Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The thyroglossal duct represents the tract in the midline neck through which the thyroid anlage descends during embryonic development. It begins at the foramen cecum at the base of the tongue, continues around the hyoid bone, and ends at the normal position of the thyroid gland in the pretracheal lower neck. Persistence of the thyroglossal duct postnatally can give rise to cysts (thyroglossal duct cysts), fistula tracts, and sinuses anywhere along the tract.
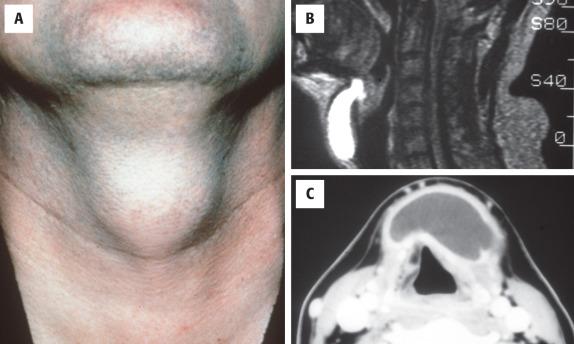
Thyroglossal duct cysts represent the most common midline mass in children but may also be found in adults and in the elderly. At least one-third occur after the age of 30 years. There is no sex predilection. The classic presentation is of a midline, mobile neck mass located either at or immediately below the hyoid bone (75%) ( Fig. 23.1 ). Less commonly, such cysts may be suprahyoid, submental, intrathyroidal, and rarely intralingual. Slightly off-midline examples may be seen; in such cases, the presence of a fibrous tract or cord emanating from the hyoid bone is a helpful finding. Cysts can be linked to the skin or foramen cecum by a sinus tract.
In most cases, the patient is asymptomatic but may have pain, a draining sinus, a fistula (10% to 20%), dysphagia, or rarely airway obstruction. Infection occurs in one-third of cysts. The cysts may fluctuate in size, especially if infected. Thyroglossal duct cysts move vertically with swallowing or protrusion of the tongue.
Cystic change of a persistent tract representing the embryologic migratory path of the thyroid anlage in the midline anterior neck
Most common pediatric midline neck mass; at least one-third occur after the age of 30 years
75% in anterior midline of neck at or immediately below the hyoid bone; the remainder are found in intralingual, suprahyoid (submental), and intrathyroidal loci
May become secondarily infected
Thyroid carcinoma, usually papillary type, develops in up to 3% of cases; this is associated with a favorable prognosis
Equal sex distribution
Most common in children or young adults
Asymptomatic midline neck mass
Draining sinus or fistula and pain, if infected
Preferred treatment is the Sistrunk procedure
4%–6% recurrence rate with Sistrunk procedure
Radiographic studies usually show a cystic mass, often with bright signal on magnetic resonance imaging (MRI) studies. These cysts are usually found at midline, with the point of origin frequently identified.
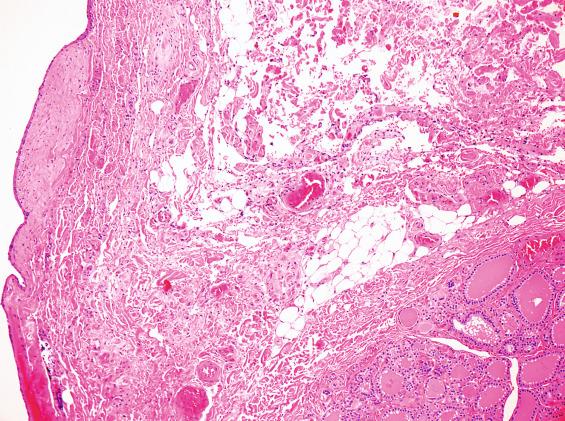
The surgical specimen usually consists of the thyroglossal duct cyst as well as the fibrous tract/duct extending to the foramen cecum and the central 1 to 2 cm of the hyoid bone ( Fig. 23.2 ). The mean cyst size is around 2.5 cm, although cysts up to 8.5 cm have been reported. The cyst may contain clear mucoid fluid or, if infected, purulent material. Solid or firm areas should be carefully sampled to exclude an associated neoplasm.
