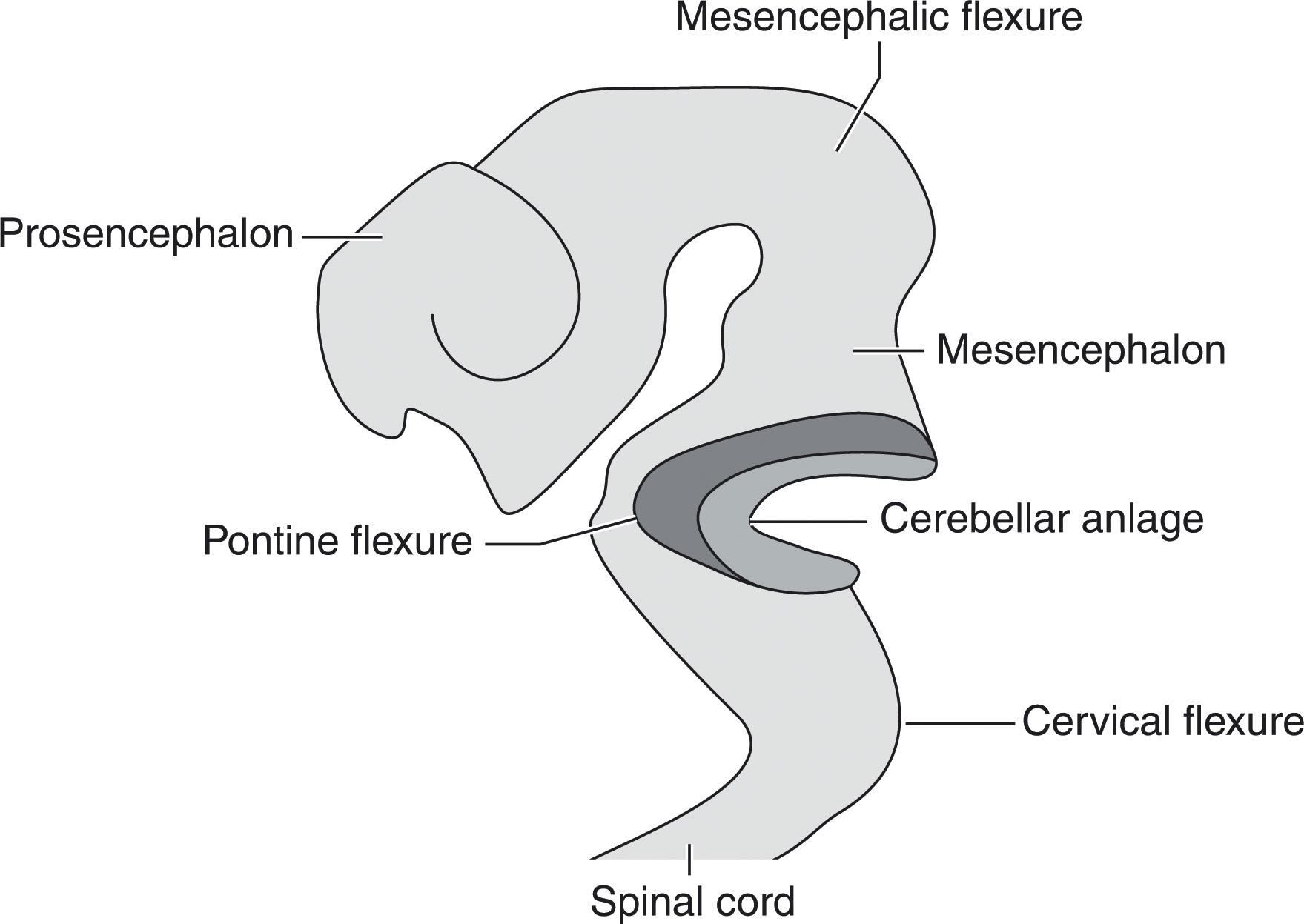
Completion of neural tube closure toward the end of 4 weeks postconception (p/c) is followed by several critical steps in brain development ( Chapter 1 ), namely the onset of dorsoventral patterning and the development of three primary vesicles and three flexures in the rostral neuroaxis. These developmental events play an important role in defining the territory of the future brainstem and rhombencephalic structures ( Fig. 4.1 ). The onset of dorsoventral patterning starts with the appearance of the sulcus limitans in the lateral wall of the neural tube (~4 weeks p/c), dividing it into the basal (ventral) plates and the alar (dorsal) plates. This division is followed by the appearance of three primary vesicles (“superventricles”) (see Chapter 3 ): the prosencephalic, mesencephalic, and rhombencephalic vesicles. These superventricles later develop into five secondary vesicles: the prosencephalic vesicle forms the telencephalic and diencephalic vesicles, and the rhombencephalic vesicle forms the metencephalic and myelencephalic vesicles. Three flexures develop in the anterior neural tube (~5 weeks p/c): the pontine flexure, the mesencephalic flexure between the forebrain and brainstem, and the cervical flexure between the myelencephalon and cervical spine. These events give rise to a Z-shaped embryonic brainstem; of note, persistence of these embryonic flexures and the Z-shaped brainstem beyond 15 weeks p/c is an indication of severe brain dysgenesis (see later).
4 weeks p/c: neural tube closure; sulcus limitans separates alar and basal plates
5 weeks p/c: rostral neural tube vesicles define future ventricles; anterior neural tube flexures form, elongating fourth ventricular roof
7–8 weeks p/c: neuronal proliferation accelerates in dorsomedial ventricular zone—origin of all inhibitory GABA-ergic neurons (express Ptf1 )
8 weeks p/c: granule cell precursors begin to express Atoh and enter external granular layer
10 weeks p/c: ventricular zone begins to shrink and subventricular zone to expand
10 weeks p/c: transverse crease (plica choroidea) forms in fourth ventricular roof
10 weeks p/c: foramen of Magendie begins to form
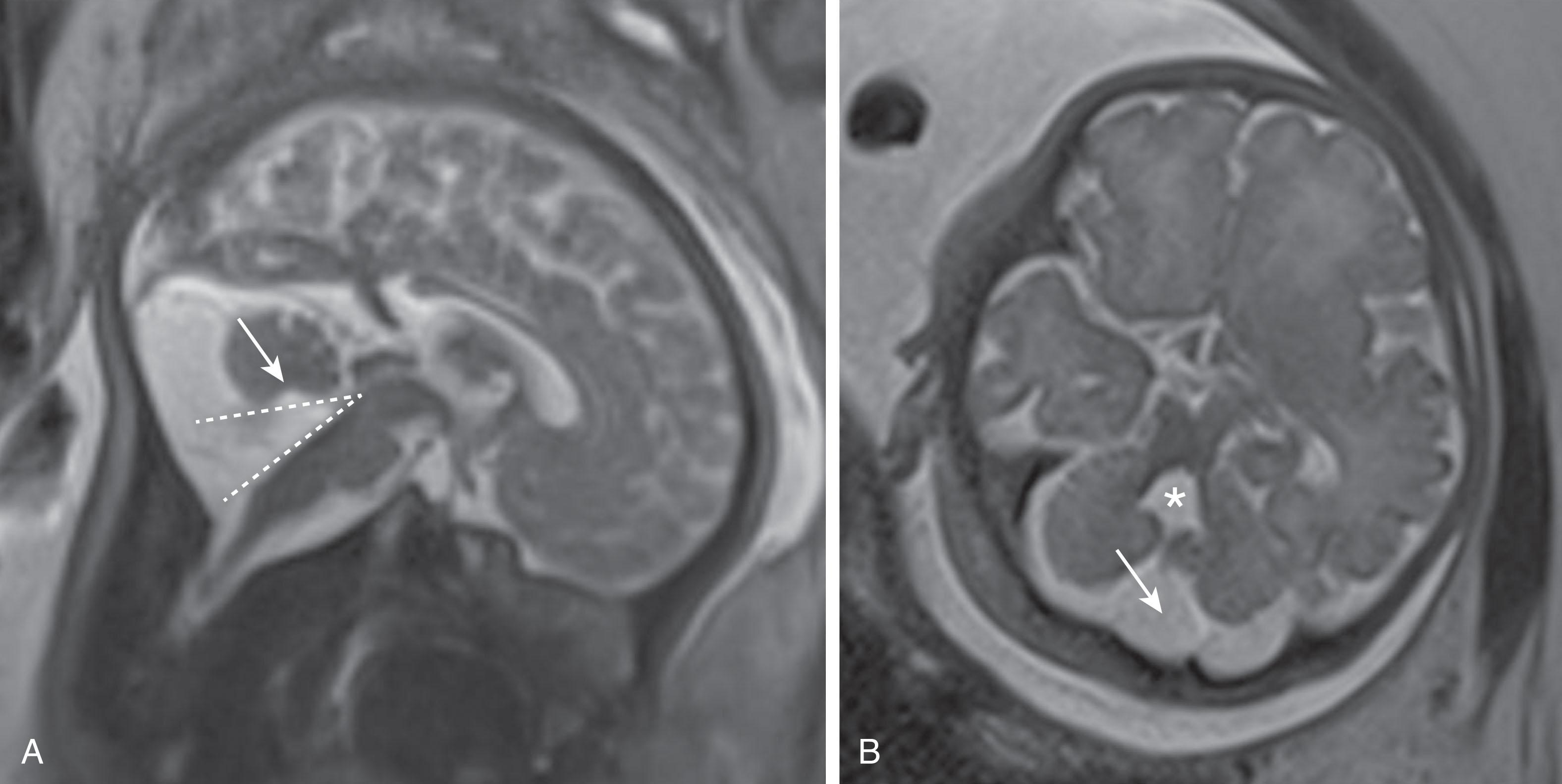
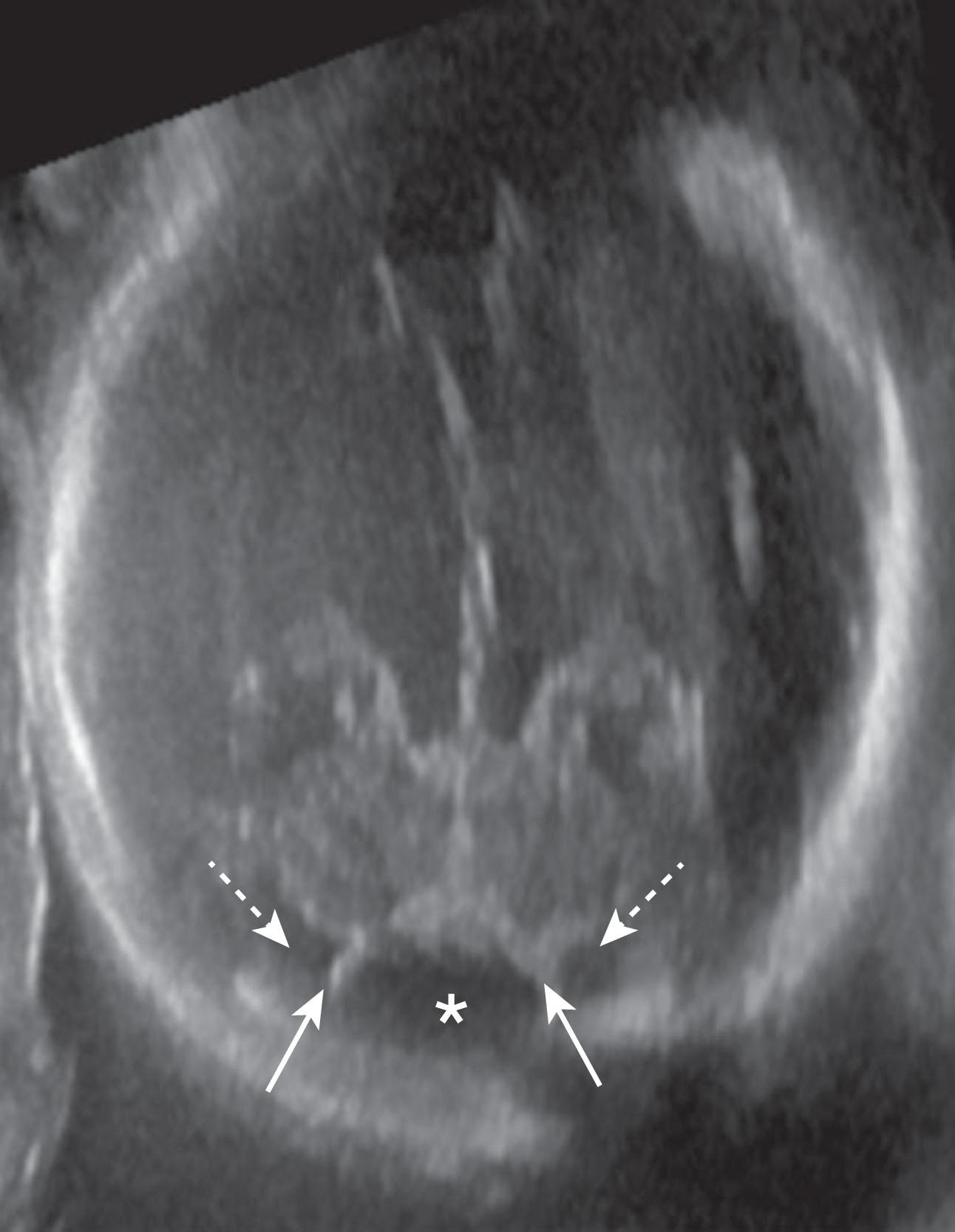
10 weeks p/c: Blake pouch begins to perforate; by 24 to 26 weeks p/c, approximately two-thirds have resolved
11 weeks p/c: primary fissure appears
12 weeks p/c: neuronal proliferation accelerates in dorsolateral subventricular zone of the rhombic lips—origin of all excitatory glutamatergic neurons (express Atoh )
