Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
From their initial emergence in the yolk sac at the beginning stages of embryogenesis to their vast proliferation in the bone marrow at the end of term gestation, the differentiation of the pluripotent hematopoietic stem cell into a mature, segmented neutrophil is a tightly controlled process where the transcriptional regulators C/EBP-α and C/EBP-ε have essential roles. Despite their relatively short life span, these intriguing cells are not only vital for pathogen elimination during early infection, but also link innate and adaptive immune responses to promote resolution of inflammation and wound healing. In this chapter, neutrophil granulopoiesis, commencing at the earliest stages of embryogenesis, will be examined. Mechanisms controlling granular sorting, trafficking, and degranulation, as well as neutrophil homeostasis and specific differences between neonatal and adult neutrophil biology will also be discussed.
During human development, the creation of all blood cells, or fetal hematopoiesis, is an evolutionarily conserved process that transpires over three distinct stages ( Fig. 108.1 ). Commencing in the extra-embryonic yolk sac around the third week of embryogenesis, the first, or primitive, stage is marked by the production of a transient population of primordial erythroid cells, macrophages, and megakaryocytes. The second, or pro-definitive, stage is discernable close to the fifth week of fetal growth by the appearance of the hemogenic endothelium within the yolk sac. This early phase of hematopoiesis is marked by differentiation of visceral yolk sac mesoderm into endothelial cells of the vitelline vessels and hematopoietic cells. The transcription factor stem cell leukemia (SCL) is essential for this stage of fetal hematopoiesis, as Scl null mice failed to develop yolk sac hematopoietic colonies and lacked expression of essential hematopoietic transcription factors GATA-1, GATA-2, and PU.1. This dedicated hemogenic endothelium generates a greater variety of transitory myeloid lineage precursors in addition to primitive megakaryocytes and erythrocytes. , It is not until the third, or definitive, stage of fetal hematopoiesis around the seventh to eighth weeks of human gestation, however, that genuine pluripotent hematopoietic stem cells (HSCs) are detected. These multifaceted cells co-express hematopoietic and endothelial markers, including CD34, CD144 (VE-cadherin), CD45, and RUNX1, and originate from specialized intra-embryonic endothelial cells located within the ventral wall of the descending aorta in a process that has been termed endothelial-to-hematopoietic transition (EHT). ,
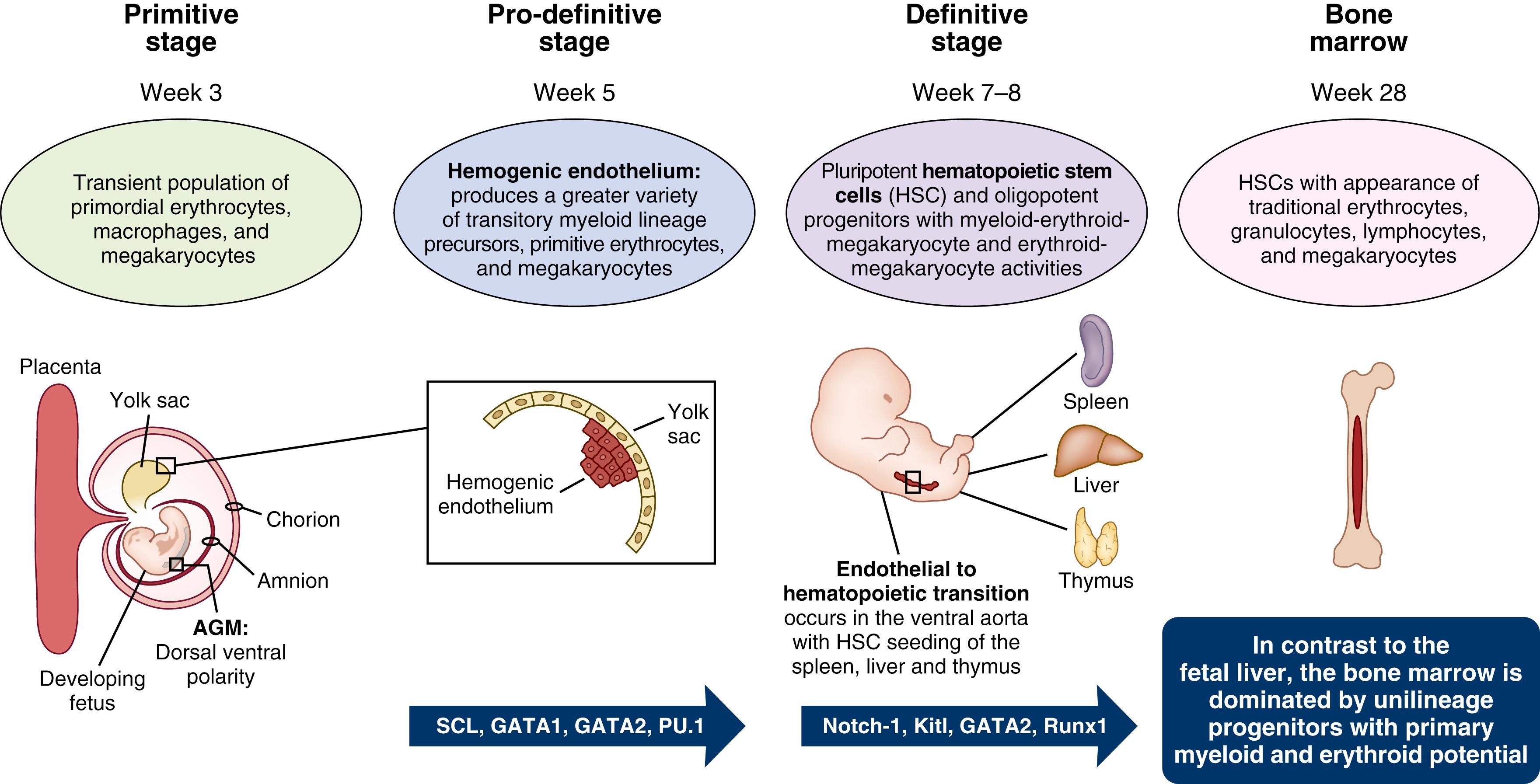
Within the distinctive hematopoietic niches of the dorsal aorta and yolk sac, the successful completion of EHT, as well as the survival and maturation (but not proliferation) of pre-HSCs, is dependent upon the production of Kit ligand (Kitl). Mice deficient for Kitl demonstrated critical reductions of yolk sac erythro-myeloid progenitors (EMPs), leading to severe anemia and late embryonic or early perinatal lethality due to a loss of fetal liver erythroid cells prior to HSC-derived erythropoiesis. , Notch-1 signaling , and GATA-2 transcription factor induction also function as essential regulators of EHT and subsequent HSC production, with inhibition of either signaling pathway resulting in serious disruptions of definitive hematopoiesis.