Cyst filled with mucoid or purulent material
Fibrous tract from area of foramen cecum to hyoid bone
One-third present as fistulas, usually due to infection
Solid areas sampled to exclude neoplasm
Cyst lined by respiratory and/or squamous epithelium
If the cyst is infected, granulation tissue may replace epithelium
Fibrosis and chronic inflammation in cyst wall
Thyroid tissue identified in at least two-thirds of cases
If carcinoma is present, at least 90% are of the papillary type
Thick mucoid or purulent material, sparsely cellular and with inflammatory material
Epidermoid and dermoid cysts, plunging ranula, degenerated adenomatoid nodules
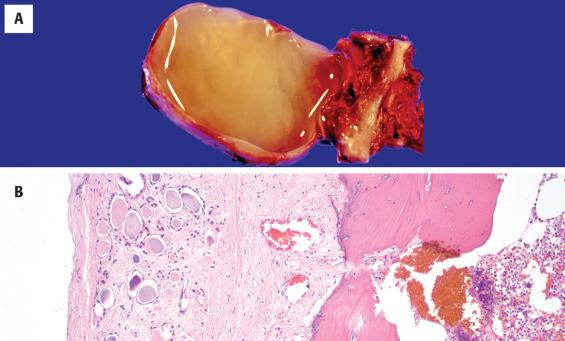
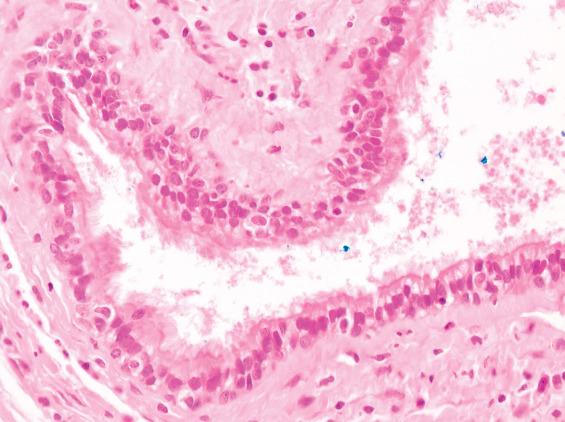
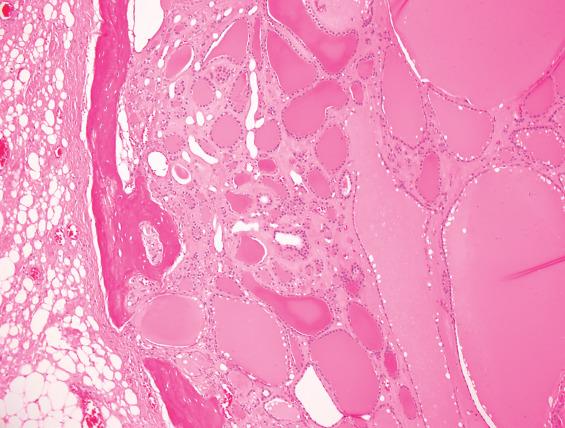
The thyroglossal duct cyst is normally lined by respiratory epithelium, but squamous metaplasia is quite common and most cysts contain a mixture of squamous and respiratory epithelium ( Figs. 23.3 and 23.4 ). About 10% are purely squamous-lined. If the cyst has been infected, the lining epithelium may be replaced by granulation tissue, sometimes with granulomatous elements such as foamy histiocytes and multinucleated giant cells ( Fig. 23.4 ). Fibrosis and chronic inflammation are often found in the cyst wall. Thyroid tissue is not invariably present but is quite common, found in up to 71% of cases with careful examination ( Fig. 23.5 ). Seromucinous glands and cartilage may also be found in the wall.
