Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neurologists have traditionally attributed neurologic symptoms and signs to lesions in particular areas of the central nervous system (CNS) or peripheral nervous system (PNS), and have used imaging studies, such as computed tomography (CT) and magnetic resonance imaging (MRI), to confirm their clinical localization. Another way to envision neurologic illnesses is in terms of disruption of neurotransmitters. This chapter reviews the synthesis, metabolism, and anatomic pathways of the most important neurotransmitters, and their altered activity in common neurologic disorders:
Monoamines:
Catecholamines: dopamine, norepinephrine, and epinephrine
Indolamines: serotonin
Histamine
Acetylcholine
Neuropeptides:
Inhibitory amino acids: gamma-aminobutyric acid (GABA), glycine
Excitatory amino acids: glutamate
Nitric oxide
Neurologists hold dopamine synthesis in preeminent regard and treasure each substrate, enzyme, and byproduct. They capitalize on its synthesis and storage, and may inhibit its breakdown or reuptake, when they treat Parkinson disease and several other neurologic illnesses. Conversely, psychiatrists base treatment of many disorders partly or predominantly on blocking dopamine receptors.
Dopamine synthesis, which takes place entirely within the presynaptic neuron, begins with the amino acid phenylalanine, and proceeds sequentially through tyrosine, dihydroxyphenylalanine (DOPA), and then dopamine. Tyrosine hydroxylase , which hydroxylates tyrosine to form DOPA , is the rate-limiting enzyme in this pathway. Another important enzyme is DOPA decarboxylase , which decarboxylates DOPA to form dopamine. That same enzyme acts on both naturally occurring DOPA and the Parkinson disease medicine, levodopa (L-dopa). In addition, decarboxylase is crucial in the synthesis of other monoamine neurotransmitters.
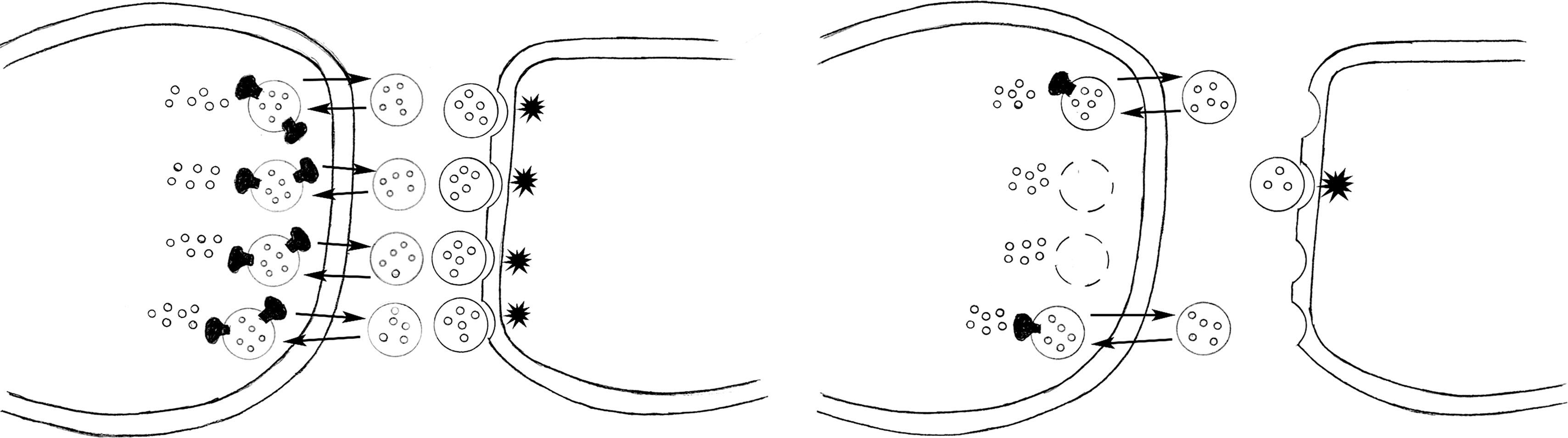
Within the presynaptic neuron, the protein vesicular monoamine transporter-2 (VMAT2) carries dopamine and other monoamines into vesicles. Positioned in the presynaptic side of the cleft, the vesicles protect the neurotransmitters from intracellular metabolic enzymes and position them for discharge as discrete quanta.