A common stem/progenitor pool, rather than two independent lineages of germinal and HSC cells, has also been proposed. Primordial germ cell (PGC) populations, which are first observed in the epiblast, and then within the allantois at the base of the yolk sac around the fifth week of fetal development, may link the traditional second and third stages of fetal hematopoiesis. PGCs will eventually settle in the aorta-gonad-mesonephros (AGM) region of the developing fetus. Here these cells maintain their differentiation plasticity and continue to undergo extensive proliferation and genome reprogramming as long as their expression of the transcription factor Oct3/4 remains low. PGCs destined to differentiate into gametes within the emerging gonads will ultimately express high levels of Oct3/4, while a subset of these mesodermal progenitors with continued low Oct3/4 concentrations will enter various lineages, including the hematopoietic pathway. PGCs destined to become pluripotent HSCs, therefore, show distinct expression of PGC markers (BLIMP-1, AP, TG-1, and STELLA), co-expressed proteins (CD34, CD41, and FLK-1), and genes ( Brachyury, Hox-B4, Scl/Tal-1, and GATA-2 ), but remain weakly positive for Oct3/4.
Irrespective of mechanisms by which HSCs come to occupy the AGM region of the ventral wall of the descending aorta, these self-renewing cells will subsequently come to seed the liver, thymus, and spleen, where hematopoiesis will continue until the third trimester of human pregnancy. , Within the fetal liver, specialized hematopoietic tissue-derived endothelial cells lining the walls of hepatic sinusoids, known as fetal liver sinusoidal endothelial cells (FLSECs) , secrete principal growth factors and Notch receptors to expedite HSC proliferation and expansion. In neonates less than 32 weeks’ gestational age (GA), fetal liver HSCs generate oligopotent progenitors with myeloid-erythroid-megakaryocyte and erythroid-megakaryocyte activities. During this developmental stage in mice and humans, myeloid lineages generally originate from HSC/multipotent progenitors (MPPs) via three intermediates including (1) a neutrophil-myeloid progenitor expressing CD34, SPINK2 (serine protease inhibitor Kazal-type 2), AZU1 (azurocidin), PRTN3 (proteinase 3), ELANE (neutrophil elastase), MPO (myeloperoxidase), and LYZ (lysozyme), (2) monocyte precursors, and (3) dendritic cell precursors. Although these early progenitors maintain a high proliferation potential, the fraction of fetal liver HSC/MPPs becoming quiescent steadily rises throughout fetal development. , This transformation occurs in conjunction with a surge in heat shock protein 1 (HSPA1A) expression, which may function to maintain cellular genome and proteome integrity. Concurrent to the surge in HSPA1A, MHC-1 (HLA-B) levels decline and may potentially cause impaired antigen-presenting potential in fetal liver–derived (compared with cord blood or adult bone marrow) HSC/MPPs. As fetal development progresses, hematopoietic composition of the fetal liver exhibits a coinciding shift away from predominately erythroid production, with a parallel escalation in the differentiation potential of HSCs/MPPs. By the end of term gestation, hematopoiesis transitions to the bone marrow, which becomes the only site for platelet, red, and white blood cell development during one’s lifetime. , The bone marrow, in contrast to the fetal liver, is dominated by unilineage progenitors with primary myeloid and erythroid potential.