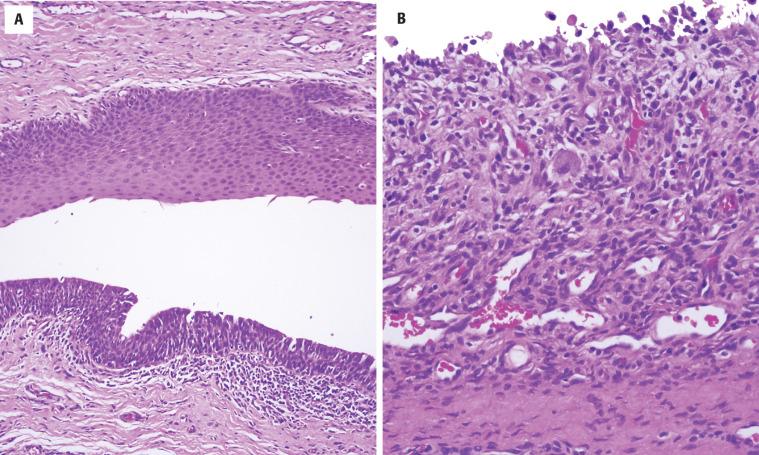
The associated thyroid tissue may harbor any inflammatory, hyperplastic, or neoplastic alteration that can be seen in the normal follicular epithelial component of the thyroid gland. Up to 3% of thyroglossal duct cysts will have an associated carcinoma, with papillary thyroid carcinoma (PTC) representing at least 90% of cases. Thyroglossal duct cysts in elderly patients are much more likely to harbor malignancies. No examples of medullary carcinoma have been documented, presumably due to the different embryologic origin of the C cells from the ultimobranchial body.
Fine needle aspiration (FNA) is not widely used in the diagnosis of thyroglossal duct cysts because of the limited cytologic findings, but it is useful in documenting other lesions that may form midline neck masses or to confirm a clinically suspected malignancy in a thyroglossal duct cyst. Aspirates consist of thick mucoid or purulent material and are sparsely cellular; inflammatory cells tend to outnumber the benign squamous or respiratory epithelial cells.
Epidermoid cysts of the thyroid, plunging ranula of salivary origin, or degenerated cystic adenomatoid nodules may be considered in the differential diagnosis; but the midline location and respiratory and/or squamous epithelial lining are key elements in making the diagnosis of thyroglossal duct cyst. When a thyroid carcinoma arising in a thyroglossal duct cyst is under consideration, the possibility of a metastatic tumor from the thyroid proper should be excluded.
The preferred treatment for a thyroglossal duct cyst is the Sistrunk procedure, which requires resection of the entire tract of the thyroglossal duct along with the cyst or fistula and the central 1 to 2 cm of the hyoid bone. The recurrence rate after this procedure is 4% to 6%; if the hyoid bone segment is not removed, the recurrence rate increases to above 25%. Well-differentiated thyroid carcinoma arising in a thyroglossal duct cyst has an excellent prognosis with the Sistrunk procedure. Total thyroidectomy is not usually required for benign cysts except in high-risk patients or in the instance of carcinoma arising in the thyroglossal duct cyst.
As the embryonic thyroid descends down the anterior neck, remnants of thyroid tissue, usually microscopic, can be distributed along the tract in the neck soft tissue or even in the hyoid bone (ectopic thyroid tissue) ( Fig. 23.6 ). Less commonly, ectopic thyroid tissue has been reported at other sites in the neck and exceptionally in the mediastinum, abdomen, or other sites. Its existence in the lateral neck is controversial. If thyroid tissue is encountered lateral to the jugular vein, especially if intranodal, it most likely represents a metastasis from a well-differentiated PTC. Rarely, the thyroid gland fails to migrate down the thyroglossal duct and remains at the foramen cecum, forming a submucosal mass at the base of tongue known as lingual thyroid, a type of thyroid ectopia.
