Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
“Study with me, then, a few things in the spirit of truth alone so we may establish the manner of Nature’s operation. For this essay which I plan, will shed light upon the structure of the kidney. Do not stop to question whether these ideas are new or old, but ask, more properly, whether they harmonize with Nature. I never reached my idea of the structure of the kidney by the aid of books, but by the long and varied use of the microscope. I have gotten the rest by the deductions of reason, slowly, and with an open mind, as is my custom.” —Marcello Malpighi, 1666
In keeping with the spirit of Marcello Malpighi, this chapter also aspires to reveal “the manner of Nature’s operation” as it affects the kidney. However, unlike Malpighi, today’s knowledge draws extensively on the labors, discoveries, and insights of investigators of the past centuries.
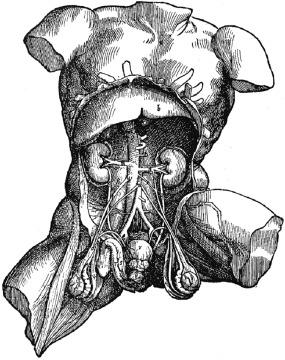
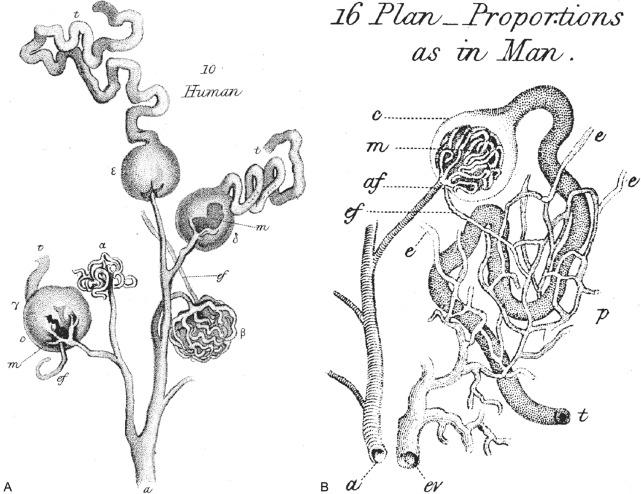
Knowledge of the normal structure and function of the kidney has been acquired over centuries of scholarly effort. We have come a long way since Aristotle taught that urine was formed by the bladder and that kidneys were present “not of actual necessity, but as matters of greater finish and perfection.” Reference to the excretory functions of the bladders and kidneys can be found in early Indian Ayurveda, Chinese, or Egyptian literature. Some of the earliest scientifically valid experimental methods are described in The Canon of Medicine by the famous Persian Muslim physician Abu Ali Sina, also known as “Avicenna.” He meticulously described the layers of the bladder and its two-stage function, the intramural ureter and antireflux mechanisms, and scientifically classified urethral and bladder diseases, notably calculi. The foundation of modern urology was established in the sixteenth century by Leonardo da Vinci and Vesalius, who provided the first accurate and detailed drawings of the female and male genitourinary tracts ( Fig. 1.1 ). More than 300 years passed before William Bowman, in 1842, coupled intravascular dye injection with microscopic examination to demonstrate the structural organization of the nephron and its vascular supply ( Fig. 1.2 ). Bowman’s observations provided morphologic support for Malpighi’s seventeenth-century speculation of a filtration function for the malpighian body (the glomerulus). Sixty years later the embryologic development of the nephron was demonstrated by Huber in a thin-section serial reconstruction study of embryos ( Fig. 1.3 ). Huber’s observations were refined and elegantly illustrated by Brödel in Kelly and Burnam's Diseases of the kidneys, ureters and bladder published in 1914. Potter and Osathanondh validated the findings in a series of microdissection studies of developing kidneys, which were published in the 1960s.
The ultrastructural features and immunohistochemical profiles of the normal kidney and of many diseases were elucidated in the 1970s and 1980s after refinement of the percutaneous biopsy technique and advances in morphologic analyses. Since 1990 there has been an explosion of new information about the genetic basis of normal and abnormal renal development, and about numerous disease processes.
This chapter begins with a brief review of the embryology and normal gross and microscopic structure of the kidney. For more in-depth coverage of these topics, several excellent resources are available.
The development of the urinary and genital tracts is closely related ( Fig. 1.4 ). These tracts both develop from paired longitudinal cords of tissue lateral to the aorta that are known as the intermediate mesoderm. From the portion caudal to the seventh somite, known as the nephrogenic mesoderm (or nephrogenic cord), three nephronic structures develop in quick succession: the pronephros, the mesonephros, and the metanephros. Although the pronephros and the mesonephros are transient organs, they are crucial for the proper development of both the urinary and the reproductive tracts.
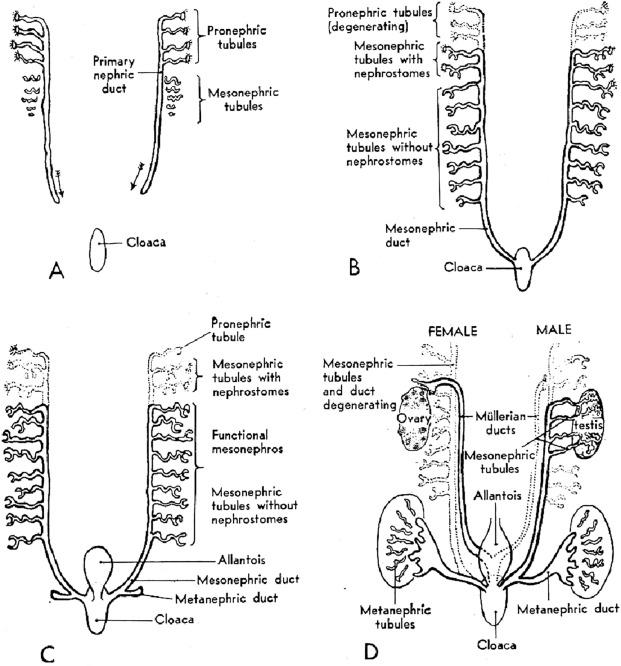
The first embryologic derivative of the nephrogenic cord is the pronephros, a structure functional only in the lowest forms of fish. It arises from the most cranial portion of the nephrogenic cord during the third week of gestation (1.7 mm stage; 7th to 14th somite stage). Approximately seven pairs of tubules form, only to regress 2 weeks later ( Figs. 1.4 and 1.5 ). The pronephros is important because the pronephric tubules grow caudally and fuse with the next pronephric unit, which gives rise to the pronephric duct. The pronephric duct is the only remnant of the pronephros, and henceforth is called the mesonephric duct.
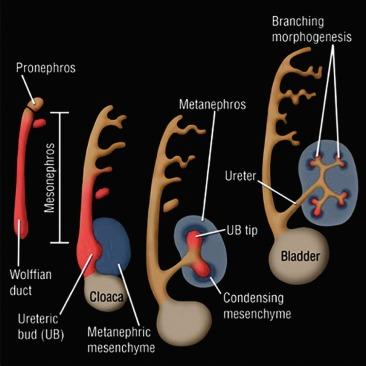
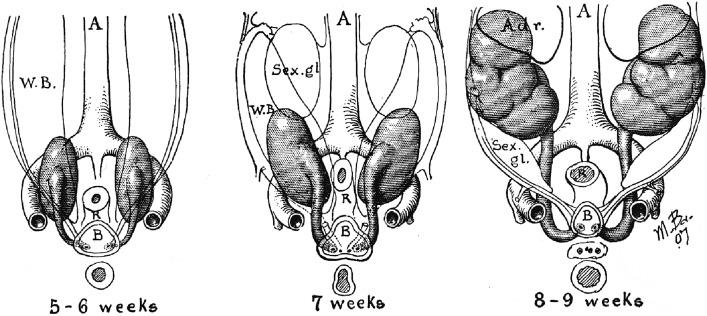
The mesonephros develops from the dorsolumbar segments of the nephrogenic cord from day 24 of gestation. Cells of the mesonephric duct proliferate caudally ( Fig. 1.4 ) and begin to form the mesonephric kidney during the fourth week of gestation (4 mm; 26th to 28th somite stage). The mesonephros is a highly differentiated structure and is the functional kidney of higher fishes and amphibians.
The mesonephric kidney consists of approximately 40 pairs of nephrons. The cranial nephrons sequentially regress while caudal nephrons form, with 7 to 15 nephrons functional at all times ( Figs. 1.4 and Fig. 1.5 ). The nephrons are induced in a fashion analogous to their metanephric counterparts.
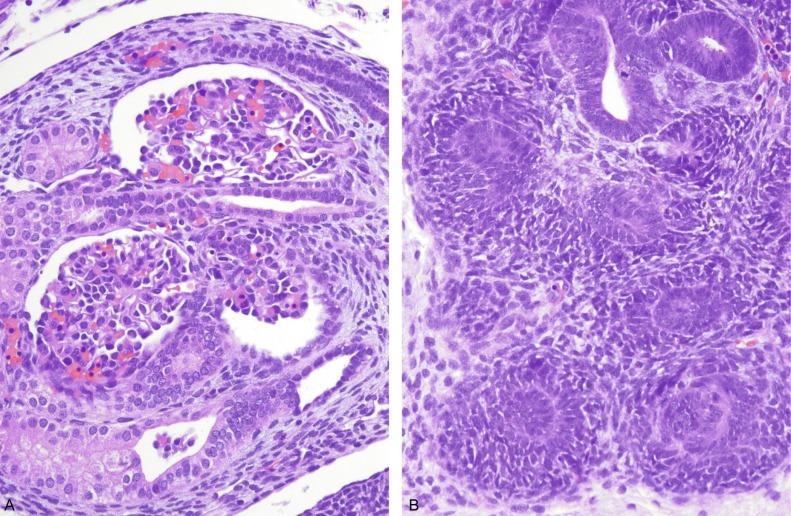
A fully developed mesonephric nephron consists of a glomerulus connected to the mesonephric duct by a convoluted proximal and distal tubule ( Figs. 1.6A and 1.7A ). The glomerulus is vascularized by capillaries that branch from small arterioles originating from the aorta, and its efferent arteriole empties into the posterior cardinal vein. The glomerulus appears to filter plasma. Its proximal tubule possesses a brush border; the proximal and distal tubules appear capable of nutrient resorption, as well as concentration and dilution of urine. The tubules connect with the mesonephric duct, which extends distally to connect to the cloaca at about 4 weeks postconception. The mesonephric kidney remains functional until the end of the fourth month of gestation.
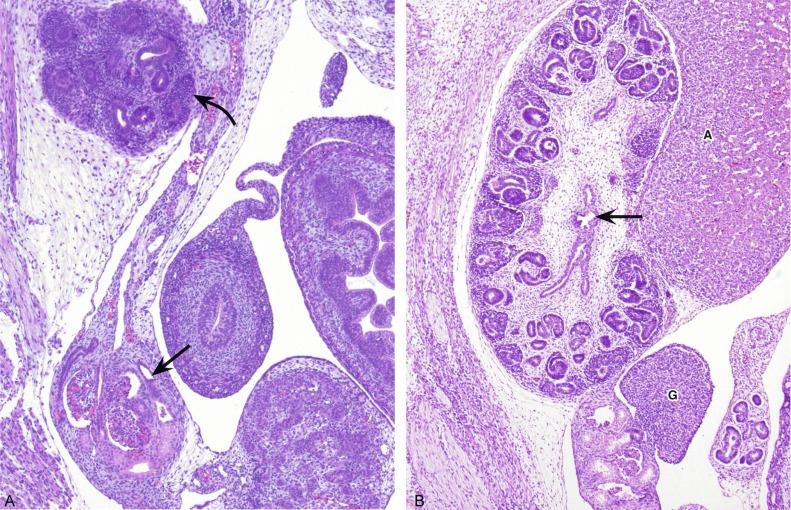
Portions of the mesonephric kidney can be easily identified in small embryos (1 to 3 cm), which are occasionally encountered in surgical specimens such as those from ectopic pregnancies. In the male, some of the caudal mesonephric tubules develop into the efferent ducts of the epididymis, while the mesonephric duct becomes the epididymis, the seminal vesicle, and ejaculatory duct. In the female, the entire mesonephros degenerates during the end of the first trimester; however, vestigial structures such as the epoophoron, paroophoron, and Gartner duct, as well as mesonephric remnants, can occasionally be seen in surgical specimens from the ovary and fallopian tubes.
The metanephric kidney is the product of a complex orchestration of embryologic processes. Although discussed separately, it must be appreciated that while the collecting system and renal pyramids are forming there is simultaneous induction of thousands of nephrons, and neurovascular and lymphatic components ramify in a carefully organized architecture throughout the cortex.
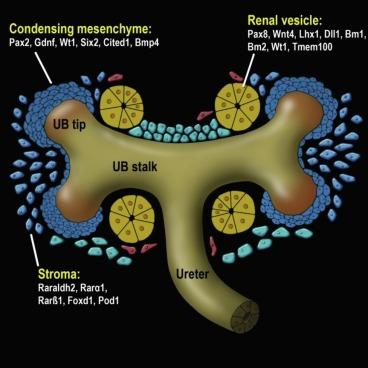
The formation of the adult metanephric kidney begins during the fourth to sixth weeks of gestation (4 to 5 mm), after the mesonephric duct has established communication with the urogenital sinus ( Fig. 1.5 ). A diverticulum, known as the ureteric (or ampullary) bud, forms on its posterior medial aspect ( Figs. 1.4 and Fig. 1.5 ) and then establishes contact with the sacral portion of the nephrogenic mesoderm, the nephrogenic blastema. A complex reciprocal inductive process results in dichotomous ureteric bud branching and nephron induction that eventually culminate in the adult metanephric kidney. The metanephros is therefore a product of two embryonic derivatives; the nephrons are of blastemal origin, whereas the ureter, pelvis, calyces, and cortical and medullary collecting ducts are derived from the ureteric bud.