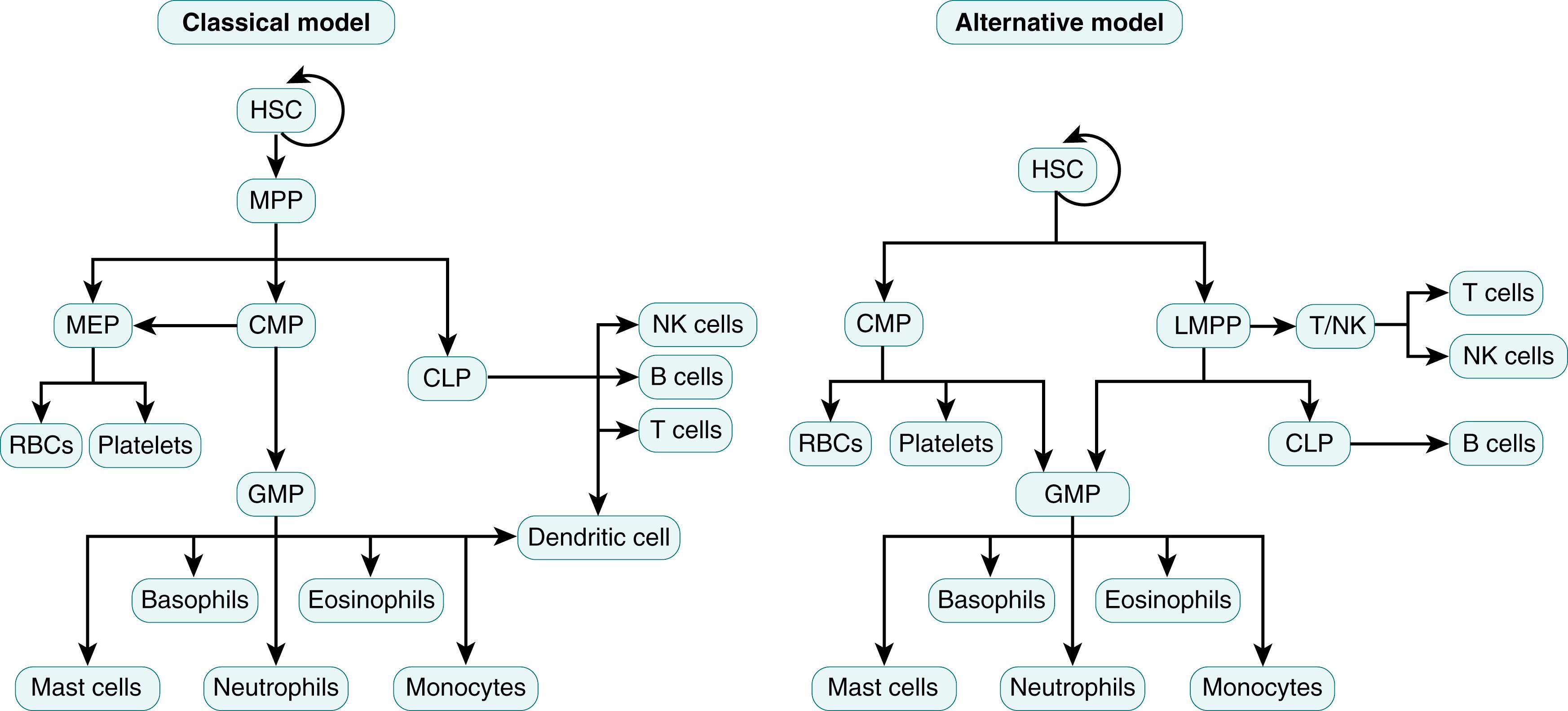
Even though in situ genetic labeling and barcoding of HSCs enable direct quantitative and qualitative measurements to illustrate the origins of blood cells from the common progenitor HSC, the initiating factors that determine whether a HSC will differentiate into a myeloid or lymphoid precursor remain controversial. In the “ classical” or “hierarchical model,” all HSCs have equal multi-lineage differentiation potential, but the fate of the HSC is predetermined prior to single lineage commitment and differentiation ( Fig. 108.2 ). In this model, bone marrow HSCs can transform into either common lymphoid progenitor (CLP) or a common myeloid progenitor (CMP), but after this transformation the cell loses its ability to differentiate into any other blood cell type. , CLP precursors will become either T or B lymphocytes, NK cells, or dendritic cells, while CMPs will differentiate into either granulocyte monocyte progenitors (GMPs) or megakaryocyte erythroid progenitors (MEPs). In contrast, the “ alternative model” postulates that common myeloid and lymphoid progenitor cells have mixed lineage potential with transcriptional and functional heterogeneity, and cell fate is principally determined by the availability of differentiation and survival factors ( Fig. 108.3 ). This model of HSC differentiation is supported by observations that HSCs can directly differentiate into CMPs, MEPs, megakaryocytes, or lymphoid-primed multipotent progenitors (LMPPs). In addition, CMPs and LMPPs can subsequently transform into CLPs or GMPs but lack the potential of becoming megakaryocytes or erythrocytes. ,
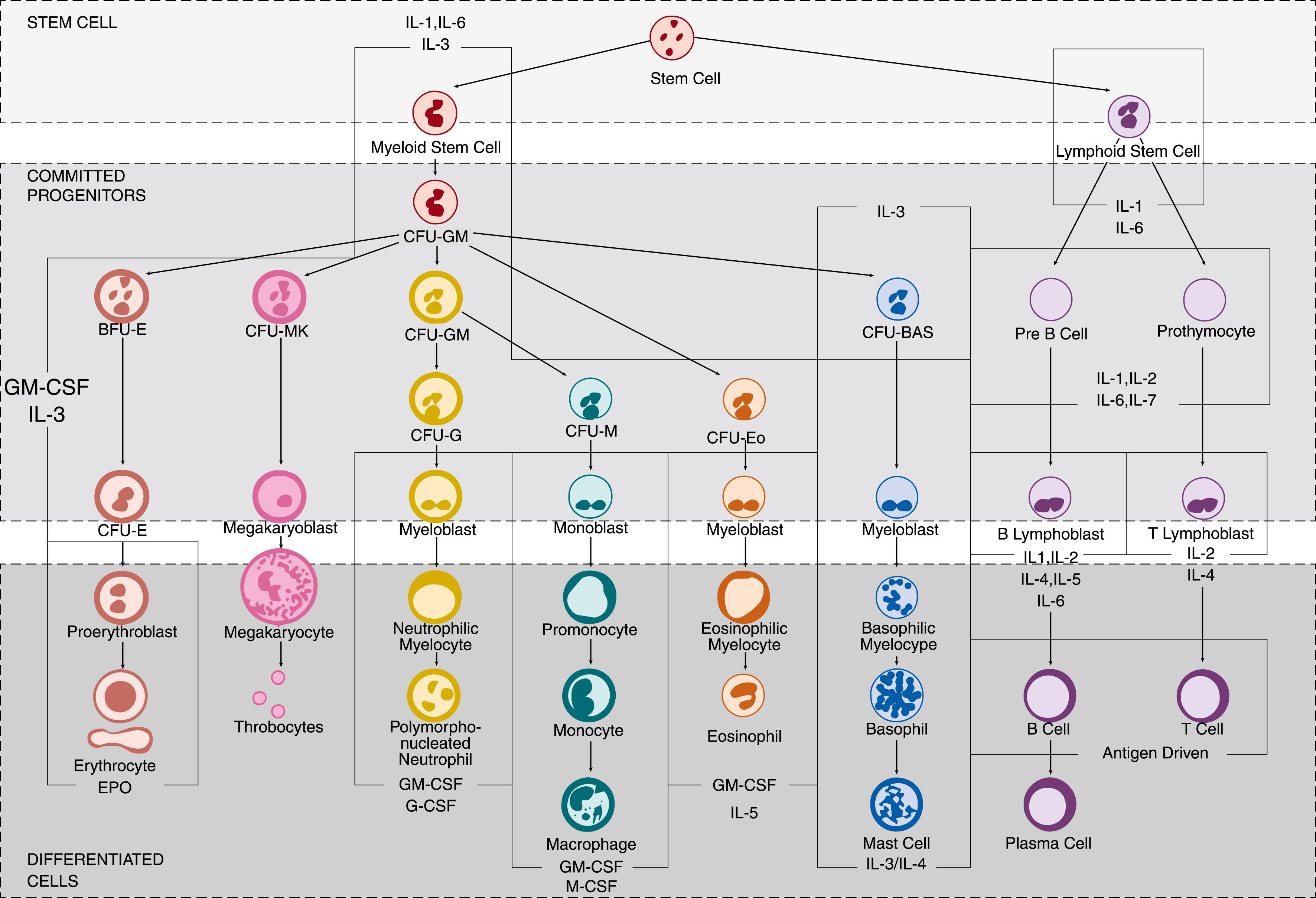
Irrespective of which hematopoietic pathway is correct, the differentiation of myeloid cells from HSCs is a well-described, closely regulated process dependent upon several transcription factors, including CCAAT/enhancer binding proteins (C/EBP), GATA-1, and PU.1. , C/EBPs consist of a family of six transcription factors (-α, -β, -γ, -δ, -ε, -ζ) that function to modulate numerous biologic processes involving cell motility, proliferation, differentiation, growth arrest, and cell death, in various tissues, including central nervous system, lung, adipose, and bone marrow. Once destined to become a myeloid cell, the