12–13 weeks p/c: cerebellar hemispheres and vermis ( Box 4.2 ) begin to emerge
14 weeks p/c: pontine growth accelerates; midbrain growth slows
14–17 weeks (as late as 26 weeks): foramina of Luschka form
GABA-ergic interneurons undergo radial migration from ventricular zone to form deep cerebellar nuclei and Purkinje cell layer
Glutamatergic cells undergo (1) tangential migration from rostral rhombic lip to form external granular layer, (2) migration from caudal rhombic lip to form pontine and inferior olivary nuclei, and (3) rhombic lip stem cell zone incorporation into posterior vermis
26–32 weeks p/c: rapid expansion (transit amplification) of EGL and early foliation begins (surges from 24–40 weeks p/c)
At peak thickness EGL is six to nine cells deep, divided into inner and outer layers separated by a vascular layer
29 weeks p/c: external granule cell precursors cover entire cerebellar surface
EGL neurons begin inward radial migration along Bergmann glial cells to form IGL
Purkinje cells differentiate and secrete Shh that stimulates proliferation of precursor cells in the EGL
Massive EGL proliferation and inward migration leads to fivefold volumetric growth of the cerebellum
EGL gradually dissipates throughout first postnatal year, and IGL cells become greatly compacted
Purkinje cells enlarge and differentiate as major outflow to dentate nuclei
Purkinje cell differentiation enlarges the molecular layer severalfold
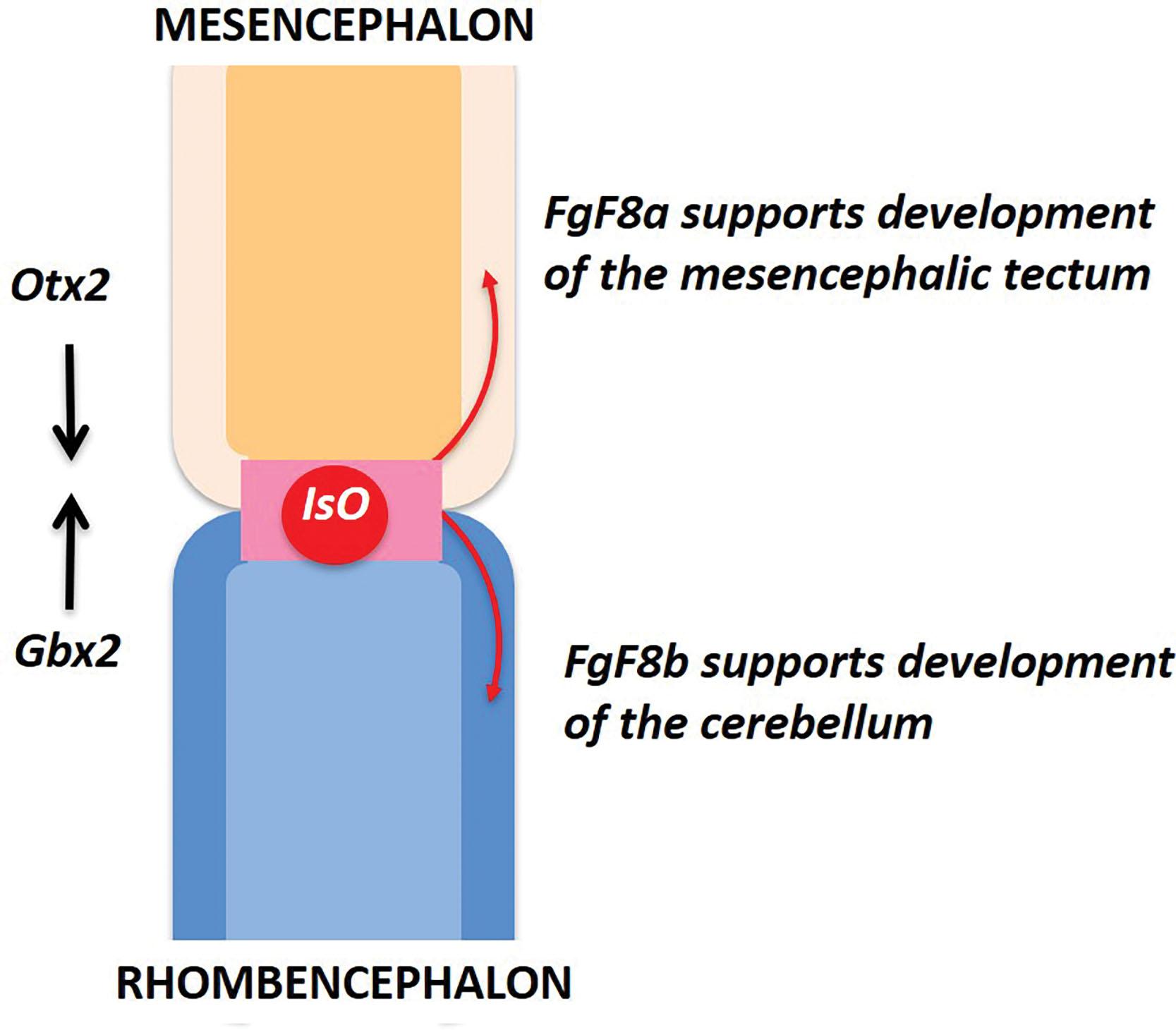
Development of the brainstem and cerebellum is the result of highly coordinated and complex patterning events in rostrocaudal and dorsoventral directions. Bidirectional signaling centers become situated at the boundaries of future segmented brainstem compartments, from where they coordinate cell lineage restriction that defines the regions under their influence. The precise position and stabilization of these signaling centers is critical for normal development of the brainstem and cerebellum and is coordinated by specific homeobox ( Hox) genes. Two signaling centers relevant to this discussion are situated at the future diencephalic-mesencephalic and rhombencephalic-mesencephalic junctions. The latter center, known as the isthmic organizer (IsO), coordinates the differentiation of the rhombencephalon into eight rhombomeres. Much less is known about signaling at the diencephalic-mesencephalic junction, although alterations in signaling likely underlie the diencephalic-mesencephalic spectrum of anomalies discussed later.