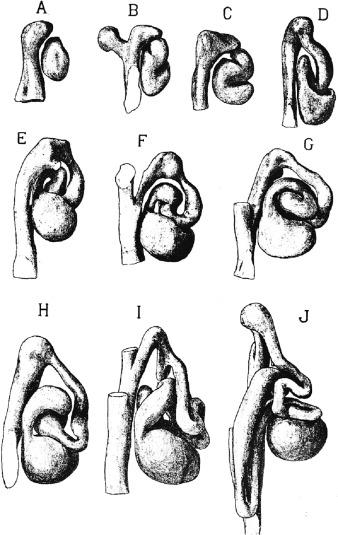
On contact with metanephric blastema the ureteric bud undergoes a rapid sequence of dichotomous branching and fusion, forming the renal collecting system by the 14th week ( Figs. 1.8 and 1.9 ). The initial two branches form the renal pelvis, the third to sixth branches form the major and minor calyces, and the sixth to eleventh branches form the papillary ducts ( Fig. 1.9 ). Because ureteric bud branching is more rapid in the upper and lower poles, the calyces and papillae in those regions are more numerous.
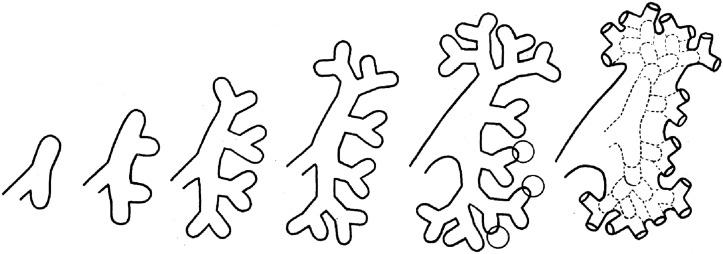
While the collecting system is forming, nephron induction has already begun ( Figs. 1.6B , 1.7B , 1.8, 1.10, and 1.11 ). The kidneys have moved into the flanks because of a combination of migration out of the pelvis and rapid caudal growth of the embryo ( Fig. 1.12 ). The kidney also has rotated from its original position with the pelvis anterior, to its final position with the pelvis medial. By week 13 or 14, the minor calyces and renal pyramids are well formed and the lobar architecture can be appreciated grossly ( Figs. 1.13 through 1.15 ). At this time, the cortex contains several generations of nephrons, and the lateral portions of adjacent lobes begin to merge to form the columns of Bertin.
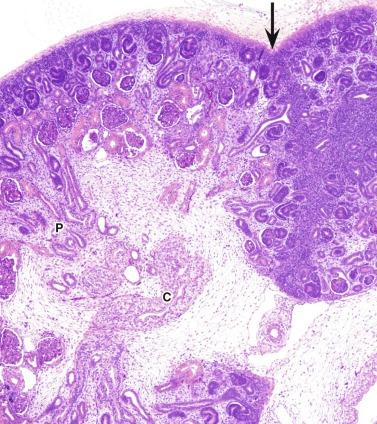

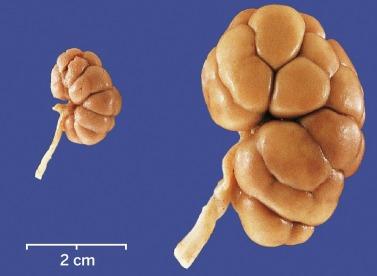
By weeks 20 to 22 the renal lobes are well formed, and the kidney is a miniature of the adult kidney ( Fig. 1.15 ). The ureteric bud has ceased branching, but the branches continue to lengthen. As they lengthen they induce arcades of four to seven nephrons, which are connected to the collecting duct by a connecting tubule ( Fig. 1.16 ). Additional groups of three to seven nephrons then form, each attached directly to a collecting duct without a connecting tubule. Therefore each cortical collecting duct will have 10 to 14 generations of nephrons attached, with the most recently formed and least mature nephrons located beneath the renal capsule.
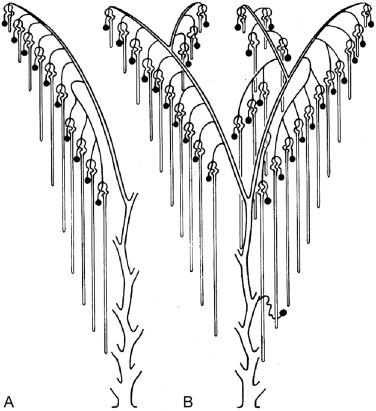
The formation of individual nephrons begins as early as 7 weeks of gestation and results in a limited degree of “renal function” by 9 weeks. In the subcapsular nephrogenic zone of any immature kidney ( Fig. 1.17 ) the sequence of nephron induction can be observed in its various stages of completion. The historic wax models and illustrations made by Huber ( Fig. 1.18 ), the drawing by Brödel ( Figs. 1.3 and 1.14 ), and the illustrations by Dressler and Jain ( Figs. 1.5, 1.8, 1.10, and 1.11 ) provide a three-dimensional perspective useful in understanding the cellular events during renal development as demonstrated in Fig. 1.17 .
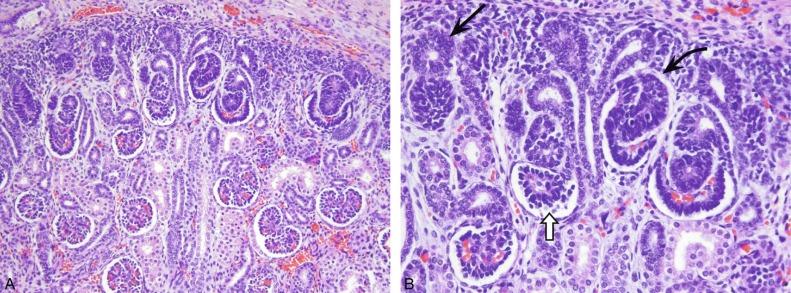
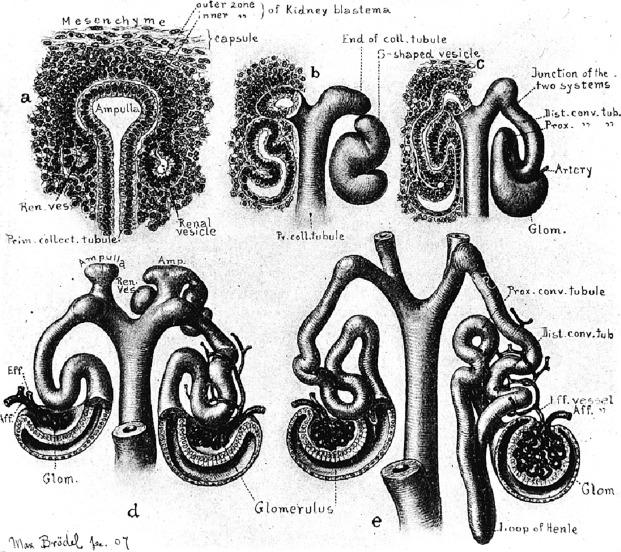
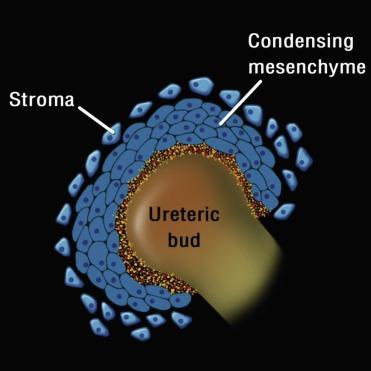
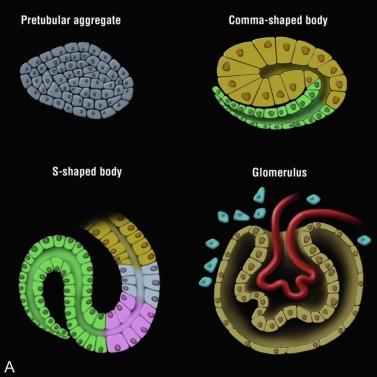
An individual nephron begins to form when the metanephric blastema aggregates adjacent to the ureteric bud to form a hollow vesicle ( Fig. 1.10 ). The molecular basis for this event is complex and involves the coordinated induction of numerous genes that encode for growth factors, adhesion molecules, matrix components, and other regulatory proteins ( Table 1.1 and Figs. 1.10 and 1.11 ). The cells within the vesicle grow differentially, first forming a comma-shaped aggregate of cells that then elongate and eventually develop two indentations creating an S-shaped structure ( Fig. 1.11 ). The distal portions of the S-shaped body (segments attached to the ureteric bud) are destined to become the proximal and distal tubules ( Fig. 1.11 ). They form tubular structures and establish communication with the collecting duct. The proximal part of the S-shaped body (segment away from the ureteric bud) gradually broadens and separates into two cell layers: the outer layer becomes the parietal epithelium of the Bowman capsule, whereas the inner layer becomes the visceral epithelium (podocytes). Endothelial cells migrate into the indentation in the proximal part and eventually form a podocyte-invested and vascularized glomerular tuft within Bowman capsule.
| Name | Role in Development | Effect of Mutations | Expression in Normal Kidney | Comment |
|---|---|---|---|---|
| PAX2 (paired box 2) | Acts as a survival signal in ureteric bud/collecting duct lineage | Renal agenesis in null mutant; large gene deletions implicated in 3% of cases of renal-coloboma syndrome | Intermediate mesoderm, nephric duct, mesonephros, ureteric bud, induced metanephric mesenchyme | Nuclear stain; can be used to confirm renal, Müllerian, or Wolffian duct origin of cells |
| N-myc (avian myelocytomatosis viral oncogene homologue) | Differentiation and organogenesis | Hypoplastic mesonephros, decreased ureteric bud tips and nephrons, renal hypoplasia | Induced metanephric mesenchyme | Amplification (> 10 copies) associated with poor prognosis in neuroblastoma |
| HNF1B (hepatocyte nuclear factor 1β) | Maintains differentiated state of renal epithelia | Null mutants do not survive; renal cysts, single kidneys, renal hypoplasia, electrolyte abnormalities | All tubular epithelia and collecting ducts | Renal tubules become cystic if HNF1B is mutated |
| WT1 (Wilms tumor 1) | Transcription factor | Renal agenesis in null mutant; proteinuria in heterozygous mice; disturbed podocyte differentiation, glomerulosclerosis | Intermediate mesoderm, mesonephros, uninduced and induced metanephric mesenchyme, comma- and S-shaped bodies; restricted to podocytes in adult kidney | Marker in tumors |
| FOXD1 (forkhead box D1) | Expressed in interstitial progenitor cells in the cortex and medulla | Fused kidneys | FOXD1 + progenitors form stroma that becomes vascular smooth muscle, juxtaglomerular cells, mesangial cells, pericytes, and resident fibroblasts | Marker of interstitial progenitor cells that form renal stroma |
| RARα/RARβ2 (retinoic acid receptor) | Ureteric bud branching, stroma signaling | Kidney and ureter malformations | RARα: ureteric bud, metanephric mesenchyme, stromaRARβ: stroma only | Marker in tumors |
| GDNF (glial cell line–derived neurotrophic factor) | Growth factor | Ureteric bud fails to form or has abnormal branching; renal hypoplasia or agenesis | Intermediate mesoderm, mesonephros, induced metanephric mesenchyme, pretubular aggregate | |
| Angiotensinogen | Renin substrate | Hypoplastic papillae, hydronephrosis, thickened blood vessels, hypotension | Ureteric bud, stroma, glomeruli, proximal tubules | |
| Angiotensin receptor types 1a and 1b | Angiotensin receptor | Hypoplastic papillae, hydronephrosis, thickened blood vessels, hypotension | Ureteric bud, stroma, proximal tubules | |
| Angiotensin receptor type 2 | Angiotensin receptor | Duplicated collecting system, hydronephrotic upper pole | Stroma adjacent to ureteric bud stalk | |
| Fibroblast growth factor | Growth factor | Increased apoptosis, truncated nephrons, renal hypoplasia | Pretubular aggregates, vesicles, tubule progenitors | |
| Platelet-derived growth factor (PDGF) receptor b | Growth factor (PDGF) receptor | Dilated glomerular capillaries with no mesangial cells | Glomerular mesangial cells | |
| Vascular endothelial growth factor | Growth factor | Small glomeruli, lack capillary loops, few endothelial cells | S-shaped body, podocytes, collecting ducts | |
| Laminin α 5 | Basement membrane protein | Abnormal glomeruli with displaced endothelial and mesangial cells, and clustered podocytes | Basement membrane of ureteric bud, developing tubules, and glomeruli | |
| Laminin β 2 | Basement membrane protein | Absence of podocyte foot processes, proteinuria | Glomerular basement membrane | Nephrotic syndrome |
| Laminin α 3 β 2 | Transmembrane adhesion receptor | Dilated and fewer capillary loops; loss of podocyte foot processes, dual GBMs | Ureteric bud, collecting ducts, podocytes | |
| Nephrin | Transmembrane protein | Foot process effacement, absence of filtration slit diaphragm, proteinuria | Podocyte filtration slit diaphragm | Congenital nephrotic syndrome of the Finnish type |
| PKD1 and PKD2 (polycystin 1 and 2) | Transmembrane proteins | Metanephric cysts in null mutants; postnatal PKD in heterozygous mice | Developing nephron segments and collecting ducts | Mutated in autosomal dominant PKD |
| UP II and III (Uroplakin) | Transmembrane proteins | Hydronephrosis; vesicoureteric reflux (UPII); ureteric obstruction (UPIII) | Superficial umbrella cells of urothelium |
Cells of the upper layer continue to proliferate to form a connecting duct and the distal convoluted tubule, whereas cells of the middle limb produce the proximal convoluted tubule and the limb of Henle. Finally, the limb of Henle grows down along the collecting duct to form the medullary rays. Nephrogenesis is usually complete by 32 to 36 weeks of gestation. Maturation occurs beyond this period and continues until adulthood, with resulting renal enlargement that reflects tubular elongation and cellular enlargement of the tubular portions of the nephron. There appears to be some correlation ( Table 1.2 ) between fetal gestational age and layers/rows of mature glomeruli, which can be useful in forensic assessment or to correlate with development in other organs.
