Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Non-neoplastic lesions of the neck can be broadly classified into developmental cystic anomalies; infections and related diseases/lesions; and reactive, inflammatory, and tumor-like lesions.
Branchial cleft anomalies are divided according to the branchial apparatus involved and are further divided into cysts, sinuses, or fistulas. When the name branchial cyst is used without further qualifications, it generally refers to a cyst of second branchial cleft origin, which accounts for 80% to 90% of all branchial cleft anomalies. Branchial cleft cysts constitute 17% of all congenital cervical cysts in children and encompass branchial cysts, sinuses, and/or fistulas.
Patients usually present with a painless mass measuring up to 6 cm. The location is dependent on the cleft origin, and second-cleft anomalies are characteristically located along the anterior border of the sternocleidomastoid muscle, from the hyoid bone to the suprasternal notch. There is no sex difference and 75% of patients are between 20 and 40 years of age at the time of diagnosis. The cysts are usually nontender masses unless they become secondarily inflamed or infected. They may be bilateral, especially when they are associated with congenital syndromes.
The radiology will characteristically show a well-circumscribed cystic mass with a smooth cavity and a dense wall.
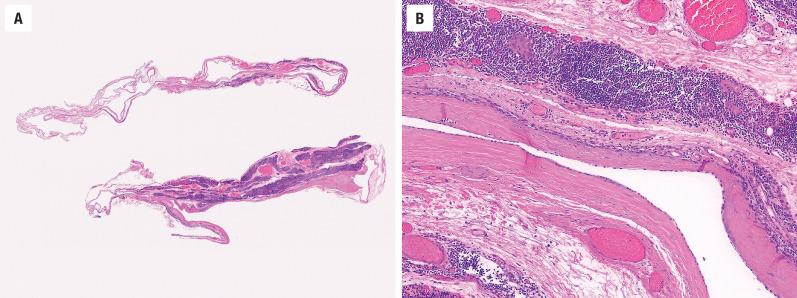
Grossly, the cysts are unilocular and between 2 and 6 cm in diameter; they usually contain clear to grumous material ( Fig. 20.1 ).
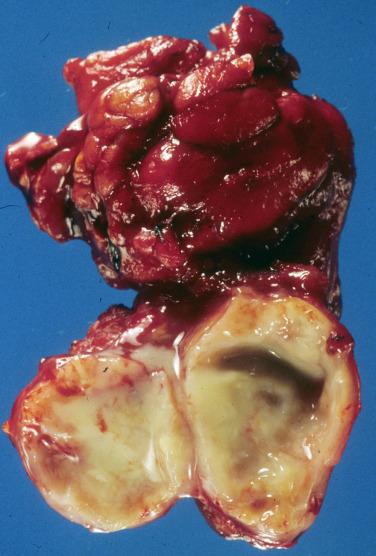
A lateral cervical cyst that results from congenital/developmental defects arising from the primitive second branchial apparatus
17% of all congenital cervical cysts
Lateral neck, with most near the mandibular angle
Equal sex distribution
75% of patients are 20–40 years of age
Less than 3% occur in patients > 50 years
Mass along the anterior border of the sternocleidomastoid muscle
Painless mass of long duration
May become secondarily infected, which will bring it to clinical attention
Complete excision
Recurrence rate about 3%
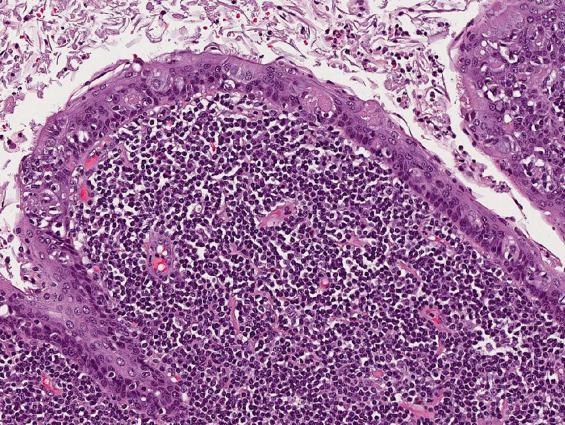
A branchial cleft cyst is usually lined by stratified squamous epithelium (90%), occasionally by respiratory epithelium (8%), or, rarely, by both (2%) ( Fig. 20.2 ). Lymphoid aggregates with or without reactive germinal centers are found beneath the epithelial lining in some 70% to 85% of cysts ( Figs. 20.2 and 20.3 ). However, as no true lymph node architecture is present, there are no sinuses, medullary regions, or interfollicular zones. Keratinaceous debris may be present within the cavity. Acute and chronic inflammation, foreign-body giant cell reaction, and fibrosis are secondary changes often seen in the wall of the cyst.