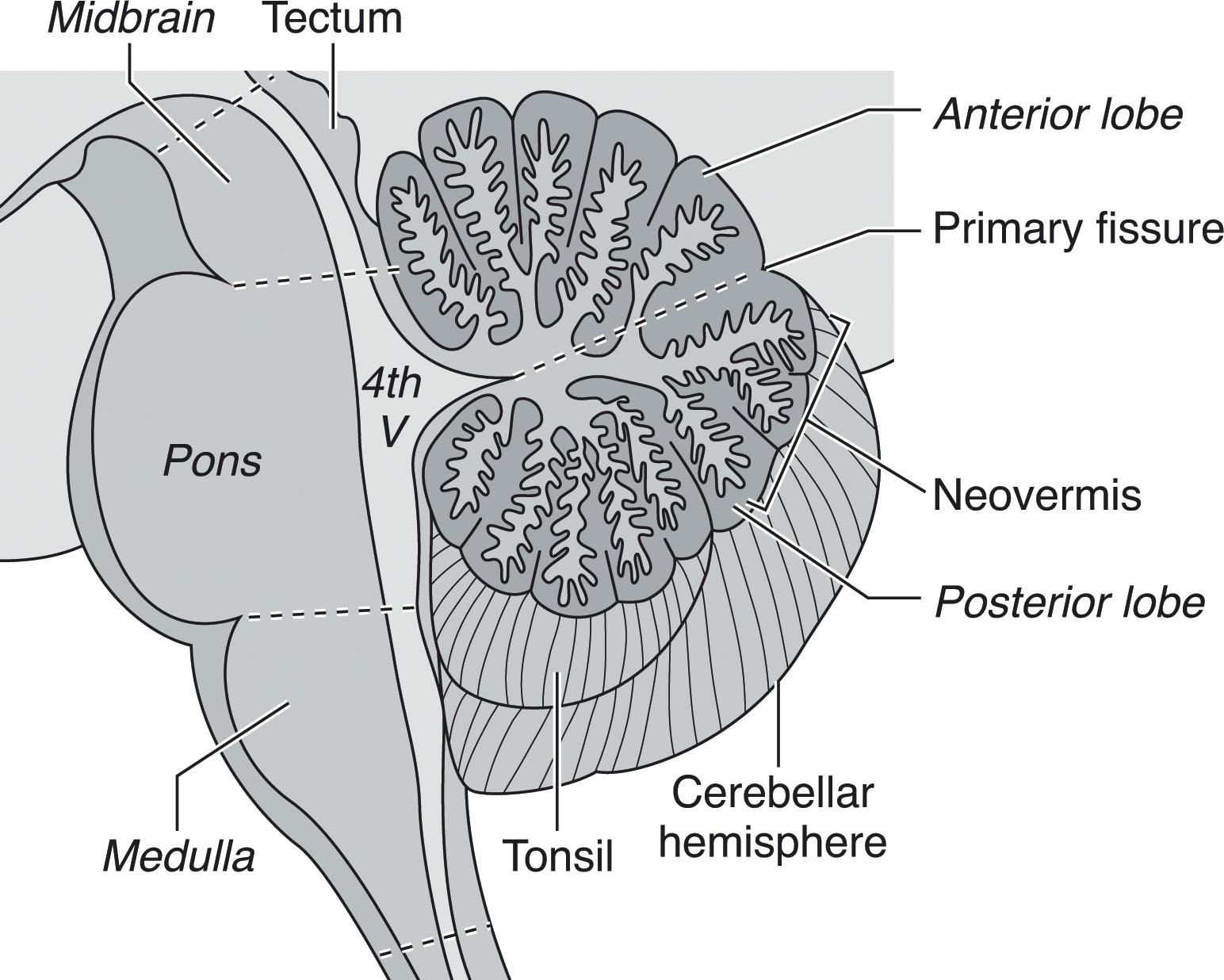
The cerebellum develops around the mesencephalic-rhombencephalic junction (MRJ), where a relative narrowing, the isthmus, defines the location of the IsO, a critical transient patterning center that will orchestrate events defining the territories of the cerebellar anlage and related brainstem structures ( Fig. 4.2 ). The future cerebellar anlage develops from the dorsolateral alar plates of the first rhombomere, appearing as bilateral thickenings on the dorsolateral surface of the rhombencephalon, known as the rhombic lips. Conversely, the pontine tegmentum develops from the basal plates. Subsequent development of the cerebellar hemispheres and vermis results from a series of proliferative and migratory events in the germinal matrices along the fourth ventricular surface and rhombic lips.
We will review brain development around the MRJ as three broad events in temporal sequence, although there is overlap between them. These are (1) development of the IsO and patterning of the MRJ and cerebellar anlage; (2) development of the fourth ventricular roof; and (3) development of the cerebellar hemispheres and vermis.
The cerebellar anlage develops out of the first rhombomere, which is bounded rostrally by the MRJ and caudally by the boundary of the second rhombomere. The IsO is critical for coordinating a precise sequence of events that define structural domains around the MRJ and will become the cerebellar anlage ( Fig. 4.2 ).
The initial critical event is the development and precise positioning of the IsO at the MRJ ( Fig. 4.2 ). This event is under the stewardship of two mutually suppressing Hox transcription factors: Otx2 and Gbx2 . Specifically, the IsO develops at the interface between the expression domain of Otx2 in the caudal midbrain and that of Gbx2 in the rostral hindbrain. Disturbed expression of either of these factors leads to abnormal positioning of the IsO and subsequent increased or decreased growth or transformation of structures in the MRJ region. Once established, the IsO expresses diffusible morphogens, including fibroblast growth factors (FGFs) and Wnt1, which define the “molecular boundaries” that determine the rostrocaudal morphological development of the midbrain and cerebellum. The differential expression of these morphogens along a rostrocaudal axis underlies the distinct spatiotemporal growth patterns of the brainstem structures, such as the relative increase in pontine growth and decrease in midbrain growth described in human fetuses after 15 weeks p/c. These factors set the stage for subsequent developmental events including specific cell differentiation and migration in the mesencephalic tectum and cerebellum. The first rhombomere (R1) has its rostral and caudal boundaries defined by complex molecular signaling. At the rostral border, the IsO releases a critical morphogen, Fgf8, of which there are two forms. Cerebellar development is dependent on Fgf8b, which induces the expression of Gbx2 , thereby suppressing Otx2 expression ( Fig. 4.2 ), whereas secretion of Fgf8a stimulates Otx2 expression, thereby suppressing Gbx2 and enabling development of the mesencephalic tectum ( Fig. 4.2 ). At the caudal boundary of R1, Fgf8 suppresses the expression of Hox2a , confining it to R2. In this manner R1 is maintained as an “Otx2/Hox2a exclusion zone” necessary for development of the primordial cerebellum. Development of the medial cerebellar structures, especially the vermis, is exquisitely dependent on appropriate Fgf8 expression. Consequently, lesions that include vermian hypoplasia likely involve early disturbances in IsO function. Given this complex series of patterning events, it is not surprising that developmental anomalies in different brainstem compartments often occur in combination (see later).
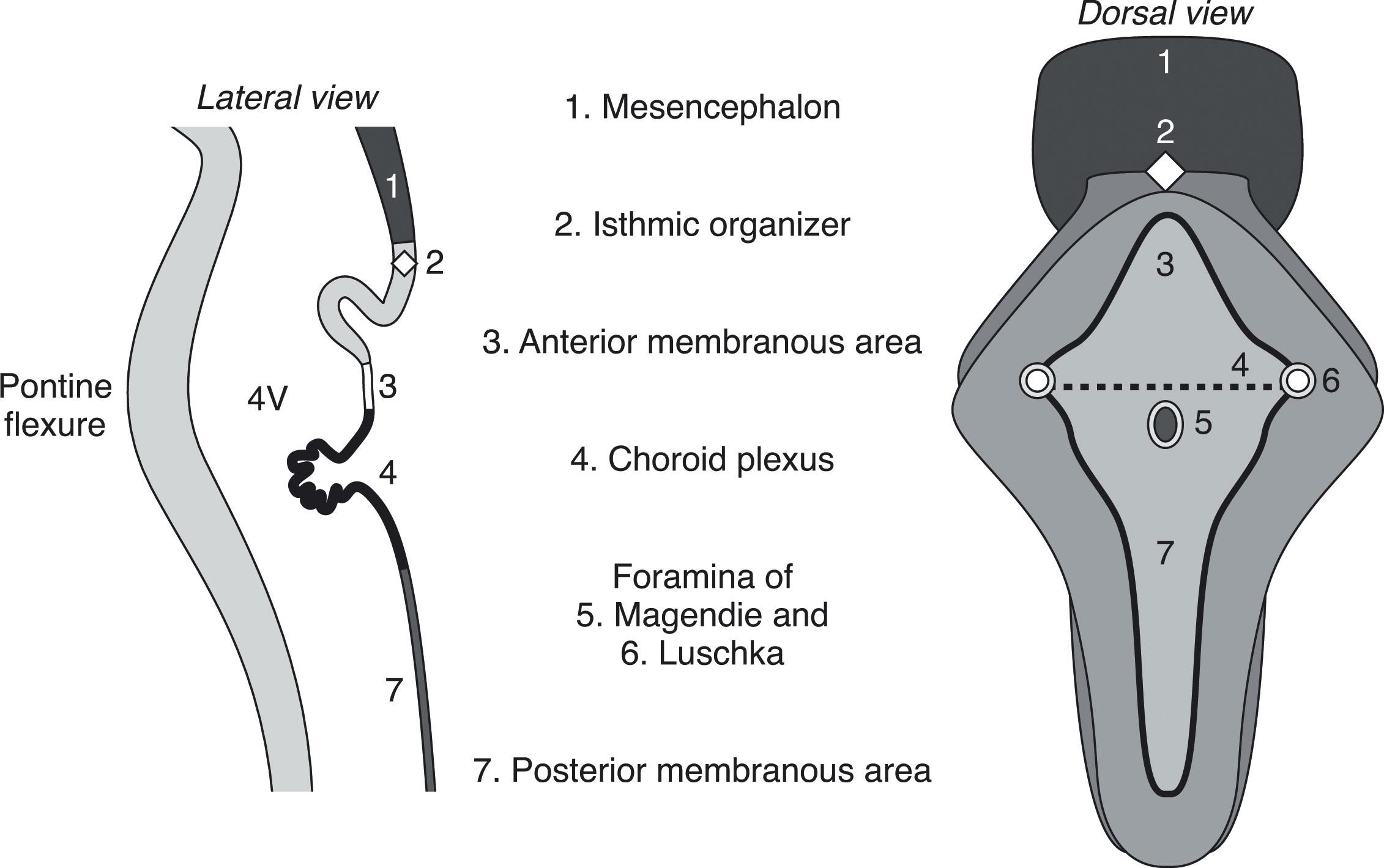
The second major event in development of the cerebellum is complex ( Fig. 4.3 ), and developmental disturbances in this region account for many of the posterior fossa anomalies seen in clinical practice. With development of the pontine flexure (~5 weeks p/c), the physical forces exerted on the neural tube widen its central lumen, forming the fourth ventricle, and stretch the dorsal rhombencephalon into the thin, diamond-shaped fourth ventricular roof ( Fig. 4.3 ). At about 10 weeks p/c, the fourth ventricular roof is transected by a transverse crease, the plica choroidea (the future fourth ventricular choroid plexus), into the anterior (AMA) and posterior membranous (PMA) areas. The AMA and PMA are also known as the superior medullary velum and inferior medullary velum, respectively. The roof of the fourth ventricle has two layers: an inner ependymal layer and an outer pial layer. The tela choroidea is vascularized tissue comprising pia and adherent ependyma that stretches from the nodulus to the flocculus in the PMA. The tela choroidea is connected to the taenia (white matter protuberances delimiting the rhomboid fossa margins) and houses the choroid plexus. The AMA also contains neurons, whereas the PMA does not. During normal development the vermis overgrows and incorporates the AMA; the PMA persists and undergoes a series of perforations. Around 10 weeks p/c the outer pial layer becomes fenestrated in the midline just caudal to the choroid plexus, followed soon thereafter by perforation of the underlying ependymal layer, to form the foramen of Magendi. If there is a delay in ependymal perforation, a small pouch of ependyma, the Blake pouch (a normal structure), herniates through the pial perforation, and then subsequently perforates. More delayed perforation of the Blake pouch may lead to increasing encysted fluid (Blake pouch cyst) in the posterior fossa (see later; Figs. 4.16 to 4.18 ). Usually by around 14 to 17 weeks p/c, but as late as 26 weeks p/c, the foramina of Luschka develop in the lateral angles of the PMA ( Fig. 4.3 ). However, the foramina of Luschka may remain atretic in up to 20% of humans.