| Gestational Age (weeks) | Rows of Glomeruli in Cortex From Medulla to Capsule | Number of Mature Glomerular Layers |
|---|---|---|
| 16-23 | 3 | – |
| 24 | 3-5 | 4.3 ± 0.8 |
| 25 | 4-6 | 4.6 ± 0.7 |
| 26 | 5-7 | – |
| 27 | 6-8 | 6.1 ± 1.1 |
| 28 | 7-9 | 6.0 ± 1.2 |
| 29 | 8-10 | 6.3 ± 1.3 |
| 30 | 9-11 | – |
| 31 | 10-12 | 7.1 ± 0.9 |
| 32 | 11-13 | – |
| 33 | 12-13 | 7.7 ± 0.8 |
| 34 | 12-14 | – |
| 35-42 | 12-14 | 7.6 ± 0.4 to 8.6 ± 1.3 |
| Newborn to adult | 12-14 | – |
| Cortex between columns of Bertin | Radial counts; excludes columns of Bertin |
The kidneys are paired retroperitoneal organs that normally extend from the 12th thoracic vertebra to the 3rd lumbar vertebra. The upper poles are tilted slightly toward the midline, and the right kidney is slightly lower and shorter than the left kidney. The average adult kidney is 11 to 12 cm long, 5 to 7 cm wide, and 2.5 to 3 cm thick, and it weighs 125 to 170 g in men and 115 to 155 g in women. The combined mass of the kidneys correlates with body surface area, whereas age, sex, and race have relatively less influence. Its volume can increase or decrease by 15% to 40% with major fluctuations in blood pressure (BP), hydration, or interstitial expansion by edema.
The posterior surfaces are flatter than the anterior, and the medial surface is concave with a 3-cm slitlike space called the hilum . The hilum is the vestibule through which the collecting system, nerves, arteries, veins, and lymphatics pass. In the adult, these structures are invested by fat within the renal sinus and are usually arranged from anterior to posterior as artery and vein and ureter.
The subcapsular surface of the renal cortex may be smooth and featureless, or may show grooves corresponding to the individual renal lobes ( Fig. 1.19 ). The persistence of distinct fetal lobes is common and is a normal anatomic variant. In some kidneys, three zones are created by two shallow superficial grooves that radiate from the hilum to the lateral border ( Fig. 1.20 ). The three regions define the upper pole, middle zone, and lower pole, and usually reflect regions drained by the three lobar veins.
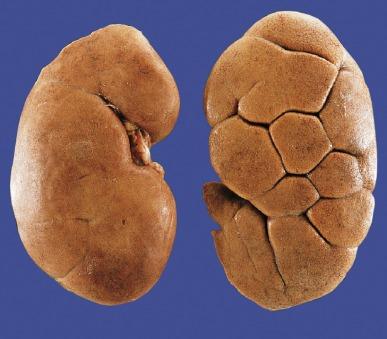
The normal adult kidney has a minimum of 10 to 14 lobes, each composed of a central conical medullary pyramid surrounded by a cap of cortex ( Fig. 1.21 ). Often there are six lobes in the upper pole and four lobes each in the middle zone and lower pole. However, substantial variability occurs both in the number of lobes in the adult kidney and in their visibility when the renal capsule is removed.
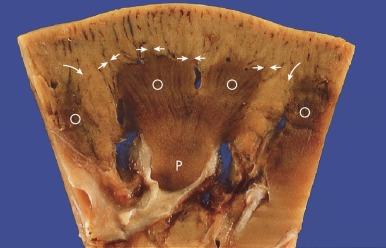
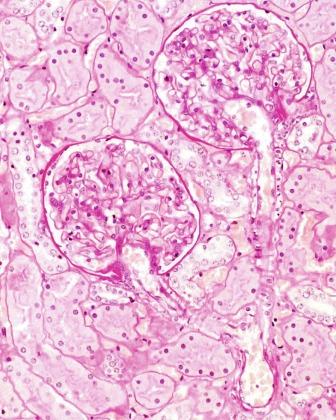
The renal parenchyma consists of the cortex and the medulla, which are grossly quite distinct ( Fig. 1.21 ). The renal cortex is the nephron-containing parenchyma. It forms a 1.0-cm layer between the renal capsule and medulla (also known as the renal pyramids) and extends down between the renal pyramids forming the columns of Bertin. The midplane of a column of Bertin is the line of fusion of two renal lobes. The renal medulla is divided into an outer medulla and the inner medulla or papilla ( Fig. 1.21 ). The outer medulla is further divided into an outer stripe and an inner stripe. Each segment of the renal medulla is defined by its unique tubular components, as discussed later. The outer medulla receives input from nephrons in the overlying cortex and nephrons in the adjacent half of a column of Bertin. The papilla protrudes into a minor calyx. Its tip has 20 to 70 openings of the papillary collecting ducts (Bellini ducts).
The arterial supply to the kidney follows a general overall blueprint, and knowledge of its details is useful when evaluating lesions in a kidney affected by vascular abnormalities. In 1901, Brödel first appreciated the distinctive renovascular segmentation of the kidney. The nomenclature used here was established by Graves in 1954.
The main renal artery arises from the aorta and divides into an anterior and a posterior division, and five segmental arteries are usually derived from these two divisions ( Figs. 1.20 and 1.22 ). The anterior division supplies most of the kidney and often divides into four segmental arteries: the apical, upper, middle, and lower segmental branches. The apical and lower segmental arteries supply the anterior and posterior aspects of the upper and lower poles, respectively ( Fig. 1.22 ). In 20% to 30% of kidneys, one or both arteries will arise separately from the aorta to form supernumerary arteries (also known as aberrant, accessory, or polar arteries). The posterior division becomes the posterior segmental artery. It passes behind the pelvis and supplies the middle two-thirds of the posterior surface. The five segmental arteries and all their branches are end arteries with no collateral blood flow. Thus occlusion of a segmental artery or any of its subsequent branches results in infarction of the zone of parenchyma it supplies.
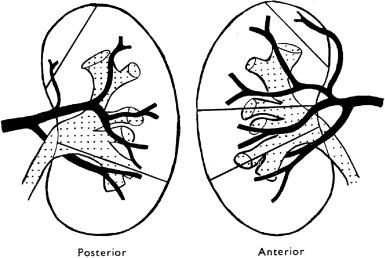
From segmental arteries, the interlobar arteries, arcuate arteries, interlobular arteries, and arterioles are sequentially derived. A segmental artery branches within the renal sinus and creates several interlobar arteries. An interlobar artery enters the parenchyma in a column of Bertin between two renal pyramids (i.e., at the junction of two lobes) and forms a splay of six to eight arcuate arteries. The arcuate arteries course along the corticomedullary junction and terminate at the midpoint of a renal lobe. At perpendicular or slightly oblique angles, the interlobular arteries arise from an arcuate artery and may branch as they pass through the cortex toward the renal capsule. The interlobular arteries course between medullary rays and are encircled by tiers of five to six glomeruli, which they supply with afferent arterioles ( Fig. 1.23 ). The glomerular efferent arteriole forms a portal system of capillaries, which supply the adjacent tubules that arise from more than one glomerulus ( Fig. 1.2B ).
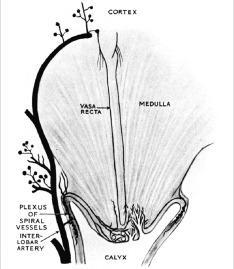
The renal medulla has a dual blood supply. Its principal blood supply arises from the efferent arterioles of the juxtamedullary glomeruli, which course directly into the medulla to form the vasa recta ( Fig. 1.24 ). In addition, as an interlobar artery courses along a minor calyx it gives rise to several spiral arteries, which supply capillaries to the papillary tip. These capillaries anastomose freely with capillaries from the opposite side and form a plexus around the ducts of Bellini.
The interlobular, arcuate, and interlobar veins parallel the arteries. Unlike the arcuate arteries, the arcuate veins have abundant anastomoses. They combine to form three large segmental veins that drain the three poles of the kidney. The veins lie anterior to the pelvis and unite to form the main renal vein.
The lymphatic drainage is a dual system. The major lymphatic drainage follows the blood vessels from parenchyma to the renal sinus, to the hilum, and terminates in lateral aortocaval lymph nodes. In addition, minor capsular lymphatic drainage from the superficial cortex courses into the capsule and then around to the hilum to join the major lymphatic flow.
The cortex is organized into two regions: the cortical labyrinth and the medullary rays ( Fig. 1.25 ). The labyrinth contains glomeruli, proximal and distal convoluted tubules, connecting tubules, and the initial portion of the collecting ducts, as well as interlobular vessels, arterioles, capillaries, and lymphatics. The principal components of the labyrinth are the proximal tubules. In the normal cortex, the tubules are closely packed with closely apposed basement membranes ( Figs. 1.23 and 1.25 ). The interstitial space is scant. It contains the peritubular capillary plexus and inconspicuous numbers of interstitial fibroblasts and reticulum cells. A medullary ray consists of collecting ducts and the proximal and distal straight tubules that course down into and back up from the medulla. The nephrons that empty into the collecting ducts of a single medullary ray comprise a renal lobule, the functional unit of the kidney.
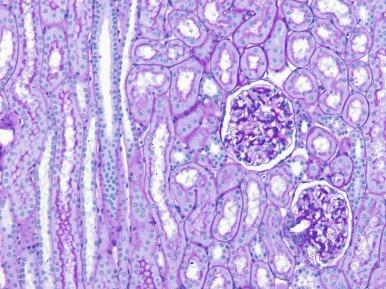
The medulla is divided into an outer medulla, composed of an outer stripe and an inner stripe, and the inner medulla or papilla. Each zone contains specific tubular segments arranged in an elaborate architecture to create the countercurrent concentration system. The outer stripe contains the straight portions of the proximal tubule, thick ascending limb of loop of Henle and collecting ducts. The inner stripe contains the thin descending and thick ascending limbs of loops of Henle and collecting ducts. The inner medulla contains the thin descending and ascending limbs of loops of Henle and collecting ducts of Bellini. For further details of the microscopic anatomy of the medulla, or for the ultrastructural features of all nephron components , several excellent resources are available.
“The more complicated an organ in its development, the more subject it is to maldevelopment, and in this respect the kidney outranks most other organs.” —Edith Potter
Developmental anomalies and cystic kidney diseases occur in approximately 10% of the population. They encompass a vast number of complex entities that may be limited to the kidney or part of a multiorgan malformation syndrome. These diseases may be sporadic, hereditary and syndromic, or acquired, and they include several that are associated with a neoplastic diathesis. Enormous progress has been made in unraveling the pathogenesis of many entities with delineation of their genetic and molecular basis. This knowledge has minimized the validity of the simplistic anatomic contribution of urinary tract obstruction popular for so many years by placing it within a larger paradigm of sequential genetic and molecular misadventures that culminate in the malformed kidney and urinary tract.
These diseases can be separated into two large categories based on pathogenesis. A heterogeneous group of diseases results from mutation of one or more key master genes crucial for proper development of the kidneys and lower urinary tract. Collectively, these are referred to as congenital anomalies of the kidneys and urinary tract (CAKUT). These lesions may be sporadic or hereditary and syndromic. They are common and very important because they account for up to 50% of cases of renal failure in children ( Table 1.3 ).