HSC will develop into both megakaryocyte/erythroid and granulocyte/macrophage lineages from a pluripotent CMP cell. , Whereas C/EBP-α and PU.1 will induce CMPs to differentiate into monocytes and macrophages, C/EBP-ε and Gfi1 will generate neutrophils and eosinophils. , Differentiation of CMPs into neutrophils instead of eosinophils, however, ultimately depends upon the acetylation of C/EBP-ε at specific lysines (K121 and K198) and absence of GATA-1. , The importance of C/EBPs -α, -β, and -ε in neutrophil development is demonstrated by mutations of C/EBP-α and -β that lead to a variety of lymphocytic and myeloid leukemias, and absence of C/EBP-ε, which is associated with inhibition of neutrophil differentiation and maturation out of the progenitor compartment. In contrast, high levels of C/EBP-δ and PU.1 are observed only in mature and circulating neutrophils.
Posttranscriptional regulators of hematopoiesis are also necessary. For example, microRNAs (miRNAs), or small, non-coding, single-stranded RNA molecules measuring approximately 19 to 25 nucleotides in length, are involved in most aspects of human and murine development, cellular differentiation, and immune modulation. , miRNAs bind to messenger RNA and alter gene expression through inhibition of RNA degradation or blockage of translation. In early stages of hematopoiesis, an increase in miR-142-3p is necessary for myeloid lineage differentiation, while miR-16 is essential for erythropoiesis. Low levels of mi-R125b are also required for granulocytic differentiation and cell maturation following induction by granulocyte-colony stimulating factor (G-CSF) in both mice and humans. Proper regulation of granulopoiesis erythropoiesis and myelopoiesis is also facilitated by transcriptional control of miR-223 by C/EBP-β, NFI-A, PU.1, and C/EBP-α, with genetic deletion of miR-223 resulting in neutrophilia due to expanded myeloid progenitor precursors.
In humans, neutrophil precursors can be detected in the bloodstream by the end of the first trimester (10 to 11 weeks of gestation), while mature cells appear by 14 to 16 weeks of postmenstrual age. , The generation of neutrophils from HSCs occurs in specialized niches in the trabecular regions of long bones near the endosteum in close proximity to osteoblasts. , Retention of