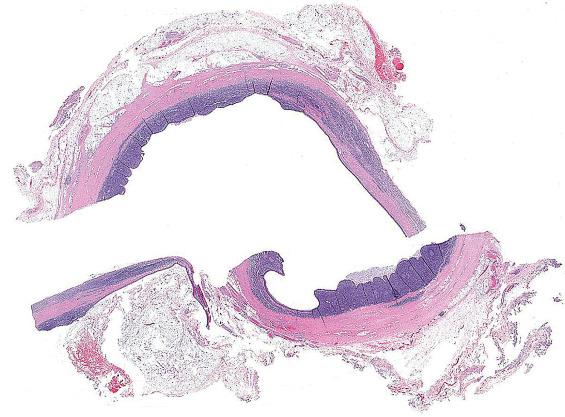
Cystic mass up to 6 cm, containing fluid
Cysts lined by squamous epithelium (90%), respiratory epithelium (8%), or both (2%)
Keratinaceous debris
Lymphoid tissue, nodular or diffuse (70%–85%)
Fibrosis and secondary changes in the cyst wall
Mature squamous epithelium
Anucleate squames
Debris, including macrophages
Lymphoid infiltrate
Cytokeratins-positive (type dependent on lining)
Negative for ISH high-risk HPV, though p16 may be positive
Metastatic cystic squamous cell carcinoma, thymic cyst, bronchial cyst, thyroglossal duct cyst
HPV , Human papillomavirus.
The epithelial lining expresses cytokeratins of different types, depending on the type of lining—pseudostratified respiratory, transitional, stratified keratinizing, or nonkeratinizing. However, these cysts are negative for high-risk human papillomavirus (HPV), a useful feature for distinguishing them from metastatic cystic squamous cell carcinoma. It is important to realize that up to 50% of benign branchial cleft cysts may show p16 immunoexpression, necessitating the use of a more specific HPV testing method (e.g., in situ hybridization).
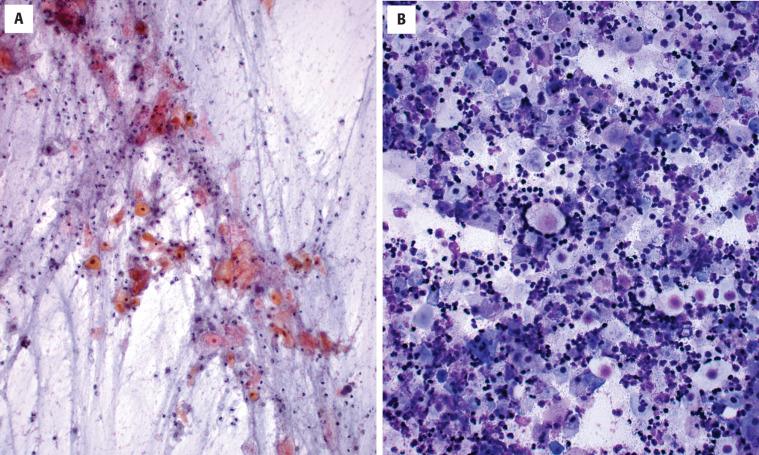
Fine needle aspiration (FNA) of a branchial cleft cyst will yield a thick, yellow, pus-like material, which microscopically is composed of anucleate squames, amorphous debris and macrophages, and variable lymphoid cells ( Fig. 20.4 ). Viable mature squamous epithelial cells and occasionally columnar respiratory epithelial cells are often noted. Significant epithelial atypia should not be present, and, in the right clinical setting, atypia would suggest potential malignancy.
Thymic cysts contain thymic tissue within the wall, including Hassall corpuscles (concentric island of squamous cells with central keratinization; Fig. 20.5 ). A thyroglossal duct cyst occurs in the midline and is often associated with thyroid tissue. Bronchial cysts are more common in the subcutaneous tissue of the supraclavicular region and are lined by respiratory mucosa with smooth muscle and bronchial glands in the wall. Dermoid cysts can also be in the differential, although these will present in a midline location and, histologically, will show adnexal structures within the wall. The most important differential diagnostic consideration, however, particularly if the lesion is in an adult, is metastatic cystic squamous cell carcinoma . These lesions usually have a malignant epithelial lining and may be positive for p16 and HPV (by immunohistochemistry and in situ hybridization, respectively), especially when the primary tumor is from the oropharynx. Metastatic HPV-related oropharyngeal carcinomas may even rarely retain cilia, further mimicking a benign cyst. Confusion and controversy exists between the diagnosis of metastatic squamous cell carcinoma and that of a carcinoma arising in a branchiogenic cyst. Evidence suggests that cases of purported branchiogenic carcinoma almost always represent metastatic HPV-related squamous cell carcinomas from the oropharynx that are clinically occult.