Most thyroid ectopia is clinically occult and discovered incidentally in patients undergoing surgery for another indication. Lingual thyroid is the most common type of clinically detected thyroid ectopia (90% of cases). In addition, most patients have no other thyroid tissue in the neck (complete thyroid ectopia). It can present at any age and is more common in women. The male-to-female ratio is 1 : 5.
Patients with lingual thyroid may present with symptoms including cough, hoarseness, and sleep apnea, but most are asymptomatic. Airway obstruction is rare. Over half of patients are hypothyroid. Clinically visible lingual thyroids appear as a submucosal, smooth mass at the base of the tongue.
Thyroid tissue located outside the normal pretracheal location of the thyroid gland; lingual thyroid is a type of thyroid ectopia located at the base of tongue
Usually an incidental finding in the central neck
Approximately 90% of clinically detected cases are located at the base of the tongue (lingual thyroid)
Lingual thyroid is associated with hypothyroidism
Patients may have cough, hoarseness, and sleep apnea
Lingual thyroid is more common in females than males (5 : 1)
Can occur at any age
Lingual thyroids appear as smooth domed, submucosal masses at the base of tongue
No treatment is necessary unless the patient is symptomatic
Hypothyroidism is treated with hormone replacement
Radionucleotide scanning either with 99m Tc pertechnetate, 123 I, or 131 I is virtually diagnostic of thyroid ectopia.
Although most thyroid ectopias are not grossly visible but rather encountered incidentally under the microscope, those that form a mass appear similar to normal thyroid tissue. They are well circumscribed with a glistening brown cut surface. Nodular hyperplasia (adenomatoid nodules) may be present.
Well-circumscribed brown glistening mass
Often not grossly visible
Similar to normal thyroid tissue
Not usually indicated unless the diagnosis is clinically unsuspected
Clusters of thyroid follicular epithelium in a background of watery colloid
Metastatic well-differentiated thyroid carcinoma
Thyroid ectopia is histologically identical to normal thyroid ( Fig. 23.7 ). Lingual thyroids often have intervening skeletal muscle. Changes typical of nodular hyperplasia, variability in follicle size, cyst formation, fibrosis, and calcification may be present. Thyroid carcinoma is rare (1%).
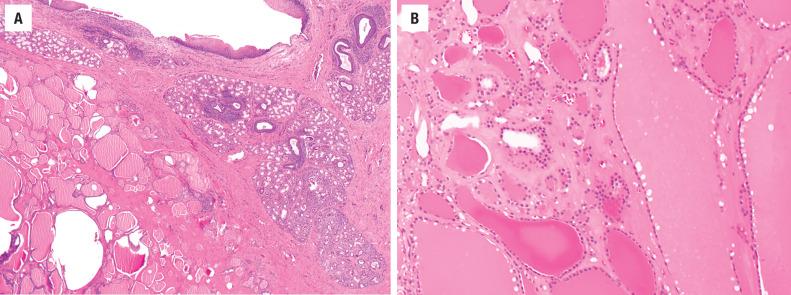
FNA is rarely necessary to establish the diagnosis of thyroid ectopia but may be used when the diagnosis is not suspected. Aspirates usually consist of watery colloid and clusters of follicular epithelial cells.
Metastatic well-differentiated PTC is the main consideration in the differential diagnosis of thyroid ectopia. Thyroid ectopia lacks nuclear features of PTC, but metastases of papillary thyroid carcinoma can be deceptively bland; therefore, careful examination is important. In the lateral neck, thyroid tissue usually represents metastatic disease rather than ectopia, especially in a lymph node.
No treatment is required for asymptomatic thyroid ectopia/lingual thyroid. If symptomatic, thyroid hormone replacement may be used to shrink the thyroid tissue. Surgery or radioactive iodine ablation can be used to treat more severe symptoms in patients who are not surgical candidates. Associated hypothyroidism is treated by hormone replacement. Death from airway obstruction is very rare.