neutrophils within the bone marrow is dependent upon osteoblast and perivascular cell expression of the chemokine CXCL12, a ligand for the neutrophil cell membrane chemokine receptor CXCR4. As the neutrophil matures, simultaneous declines of CXCR4 levels and increased expression of the chemokine CXCL2, and its cell membrane receptor CXCR2, facilitate the neutrophil’s release from the bone marrow as the cell becomes less responsive to CXCL12. Neutrophils become available for release into the circulation after a transit time through the post-mitotic pool of 4 to 6 days. To exit the bone marrow, neutrophils must pass through the cell bodies of the endothelium, rather than through cell-cell junction, in a process known as transcellular migration . Conventional dendritic cells (cDCs) are also important regulators of neutrophil homeostasis between the bone marrow, bloodstream, and organs through controlled production of chemokines CXCL1, CCL2, and CXCL10 and growth factor G-CSF. Although the exact mechanism of their involvement remains unclear, depletion of cDCs in murine models causes a surge in neutrophil numbers, while cDC expansion leads to low circulating neutrophil numbers, or neutropenia, due to bone marrow neutrophil loss. ,
To summarize, neutrophil differentiation from progenitor cells involves an intricate interaction between modulators and transcription factors, including C/EBPs, PU.1, and Gfi1. Generation, regulation, and maintenance of HSCs is supported by osteoblasts and perivascular cells within dedicated niches within the trabecular regions of long bones and their secretion of growth factors, such as angiopoietin, thrombopoietin, and stem cell factor. A delicate interplay between production of CXCL12 by perivascular cells and osteoblasts and its receptor, CXCR4, on the neutrophil cell membrane confines developing neutrophils to the bone marrow. Declining CXCR4 levels during neutrophil maturation and increases in CXCL2/CXCR2, however, facilitate their release into the bloodstream. ,