After neurons release dopamine, a transmembrane protein, dopamine transporter (DaT) draws dopamine back from the synaptic cleft into the cell. However, in Parkinson disease and other conditions where presynaptic dopamine neurons degenerate, dopamine synthesis stops and DaT is lost. In contrast, in medication-induced parkinsonism, dopamine synthesis continues and DaT remains. A recently developed nuclear medicine technique identifies the presence or absence of DaT to assist physicians in distinguishing the idiopathic, neurodegenerative form of Parkinson disease, where DaT is absent, from medication-induced parkinsonism, where it is present (see Chapters 18 and 20 ).
Although reuptake is the primary way that cells terminate dopamine activity, two different enzymes metabolize dopamine: catechol-O-methyltransferase (COMT) , mostly an extracellular enzyme, and monoamine oxidase-B (MAO-B) , mostly an intracellular enzyme. Certain medicines for Parkinson disease and related illnesses preserve dopamine by inhibiting these metabolic enzymes (see later).
Dopamine metabolism by COMT and MAO-B yields homovanillic acid (HVA ). Cerebrospinal fluid (CSF) concentration of HVA corresponds roughly to dopaminergic activity in the brain.
Three “long dopamine tracts” hold the greatest clinical importance in neurology and psychiatry:
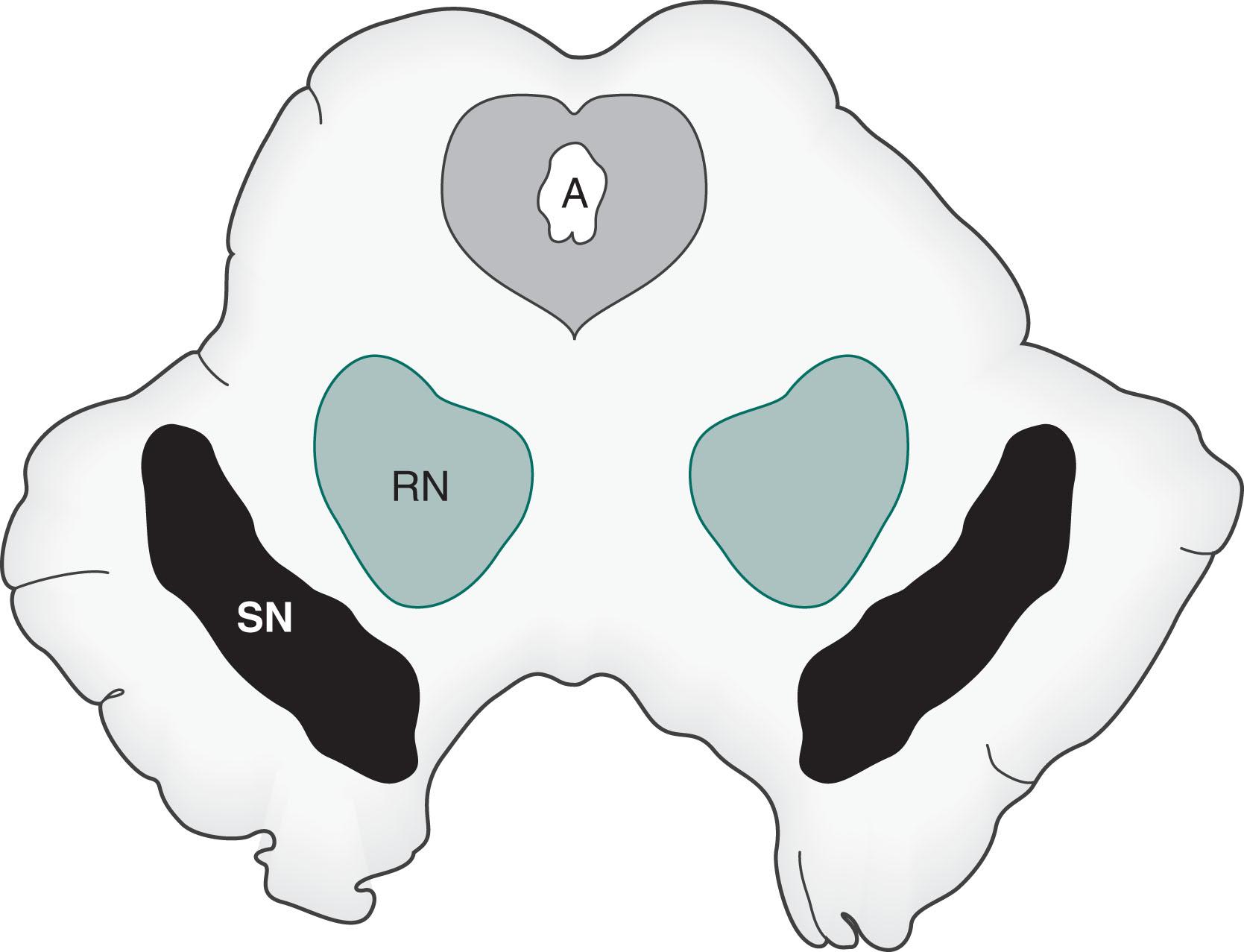
The nigrostriatal pathway , a major component of the extrapyramidal motor system, carries most of the dopamine in the brain. This tract projects from the substantia nigra, the crescentic pigmented nuclei in the midbrain ( Fig. 21.1 ), to the striatum (the caudate nucleus and putamen [see Chapter 18 ]).