Small remnants of the ultimobranchial apparatus, or “solid cell nests,” are associated with the embryologic development of the C-cell (calcitonin-producing) population of the thyroid. They are encountered as an incidental finding, usually in the posteromedial and posterolateral lobes but never in the isthmus.
Solid cell nests, which are fairly common, are of no clinical significance. They are identified in some 25% of thyroid resection specimens; their discovery is largely related to the generosity of sampling of “normal” thyroid tissue.
Small remnant of the ultimobranchial apparatus that delivers C-cells to the thyroid gland
Incidental finding in some 25% of thyroid specimens
Posteromedial and posterolateral areas of lobes; never in the isthmus
None (incidental microscopic finding)
No clinical significance
Ultimobranchial remnants (solid cell nests) are quite small, most measuring no more than 0.1 mm. They are represented by a cluster of small, round, or lobulated epithelial nests and may be solid or partially cystic ( Fig. 23.8 ). Occasionally, several foci may be found in a gland. The epithelial cells are small and ovoid to polygonal, with slightly elongated nuclei; they are called “main cells.” The chromatin is finely granular and evenly dispersed. A longitudinal nuclear groove is often present ( Fig. 23.9 ). The overall histologic pattern is very similar to that of immature squamous metaplasia, but keratinization and intercellular bridges are not visible. A minor population of cells with clear cytoplasm comprises C-cells. Degenerative changes are thought to account for the occasional presence of mucicarmine-positive material in ultimobranchial remnants.
Small, round, or lobulated nests of polygonal epithelial cells resembling immature squamous metaplasia, usually ≤0.1 mm
Some examples are partially cystic
No keratinization or intercellular bridges
Nuclei ovoid, with evenly distributed chromatin and frequent longitudinal nuclear grooves
Occasional clear cells and mucoid material may be seen
Main cells p63-positive but negative for thyroglobulin, thyroid transcription factor-1, and calcitonin
Scattered C-cells positive for neuroendocrine markers, calcitonin, and carcinoembryonic antigen (CEA)
Incidental papillary thyroid carcinoma, C-cell hyperplasia/small medullary carcinoma, and squamous metaplasia
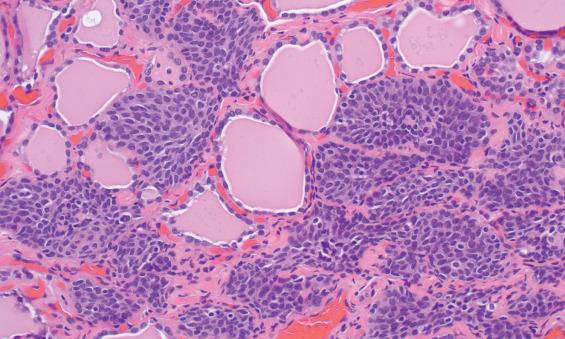
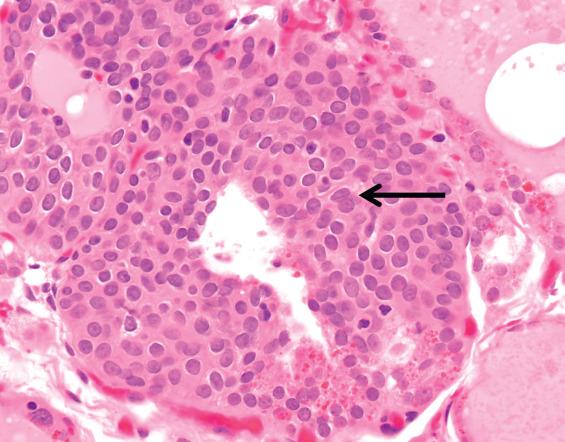
Although immunohistochemical studies are certainly not necessary for making a diagnosis, the staining pattern of ultimobranchial remnants is fairly distinctive. Main cells are positive for p63, bcl-2, CK19, and galectin-3 but negative for thyroglobulin, thyroid transcription factor-1 (TTF1), and calcitonin. The scattered C-cells are positive for neuroendocrine markers, calcitonin, carcinoembryonic antigen (CEA), and galectin-3.