Beginning between the myeloblast and promyelocyte stages of neutrophil development, granulopoiesis, or the formation of granules within the developing neutrophil, occurs over approximately 4 to 6 days ( Fig. 108.4 ). High concentrations of C/EBP-α and low amounts of PU.1 in myeloblasts favor granulocytic rather than monocytic differentiation. Other major transcription factors that regulate neutrophil granulopoiesis include Runx1, Gfi-1, and C/EBP-ε. In murine models, Runx1 plays a crucial role in HSC development in the AGM region, with fetal runx1 murine knockouts exhibiting impaired endothelial to EMP/HSC transition prior to liver colonization and subsequent blockage of myeloid hematopoiesis. , Deletion of the runx1 gene in adult mice also leads to primary monopoiesis at the expense of granulopoiesis. Bias towards myelopoiesis can also be induced via the transcription factor Trim 27, a zinc Ret finger protein (RFP), through upregulation of myeloid master genes Runx1 and Cbfb without expanding HSC baseline populations.
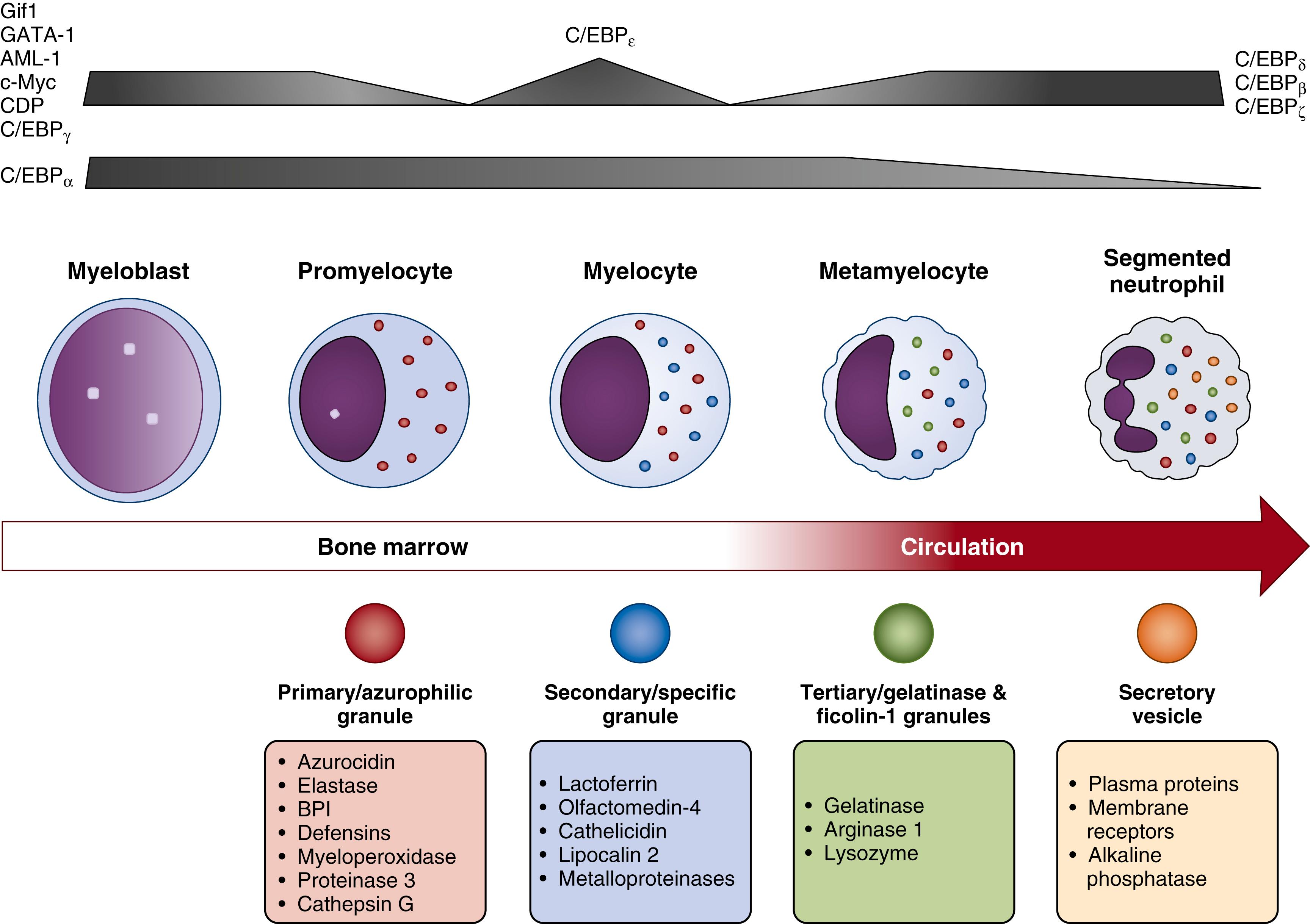
Gfi-1 is required to maintain the functional integrity of HSCs, with gfi-1 ablation in mice resulting in impaired HSC self-renewal capabilities. Gfi-1 also promotes neutrophil development and antagonizes monocyte development through repression of c-FOS, Egr-1, and Egr-2, and inhibition of the ERK1/2 signaling pathway. Gfi-1knockout in mice hinders neutrophil maturation at the promyelocyte stage, with cells exhibiting developmental failures of gelatinase granules and secretory vesicles. , Moreover, loss of gfi-1 causes expansion of GMP precursor cells with appearance of immature monocyte-like myeloid cells. The promyelocyte to myelocyte transition is also controlled by C/EBP-ε, an essential transcription factor for the formation of specific and gelatinase granules. Even though inhibition of C/EBP-ε does not affect initial neutrophil commitment, its absence impedes neutrophil maturation and terminal differentiation, leading to an aberrant differentiation of GMPs to the monocyte lineage. ,
Severe congenital neutropenia (SCN), or Kostmann syndrome, is a life-threatening congenital condition caused by defects in neutrophil production. Mainly characterized by recurrent invasive fungal and bacterial infections, SCN is also associated with a predisposition for acute myeloid leukemia and myelodysplastic syndrome, with a rate of malignant transformation generally exceeding 20%. , More than half of all SCN cases occur as secondary mutations in genes encoding neutrophil elastase ( ELA2) . SCN