The recurrence rate is about 3% for cases with no history of infection or prior surgery. However, recurrence is more common in second operations or when the lesion is infected at the time of operation (14% and 21%, respectively). A complete excision of the cyst is indicated.
Many infectious agents can affect the skin, soft tissues, and lymph nodes of the neck. Cat scratch disease (CSD; cat scratch fever, cat scratch adenitis, Debre syndrome, Foshay-Mollaret syndrome) will be highlighted as a single example. CSD is a zoonotic infection caused by any one of a group of rickettsial microorganisms of the alpha-2 subgroup of alpha-protobacteria, specifically Bartonella henselae, with some taxonomic overlap with Afipia felis and B. quintana . B. henselae is a gram-negative pleomorphic rod-shaped bacillus. CSD occurs worldwide and is more common in autumn and winter, especially in dwellings where cats are kept as pets.
The characteristic clinical syndrome consists of an initial lesion that develops at the site of inoculation followed by enlargement of regional lymph nodes. The primary lesion, a cat scratch or bite, will cause skin erythema within 3 to 5 days and may be slightly painful (in some cases, this initial injury goes unnoticed). Within 3 weeks of inoculation, acute regional lymphadenopathy develops proximal to the inoculation site. The affected lymph nodes are those that drain the primary lesion; they become enlarged and tender. Occasionally they drain to the surface, forming a fistula. Lymphadenopathy involves only one nodal region in about 85% of patients, while matted, suppurative lymph nodes are seen in some 15% of patients. Constitutional symptoms may be present; these include a low-grade fever, headaches, and malaise. Most infections occur in children and young adults, usually less than 21 years of age. In general, most cases are self-limited and resolve within 3 to 4 months, although lymphadenitis may persist for years. Atypical presentations include ocular, neurologic, or visceral organ involvement. Erythema nodosum, an erythematous nodular panniculitis associated with a variety of conditions, is seen in only 2% of patients. Serology for B. henselae antibodies does not work well, and culture, using brain-heart infusion agar with incubation at 32°C, is difficult to perform owing to the organism's fastidious and slow growth.
A zoonotic infection caused by a group of gram-negative rickettsial bacilli, specifically Bartonella henselae, Afipia felis, and B. quintana
Worldwide; most common in autumn and winter
Found especially in dwellings where cats are kept as pets
No mortality unless patients are immunosuppressed
Equal sex distribution
Children and young adults between 3 and 21 years (majority < 8 years)
Usually associated with a cat scratch or bite
Solitary, tender lymphadenopathy
Constitutional symptoms including low-grade fever, malaise, and headaches
Excellent prognosis
Supportive therapy is adequate, although ciprofloxacin has been advocated by a few
Excision and drainage plus antibiotics may yield best overall results
The lymph nodes are soft and swollen and have foci of necrosis on the cut surface. They measure up to 10 cm.
The histology can be divided into three stages of progression. Histologically, early lesions show foci of swollen capillaries, which have a pink hyaline appearance and are associated with lymphoid follicular hyperplasia. As the foci of suppuration grow, they coalesce to form stellate abscesses ( Fig. 20.6 ), which become surrounded by histiocytes, epithelioid cells, and occasionally giant cells. Eventually a granulomatous perimeter surrounding a central area of caseation remains. The caseous center, unlike the centers of tuberculosis lesions, is rarely calcified. Obviously these changes are nonspecific, requiring additional studies to document the causative agent.