Ultimobranchial remnants are sometimes mistaken for squamous metaplasia, incidental microscopic PTC , or a small medullary carcinoma/C-cell hyperplasia . Although nuclear grooves are frequent in ultimobranchial remnants, these lesions lack the other nuclear features of PTC (overlapping nuclei, ground-glass chromatin pattern, intranuclear inclusions) as well as an adjacent stromal desmoplastic reaction.
C-cell hyperplasia or a small medullary carcinoma may have similar nodular growth. However, C-cells (which give rise to both hyperplasia and medullary carcinoma) are plasmacytoid; they have granular chromatin and more abundant pale pink to clear cytoplasm than solid cell nests. Small medullary carcinomas show expansile growth, stromal fibrosis, and/or amyloid deposition as well. Scattered calcitonin or neuroendocrine marker staining should not be mistaken for C-cell neoplasia, as C-cells are normally present in solid cell nests.
While not necessarily within the differential diagnosis, endodermally derived tissues may be encountered in the thyroid gland, including cartilage, parathyroid gland ( Fig. 23.10 ), thymic tissue, and salivary gland tissue ( Fig. 23.11 ). These “inclusions” are usually of no clinical significance, typically representing incidental findings in surgical resection specimens. Thymic tissue retains its lobulated appearance, has small cystic islands of squamous epithelium (Hassall corpuscles) with an abundant lymphoid component that may be mistaken for lymphocytic thyroiditis or a lymph node ( Fig. 23.11 ). Parathyroid tissue , particularly if it is hyperplastic or neoplastic, can be difficult to distinguish from a cellular adenomatoid nodule or follicular thyroid neoplasm. Oxyphilic change makes separation a challenge. Parathyroid cells often have perinuclear clearing and the nuclei are very small, round, and hyperchromatic, with rather coarse chromatin that suggests a neuroendocrine cell type.
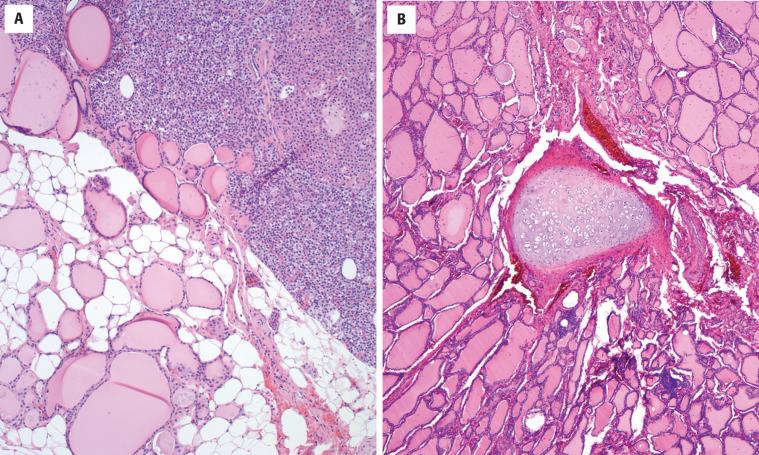
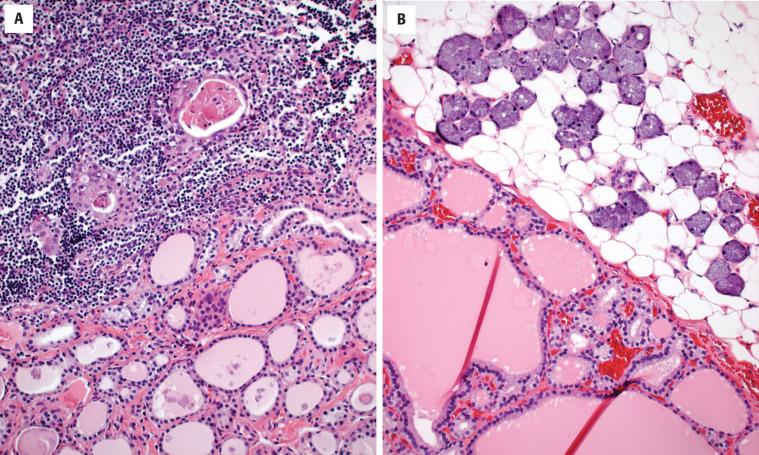
No therapy is necessary as this is an incidental finding.