is, however, also associated with genetic defects in HCLS1 associated protein X-1 ( HAX1) , GIF1, or inhibition of secretory leukocyte protease inhibitor (SLPI). Many causes of SCN originate at the earliest phases of GMP commitment to neutrophil differentiation, or at the myeloblast-to-promyelocyte maturational stage. Defects of ELA2 , for example, lead to neutrophil elastase mis-folding, prompting its intracellular accumulation and mis-localization. This anomaly subsequently causes endoplasmic reticulum stress and induction of apoptosis through activation of the unfolded-protein response (UPR). For cases resulting from SLPI disruption, G-CSF phosphorylation of STAT5, ERK1/2, and lymphoid enhancer-binding factor-1 (LEF-1) is blocked in bone marrow myeloid progenitor cells, leading to downstream inhibition of LEF-1 target genes that are essential for neutrophil survival and proliferation, such as c-myc, survivin, and cyclin D1 . , LEF-1 also induces C/EBP-α binding to the SLPI promotor to boost SLPI gene expression and neutrophil differentiation. Inhibition or loss of LEF-1 blocks neutrophil maturation and proliferation, even though the production of red blood cells and monocytes remain unhindered due to a decline in C/EBP-α and heightened c-Myc levels. The HAX1 gene, on the other hand, directly controls mitochondrial proteases that regulate the accumulation of BL-2-associated X protein (BAX), a proapoptotic protein in the outer mitochondrial membrane. Mitochondrial functions, indispensable for cell survival, are abrogated when cytoplasmic levels of BAX exceed that of their anti-apoptotic counterparts MCL-1. This facilitates BAX oligomerization and the formation of pores in the outer mitochondrial membrane, which enables cytochrome c release into the cytoplasm and cell death through caspase activation via the intrinsic cell death pathway .
To summarize, the transformation of CMPs to GMPs is dependent upon the suppression of c-Myc by the differentiation factor C/EBP-α. Conversely, c-Myc is primarily involved in progenitor cell proliferation, which if silenced will result in the absence of all hematopoietic lineages except megakaryocytes. Failure to appropriately repress the c-myc gene at the correct maturational state may, therefore, elicit the development of myeloid leukemias.