The mesolimbic pathway projects from the ventral tegmental area, situated in the inferior medial portion of the midbrain, to the amygdala and other portions of the limbic system. Its receptors are predominantly D4. The mesolimbic tract appears to underlie the positive symptoms of psychosis; many antipsychotic agents block dopamine transmission in this tract, thereby reducing dopamine activity in the limbic system and thus suppressing psychotic symptoms.
The mesocortical pathway also projects from the ventral tegmental area, but it terminates primarily in the frontal cortex. It also terminates in the cingulate and prefrontal gyrus, creating an overlap with the mesolimbic system. The mesocortical tract likely underlies negative symptoms of psychosis. Because illicit drugs affect both the mesolimbic and mesocortical tracts, these tracts also serve as neural substrates for drug addiction.
In addition to these long tracts, several “short dopamine tracts” have clinical significance. The tubero-infundibular tract connects the hypothalamic region with the pituitary gland. Dopamine in the tubero-infundibular tract suppresses prolactin secretion and thus inhibits galactorrhea. As a corollary, blockade of the tubero-infundibular tract promotes galactorrhea, as seen with many antipsychotic agents. Another short tract exists within the retina.
Although neuroscientists have identified many dopamine receptors, the most important ones are the D1, D2, and closely related receptors. The D1 receptor group includes both the D1 and D5 receptor subtypes. The D2 receptor group includes the D2, D3, and D4 receptor subtypes. These dopamine receptors are coupled to guanine nucleotide-binding proteins (G proteins). Because they exert their effects through second messengers, such as cyclic adenosine monophosphate (AMP), neuroscientists consider them “slow” neurotransmitters. The scientists Brian K. Kobilka and Robert J. Lefkowitz, who elucidated G protein-coupled receptors, received the Nobel Prize in Chemistry in 2012.
The D1 and D2 receptors represent the most important ones in extrapyramidal disorders and psychosis ( Table 21.1 ). With several exceptions, the effectiveness of antipsychotic agents hinges on their ability to block D2 receptors. Clozapine and quetiapine, in contrast, bind more strongly to D1 than D2 receptors. Some second-generation antipsychotic agents, such as quetiapine, risperidone, and ziprasidone, block serotonin as well as dopamine receptors, but aripiprazole and brexpiprazole stimulate D2 and one serotonin receptor while blocking another.
| D1 | D2 | |
|---|---|---|
| Effect of stimulation on cyclic AMP production | Increased | Decreased |
| Greatest concentrations | Striatum, limbic system, cerebral cortex | Striatum, substantia nigra |
| Effect of dopamine | Weak agonist | Strong agonist |
| Effect of phenothiazines | Strong antagonist | Strong antagonist |
| Effect of butyrophenones | Weak antagonist | Strong antagonist |
| Effect of clozapine | Weak antagonist | Weak antagonist |
When antipsychotics block D2 receptors, the reduced dopamine activity potentially induces parkinsonism, raises prolactin production (inducing galactorrhea), and places patients at risk for tardive dyskinesia. Some atypical antipsychotic agents, particularly risperidone and its active metabolite paliperidone (Invega), raise prolactin serum concentration and induce galactorrhea. Some antiemetic medications, such as metoclopramide (Reglan) and prochlorperazine (Compazine), also can block D2 receptors, increase serum prolactin concentration, and induce parkinsonism. On the other hand, aripiprazole, clozapine, and ziprasidone reduce or only slightly increase prolactin concentrations.