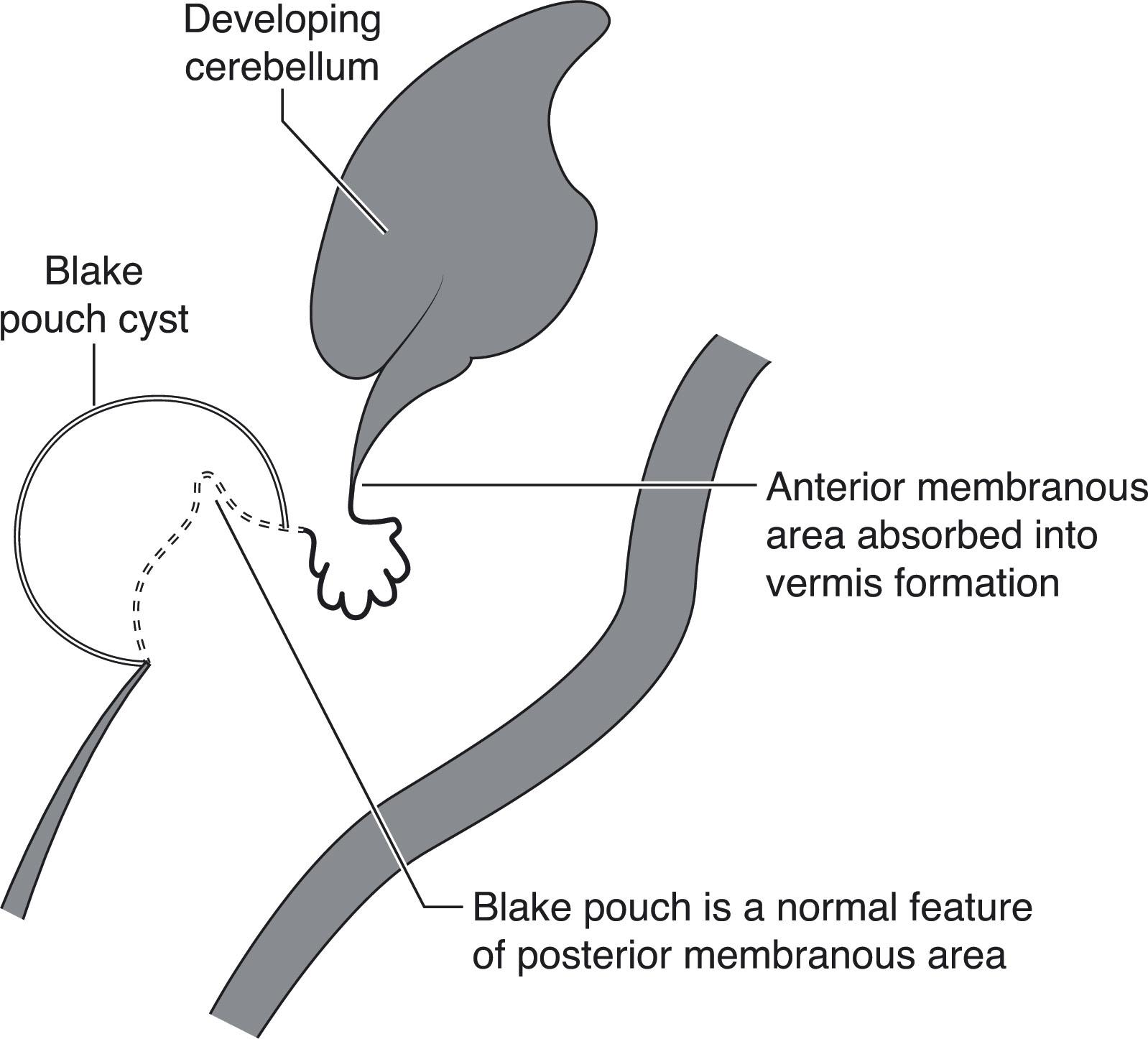
The dorsal regions of the anterior neural tube are initially transiently populated by future neural crest cells but subsequently develop into the definitive roof plate patterning center, which expresses bone morphogenic protein (BMP) and Wnt-secreted factors through which dorsoventral territory patterning is mediated. Additional signaling support for developmental events in and around the fourth ventricular roof comes in part from genetic expression in the overlying mesenchyme. Forkhead box (Fox) genes are expressed by the mesenchymal tissues but not by the underlying rhombencephalic tissues. Nonetheless, mutations in the Fox gene C1 (Foxc1) are associated (see later) with cerebellar hypoplasia, hypoplasia and dysplasia of the vermis, mega cisterna magna, and the Dandy-Walker malformation (DWM). Furthermore, disturbances in these signaling pathways may underlie conditions in which mesenchymal and neuroepithelial defects are associated (see later).
Although earlier investigators proposed that up to one-third of the cerebellum derives from the rostral mesencephalon, it is now known that all cell types composing the cerebellum are derived from the alar plate at the junction of the IsO and the first rhombomere. With the fundamental structures of the cerebellar anlage in place, the growth and development of the cerebellar hemispheres and vermis unfold through a complex series of programmed events involving neuronal proliferation, migration, and differentiation. The major events in cerebellar histogenesis are illustrated in Fig. 4.4 .
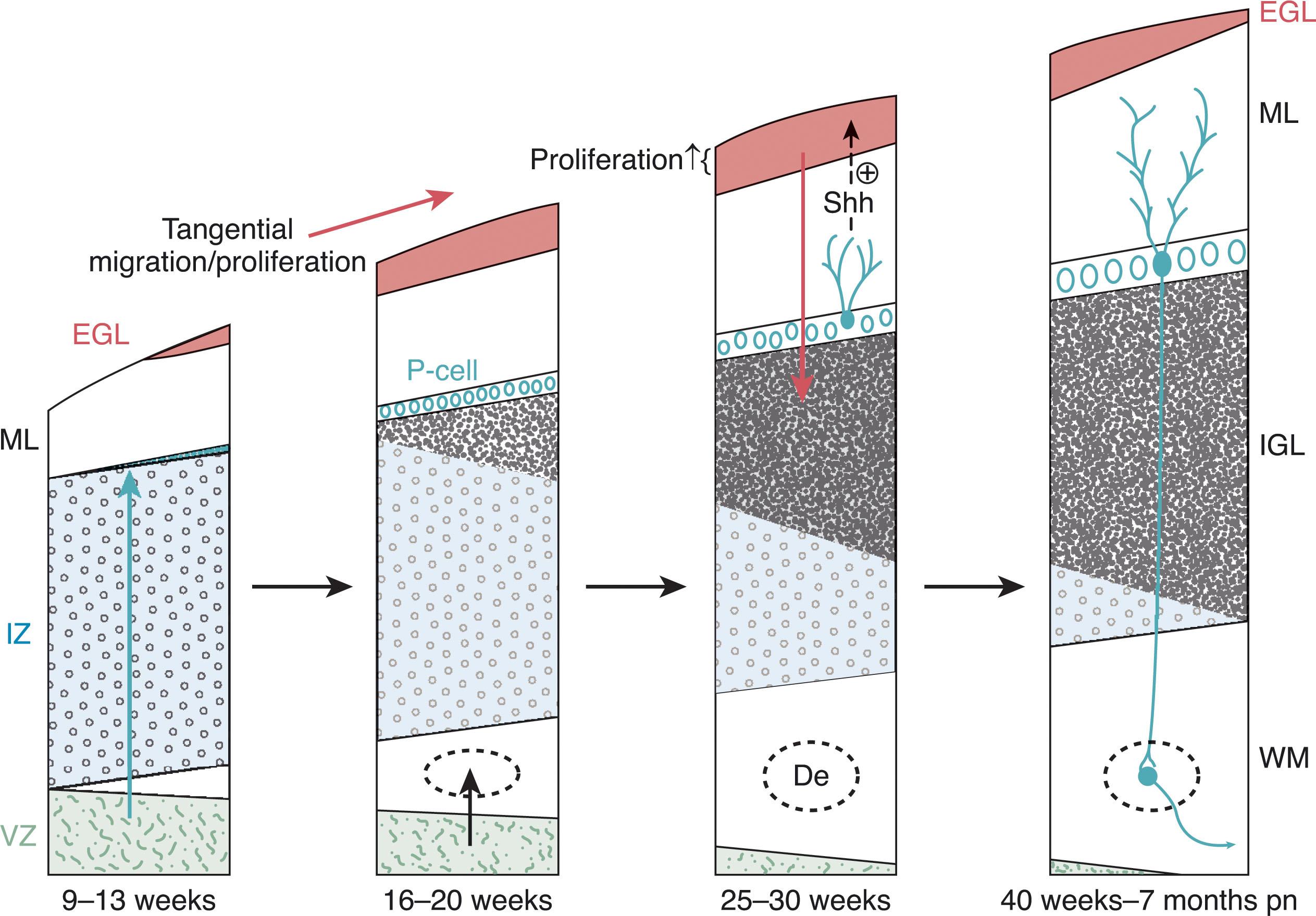
Cerebellar neural progenitors originate in two primary proliferative zones, the ventricular zone and the rhombic lip. In addition, the rhombic lip generates two secondary proliferative regions, one in the external granular layer and one in the posterior vermis (see later). The ventricular zone generates all GABA-ergic neurons, and the rhombic lip generates all glutamatergic precursors of the cerebellum ( Fig. 4.5A ). In humans, both zones are now known to develop a subventricular zone, similar to that of the cerebral hemispheres, with a vascular layer between the ventricular zone and subventricular zone.