Autosomal recessive polycystic kidney disease
Classic in neonates and infants
Childhood with hepatic fibrosis
Autosomal dominant polycystic kidney disease
Classic adult form
Early-onset childhood form
Glomerulocystic kidney
Primary GCKD
Sporadic GCKD
Familial GCKD
Hereditary GCKD associated with UROM or HNF1B mutations
Secondary glomerular diseases in which glomerular may be present
Associated with ADPKD/ARPKD/TSC
Syndromic nonhereditary glomerulocystic kidney
Ischemic glomerular atrophy
Renal dysplasia
Acquired cystic kidney disease
Renal agenesis and dysplasia
Agenesis
Sporadic: unilateral or bilateral
Syndromic
Nonsyndromic multiple malformation syndromes
Renal dysplasias
Sporadic: unilateral or bilateral
Syndromic
Nonsyndromic multiple malformation syndromes
Hereditary adysplasia
Renal hypoplasias
Simple hypoplasia: unilateral or bilateral
Oligomeganephronic hypoplasia
Cortical hypoplasia (reduced nephron generations)
Reduced nephron numbers (premature and low birth weight risk for hypertension)
Abnormalities in form, position, and number
Rotation anomaly
Renal ectopias
Renal fusions
Supernumerary kidney
In combination with A, B, or D
Ureteral and urethral abnormalities
Ureteropelvic junction obstruction
Ureteral duplication/bifid ureter
Vesicoureteral reflux
Primary megaureter
Ureteral ectopia
Posterior urethral valves
In combination with A, B, or C
Nephronophthisis
Autosomal dominant tubulointerstitial disease
UROM kidney disease
REN kidney disease
HNF1B kidney disease
MUC1 kidney disease
Renal tubular dysgenesis
Bardet-Biedel syndromes
Mixed epithelial and stromal tumor family (includes cystic nephroma)
Cystic partially differentiated nephroblastoma
Multilocular cystic renal cell carcinoma of low malignant potential
Tubulocystic renal cell carcinoma
von Hippel–Lindau disease
Lymphangioma/lymphangiectasia
Simple cortical cysts
Medullary sponge kidney
Localized cystic kidney disease
A second category encompasses a “family” of cystic kidney diseases, the ciliopathies, which result from mutation of genes that encode for certain proteins crucial to the formation and function of the primary cilium of renal tubular cells. Most renal tubular cells have a single primary cilium, a slender organelle that originates from the basal body and extends from the apical surface of tubular cells. It is a structure long regarded as vestigial, but it is now apparent that the primary cilium has critical sensory and cell signaling functions that affect cell proliferation, polarity, and differentiation. The ciliopathies are hereditary diseases. Several have associated liver diseases that include bile duct cysts and bile duct plate malformations that may lead to congenital hepatic fibrosis. The ciliopathies include one of the most common genetic diseases, autosomal dominant polycystic kidney disease (ADPKD), as well as numerous other uncommon syndromic disorders. The members of this family of diseases are listed in Table 1.4 . However, this list is likely not complete because new entities are regularly added.
Autosomal dominant polycystic kidney disease
Von Hippel–Lindau disease
Uromodulin-associated kidney diseases (medullary cystic kidney disease type II, familial juvenile hyperuremic nephropathy, glomerulocystic kidney disease)
Autosomal recessive polycystic kidney disease
Nephronophthisis (with or without renal-retinal dysplasia, Joubert syndrome, or Senior-Loken syndrome)
Bardet-Biedl syndrome
Meckel-Gruber syndrome
Orofacial-digital syndrome
Jeune syndrome
Finally, there are miscellaneous other cystic kidney diseases of uncertain pathogenesis. These include the common simple cortical cyst, the uncommon isolated polycystic kidney disease that resembles ADPKD, and acquired cystic kidney disease, which is of great importance because of its neoplastic diathesis.
Construction of a classification system designed to logically organize this vast compendium of developmental and cystic diseases is challenging. Many schemas have been proposed. The ideal scheme would account for morphologic features, their clinical importance, and their pathogenesis. Although knowledge of the embryologic development of the kidney provides a tempting basis for explaining departures from the normal renal development, it must be accepted that little experimental evidence exists to defend such conjectures.
Classification of developmental anomalies and cystic kidney diseases based on their underlying genetic defects will likely gradually replace current schemes. However, even with a more thorough understanding of the genetic basis of these diseases, organizing these entities will remain difficult. For instance, CAKUT lesions may affect a single kidney–lower urinary tract unit with a completely normal contralateral kidney. Conversely, CAKUT lesions may show distinctly different types of anomalies that affect each kidney–lower urinary tract unit. Finally, the spectrum of CAKUT diseases may arise in both syndromic and nonsyndromic contexts. Similarly, although the diseases associated with mutation of ciliary proteins are all hereditary, the inheritance can be dominant or recessive. In addition, the renal diseases encountered in the ciliopathies range from cystic diseases that arise in normally formed kidneys, to cystic kidney disease resulting from metanephric maldevelopment identical to several CAKUT abnormalities, to chronic progressive tubulointerstitial diseases that may or may not form cysts. Another complicating factor is the polygenetic nature of many disorders, in which the variable presence of, or accumulation of, multiple minor genetic defects affects susceptibility and influences the nature of the malformation expressed. This conundrum of developmental misadventures prompted Edith Potter to offer the comment quoted earlier. The classification scheme offered in this chapter is more of a tabulation of entities based on a selected major anatomic feature that is the avenue through which pathologists encounter these entities ( Table 1.5 ).
Rotation anomaly
Ectopia
Fusion
Supernumerary kidney
Renal hypoplasia
Simple hypoplasia
Oligomeganephronia
Cortical hypoplasia
Segmental hypoplasia (Ask-Upmark kidney)
Renal agenesis
Unilateral renal agenesis
Bilateral renal agenesis (Potter syndrome)
Syndromic and hereditary renal agenesis
Multicystic and aplastic dysplasia
Segmented dysplasia
Dysplasia associated with lower tract obstruction
Dysplasia associated with hereditary syndromes
Hereditary renal dysplasia and urogenital dysplasia
Autosomal recessive polycystic kidney disease
Autosomal dominant polycystic kidney disease
Nephronophthisis
Medullary cystic disease
Von Hippel–Lindau disease
Tuberous sclerosis
Glomerulocystic kidney
Renal tubular dysgenesis
Acquired cystic kidney disease
Localized cystic kidney disease
Medullary sponge kidney
Simple cortical cyst
Pyelocaliceal ectasia and diverticula
It is useful to group abnormalities of form and position because they often occur in combination. For instance, fused kidneys are always ectopic, and most ectopic or fused kidneys also are abnormally rotated. Each anomaly may occur in isolation or may represent one component of a more serious complex of malformations affecting other urologic sites or other organ systems. Each may be completely innocent and asymptomatic; however, if urinary tract symptoms develop, they invariably result from impaired urinary drainage, which may cause hydronephrosis or pain, and may be complicated by infection or nephrolithiasis.
During ascent of the kidney to a lumbar location, the renal pelvis rotates 90 degrees from an anterior to a medial position ( Fig. 1.12 ). Failure of the pelvis to assume a medial orientation, reverse rotation, and overrotation to a posterior or even lateral location comprises a spectrum of orientation abnormalities known as rotation anomalies. Some degree of malrotation occurs in 1:400 to 1:1000 individuals. The most common rotation anomaly is nonrotation or incomplete medial rotation resulting in an anterior location of the pelvis and ureter ( Figs. 1.20 and 1.26 ). This may occur as an isolated abnormality in an otherwise normal kidney. It always accompanies renal ectopia or renal fusion. Ureteropelvic obstruction may on occasion result from a crossing vessel ( Fig. 1.26 ). Excess rotation and reverse rotation with the pelvis posterior or lateral are rare.
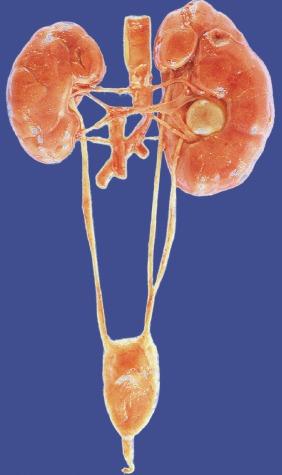
Failure of the kidney to assume its proper location in the renal fossa is known as renal ectopia. The several varieties are named according to location ( Table 1.6 ). Renal ectopia should be distinguished from renal ptosis in which a normally situated kidney shifts to a lower position. The origin of the renal artery from a normal aortic location identifies a lower-situated kidney as ptotic rather than ectopic. The incidence of ectopia at autopsy ranges from 1:660 to 1:1200. Renal ectopia is bilateral in 10% of cases.
Pelvic: opposite sacrum
Iliac: opposite sacral prominence
Abdominal: above iliac crest
Cephaloid: subdiaphragmatic
Thoracic: supradiaphragmatic
Crossed: contralateral
With fusion (90%)
Without fusion (10%)
Solitary crossed (rare)
Bilateral crossed (rarest)
The three most common forms of renal ectopia are pelvic, iliac, and abdominal, all of which are inferiorly located. The kidney may be nonreniform in shape, its pelvis and ureter are anterior (nonrotated), and the ureter is short and usually placed in the bladder, but it may have a high insertion on the pelvis that leads to obstruction. The vascular supply is influenced by the final location of the kidney, arising from the aorta or from the common iliac, internal or external iliac, or inferior mesenteric arteries ( Fig. 1.27 ). The contralateral kidney may be normal or occasionally may be absent or even dysplastic. Other anomalies of urologic organs and cardiovascular, skeletal, and gastrointestinal systems are frequent in both sexes.
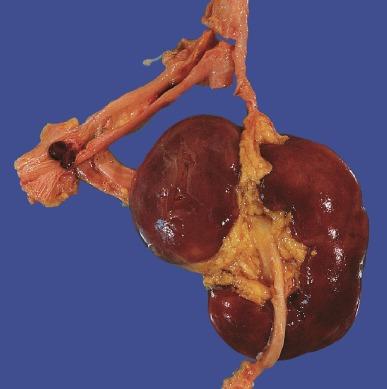
Cephaloid ectopia is usually associated with an omphalocele. The kidney appears to continue its ascent when the abdominal organs herniate into the omphalocele sac. The ureter and pelvis are typically normal. Thoracic ectopia is rare and usually involves the left kidney. The kidney resides in an extrapleural location in the posterior mediastinum. The diaphragm must be intact to distinguish this anomaly from herniation of the kidney and possibly other abdominal organs into the thorax secondary to diaphragmatic hernia. The lower lobe of the lung may be hypoplastic, but other anomalies are not present. Thoracic ectopia is usually asymptomatic, with a normal ureter and pelvis.
In crossed ectopia the kidney is situated opposite the side of insertion of its ureter in the trigone. Four combinations are possible ( Table 1.6 ). In 90% of cases there is also fusion to the other kidney. In crossed fused ectopia the kidneys may assume a variety of shapes and positions giving rise to six “types”: inferior, superior, lump, sigmoid, disk, and L-shaped. The kidneys function normally and their ureters are normally located within the bladder, but their pelves are nonrotated. Extrarenal anomalies (genital, skeletal, and anorectal) occur in 20% to 25% of patients.
Horseshoe kidney is the most common form of renal fusion. It is the midline fusion of two distinct renal masses, each with its own ureter and pelvis ( Figs. 1.28 and 1.29 ). Horseshoe kidney is relatively common (1:400 to 1:2000) with a 2:1 male predominance. Horseshoe kidney is commonly seen as part of other anomalies such as trisomy 18 (25%), caudal dysplasia syndrome, and Zellweger syndrome. The fusion is typically at the lower poles but can vary greatly in the quantity of fused parenchyma. A horseshoe kidney is ectopic and usually situated anterior to the aorta and vena cava. Occasionally the fusion is posterior to the vena cava or posterior to both the aorta and vena cava. The ureters and pelves are always anterior. This placement, coupled with commonly encountered high insertion of the ureter on the pelvis, can result in obstruction ( Fig. 1.28 ). Approximately 30% of patients also have other anomalies of the urinary tract, central nervous system, heart, gastrointestinal tract, or skeletal system.
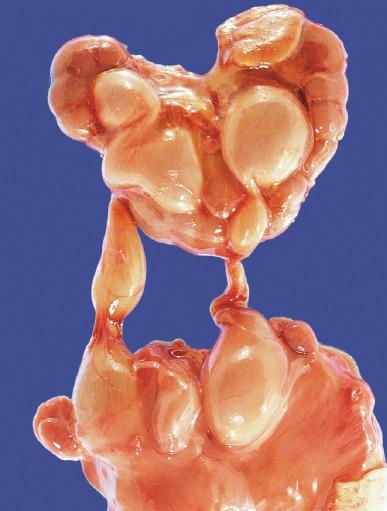
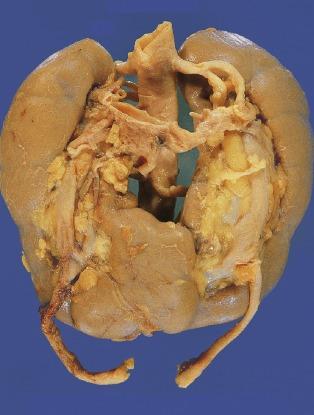
The following group of anomalies is much less common than those described in the preceding section. Hypoplasia is usually bilateral, whereas supernumerary kidney is usually unilateral, and neither is hereditary. The renal parenchyma in each is normally formed. In contrast, renal agenesis can be either unilateral or bilateral, and may be hereditary.
A supernumerary or duplicated kidney is one of the rarest disorders. It has been defined as “a free accessory organ that is a distinct, encapsulated, large or small parenchymatous mass topographically related to the usual kidney by a loose, cellular attachment at most and often by no attachment whatsoever.” It may be located below (most common), above, or adjacent to the kidney and is rarely bilateral. It is connected to the lower urinary tract by either a bifid ureter or its own separate ureter ( Fig. 1.30 ). In half of the reported cases, complications have developed related to obstruction and infection.
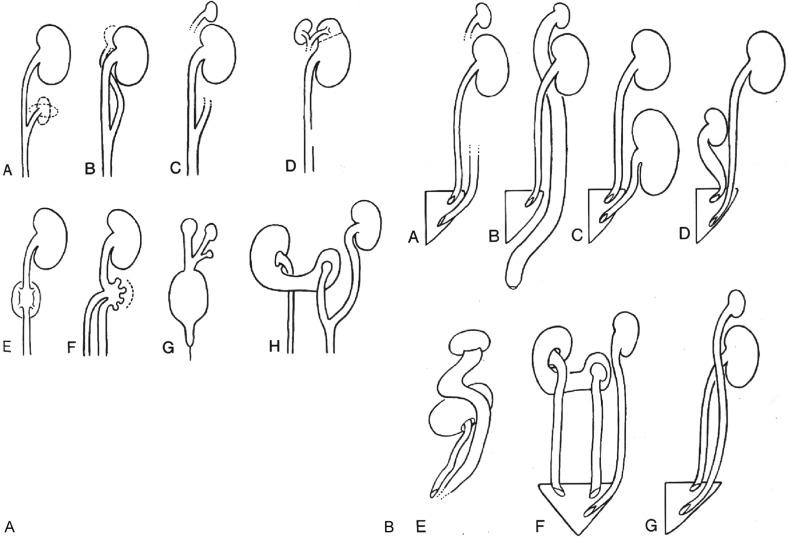
Hypoplasia refers to a small (< 50% of normal) but otherwise normally developed kidney. By definition, nephron formation is normal, albeit deficient, in quantity, and dysplastic elements (metanephric dysgenesis) are absent. There are four types of hypoplasia ( Table 1.7 ).