Acute inflammation with a neutrophilic inflammatory infiltrate in the thyroid parenchyma is rare and accounts for less than 1% of all thyroid disease. A wide spectrum of bacterial, fungal, and rarely parasitic pathogens are responsible. Routes of infection include congenital piriform sinus fistula (usually presenting in the 1st decade of life), direct extension from a neck or retropharyngeal abscess, penetrating trauma (including fine needle aspiration), hematogenous spread from a systemic infection, and esophageal perforation. The immunosuppressed population and patients with underlying thyroid abnormalities (e.g., malignancies, chronic lymphocytic thyroiditis, or goiter) are at increased risk.
Patients are usually febrile and complain of chills, malaise, and pain and swelling of the anterior neck. Dysphagia or hoarseness may also be noted. While most patients are euthyroid, occasional patients demonstrate clinical and laboratory evidence of hyperthyroidism or hypothyroidism.
Acute inflammation of the thyroid parenchyma associated with a local or systemic bacterial or fungal infection
Rare (< 1% of all thyroid disease)
Most cases are associated with congenital piriform sinus fistulae, generalized sepsis, immunosuppression, or trauma
Fever, chills, malaise, pain, swelling of anterior neck
Occasionally dysphagia or hoarseness
Most patients are euthyroid; occasionally hypothyroid or hyperthyroid
Prognosis related to underlying condition (sepsis, trauma, etc.)
Cultures to determine causative agent
Aggressive antibiotic or antifungal therapy, with surgery reserved for abscess drainage, injury debridement, or excision of piriform sinus fistula tract to prevent recurrence in such cases
The thyroid is seldom removed for acute thyroiditis. A specimen is usually submitted as part of an incision and drainage of an abscess or debridement following neck trauma. The gland is erythematous and soft and may contain pockets of purulent exudate or areas of necrosis.
Thyroid erythematous and soft, with pockets of purulent exudate or necrosis
Infiltration of parenchyma by neutrophils
Microabscesses, foci of necrosis, vasculitis common
Organisms may be identified on histology in some cases, highlighted with special studies
Subacute (granulomatous) thyroiditis, acute inflammation associated with malignancy
The key finding is the presence of polymorphonuclear leukocytes infiltrating the thyroid parenchyma. Microabscesses, foci of necrosis ( Fig. 23.12 ), and vasculitis may be seen. Identification of a causative organism may be possible with histochemical stains, such as tissue Gram stains, Gomori methenamine silver, or periodic acid–Schiff (PAS) stains for fungi or mycobacterial stains (Ziehl–Neelsen). Immunosuppressed patients may develop an acute infectious thyroiditis rather than the typical granulomatous type in response to fungal or mycobacterial organisms. Tissue cultures are invaluable in identifying the organism and in determining its sensitivity to antibiotic therapy.
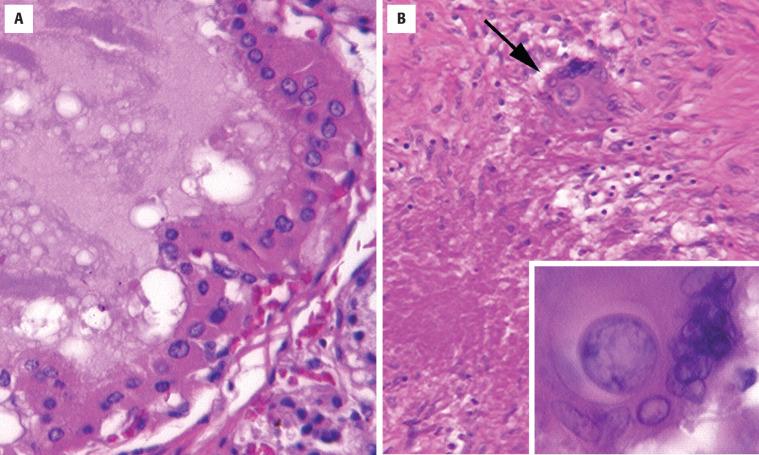
FNA is useful to obtain material for culture and for therapeutic drainage of fluid collections. Aspirates typically show neutrophils and cellular debris.