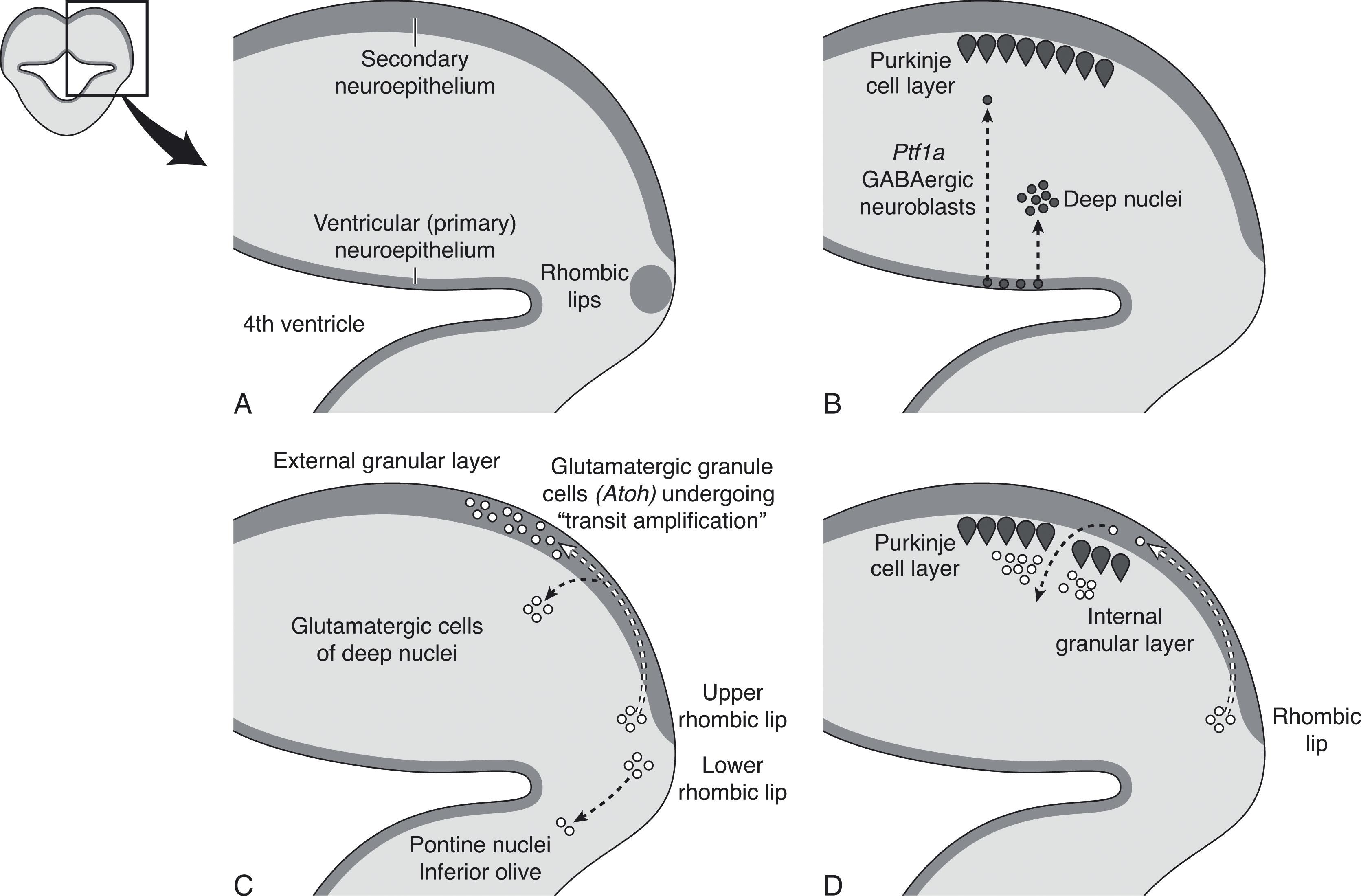
The primary subependymal neuroepithelium (dorsomedial ventricular zone) forms the ventricular layer around the fourth ventricle and is responsible for generating precursors for all the inhibitory GABA-ergic neurons and interneurons of the future cerebellum ( Fig. 4.5B ). A number of signals stimulate proliferation in this zone, including Wnt signaling from the roof plate signaling center and the diffusible mitogen, sonic hedgehog (Shh). Expression of Ptfa1 by these neuronal precursors is stimulated by Shh signaling, as well as BMP from the rhombic lip. Neuronal proliferation in the subependymal ventricular zone accelerates around 7 to 8 weeks p/c, followed by radial migration of neurons into the body of the future cerebellar anlage where these cells make up the inhibitory interneurons of the deep cerebellar nuclei and the Purkinje cell layer (i.e., the primary efferent neurons of the cerebellum) ( Fig. 4.5B ). Later, this zone generates the inhibitory GABA-ergic neurons that become the Golgi cells of the internal granular layer and the basket and stellate cells of the molecular layer. The ventricular zone continues to grow until 10 weeks p/c and then begins to shrink as the subventricular zone begins to expand.
This transient progenitor zone generates all future excitatory glutamatergic neurons of the cerebellum ( Fig. 4.5C ). The rhombic lips are neuroproliferative zones along the dorsolateral margins of the fourth ventricle, comparable to the ventricular zone of the developing cerebrum ( Chapter 5 ). The rhombic lips are responsible for the accelerated third trimester growth of the cerebellum, primarily through rapid expansion of the external granule cell layer, but also contribute to certain deep nuclei of the cerebellum and brainstem, unipolar brush cells, and a transient posterior vermian proliferative zone (see later). Granule cell precursors generate the dominant cell population of the cerebellum and originate only from the rhombic lips. The rhombic lips develop a ventricular and subventricular zone separated by a prominent vascular layer. The first cells to leave the rhombic lips are those migrating into the substance of the future cerebellum to become glutamatergic projection neurons of the cerebellar nuclei ( Fig. 4.5C ). It is of interest that the origin of the glutamatergic and GABA-ergic neurons differ in the cerebellum and cerebrum where glutamatergic neurons originate from the ventricular zone and the GABA-ergic neurons from the subventricular zone ( Chapter 5 ). Once these excitatory glutamatergic precursors in the subventricular zone begin to express Atoh1 , they migrate out of the rhombic lips into the external granule layer, beginning at 8 weeks p/c, and then embark on a complex tangential migration pathway over the subpial surface of the cerebellum in a rostrocaudal direction. Given the configuration of the developing cerebellum and fourth ventricle, the lateral subventricular zone origin of these future granule cells is in proximity to the subpial spaces along which they migrate away from the rhombic lips. Their expression of Atoh1 is transient, and they do not have a prolonged clonal expansion phase.