Simple hypoplasia
Oligomeganephronia
Cortical hypoplasia
Segmental hypoplasia/Ask-Upmark kidney
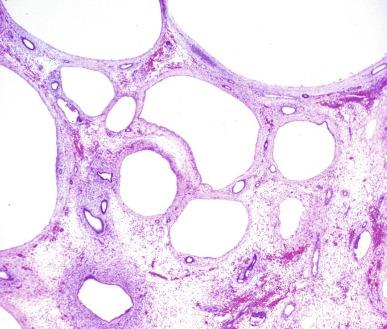
Simple hypoplasia is a rare, usually bilateral, and often nonhereditary disease in which the small size of the kidney usually reflects a marked reduction in the number of renal lobes. Frequently only one to five lobes are present ( Fig. 1.31 ). Dysplastic elements, by definition, are absent. When the condition is unilateral, the contralateral kidney may be hypertrophied. When it is bilateral, the small kidneys may eventually fail to provide renal function with body growth, and renal failure and nephron sclerosis may develop, the onset of which is determined by the degree of hypoplasia.
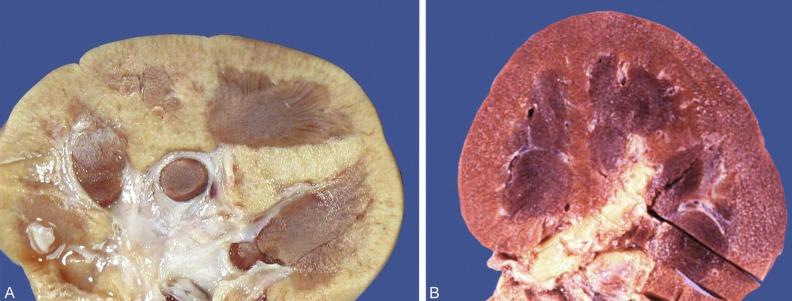
Oligomeganephronia may be the most common form of renal hypoplasia. It is a bilateral, nonhereditary disorder. Most cases are sporadic; however, few associations have been described with mutations in transcription factors involved in renal development including PAX2, HNF1B, and SIX. The kidneys are small because of a reduction in the number of renal lobes and the number of nephrons within each lobe. Microscopically, the nephrons present are tremendously enlarged ( Fig. 1.32 ). Glomerular and tubular volumes have been measured to be 12 times and 17 times normal, respectively.
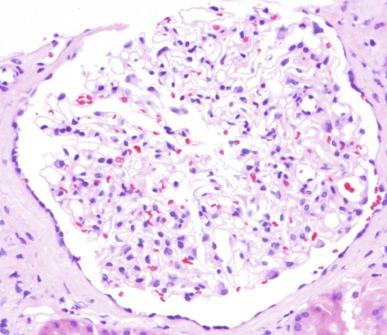
Children with oligomeganephronia present with a concentration defect causing polyuria, polydipsia, and salt wasting, resembling patients with nephronophthisis. Renal insufficiency and proteinuria gradually develop with body growth as progressive glomerular and tubulointerstitial scarring occur. The absence of a family history of renal disease, the presence of proteinuria, and imaging studies revealing symmetrically small noncystic kidneys usually permit separation from nephronophthisis.
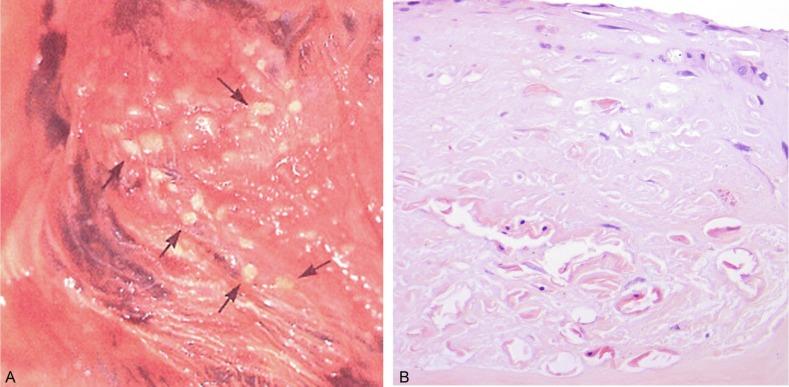
Cortical hypoplasia is a type of hypoplasia not generally recognized; it does not appear in standard texts of urologic pathology. Examples of cortical hypoplasia, however, are amply demonstrated in the Atlas of Medical Renal Pathology by Bonsib. Cortical hypoplasia refers to a reduction in the number of nephron generations that results in cortical thinning, reduction in overall renal size, and, if severe, a clinically significant reduction in nephron endowment, a major risk factor for hypertension and renal insufficiency. The “normal” number of generations ranges from 10 to 14, although rarely are more than 9 to 10 generations evident even in a well-oriented section. Determination of nephron generations is best performed with a nephrectomy specimen with sections oriented along medullary rays. Nephron generation counting is admittedly imprecise. However, if there is a 50% reduction in nephron generations, that is, four to five generations in a properly oriented section, then the reliability of this assessment is reasonable ( Fig. 1.33 ). This form of hypoplasia may coexist with other forms of hypoplasia, especially Ask-Upmark segmental hypoplasia.
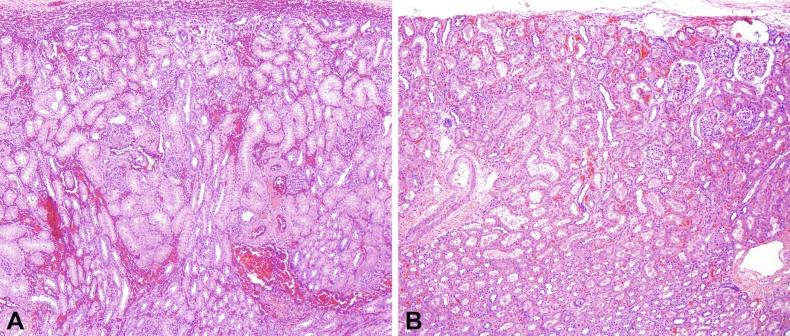
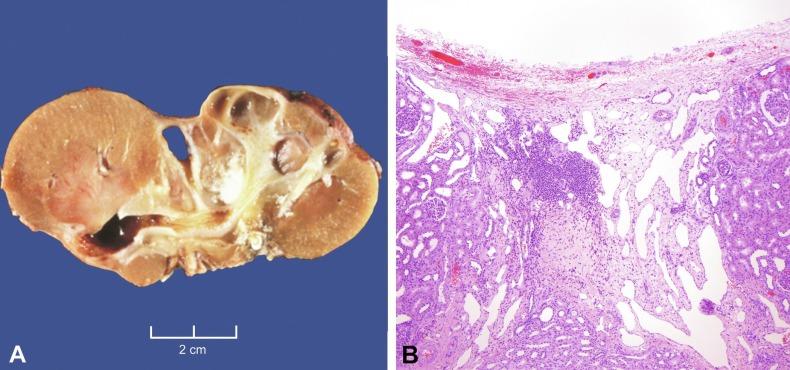
Segmental hypoplasia may manifest in neonates or adults and is often associated with hypertension. There is widespread agreement that vesicoureteral reflux is the fundamental injury. Some investigators regard it as an acquired lesion because its evolution over time has been radiographically documented in a few cases. Others agree with reflux-related injury but believe that most cases are developmental in origin secondary to in utero reflux that damages the developing renal lobe. That this condition often manifests in neonates and children supports a developmental basis. Furthermore, it is associated with renal vascular anomalies in 40% of cases and may be coexistent with lobes that show cortical hypoplasia. Segmental hypoplasia is defined as a small kidney with a deep cortical groove and dilatation of adjacent calyx ( Fig. 1.34A ). Microscopic features include sharply delineated cortical lesions ( Fig. 1.34B ). The cortex contains few tubules, with no or only rare glomeruli. There is little or no inflammation, nor is glomerulosclerosis or tubular atrophy present to indicate a regressive lesion. Finally, the kidney should have no evidence of metanephric dysgenesis. The medulla is characteristically absent. If present it may be rudimentary or flattened, with no loops of Henle, and may contain a distinctive cellular interstitial mesenchymal tissue not present in the normal renal pyramid.
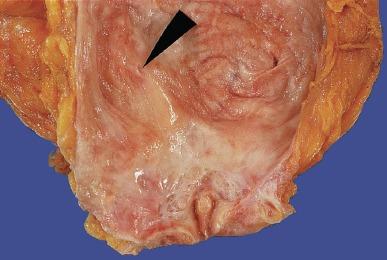
Absence of the kidney and its corresponding ureter is known as renal agenesis ( Table 1.8 ). The corresponding bladder hemitrigone is also absent because it represents the distal continuation of the ureteral smooth muscle ( Fig. 1.35 ). Failure to identify a kidney in a child or an adult does not prove congenital absence of the kidney because cystic dysplastic kidneys identified in newborns have been shown radiographically to regress further over time and may become undetectable.
Unilateral renal agenesis
Bilateral renal agenesis (Potter syndrome)
Chromosomal anomalies (trisomy 13 and 18)
VATER association
Müllerian aplasia syndrome (MURCS syndrome)
Sirenomelia (caudal regression syndrome)
Cloacal exstrophy
Fraser syndrome
Williams syndrome
Multiple malformation syndromes, not otherwise specified
Hereditary renal adysplasia
In unilateral renal agenesis the contralateral kidney may be hypertrophic up to twice the normal size. The overall renal function may be normal, and the condition may be entirely asymptomatic ( Fig. 1.27 ). Several genetic and environmental factors have been associated with an increased risk, such as African American race, maternal diabetes mellitus, and maternal age younger than 18 years. In up to 70% of patients, renal agenesis is associated with additional anomalies, most often affecting the genital tract. This presumably reflects a common abnormality affecting development of both the mesonephric duct– and Müllerian duct–derived structures. Female genital anomalies include absence of the ipsilateral fallopian tube, uterine horn, and proximal vagina or uterine didelphia or vaginal septum. Male genital anomalies may include absence of the ipsilateral epididymis, vas deferens, or seminal vesicle, or a seminal vesicle cyst may be encountered. Identification of a patient with a unilateral genital anomaly or renal agenesis should therefore prompt evaluation of the other organ system.
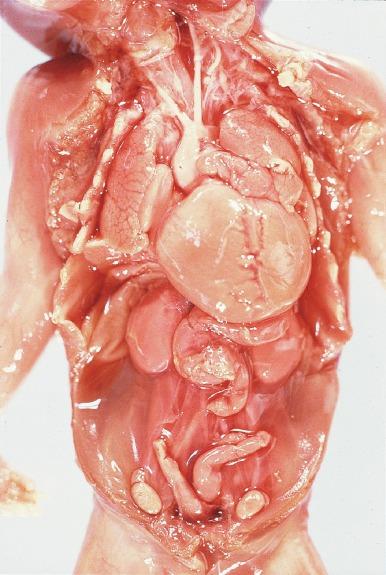
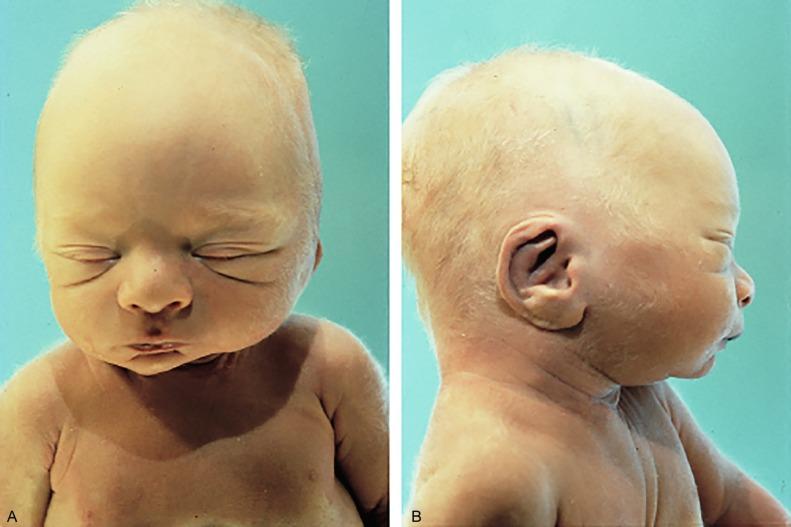
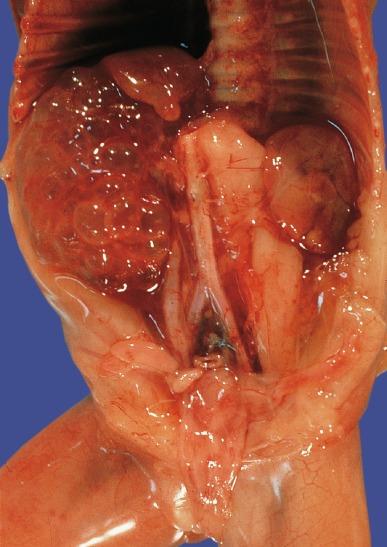
Bilateral renal agenesis is a uniformly fatal disorder known as Potter syndrome ( Fig. 1.36 ). Both ureters are also absent, so the bladder has no ureteral orifices. Approximately 40% of affected fetuses are stillbirths, and those born alive die of pulmonary failure within 48 hours. Mothers present with severe oligohydramnios because fetal urine normally accounts for most of the amniotic fluid in the second half of gestation. Oligohydramnios impairs pulmonary development that results in pulmonary hypoplasia and produces a variety of distinctive gross features known as the Potter phenotype or oligohydramnios phenotype ( Table 1.9 ). Figs. 1.37 and 1.38 demonstrate some of the characteristic facial and placental findings. Some urologists refer to any fetus born with the oligohydramnios phenotype as having Potter syndrome, rather than reserving the term for the entity of bilateral renal-ureteral agenesis as initially described. This can be confusing because oligohydramnios has other causes ( Table 1.10 ).