Physicians should bear in mind that finding an elevated serum prolactin level does not always indicate use of a dopamine-blocking medication or a pituitary tumor (see Chapter 19 ). The most common cause of serum prolactin elevation is pregnancy. Generalized tonic-clonic seizures raise serum prolactin concentrations in the postictal period. Detection of a prolactin elevation helps distinguish these seizures from psychogenic nonepileptic seizures (see Chapter 10 ).
Deficiencies in dopamine synthesis enzymes underlie several well-known neurologic disorders. A genetically determined absence of phenylalanine hydroxylase , the initial enzyme in catecholamine synthesis, leads to phenylketonuria (PKU) (see Chapter 13 ). A multifactorial genetic deficiency of a cofactor for both phenylalanine hydroxylase and tyrosine hydroxylase leads to dopa-responsive dystonia (DRD) (see Chapter 18 ). Although studies have not yet established the definitive basis of restless legs syndrome, dopamine agonists are an effective treatment (see Chapter 17 ).
In Parkinson disease, the progressive degeneration of dopamine-synthesizing neurons in the substantia nigra leads to increasingly severe dopamine deficiency. As the disease progresses, the presynaptic neurons degenerate and can no longer synthesize, store, and appropriately release dopamine. Neurologists treating Parkinson disease patients attempt to enhance dopamine activity in primary ways:
They administer the dopamine precursor levodopa. As long as enough nigrostriatal (presynaptic) neurons remain intact, which is generally the case during the first 5 years of the illness, DOPA decarboxylase converts levodopa to dopamine in sufficient quantities to treat the symptoms (see Fig. 18.3 ).
As the disease progresses, the presynaptic neurons degenerate and can no longer synthesize, store, and appropriately release dopamine. At that time, if not as a first-line treatment, neurologists may prescribe a dopamine agonist , like pramipexole, ropinirole, or rotigotine, to stimulate dopamine receptors.
Medications that inactivate enzymes that metabolize levodopa enhance dopamine activity (see Fig. 18.12 ). Carbidopa inactivates DOPA decarboxylase. Entacapone and opicapone inhibit COMT, which normally inactivates levodopa by converting it to 3-O-methyldopa. These enzyme inhibitors act almost entirely outside the CNS because they have little ability to penetrate the blood-brain barrier. Administering these enzyme-inhibitor medicines along with levodopa enables lower doses of levodopa to be effective. Another therapeutic option entails protecting dopamine from MAO-B. To enact this strategy, neurologists prescribe selegiline (Eldepryl), rasagiline (Azilect) or safinamide (Xadago), which are inhibitors of MAO-B.
In contrast to Parkinson disease, where presynaptic neurons have degenerated, medication-induced blockade of basal ganglia D2 receptors causes parkinsonism. This distinction holds great clinical importance when a patient who is under treatment with an antipsychotic agent develops Parkinson disease-like symptoms. Giving levodopa at the same time as antipsychotic agents that block D2 receptors may still increase dopamine levels, but the dopamine will not stimulate its receptors or correct the symptoms. More important, excess dopamine may over-stimulate frontal cortex and limbic system dopamine receptors to provoke or exacerbate psychosis. Thus, when a patient who has been treated with an antipsychotic agent—even for as briefly as 1 month—appears to have developed Parkinson disease, physicians should generally maintain that the patient has iatrogenic parkinsonism; search for alternative diagnoses that can cause both psychosis and parkinsonism, such as dementia with Lewy bodies or Wilson disease; and avoid administering medicines that enhance dopamine. A DaT scan greatly helps distinguish idiopathic from iatrogenic parkinsonism (see Chapter 18 ).