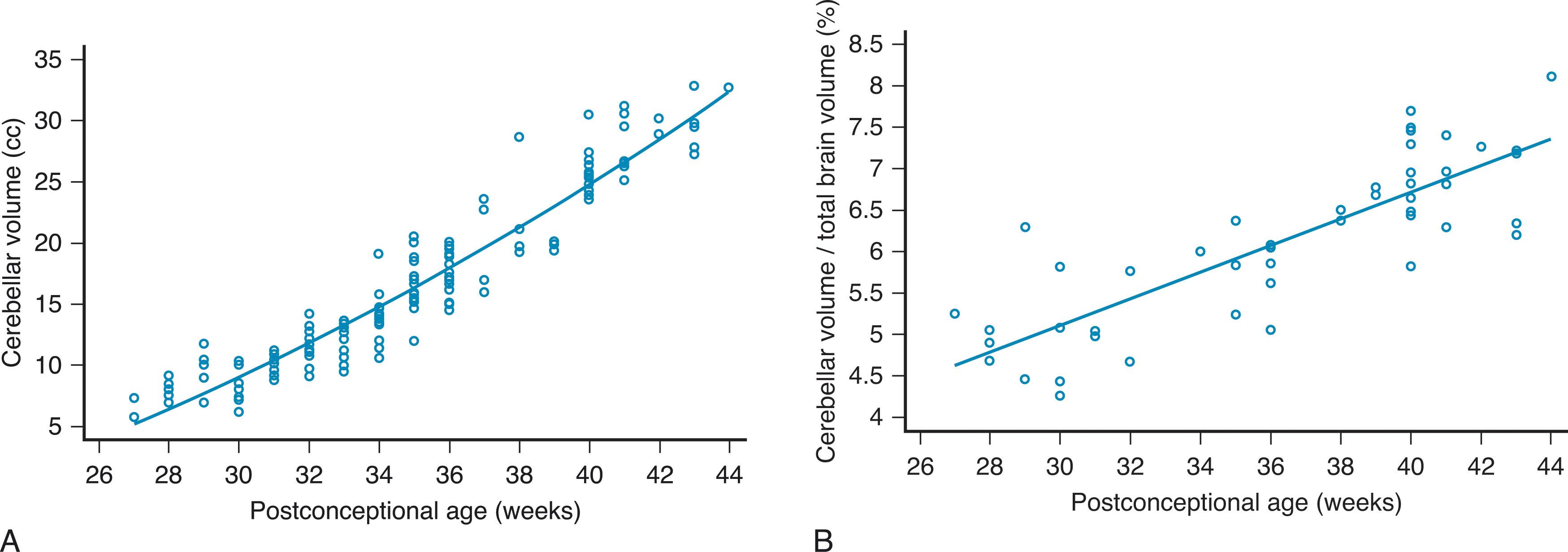
The initial thin layer of granule cell precursors undergoes waves of symmetrical cell division that result in an exponential expansion of the external granular layer, a process termed transit amplification . Proliferation of external granule cell precursors peaks between 26 and 32 weeks p/c, and by 29 weeks p/c these cells cover the entire surface of the developing cerebellum. The external granular layer develops an outer zone of active proliferation and an inner zone of postmitotic cells. Between 24 and 40 weeks p/c this external granule cell proliferation is largely responsible for the fivefold volumetric growth of the cerebellum ( Fig. 4.6 ). From the inner zone postmitotic granule cells migrate radially into the cerebellum along Bergman glial fibers, crossing the Purkinje cell layer to form the (internal) granular layer of the mature cerebellum ( Fig. 4.5D ). During and after this inward migration, important connections are made by the granule cells. As they pass through the Purkinje cell layer, they extend horizontal parallel fibers to contact the dendrites of the Purkinje cells, and soon after arrival at the internal granular layer they link with the mossy fibers ascending from the pontine nuclei. As discussed later, the proximity of the actively proliferating outer layer of granule cells to the subarachnoid space (separated only by the thin pial layer) may expose this crucial neuroproliferative zone to potentially noxious substances circulating in the cerebrospinal fluid (CSF), including the products of hemorrhage in the fetal and premature brain.
The rhombic lips also contribute to other brainstem nuclei by generating neurons that follow a different, caudal migrational path along the ventrolateral pons and rostral medulla to form specific brainstem nuclei, including the pontine and inferior olivary nuclei, among others. These nuclei subsequently generate important afferent input to the cerebellum, the pontine nuclei connecting to the internal granule cells through the mossy fibers, and the inferior olivary nuclei connecting to the Purkinje cells through the climbing fibers. The ventral pons begins to grow rapidly around 13 weeks p/c and undergoes a striking increase in dorsoventral growth in second trimester. This common origin of cellular precursors underlies the association between malformations in the cerebellum and other brainstem structures (discussed later).
Shh plays a central role in the spatial and temporal expansion of neuronal and glial cells across cerebellar development. Shh derived from the choroid plexus and roof plate is released into the CSF, “transported” in laminar flow by the ependymal cilia along and across the ventricular surface, to stimulate cell proliferation in the ventricular zone. When Shh signaling is impaired, proliferation of radial glial cells, as well as their ability to generate GABA-ergic interneuron progenitors, fails. During the phase of transit amplification, Shh secreted by the underlying Purkinje cells drives the rate of cell division in the overlying external granular layer. Later, Shh from the Purkinje cells drives the proliferation of a secondary population of inhibitory interneurons and glial cells in the future white matter of the cerebellum. Finally, Shh plays an important role in the patterning of folia in the cerebellar cortex.
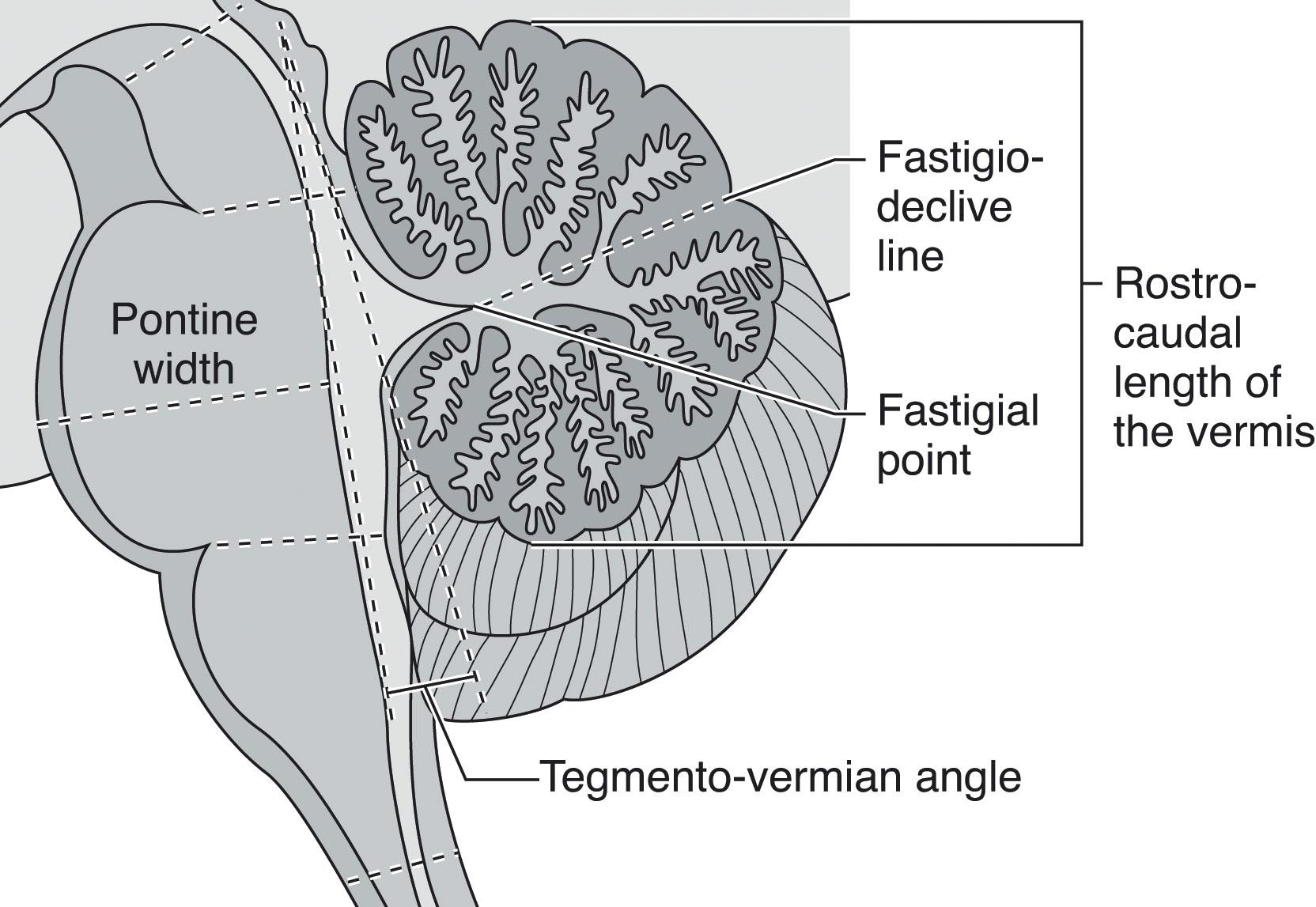
Understanding of normal vermian development is especially important because vermian anomalies are increasingly recognized for their effect on long-term outcome. Several earlier concepts of vermian development have undergone revision. Specifically, it is now known that the entire cerebellum originates from the first rhombomere, that the vermis is not of mesencephalic origin, and that it is not formed by fusion of the cerebellar hemispheres. Instead the cerebellar primordium arises as an unpaired single band of tissue in the shape of an inverted V stretched across the midline with the future vermis developing from the apex. Vermis growth is in a rostrocaudal direction ( Fig. 4.8 ); the caudalmost aspects of the posterior lobe of the vermis are among the earliest to develop, followed by the delayed expansion of the neovermis below the primary fissure that pushes the previously formed posterior vermis in an inferior direction. (It is important to note that in the literature the anterior vermis is also known as the superior or rostral vermis , and the posterior vermis as the inferior or caudal vermis; here we use anterior and posterior.) In this regard development of the vermis resembles that of the corpus callosum ( Chapter 2 ). The vermis develops from proliferative events in a distinct region at the rostral midline of the first rhombomere. Initially, vermian development lags behind that of the hemispheres but then accelerates around 12 weeks p/c. After 10 weeks p/c the rhombic lip appears as an elongated, “tail-like” structure (see image at 13 weeks p/c in Fig. 4.7 ) that trails from the posterior vermis. This tail-like aspect of the rhombic lip expands between 11 and 13 weeks p/c, and failure of this structure to enlarge sets up the “tail sign” seen on fetal magnetic resonance imaging (MRI) in half of DWMs. At around 13 to 14 weeks p/c, the dorsal stem cell zone of the rhombic lip becomes incorporated into the posterior vermis and forms a densely packed and proliferating cell mass (posterior vermian proliferative zone), which persists until 36 weeks p/c ( Fig. 4.7 ). The posterior vermis begins to exceed the anterior vermis in size only after 17 weeks p/c, and after 18 weeks p/c its growth is exponential. It is proposed that this internalized spatiotemporal expansion of the rhombic lip generates the posterior vermis during human cerebellar development and leads to its eventual 2:1 ratio with the anterior vermis. Failure of these events has been implicated in the posterior (inferior) vermian hypoplasia. Specifically, posterior vermian hypoplasia is detectable by prenatal US and MRI by 16 to 18 weeks p/c, well before the start of the major proliferation of external granule cell precursors (transit amplification) around 26 weeks p/c. During earlier gestation the fastigial angle at the ventral junction of the anterior and posterior lobes of the vermis is markedly obtuse, but with expansion of the posterior lobe this angle becomes more acute; failure of accelerated expansion of the posterior lobe after 16 to 18 weeks p/c in certain cerebellar malformations leaves the fastigial angle obtuse (flattened).
a Haldipur P, Aldinger KA, Bernardo S, et al. Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science . 2019;366:454–460.