Potter facies
Increased interocular distance
Broad, flattened nose
Prominent inner canthic folds (sweeping downward and laterally)
Receding chin
Large, low-set ears with little cartilage
Positional deformities (flexion of hips and knees, clubbed feet)
Dry skin
Hypoplastic lungs
Small bladder with absent trigone
Placenta: amnion nodosum
Potter syndrome (bilateral renal agenesis)
Bilateral renal dysplasia
Distal (complete) urinary tract obstruction
Autosomal recessive polycystic kidney disease
Glomerulocystic kidney disease
Renal tubular dysgenesis
Chronic amniotic fluid leak
In utero acute renal failure
Idiopathic conditions
Many syndromes are characterized by absence of one kidney or, rarely, both kidneys as a component of a constellation of congenital anomalies. The list includes chromosomal anomalies, several malformation syndromes, and multiple malformation events affecting the gastrointestinal, cardiac, central nervous system, or skeletal system that do not conform to a specific syndrome. Finally, renal agenesis may also occur in a familial disorder with renal dysplasia (see Hereditary Renal Adysplasia section later in this chapter). In each disorder, identification of extrarenal components and a detailed family history are essential for proper classification and appropriate genetic counseling. The extrarenal anomalies are responsible for many complications and for the lethal nature of many of the syndromes.
A dysplastic kidney is a metanephric structure with aberrant nephronic differentiation. The term dysplasia is used in a developmental sense and does not connote any relationship with neoplasia. Dysplastic kidneys should not be confused with hypoplastic kidneys, which are small but have normal nephron development, or with polycystic kidney diseases, which although cystic do not contain dysplastic elements. Dysplastic kidneys are, by definition, maldeveloped ( Figs. 1.39 to 1.41 ). They are usually not reniform, can vary greatly in size and appearance, and occur in several patterns: unilateral, bilateral, or confined to the upper pole of a duplex kidney ( Table 1.11 ). Approximately 90% of cases have a ureteral abnormality or are associated with distal obstruction resulting in ureteral stenosis or dilation and megacystis or bladder hypertrophy. Renal dysplasia most commonly is sporadic, but it may be familial, part of a multiple malformation complex, or a component of a hereditary malformation syndrome ( Table 1.12 ). Table 1.13 and Fig. 1.39 compare the major anatomic and clinical features of renal dysplasias with the autosomal recessive polycystic kidney disease (ARPKD) and ADPKD discussed in the following sections.
Multicystic and aplastic dysplasia
Segmental dysplasia
Dysplasia associated with lower urinary tract obstruction
Dysplasia associated with malformation syndromes
Hereditary adysplasia and urogenital adysplasia
VATER (VACTERL) association
MURC syndrome
Prune-belly syndrome
Caudal regression syndrome
Cloacal exstrophy
Urogenital sinus syndrome
Urorectal septum syndrome sequence
Meckel-Gruber syndrome a
a Autosomal recessive inheritance.
Dandy-Walker syndrome a
Short rib–polydactyly syndrome a
Elejalde syndrome
| Agenesis/Aplasia | Hypoplasia | Dysplasia | |
|---|---|---|---|
| Definition | Agenesis: no kidneys Aplasia: rudimentary kidneys |
Small, architecturally normal kidneys, weight < 50% expected; decreased number of nephrons (< 2 SD normal) | Architecturally abnormal kidney with immature nephrons |
| Incidence | Unilateral: 1:1000 Bilateral: 1:10,000 |
Unilateral: 1:1000 Bilateral: 1:4000 |
Unilateral: 1:7500 Bilateral: 1:7500 |
| Etiology | Defect in formation of Wolffian duct and/or ureteric bud | Slow induction or incorrect position of ureteric bud, decreased branching | Defective branching |
| Clinical presentation | Unilateral: M = F; may be asymptomatic, risk of FSGS Bilateral: M > F; perinatal death |
May be asymptomatic (if unilateral) or symptomatic:
|
May be asymptomatic (if unilateral) or symptomatic:
|
| Radiologic features | Absent kidney | Small kidney | Small kidney with noncommunicating hypoechogenic cysts |
| Gross appearance | No kidneys Earlobe-shaped, elongated adrenals |
Small kidneys Decreased number of pyramids |
Nonreniform, multicystic mass Small, normal, or large size |
| Microscopic features | Normal or compensatory hypertrophy in unilateral agenesis |
|
|
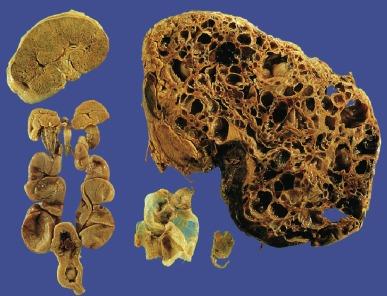
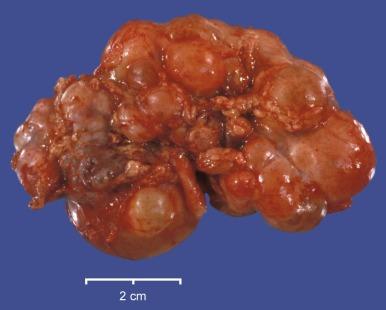
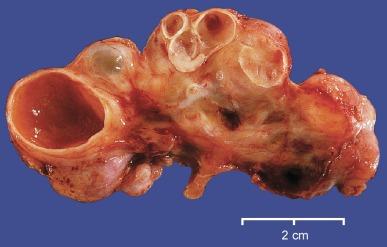
Dysplastic kidneys vary tremendously in gross appearance, ranging from the large multicystic kidney to the small aplastic kidney ( Fig. 1.39 ). The typical multicystic kidney is composed entirely of variably sized cysts and is the most common cause of a unilateral renal mass in a child ( Fig. 1.40 ). The cysts contain serous fluid. Typically, there is no corticomedullary differentiation, and a collecting system is often absent ( Fig. 1.41 ). The typical aplastic dysplasia is tiny and contains no cysts or only microscopic cysts ( Fig. 1.39 ). Corticomedullary differentiation and a collecting system are again absent. The multicystic and aplastic dysplasias represent extremes of a morphologic continuum differing only in the extent of cyst formation. Intermediate forms commonly occur. Most often renal dysplasia is a unilateral process, and the contralateral kidney is normal or larger than normal. When multicystic dysplasia or aplastic dysplasia is bilateral, the neonate presents with a Potter phenotype and dies of pulmonary hypoplasia.
The histologic appearance of a dysplastic kidney can also be quite varied. The kidney may be composed entirely of large cysts with little or no metanephric tissue represented ( Fig. 1.42 ). The cysts are of variable size and usually lined by flattened cells. Immature or dysplastic ducts are commonly present. They are lined with columnar epithelium and surrounded by collars of spindle cells that express estrogen receptor and/or progesterone receptor ( Fig. 1.43 ). Immature-appearing cartilage may be also present but is far less frequent than dysplastic ducts ( Figs. 1.43B and 1.44A ). The dysplastic ducts are thought to originate from the ampullary bud, whereas the immature cartilage is regarded as blastemal derived. Immature tubules and aberrantly formed glomeruli may be present, or relatively normal-appearing tubules and well-formed glomeruli may be present but not sufficiently organized to contribute appreciably to renal function ( Fig. 1.44 ).
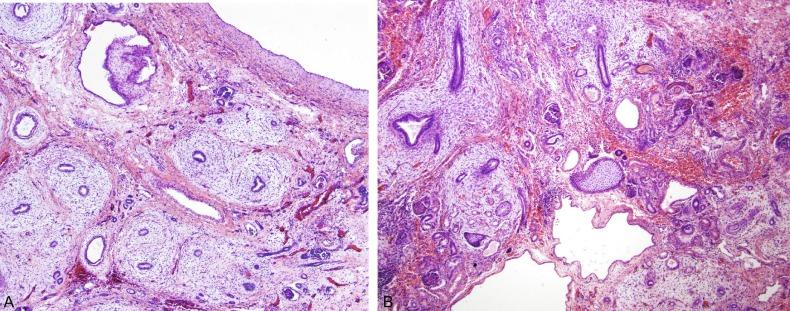
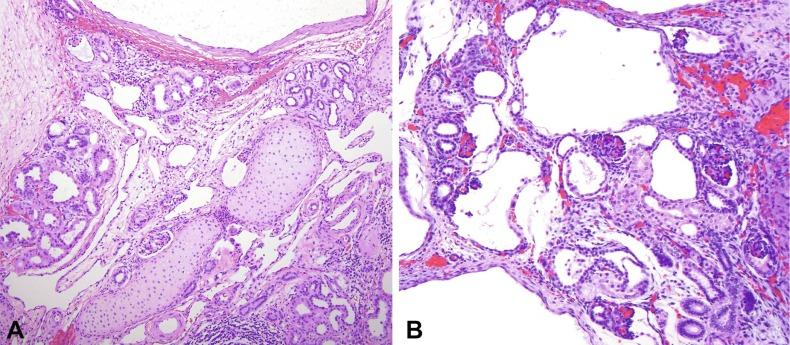
Occasionally an infant presents with renal insufficiency and small reniform kidneys with normal ureters and pelves. Simple hypoplasia may be suspected, but biopsy reveals an admixture of normal nephrons and aberrantly formed nephrons with microcysts and cartilage or dysplastic ducts. The renal prognosis is bleak, and the infant usually develops progressive renal failure with further growth.
Segmental forms of dysplasia occur in kidneys with duplication of the collecting system (duplex kidney). Usually the duplication is complete with two separate ureters. The upper pole moiety is affected, and histologic examination shows the same range of aberrant nephrogenesis encountered in aplastic and multicystic dysplasia ( Fig. 1.45 ). The upper pole ureter is usually ectopic, in a more cranial or caudal location relative to the normally situated lower pole ureter. The incidence and severity of dysplasia increase with the severity of the ectopia.
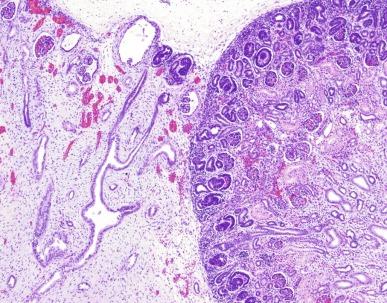
Bilateral renal dysplasia can be associated with distal obstruction resulting from urethral stenosis, posterior urethral valves, or bladder neck obstruction. This form of dysplasia may have a distinctive gross appearance. The kidneys are typically reniform, and they may be large or small, but they often show corticomedullary differentiation. The bladder is either hypertrophic or greatly dilated, and the ureters are dilated and tortuous ( Figs. 1.39 and 1.46 A ). There may be a severe degree of dysplasia with scant nephronic elements ( Fig. 1.46B ) or only a peripheral zone of dysplastic elements with normal deeper nephrons.
Renal dysplasia may develop in many multiple malformation syndromes, chromosomal anomalies, and hereditary malformation syndromes ( Table 1.12 ). When multiple malformations are encountered in a pediatric autopsy, it is important to obtain tissue for karyotype or genetic analysis, and to meticulously document all anomalies. Consultation with specialists in pediatrics and genetics is advisable to provide the proper classification of the disease so that appropriate family counseling can be provided.
The major features of renal aplasia, hypoplasia, and dysplasia are compared in Table 1.13 . Multicystic dysplasia, aplastic dysplasia, and renal agenesis are the most severe forms of metanephric maldevelopment. When they are not associated with extrarenal anomalies of a multiple malformation syndrome, they usually are sporadic events with low risk of a subsequently affected sibling. Rarely, however, renal agenesis or renal dysplasia, either unilateral or bilateral ( Figs. 1.39 and 1.47 ), or combined agenesis and dysplasia may be familial (usually autosomal dominant). This is known as hereditary renal adysplasia. There also may be concomitant malformation of Müllerian structures, a condition referred to as hereditary urogenital adysplasia. Unfortunately, neither syndrome can be anticipated until a second family member is identified with either agenesis or dysplasia.