An acute absence of dopamine activity causes the parkinsonism-hyperpyrexia syndrome, which neurologists still call neuroleptic malignant syndrome (NMS) (see Chapter 6 ). This occurs most commonly as an adverse effect of dopamine receptor-blocking agents (especially neuroleptics) but may also follow abrupt withdrawal or reduction of dopaminergic anti-Parkinson’s medication. Cardinal features include confusion, fever, muscle rigidity with elevated creatine kinase (CK), and autonomic instability. Administering dopamine agonists, such as bromocriptine, may compensate for the absence of dopamine activity and alleviate some of the symptoms, but treatment is generally supportive.
Of the several mechanisms that might lead to excessive dopamine activity, the most common is the administration of levodopa. Stimulants, such as cocaine, also increase dopamine activity, if only in a burst, by provoking dopamine release from its presynaptic storage sites, blocking its reuptake, or both (see later). Some psychiatric medications, such as bupropion (Wellbutrin), also block dopamine reuptake. In a different mechanism that possibly underlies tardive dyskinesia and Tourette’s disorder, increased sensitivity of postsynaptic dopamine receptors results in an amplified response to dopamine.
Whatever the cause, excessive dopamine activity produces symptoms ranging from psychosis to hyperkinetic movement disorders. Studies have long suggested that excessive dopamine activity causes psychosis. In a related example, Parkinson disease patients taking levodopa develop visual hallucinations, paranoia, and thought disorders that can reach psychotic proportions. Excessive dopamine activity also produces hyperkinetic movement disorders, such as chorea, tremor, tics, dystonia, and tardive dyskinesia.
As a less dramatic example of the effects of excessive dopamine, some Parkinson disease patients become overly involved with stimulating activities, such as sex and gambling. In these cases, neurologists diagnose the dopamine dysregulation syndrome or impulse control disorder (see Chapter 18 ). They often ascribe the aberrant behavior to dopamine-induced novelty seeking and inattention. On the other hand, with a small increase in dopamine activity from levodopa, individuals enjoy a temporary sense of well-being, a sensation that is a glimmer of a cocaine or amphetamine rush.
In addition to their effects on movement, dopamine and its agonists, acting through the tubero-infundibular tract, inhibit prolactin release from the pituitary gland. Neurologists and endocrinologists may prescribe bromocriptine or cabergoline, both dopamine agonists, to shrink and inactivate prolactinomas. In the opposite situation, when typical or atypical dopamine receptor-blocking neuroleptics—but not clozapine (Clozapine) or quetiapine (Seroquel)—block tubero-infundibular tract receptors, they enhance prolactin release. Patients taking these medicines often report decreased sexual drive and even galactorrhea.
Hyperkinetic movement disorders thought to arise from an excess or imbalance in dopamine activity include oral-buccal-lingual dyskinesias from chronic antipsychotic use, chorea of Huntington disease, and motor tics in Tourette’s disorder. Neurologists treat these with medicines that reduce dopamine activity, resulting in a reduction of these involuntary movements ( Fig. 21.2 ). These medicines—tetrabenazine (Xenazine), deutetrabenazine (Austedo), and valbenazine (Ingrezza)—inhibit VMAT2 and thereby prevent the accumulation of dopamine in the presynaptic vesicles. Because they deplete dopamine stores, potential side effects include parkinsonism, depression, suicidality, and very rarely NMS.
After the catecholamine synthesis pathway yields dopamine, it goes on to yield norepinephrine and subsequently epinephrine. As with dopamine synthesis, tyrosine hydroxylase remains the rate-limiting enzyme. Also, as with dopamine, reuptake and metabolism by COMT and MAO terminate their actions. However, because the MAO-A form of MAO metabolizes norepinephrine, most norepinephrine metabolism takes place outside the CNS. Norepinephrine’s primary metabolic byproduct, which appears in the urine, is vanillylmandelic acid (VMA) .