12 weeks p/c vermis growth accelerates after initially lagging behind hemispheres
10–12 weeks p/c rhombic lip is an elongated, tail-like structure
11–13 weeks p/c rhombic lip tail expands
13–14 weeks p/c rhombic lip tail becomes incorporated into posterior vermian proliferative zone; persists until 36 weeks p/c.
After 17 weeks p/c posterior vermis size begins to exceed that of the anterior vermis
Posterior vermis growth becomes exponential
Normal gestational age-appropriate rostrocaudal length b
b Bromley B, Nadel AS, Pauker S, et al. Closure of the cerebellar vermis: evaluation with second trimester US. Radiology . 1994;193:761–763.
as late as 24 weeks c
c Bronshtein M, Zimmer EZ, Blazer S. Isolated large fourth ventricle in early pregnancy—a possible benign transient phenomenon. Prenat Diagn . 1998;18:997–1000.
, d
d Imamoglu EY, Gursoy T, Ovali F, et al. Nomograms of cerebellar vermis height and transverse cerebellar diameter in appropriate-for-gestational-age neonates. Early Hum Dev . 2013;89:919–923.
Caudal edge covers the fourth ventricle and reaches the obex
Primary fissure is present between the anterior and posterior lobes of the vermis e
e Robinson AJ, Goldstein R. The cisterna magna septa: vestigial remnants of Blake’s pouch and a potential new marker for normal development of the rhombencephalon. J Ultrasound Med . 2007;26:83–95.
Fastigium-declive line divides the vermis into anterior and posterior lobes with a 1:2 ratio
Mature pattern of vermian lobules and fissures e
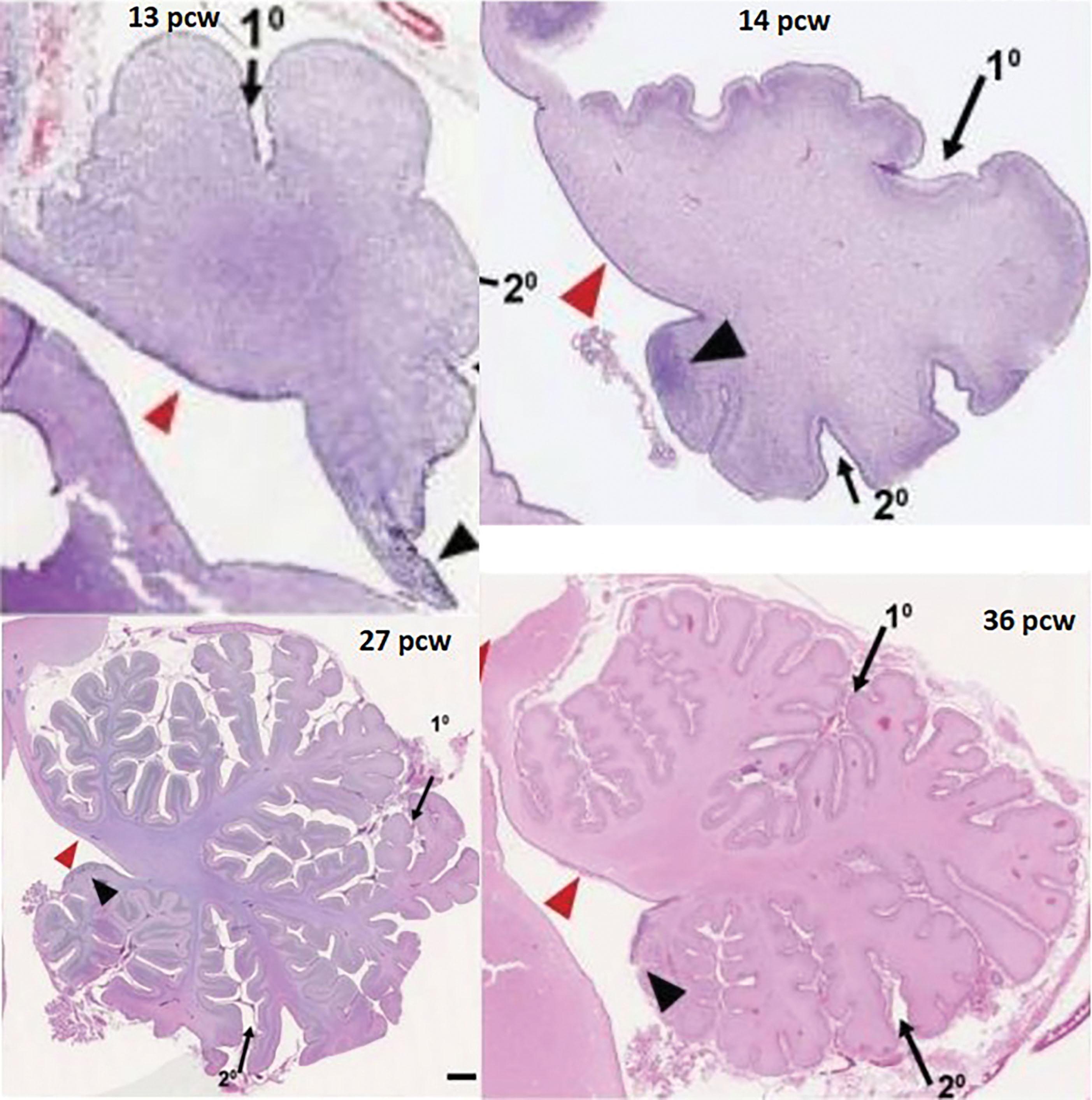
In a neuropathology study of the DWM and vermian hypoplasia cases, Haldipur and colleagues describe posterior vermis hypoplasia in all cases, with absence of the rhombic lip in half and failure of its internalization and/or undergrowth in the remainder. The authors propose that the anterior and posterior lobes of the vermis and hemispheres develop from two different rhombic lip progenitor pools. Before 13 weeks p/c, the rhombic lip contributes progenitors to the external granular layer for later expansion (between 26 and 32 weeks p/c) of the anterior cerebellum; after 14 weeks p/c, the posterior vermian proliferative zone generates granule cell progenitors that lead to the development of the posterior vermis.
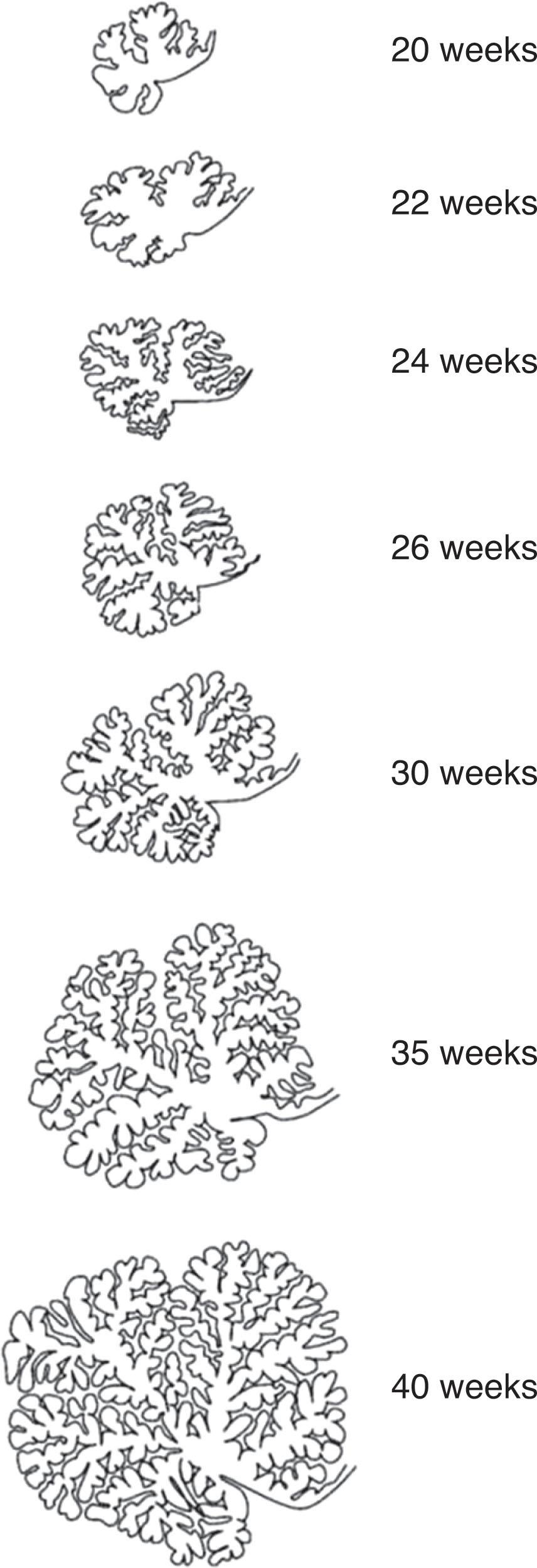
The primary fissure develops at 11 weeks p/c followed by the secondary fissure at 13 weeks p/c. The progressive complexity of cerebellar surface growth and foliation is demonstrated in Fig. 4.9 . By 22 weeks p/c the cerebellar lobules can be reliably assessed by fetal MRI, and at this point seven vermian lobules can be identified. Between 24 and 40 weeks p/c there is a major surge in the rate of foliation. Within the various diagnostic categories of posterior fossa malformations there is variability in outcome, and it has been proposed that this may occur because of quantifiable differences in vermis volumes and lobule number within each diagnostic category.
In this section we review the more common malformations of the brainstem and cerebellum. It is increasingly recognized that abnormal patterning (both rostrocaudal and dorsoventral) may result in anomalies that cross conventional anatomical boundaries and result in anomalies that involve adjacent posterior fossa structures. Anomalous differentiation of the diencephalon, mesencephalon, and rhombencephalon are often variably combined along the rostrocaudal axis. Although the diencephalon is not thought of as a posterior fossa structure, it may be involved in a spectrum of patterning malformations that involve adjacent brainstem and cerebellar structures. For this reason, we include lesions of the diencephalon and the diencephalic-mesencephalic junction in this section. We will discuss malformations in these and other posterior fossa structures in a rostrocaudal sequence.