Autosomal recessive polycystic kidney is a rare disorder that occurs in 1 in 20,000 to 50,000 births ( Table 1.14 ). In ARPKD, parents lack the disease and 25% of siblings are affected. It is associated with mutations of the polycystic kidney and hepatic disease 1 ( PKHD1 ) gene, on chromosome 6p12. The product of this gene, fibrocystin/polyductin, localizes to the primary cilium and centrosome of renal tubule epithelial cells. Organogenesis in ARPKD appears normal based on microdissection studies of severe neonatal forms. The primary lesion is fusiform ectasia of cortical and medullary collecting ducts, eventually leading to renal failure in severely affected cases. The renal lesion is accompanied by a bile duct plate malformation that develops into congenital hepatic fibrosis in surviving older patients.
| Dysplasia | ARPKD | ADPKD | |
|---|---|---|---|
| Incidence | 1:1000-2000 | 1:50,000 | 1:500-1000 |
| Bilateral | +/− | + | + |
| Segmental | +/− | − | − |
| Ureter abnormal | + | − | − |
| Reniform shape | +/− | + | + |
| Uniform cysts | − | + | − |
| Liver abnormal | +/− | + | + |
| Other malformations | +/− | − | − |
Recessive polycystic kidney disease was originally believed to be an invariably lethal neonatal disorder. Observation of some patients who survived into childhood prompted Blyth and Ockenden to propose a classification of patients into perinatal, neonatal, infantile, and juvenile forms. These forms vary in the degree of cyst formation. Although conceptually useful, it is often difficult to place a patient into a given category. More than 100 mutations of the PKHD1 gene have been identified that account for the clinical spectrum. The most severe neonatal cases manifest with pulmonary hypoplasia secondary to the massive renal enlargement that compromises pulmonary development. It appears that as the extent of cyst formation decreases, the child has better pulmonary development and a greater likelihood of survival. Unfortunately, with increasing duration of survival there is worsening of the liver disease that may culminate in congenital hepatic fibrosis. If one examines the kidneys of children with congenital hepatic fibrosis, two-thirds have a concentrating defect and have some degree of medullary cyst formation, findings indicating that ARPKD and congenital hepatic fibrosis are different manifestations of a single entity.
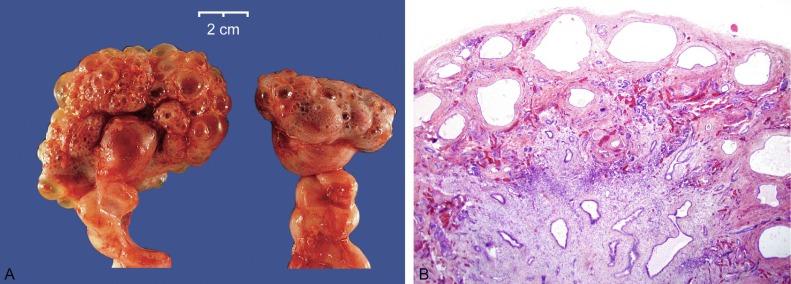
Most cases of ARPKD result in stillbirth, early neonatal death, or end-stage kidney disease by age 20 years. Affected neonates have massively enlarged and diffusely cystic kidneys that produce abdominal distention and compress thoracic organs ( Fig. 1.48A ). The lungs cannot develop normally, and death results from pulmonary hypoplasia. Despite the impressive cyst formation, the kidneys may be functional. If they are nonfunctional, oligohydramnios and a Potter phenotype may develop.
In severe cases, the cysts extend throughout the cortex and medulla in a distinctive radiating pattern imparting a spongy quality ( Fig. 1.48B ). Histologically, the cysts consist of dilated collecting ducts lined with uniform cuboidal cells ( Figs. 1.49 and 1.50 ). The nephrons between the collecting ducts appear normal.
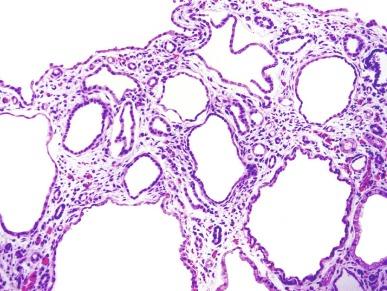
The liver in patients dying in the neonatal period shows portal bile duct proliferation that assumes a distinctive dilated and irregular branched pattern of anastomosing channels at the periphery of portal triads ( Fig. 1.51 ). There is an increase in the size of portal areas with increased fibrous tissue. In older patients, congenital hepatic fibrosis develops, resulting in portal hypertension and hepatosplenomegaly.
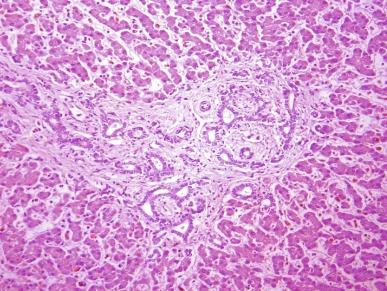
In less severely affected kidneys of older children the appearance is variable, and the diagnosis may be less obvious. The kidneys are smaller, and the cysts are fewer. Medullary cysts are always present and tend to be elongated. Cortical cysts if present are often rounded and variably distributed ( Fig. 1.52 ). The parenchyma adjacent to the cysts eventually develops atrophic changes with tubulointerstitial scarring and glomerulosclerosis. These features may create a resemblance to ADPKD. The presence of the liver lesion of congenital hepatic fibrosis therefore is a useful diagnostic feature. However, many diseases may be associated with renal cysts and liver disease, and awareness of additional anomalies is required for proper classification ( Table 1.15 ).
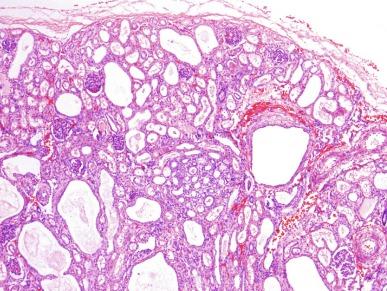
Autosomal recessive polycystic kidney disease
Autosomal dominant polycystic kidney disease
Nephronophthisis
Joubert syndrome
Bardet-Biedl syndrome
Meckel-Gruber syndrome a
a Additional malformations are present.
Oral-facial digital syndrome
Glomerulocystic kidney disease
Zellweger syndrome a
Ivemark syndrome a
Chondrodysplastic syndromes a
Trisomy C a
Trisomy D a
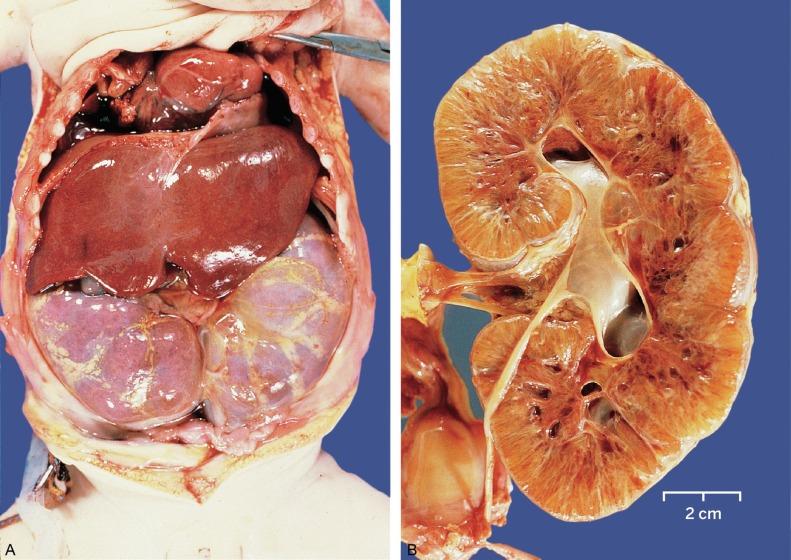
ADPKD is the most common cystic kidney disease and the most common genetically transmitted renal disease. It occurs with an estimated frequency of between 1:500 and 1:1000 ( Table 1.14 ). It is the fourth leading cause of end-stage renal disease, and affected patients comprise 5% to 10% of patients treated with dialysis. Although patients vary greatly in the age of onset of symptoms, most present in their third to fifth decade of life. Penetrance is nearly 100% if the individual survives to 80 years. Approximately 25% of affected patients lack a family history and presumably represent a new mutation. The disease results from mutations of PKD1 and PKD2 that localize to chromosome 16 in 90% of patients and to chromosome 4 in 10%, respectively. The gene product of PKD1 is polycystin-1, a transmembrane glycoprotein involved in cell signaling. PKD2 encodes for polycystin-2, a member of the transient receptor potential channel superfamily of nonselective cation channels.
Patients with ADPKD present with a variety of symptoms, most referable to the urinary tract. Chronic flank pain is the most common and correlates with renal weight and cyst size greater than 3 cm. Acute flank pain often reflects hemorrhage into a cyst. Hematuria is the second most common symptom. This may be gross, resulting in clot formation and urinary tract obstruction. Hypertension often develops early in the disease, and activation of the renin-angiotensin system secondary to intrarenal vascular occlusion by expanding cysts has been implicated. Urinary tract infection develops in 50% to 75% of patients and affects women more often than men. The infection may be confined to the collecting system or a cyst, or it may involve the parenchyma. Perinephric extension with abscess is a serious complication with a 60% mortality rate. Urate or calcium oxalate nephrolithiasis develops in 10% of patients. Extrarenal complications related to hypertension and berry aneurysms develop in 5% to 15%. Infection and cardiovascular disease represent the most common causes of death.
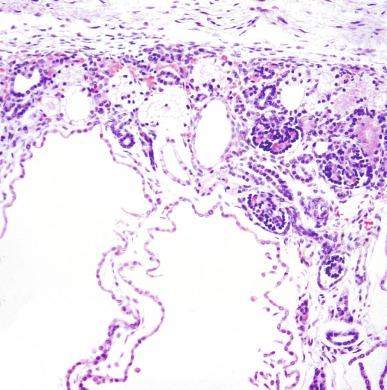
Early in the disease ( Fig. 1.53 ), the kidney may appear nearly normal with only scattered cysts in the cortex and medulla, and normal intervening parenchyma. The cysts initially are small and develop in only about 1% of nephrons. Microdissection studies have shown that the cysts develop in all segments of the nephron. Scanning electron microscopy and immunohistochemistry of cyst lining cells have confirmed these observations.
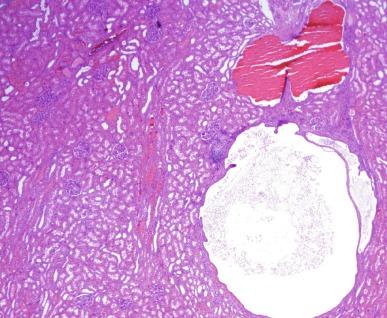
As the disease progresses, the cysts grow in size and number, with resulting massive renal enlargement ( Fig. 1.54 ). The cysts range in size from a few millimeters to several centimeters, and cyst contents vary from transparent to opaque to hemorrhagic fluid. Most cysts are lined with a single layer of flattened to cuboidal epithelium ( Fig. 1.55 ). Hyperplastic foci or polyp formation are detectable in some cysts ( Fig. 1.56 ). The cyst contents may be proteinaceous or include red cells or calcific deposits. The intervening parenchyma shows interstitial fibrosis with a lymphoid infiltrate, tubular atrophy, and glomerular and vascular sclerosis. Despite the cystic transformation, the kidneys retain a reniform shape and preserve their collecting systems.
Nephronophthisis is an autosomal recessive tubulointerstitial nephropathy in which cysts often develop. It inevitably leads to end-stage kidney disease. The first description of nephronophthisis was by Smith and Graham in 1945, who used the term medullary cystic disease to highlight the grossly visible medullary cysts. Fanconi, in 1951, coined the term juvenile familial nephronophthisis in reference to its histologic outcome; nephronophthisis is Greek for “disintegration of nephrons.” Most reports of nephronophthisis that appeared before the 1990s combined the two entities into the medullary cystic disease/juvenile nephronophthisis complex because of their morphologic similarities, a practice no longer appropriate considering differences in genetics and pathogenesis. Nephronophthisis is autosomal recessive and a ciliopathy, whereas medullary cystic kidney disease, now referred to as autosomal dominant tubulointerstitial disease (ADTID), is autosomal dominant and is not a ciliopathy as discussed later.
There are three clinical phenotypes of nephronophthisis that are distinguished by age of onset: infantile, juvenile, and adolescent forms. Affected individuals present with polyuria and polydipsia resulting from salt wasting, a concentration defect, anemia disproportionately severe for the level of renal insufficiency, and growth retardation. Although fundamentally a tubulointerstitial disease, 15% of patients have extrarenal components: retinal dystrophy (Senior-Loken syndrome), oculomotor apraxia (Cogan syndrome), situs inversus (infantile nephronophthisis), and rarely, congenital hepatic fibrosis.
Twenty mutated genes have been identified in nephronophthisis that encode for proteins expressed in the primary cilium, centrosome, and cell junctions of renal epithelial cells. These mutations, however, account for only 30% of nephronophthisis cases. Most mutations are responsible for some of the juvenile and adolescent forms, whereas NPHP2 and occasionally NPHP3 mutations are responsible for the infantile form. The juvenile and adolescent forms cannot be histologically distinguished. However, the infantile form has several distinctive features.
The kidneys in nephronophthisis are normally developed at birth. Cyst formation occurs in approximately 70% of patients but is usually delayed until advanced or end-stage disease develops. Sequential imaging shows that most patients lack cysts at presentation but many subsequently develop cysts, and that cyst frequency and size increase over time. Therefore early in the disease no cysts may be detectable to assist in the diagnosis.
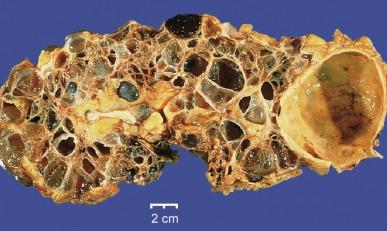
Kidney size in the juvenile and adolescent forms is usually normal or smaller than normal. When cysts develop, they congregate at the corticomedullary junction and may range from 1 to several centimeters in diameter ( Fig. 1.57 ). In the infantile form the kidneys may be larger than normal because cyst formation occurs earlier in the disease, before contraction from tubulointerstitial scarring. If cysts develop they may involve the medulla but generally are cortical in location.