Subacute (granulomatous) thyroiditis also involves a neutrophilic infiltrate in its early phase, but it is folliculocentric with accompanying histiocytes, lymphocytes, and multinucleated giant cells. No infectious agent is identified. Secondary infection and acute inflammation can also accompany an underlying malignancy.
The prognosis is favorable with treatment and in the absence of underlying predisposing factors such as immunosuppressive states or extensive neck trauma, which may carry their own risk of poor outcome. Appropriate treatment requires identification of the infectious agent, preferably by culture, and initiation of effective antibiotic therapy. If abscesses or areas of infarction are present, surgical incision and drainage or debridement may be necessary.
Also known as de Quervain thyroiditis (named after Fritz de Quervain, 1868–1940), subacute granulomatous thyroiditis is a self-limited inflammatory disorder widely suspected to be related to a systemic viral infection. Association with viral epidemics such as mumps, influenza, coxsackievirus, and measles has been reported. Some cases have also been associated with Epstein–Barr virus and with immunosuppressive therapy. Although seasonal variation corresponding to peak enteroviral incidence has been noted in the past, some recent studies show only a modest increase in cases during the spring and summer. An autoimmune component is also postulated due to the presence of thyroidal autoantibodies in some patients.
The annual incidence in the United States is estimated at 5 per 100,000 population. Women are more commonly affected than men, with a female-to-male ratio of 3.5 : 1. The mean age of onset is in the 5th decade (range, 14 to 87 years). The most common presenting symptom is pain in the thyroid region, sometimes radiating to the jaw. Other complaints include dysphagia, sore throat, low-grade fever, arthralgia, myalgia, tremor, excessive sweating, and weight loss. Physical examination is notable for pain on palpation of the thyroid. The entire gland is usually involved; however, the changes may be localized to one lobe or to a distinct nodule. Thyroid function often varies with disease activity. In the early phase, patients may be hyperthyroid owing to destruction of follicles and release of thyroglobulin. Rarely, a life-threatening thyrotoxicosis or “thyroid storm” occurs early in the disease. With disease progression, thyroid epithelium is destroyed, resulting in hypothyroidism. However, most patients regain normal thyroid function after resolution of the disease.
Self-limited inflammatory disorder thought to be related to systemic viral illness and possible autoimmune factors
Also called de Quervain thyroiditis
About 5 per 100,000 population per year
Seasonal increase in spring and summer may occur
Females > males (3.5 : 1)
Peak in 5th decade (range, 14–87 years)
Pain in the thyroid region, tender to palpation
Other symptoms include dysphagia, “sore throat,” fever, arthralgia, myalgia, tremor, excessive sweating, weight loss
Entire gland usually involved, but inflammation may be localized
Thyroid function varies: hyperthyroidism may occur early; hypothyroidism may develop in midphase
Most patients are euthyroid after resolution
Usually self-limiting disease that resolves in months, although it may recur (years after initial disease)
5% remain hypothyroid
Treatment includes aspirin, nonsteroidal anti-inflammatory drugs, steroids, and beta-blocking agents if hyperthyroidism present
Radioactive iodine (RAI) uptake is decreased.
Serum thyroid-stimulating hormone (TSH) is suppressed and total and free T3 and T4 are elevated early in the course of disease. In the subsequent hypothyroid phase, TSH is elevated and total and free T3 and T4 are low.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here