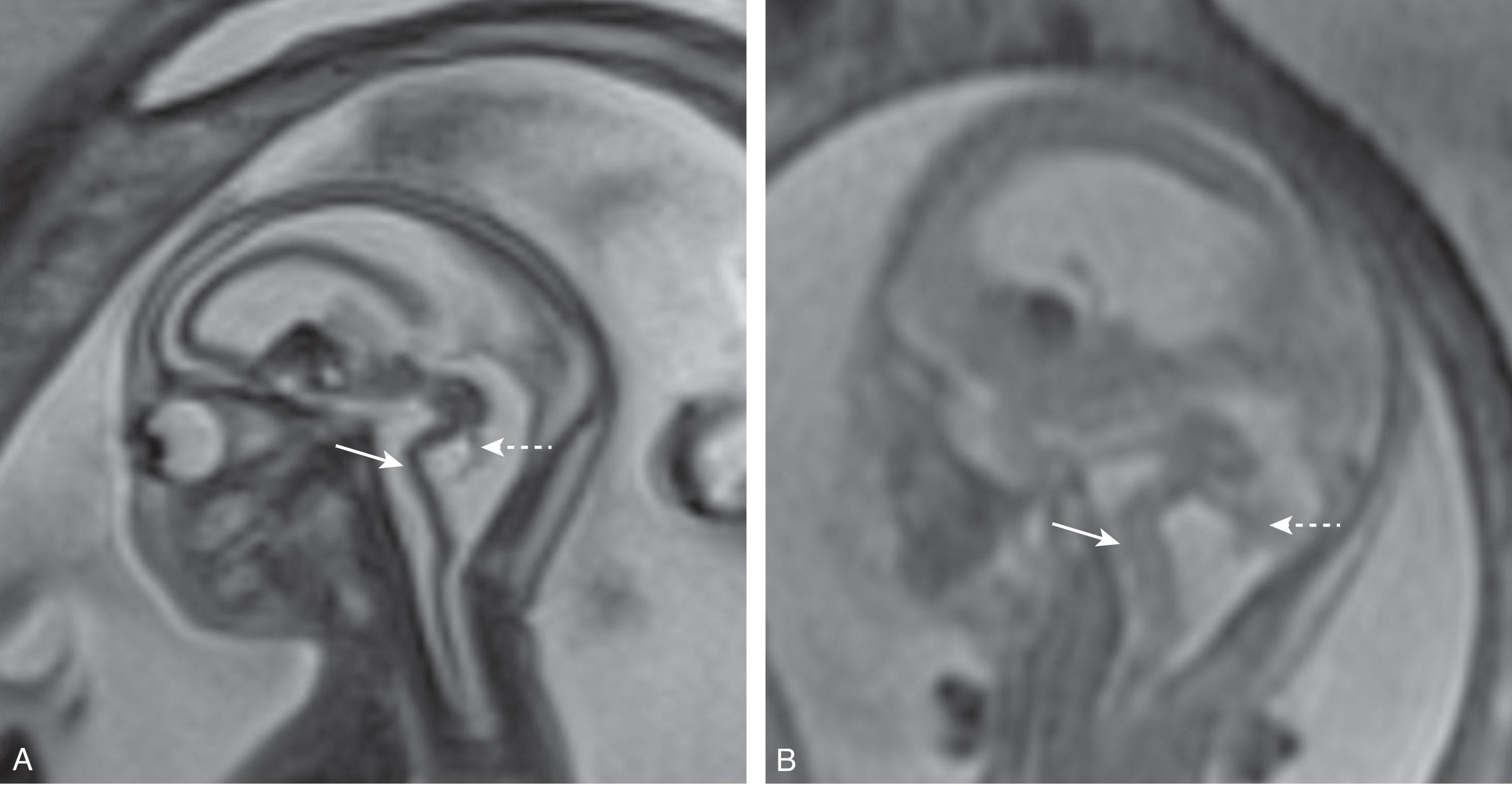
The anterior neural tube flexures usually appear around 5 weeks p/c and should not persist beyond 15 weeks p/c. Arrested development of this primitive pattern is one of the earliest and most fundamental of malformations and leaves a “kinked” or “Z-shaped” brainstem, which is commonly associated with major cerebral and cerebellar dysgenesis ( Fig. 4.11 ), including those due to dystroglycanopathies and cerebromuscular dystrophies (e.g., Walker-Warburg syndrome). Cobblestone lissencephaly (seen in the cerebromuscular dystrophies) is a disorder characterized by neuroglial overmigration into the subarachnoid space; the embryonic Z-shaped brainstem has been described as “encased” in a neuroglial outer layer and appearing like an echogenic band on prenatal US, suggesting a similar pathway in the brainstem.
Diencephalosynapsis describes a condition of thalamic fusion (nonseparation) with decreased third ventricular volume. The constriction of third ventricular volume with impaired CSF flow may lead to lateral ventriculomegaly and interhemispheric cyst formation, the latter presumably from ballooning of the third ventricular roof. Diencephalosynapsis may be associated with aqueductal stenosis, cerebellar hypoplasia, and rhombencephalosynapsis (see later).
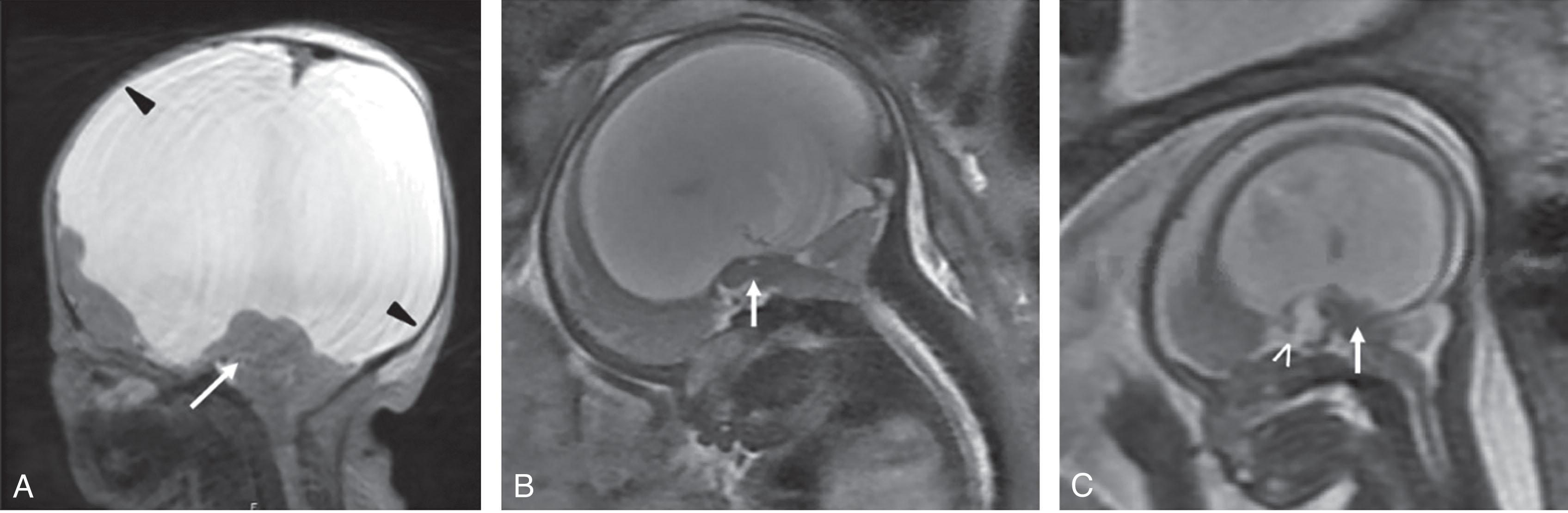
![Fig. 4.12, Diencephalosynapsis in a 22-week gestational age fetus by T2-weighted magnetic resonance imaging, showing dehiscence both lateral ventricles and large interhemispheric cyst (possibly distended third ventricle [asterisk] ), fusion of thalami (white arrow) , and shallow base of the third ventricle (black arrow) . Fig. 4.12, Diencephalosynapsis in a 22-week gestational age fetus by T2-weighted magnetic resonance imaging, showing dehiscence both lateral ventricles and large interhemispheric cyst (possibly distended third ventricle [asterisk] ), fusion of thalami (white arrow) , and shallow base of the third ventricle (black arrow) .](https://storage.googleapis.com/dl.dentistrykey.com/clinical/NormalandAbnormalDevelopmentofthePosteriorFossaStructures/14_3s20B9780443105135000048.jpg)
This spectrum of brain malformations was only recently described. The original descriptions included two phenotypes, Type A (“butterfly-shaped” midbrain/hypothalamus-midbrain fusion) and Type B (partial thalamus-midbrain fusion), to which a third form, Type C (complete/near complete midbrain-thalamic continuity), was recently added. The earlier descriptions included fetuses of consanguineous unions, leading to the unconfirmed notion of a recessive inheritance pattern. In a subsequent report of 14 diencephalic-mesencephalic junction dysplasias cases, all had variants in the Protocadherin-12 gene. A subsequent series of 13 male fetuses with diencephalic-mesencephalic junction dysplasias suggested an X-linked inheritance, but this pattern was not confirmed. In the largest series to date, Lawrence and colleagues performed a retrospective 12-year single-center review of 33 pregnancies complicated by fetal diencephalic-mesencephalic junction dysplasias. In this cohort, 70% of cases were initially referred for fetal hydrocephalus. Examples of all three diencephalic-mesencephalic junction dysplasia phenotypes were identified. Mutations of the L1CAM gene were identified in four cases and biallelic VRK1 variants in another. Of those surviving infancy, all were developmentally delayed, most with additional neurological complications, including seizures and tone abnormalities.
Anomalies of the mesencephalon include aqueductal stenosis , presenting with fetal ventriculomegaly/hydrocephalus, and “fusion” of the colliculi. Aqueductal stenosis is discussed in detail in Chapter 3 . Fusion of the colliculi is rarely described in isolation and is usually found in association with other more prominent posterior fossa anomalies discussed later in this chapter.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here