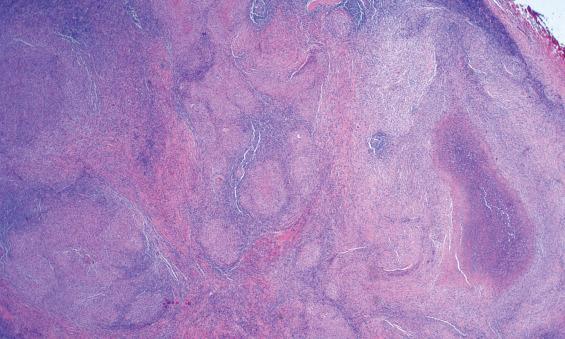
Enlarged, swollen lymph nodes with areas of necrosis
Three stages of progression are generally identified:
Early stage shows follicular hyperplasia
Stellate abscess surrounded by histiocytes, epithelioid cells, and occasionally giant cells
Late stage shows granulomas with central caseation
Granulomatous inflammation with epithelioid histiocytes and occasional giant cells
Lymphoid infiltrate
Necrotic debris, depending on stage
Warthin-Starry stain for rod-shaped organisms
Immunoperoxidase stain
DNA primer for use with polymerase chain reaction
Culture using brain-heart agar at 32°C
Primarily infectious agents (brucellosis, tuberculosis, lymphogranuloma venereum)
May also resemble other reactive nodal conditions, including Kikuchi-Fujimoto disease and Kimura disease
Lymphoma
Tissue Gram stain will demonstrate the bacillus, but a Warthin-Starry silver impregnation technique stains the 1- to 3-µm pleomorphic bacteria black, highlighting the organisms in the wall of the vessels in the early stages and in the suppurative areas in the later stages. Stains are often difficult to interpret because of high background staining in the necrotic debris.
The features on FNA are those of a granulomatous inflammation and are nonspecific. Epithelioid histiocytes, Langhans-type giant cells, inflammatory cells, and debris are present to a variable degree depending on stage. However, additional studies, including culture and/or special studies, are necessary to confirm the diagnosis.
An indirect immunoperoxidase stain has been developed to demonstrate the organisms. However, it is capricious, requiring a specific volume of cases to achieve quality, reproducible results, so it is not of much practical value.
DNA primers have been developed for use with a polymerase chain reaction (PCR) for the evaluation of CSD.
Stellate abscesses, characteristic of CSD, are also seen in a broad range of infectious etiologies , including mycobacterial infections, fungal infections, toxoplasmosis, tularemia, brucellosis, leishmaniasis, chancroid, granuloma inguinale, and lymphogranuloma. However, many of these are exceedingly rare or nonexistent in the head and neck region or have very specific culture findings. Metastatic disease will show neoplastic cells with areas of necrosis, while lymphoma will show specific atypical lymphoid elements, with ancillary studies supporting monoclonality. Sarcoidosis shows tight, well-formed, small granulomas without necrosis, while a branchial cleft cyst usually lacks granulomatous or suppurative inflammation. Kikuchi-Fujimoto disease is a histiocytic necrotizing lymphadenitis characterized by crescentic histiocytes and marked karyorrhectic debris without neutrophils. Most helpful in diagnosing CSD is a high index of suspicion based on the clinical history, leading to the demonstration of bacilli by silver impregnation or by culture. Kimura disease is a rare benign chronic inflammatory disease that usually involves deep subcutaneous tissue and lymph nodes of the head and neck with regional lymphadenopathy and salivary gland enlargement. The disease is more common in middle-aged Asian populations; elevated serum immunoglobulin E (IgE) levels and peripheral blood eosinophilia are common. The histologic features of Kimura disease include preserved nodal architecture; follicular hyperplasia with reactive germinal centers; well-formed mantle zones; eosinophilic infiltrates involving the interfollicular areas, sinusoidal areas, perinodal soft tissue, and subcutaneous tissue; and proliferation of postcapillary venules.
The prognosis is excellent with supportive treatment alone. Antibiotics are generally not required to relieve symptoms, but ciprofloxacin has been advocated by a few. Excision with drainage and antibiotics yields the best overall outcome.
Bacillary angiomatosis is a vascular, proliferative form of Bartonella ( B. henselae or B. quintana ) infection that occurs primarily in immunocompromised persons.
Bacillary angiomatosis almost always occurs in immunocompromised patients and is characterized by reddish, berry-like lesions on the skin surrounded by a collar of scale, which may bleed significantly if traumatized. Lesions can also occur on the oral mucosa, tongue, oropharynx, and nose. Infection with B. henselae is spread by fleas from cats, while B. quintana is spread by lice. Disease may spread throughout the reticuloedothelial system, causing bacillary peliosis, mainly in patients carrying human immunodeficiency virus (HIV). Patients with bacillary angiomatosis commonly have a history of HIV infection, organ transplantation, leukemia, or chemotherapy. Inoculation bartonellosis may be evident in immunocompetent individuals as a pyogenic granuloma-like nodule at the site of a cat scratch.
A vascular, proliferative form of Bartonella ( B.henselae or B. quintana ) infection
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here