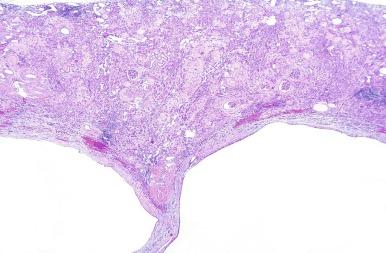
The primary histologic findings in nephronophthisis are nonspecific, so complete laboratory and clinical data are necessary. The juvenile and adolescent forms show a radial distribution of cortical injury with atrophic zones that alternate with zones of normal or hypertrophied tubules largely localized to medullary rays ( Fig. 1.58A ). Before end-stage renal disease the tubulointerstitial injury exceeds the extent of glomerulosclerosis, thus implicating a primary tubulointerstitial process ( Fig. 1.58A ). The tubules have an irregular profile sometimes described as figure-eight or T-shaped because of the tubule diverticula, particularly numerous in the limbs of Henle. Many small “cysts” are not true cysts but are localized segments of tubular dilatation with patent afferent and efferent tubule connections.
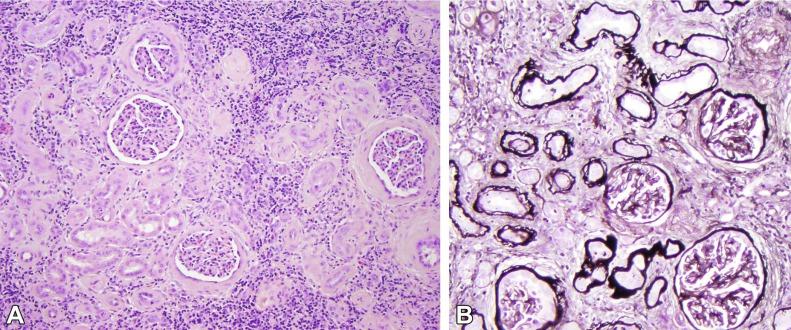
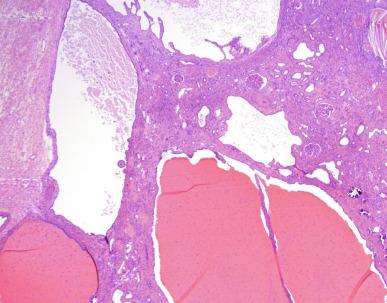
The irregularly shaped atrophic tubules show prominent multilayering of their tubular basement membranes, or the tubule basement membranes may range from thick and irregular, to thin and attenuated, to segmental absence of basement membrane ( Fig. 1.58B ). Dense interstitial fibrosis is present often with a prominent lymphoid cell infiltrate. Periglomerular fibrosis is a common finding, as it is in other chronic inflammatory interstitial diseases. As the tubulointerstitial disease progresses, glomeruli undergo sclerosis. Advanced cases show marked fibrointimal thickening of arteries and medial hypertrophy.
Medullary cysts, when present, arise from the loops of Henle and collecting ducts. The cyst cell lining is variable. A cuboidal cell lining is present in small cysts, but large cysts may have a flattened, nondescript cell lining surrounded by a rim of dense fibrous tissue. Medullary inflammation is not usually present.
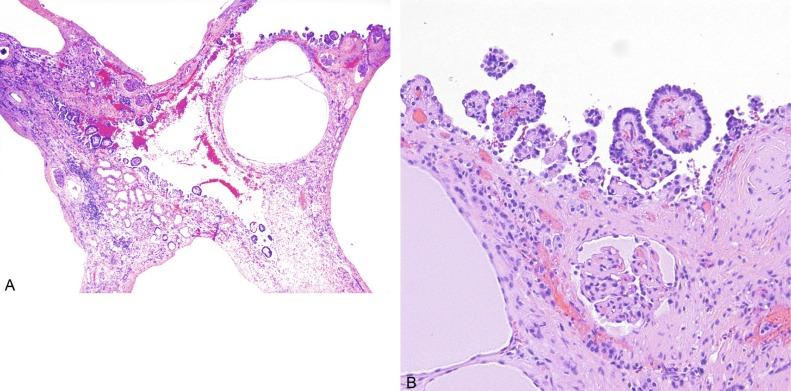
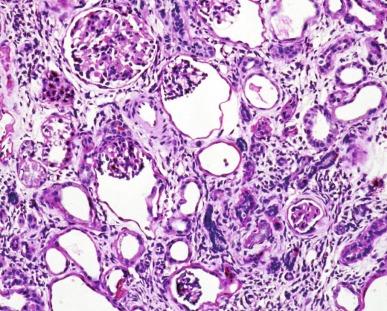
In the infantile form of nephronophthisis the cysts affect tubules, predominantly distal tubules and collecting ducts, in the form of tubular dilation or ectasia ( Fig. 1.59 ). Macrocystic dilation of tubules occurs and may be visible grossly. The ectatic and grossly cystic tubules are lined by cuboidal to columnar epithelium typical of distal tubules and collecting ducts. The tubules between the cysts may be normal or atrophic. Atrophic tubules are small and lined by inconspicuous cuboidal epithelium with thin tubular basement membranes that lack the irregular basement membrane multilayering of the juvenile and adolescence forms. Glomeruli may develop microcysts in which the Bowman capsule is enlarged two to three times normal. Although mild interstitial inflammation occurs, it is usually less than in the juvenile and adolescent forms.
ADTID, previously referred to as medullary cystic kidney disease (MCKD), is like nephronophthisis, a chronic progressive tubulointerstitial disease in which cysts may develop. It is a rare disease primarily of adults, but the age of onset overlaps with older patients with nephronophthisis. There are four known causes of ADTID due to mutation of uromodulin (UROM; Tamm-Horsfall glycoprotein), mucin 1 (MUC1; epithelial membrane antigen), renin (REN), and hepatocyte nuclear factor 1β (HNF1B; Table 1.16 ). One or more additional mutations remain to be identified. The recommended terminology for these diseases is UROM kidney disease, MUC1 kidney disease, REN kidney disease, and HNF1B kidney disease.
| Mutation | Onset/ESRD (years) | Laboratory/Clinical Results | Extrarenal Disease | Biopsy | Renal Cysts | Other Pathology |
|---|---|---|---|---|---|---|
| UROM | 20-70/avg. 54 | Gout, conc. defect | None | CTIN | 40% | UROM inclusions |
| MUC1 | 20-70/avg. 40 | Gout, conc. defect | None | CTIN | 12%-17% | None |
| REN | Childhood/30-40 | Gout, conc. defect, ↓ BP, anemia, ↑ K | None | CTIN | None | ↓ Renin in JGA |
| HNF1B | 24/Adulthood | Gout, conc. defect, DM, ↓ Mg | Pancreas hypoplasia/agenesis | CTIN | 60%-80% | CAKUT |
Most patients present in the third to fourth decade of life with polyuria and polydipsia as a result of salt wasting and a concentration defect ( Table 1.16 ). They progress to end-stage disease, usually by the fourth to seventh decade, although the rate of progression varies within and between affected families. Hyperuricemia and gout are common, especially in UROM and REN kidney disease. REN kidney disease patients have anemia refractory to treatment, hyperkalemia, and often low BP.
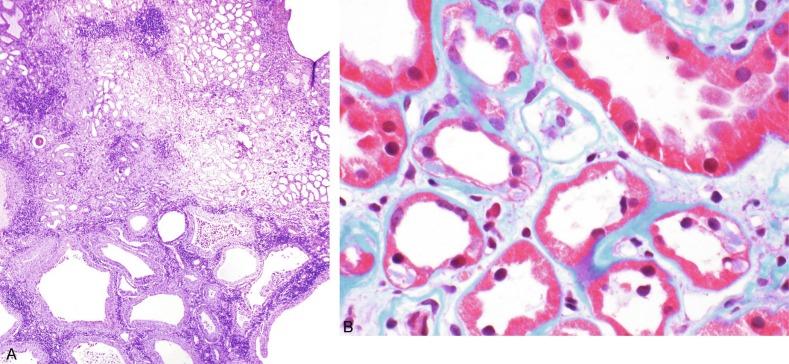
The kidneys in ADTID may be enlarged if cysts are prominent. Although medullary cysts are common, they are usually present in small numbers and develop late in the disease. Within a family not all affected individuals have cysts. The cysts congregate at the corticomedullary junction and can be several centimeters ( Fig. 1.60A ). Microscopically, all four ADTIDs show a nonspecific chronic interstitial nephritis with tubular atrophy, interstitial fibrosis, and periglomerular fibrosis similar to nephronophthisis. If cysts are present, they are lined with a flattened to cuboidal epithelium. UROM kidney disease contains uromodulin intracellular aggregates in thick ascending limb of Henle cells ( Fig. 1.60B ). REN kidney disease shows reduced to absent renin staining in cells of the juxtaglomerular apparatus. MUC1 and HNF1B kidney disease have no defining histologic findings. However, patients with HNF1B kidney disease often have congenital anomalies of the kidney and/or lower urinary tract.
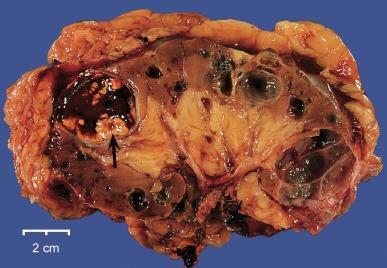
Von Hippel–Lindau disease is an uncommon autosomal dominant disorder due to germline mutation of the VHL gene in which renal and extrarenal cysts and neoplasms develop. The extrarenal manifestations include retinal, cerebellar, and spinal hemangioblastomas, pheochromocytoma, epididymal and pancreatic cysts, and cystadenomas. The renal manifestations consist of multiple and bilateral cysts that develop in 75% of patients, and renal cell carcinomas, often bilateral and multicentric, that develop in approximately 50% of patients ( Fig. 1.61 ). The mutant VHL gene has been localized to chromosome 3p25, adjacent to the gene implicated in development of sporadic clear cell renal cell carcinoma.
The renal cysts are lined with glycogen-rich cells like those of grade 1 to 2 clear cell renal cell carcinoma ( Fig. 1.62 ). These range from a benign-appearing lining of one to two cell layers of clear cells to multiple layers of cells. The broad spectrum of neoplastic proliferative lesions ranges from cysts with papillary tufts of mildly atypical cells, to cysts with solid mural nodules of clear cell renal cell carcinoma, to markedly cystic clear cell renal cell carcinoma. This morphologic spectrum represents a challenge in the classification of lesions in biopsy and nephrectomy material. Despite awareness of the high frequency of renal cell carcinoma in this syndrome, metastatic renal cell carcinoma remains the leading cause of death.
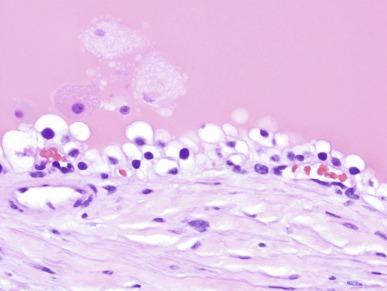
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder characterized by mental retardation, epilepsy, angiofibromas, cardiac rhabdomyomas, renal angiomyolipomas, renal cell carcinomas and renal cysts. Two genes have been identified that cause TSC, TSC1 and TSC2, mapped to chromosomes 9 and 13 that encode for hamartin and tuberin, respectively. The latter locus is within a few nucleotides of the PKD1 locus. Although renal cysts are uncommon and usually not extensive, some individuals, usually children, have dual mutations involving TSC2 and PKD1. They develop a diffuse cystic kidney disease with numerous large cortical and medullary cysts that resemble autosomal dominant polycystic disease; this disorder is known as the TSC2/PKD1 contiguous gene syndrome.
Many tubules and cysts in tuberous sclerosis are distinctive and provide diagnostic specificity in the recognition of this disorder. The cysts are lined with large eosinophilic cells with large hyperchromatic nuclei ( Fig. 1.63 ). The cyst lining cells may form papillary or polyploid masses, and may show occasional mitotic activity. Renal cell carcinomas of several types also develop in TSC but are far less frequent than in von Hippel–Lindau disease.
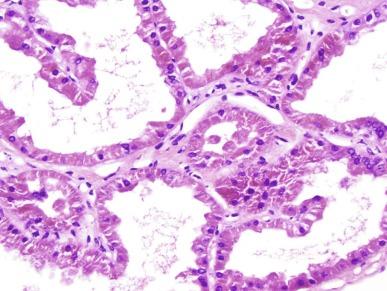
A glomerulocystic kidney (GCK) is defined as the presence of glomerular cysts in more than 5% of glomeruli in the absence of another cystic kidney disease. A GCK may be primary or secondary. Glomerulocystic kidney disease is used in reference to the primary forms that include a sporadic form, a familial form, and forms caused by mutations of UROM and HNF1B ( Table 1.3 ). Secondary forms of glomerular cysts occur in many unrelated disorders such as ADPKD, ARPKD, TSC, a variety of syndromes, ischemic glomeruli, and renal dysplasias. In these diseases, glomerular cysts may be present, but glomerular cysts are not definitional of the entity.
A glomerular cyst is defined as cystic dilation of Bowman capsule to two to three times normal. The glomerular tuft itself may be normal or abnormally formed. In most cases of GCK the glomerular cysts are widespread and affect far more than 5% of glomeruli ( Fig. 1.64 ). The Bowman capsule dilation in some cases of GCK may be massive, sufficient to result in a grossly cystic kidney. In other cases the kidneys may be small and hypoplastic.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here