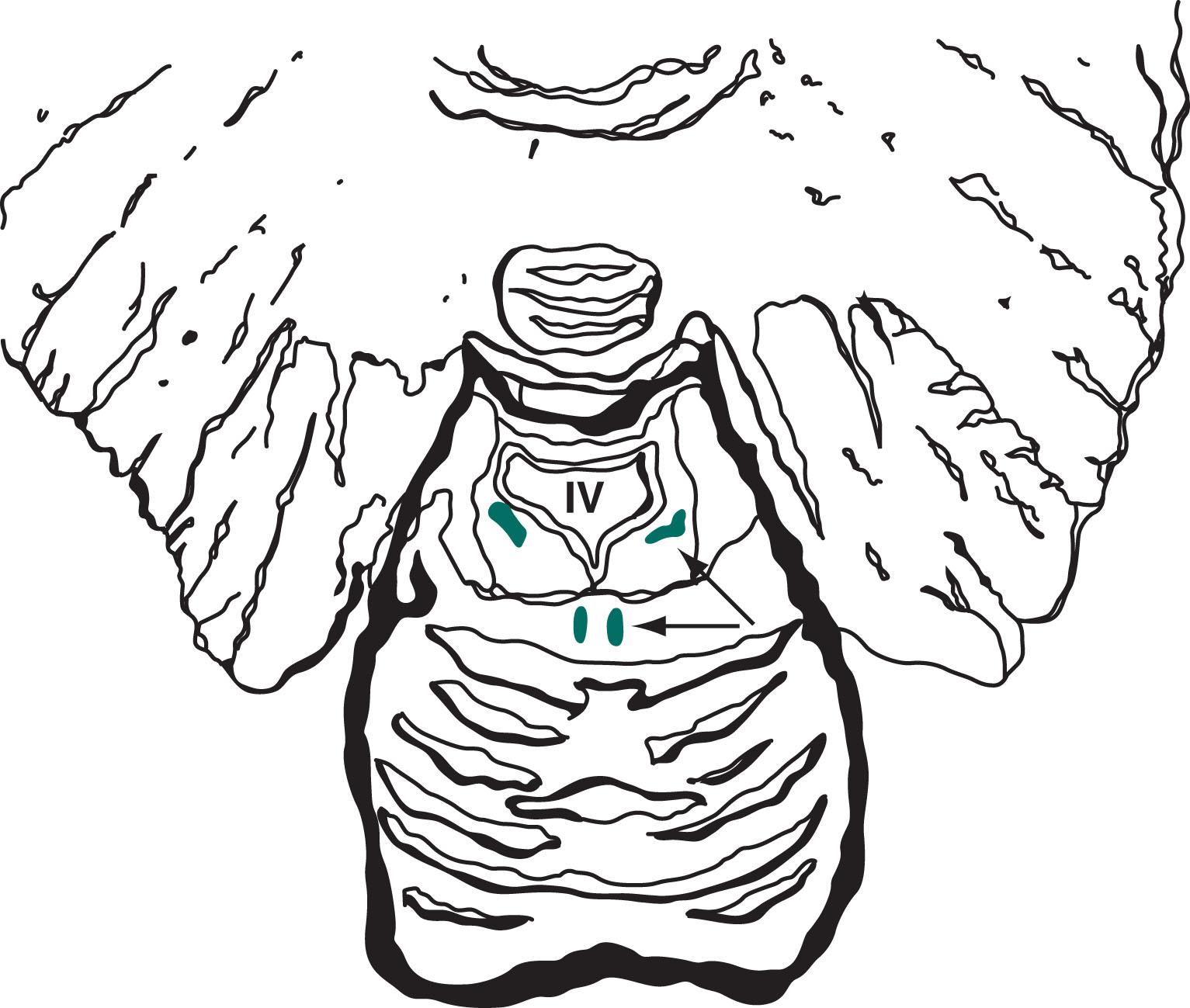
CNS norepinephrine synthesis takes place primarily in the locus ceruleus , which is located in the dorsal portion of the pons ( Fig. 21.3 ). Neurons from the locus ceruleus project to the cerebral cortex, limbic system, and reticular activating system. In addition, whereas dopamine tracts remain confined to the brain, norepinephrine tracts project down into the spinal cord.
Unlike dopamine, norepinephrine serves as the neurotransmitter for the sympathetic nervous system’s postganglionic neurons. In the adrenal gland, a pathway converts norepinephrine (noradrenaline) to epinephrine (adrenaline).