An estimated 10 11 neutrophils, or 10 9 cells/kg of body weight, are generated every day to maintain neutrophil homeostasis, or the delicate balance between granulopoiesis, bone marrow storage and release, intravascular margination, and migration into peripheral tissues. Neutrophil production in the bone marrow involves five main stages of differentiation, beginning with (1) myeloblasts that maintain proliferative capabilities for nearly 1 week prior to maturation, (2) promyelocytes/myelocytes, (3) metamyelocytes, (4) band forms, and (5) segmented neutrophils. The regulation of neutrophil production has been described in terms of steady-state versus emergency granulopoiesis and is directly dependent upon, and impacts, the individual’s health.
In steady state, human neutrophils are short-lived cells with an estimated half-life of approximately 19 hours. Aged and dying neutrophils are continually replenished by mature, segmented neutrophils, located within bone marrow reserve pools. , Turnover and replenishment of quiescent neutrophils follows the ingestion of apoptotic cells by tissue macrophages, which triggers the transcription of C/EBP-α and factors of the LXR family to suppress proinflammatory cytokine production and, in turn, diminish G-CSF levels. , Under baseline conditions in humans and mice, the transformation of a promyelocyte to mature, segmented neutrophil is accompanied by a drastic decline in transcripts and proteins related to general cellular processes, including DNA replication and repair, RNA transcription and processing, protein translation, and mitochondrial energy metabolism. , , Concurrent with this decline in cellular proteins is a drop in cell-cycle, transcriptional, mitochondrial, and metabolic activity. Despite an overall reduction in transcription and translation, components related to immune system functions and vesicle transport processes are preserved or become upregulated, including those necessary for cellular response to microbial stimuli, G-protein cell receptor (GPCR) signaling to facilitate chemotaxis, and cytoskeletal rearrangements to enable direct motility. , Finally, only modest contributions are made in histone modifications and epigenetic profiles during neutrophil development.
In contrast, emergency granulopoiesis is activated following a microbial or inflammatory challenge and results a surge of both immature and mature neutrophil forms from bone marrow storage pools into the peripheral circulation. This process leads to a clinical neutrophilia with a “left-shift,” or increased number of bloodstream neutrophils composed of a greater proportion of immature forms. This neutrophilia occurs in response to host-induced mechanisms (i.e., induction of C/EBP-β and early inflammatory mediators, such as interleukin [IL]-1β, tumor necrosis factor [TNF]-α, G-CSF, and GM-CSF) and pathogen-mediated factors, such as lipopolysaccharide (LPS) and other microbial products. ,
The abrupt biologic switch from steady-state to emergency granulopoiesis requires high energy demands, which have only recently been elucidated using murine models of Gram-negative infection. Although anaerobic glycolysis is favored by quiescent HSCs, exposure to host or pathogen inflammatory or infectious etiologies quickly shifts metabolic demands to mitochondrial oxidative phosphorylation. This transformation is facilitated by the transfer of mitochondria from bone marrow stromal cells (BMSCs) directly to specific HSCs, targeted to undergo proliferation and differentiation. This energy switch is triggered by elevations in reactive oxygen species (ROS) levels in response to infection-associated stressors. Mitochondrial transfer from BMSC to HSCs is controlled by phosphoinositide 3-kinase (PI3K) signaling and transpires through connexin 43 gap junctions. This interplay between cells of the bone marrow microenvironment, therefore, enables pluripotent HSCs to rapidly cycle and differentiate into key innate immune cells, including neutrophils, without being subjected to critical delays related to intracellular activation of mitochondrial biogenesis.
The detection of conserved pathogen-associated molecular patterns (PAMPs) by host pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), may unite the steady-state and emergency granulopoietic pathways. Under routine conditions, the interplay between direct or indirect activation of PRRs on HSCs and/or progenitor cells may function to maintain steady-state granulopoiesis. Following acute infection or inflammation, however, increased activation of PRRs on HSCs and HSC exposure to proinflammatory chemokines, including keratinocyte chemoattractant (KC), macrophage inflammatory protein (MIP)-2, G-CSF, and TNF-α, , , act as powerful stimulators for neutrophil proliferation and differentiation. Moreover, induction of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase by bone marrow myeloid cells boosts the production of ROS, which acts locally to trigger oxidation and deactivation of phosphate and tensin homolog (PTEN) in resident myeloid cells, leading to increased levels of G-CSF, upregulation of PtIns(3,4,5) P3 signaling, and induction of emergency granulopoiesis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here