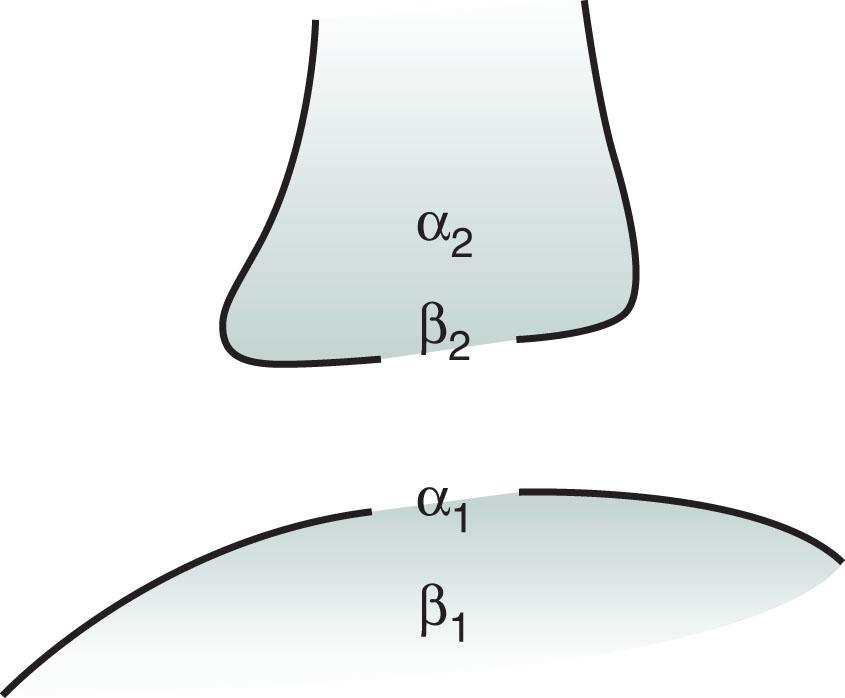
Norepinephrine receptors are located in the cerebral cortex, brainstem, and spinal cord. The α 2 and β 2 receptors, termed “autoreceptors,” are situated on presynaptic neurons. Through a feedback mechanism, these presynaptic receptors modulate norepinephrine synthesis and release ( Fig. 21.4 ). Postsynaptic receptors are also of two varieties, α 1 and β 1 , and they produce varied and sometimes almost opposite effects ( Table 21.2 ).
| Receptor a | Effect of Stimulation | Agonists | Antagonists |
|---|---|---|---|
| Presynaptic | |||
| α 2 | Vasodilation, hypotension | Clonidine | Yohimbine |
| β 2 | Bronchodilation | Isoproterenol | Propranolol |
| Postsynaptic | |||
| α 1 | Vasoconstriction | Phenylephrine | Phenoxybenzamine, phentolamine |
| β 1 | Cardiac stimulation | Dobutamine | Metoprolol |
a See Fig. 21.4 .
In Parkinson disease, the locus ceruleus, just like the substantia nigra, degenerates and loses its pigment. Loss of norepinephrine typically leads to orthostatic hypotension, sleep disturbances, and depression. Droxidopa (Northera), which the body metabolizes to norepinephrine, is useful for treatment of symptomatic orthostatic hypotension in Parkinson disease and related illnesses.
In the opposite situation, excessive stimulation of β 2 adrenergic sites leads to tremor and bronchodilation. For example, isoproterenol and epinephrine, particularly when used for asthma, cause tremor as they alleviate the bronchospasm. Conversely, β-blockers, such as propranolol, which are contraindicated in asthma patients, suppress essential tremor, stage fright, and, to a certain extent, social anxiety (see Chapter 18 ).
Pheochromocytomas secrete norepinephrine or epinephrine that cause hypertension, tachycardia, and sometimes convulsions. A similar response—the tyramine reaction—could occur when patients who are taking an MAO-A inhibitor (or an excessive dose of an MAO-B inhibitor) consume foods or wine that contain tyramine.
Some evidence supports the use of two norepinephrine agonists—clonidine and guanfacine—for the treatment of Tourette’s disorder and opioid withdrawal symptoms. In the case of Tourette’s disorder, the mechanism of action is unclear. Because these medicines’ primary use has been as antihypertensives, hypotension remains one of their primary side effects.
Serotonin (5-hydroxytryptamine, 5-HT) is another monoamine, but an indolamine rather than a catecholamine. Serotonin synthesis parallels dopamine synthesis: hydroxylation then decarboxylation. However, the rate-limiting factor in serotonin synthesis is the concentration of tryptophan rather than the hydroxylase enzyme. As with dopamine, VMAT2 packages serotonin into vesicles for protection and storage. Similarly, after discharge into the synaptic cleft, reuptake and, to a lesser extent, oxidation terminate serotonin’s activity.
One metabolic pathway converts serotonin to melatonin, which is also an indolamine. In another pathway, MAO-A metabolizes serotonin to 5-hydroxyindoleacetic acid (5-HIAA). Thus, MAO-A metabolizes both norepinephrine and serotonin, which are thought to be deficient in depression. Many antidepressants exert their benefit by inhibiting reuptake of serotonin and/or norepinephrine. Inhibitors of MAO-A are antidepressants, in part, because they preserve these two neurotransmitters. Selegiline, the first MAO-B inhibitor, not only preserves dopamine, but it also acts like an MAO-A inhibitor when given in high enough dosage. Selegiline has an antidepressant effect because it preserves serotonin, and its own metabolism yields amphetamine and methamphetamine.
Although platelets, gastrointestinal cells, and other non-neurologic cells synthesize more than 98% of the body’s total serotonin, their serotonin does not penetrate the blood-brain barrier. Thus, CSF concentrations of HIAA reflect only CNS serotonin activity.
Serotonin-producing CNS neurons reside predominantly in the dorsal raphe nuclei , which are located in the midline of the dorsal midbrain and pons (see Figs. 21.3 and 18.2 ). Serotonin tracts project rostrally (upward) to innervate the cortex, limbic system, striatum, and cerebellum. They also innervate intracranial blood vessels, particularly those around the trigeminal nerve.
Another serotonin-producing center, the caudal raphe nuclei , is located in the midline of the lower pons and medulla. It projects caudally (downward) to the dorsal horn of the spinal cord to alleviate pain (see Chapter 14 ).
Studies have identified several CNS serotonin receptors (5HT 1 –5HT 7 ) and many subtypes. Serotonin receptors differ in their function, response to medications, effect on second-messenger systems, and excitatory or inhibitory capacity. Several serotonin receptors, such as 5-HT 1D , are presynaptic autoreceptors that suppress serotonin synthesis or block its release. In an almost ironic relationship, 5HT 1 promotes production of adenyl cyclase and is inhibitory, but 5HT 2 promotes production of phosphatidyl inositol and is excitatory. Other serotonin receptors are usually G protein-linked and excitatory.
Serotonin plays a major role in the daily sleep-wake cycle. The activity of serotonin-producing cells reaches its highest level during arousal, drops to quiescent levels during slow-wave sleep, and disappears during rapid eye movement (REM) (see Chapter 17 ).
Low serotonin activity is associated with depression. In one of the most consistent findings in biologic psychiatry, low postmortem CSF concentrations of HIAA, the major serotonin metabolite, characterize suicides by violent means. Similarly, individuals with poorly controlled violent tendencies, even those without a history of depression, have low concentrations of CSF HIAA. Serotonin levels are also low in Parkinson and Alzheimer diseases. Among Parkinson disease patients, the decrease is more pronounced in those with comorbid depression.
Another problem that occurs among Parkinson patients is the development of visual hallucinations. This complication usually develops among older patients with long-standing illness and cognitive impairment. Pimavanserin (Nuplazid) suppresses these hallucinations by acting as an inverse agonist and antagonist of serotonin. (An inverse agonist reduces the basal activity of a receptor, whereas an antagonist prevents agonists from activating the receptor.) For unclear reasons, unexpected deaths have occurred with use of pimavanserin in patients with dementia-related psychosis unrelated to hallucinations and delusions associated with Parkinson disease psychosis.
Sumatriptan and other triptans, a mainstay of migraine therapy, are agonists to 5-HT 1B and 5-HT 1D serotonin receptors on nerve endings and blood vessels. Once stimulated, these serotonin receptors inhibit the release of pain-producing vasoactive and inflammatory substances from trigeminal nerve endings in the brain. Despite a theoretical fear of inducing serotonin syndrome (see Chapters 6 and 18 ) by prescribing a triptan to a patient who is taking a serotonin reuptake inhibitor, the number of such adverse reactions is miniscule.
Sumatriptan and other triptans, a mainstay of migraine therapy, are agonists to 5-HT 1B and 5-HT 1D serotonin receptors on nerve endings and blood vessels. Once stimulated, these serotonin receptors inhibit the release of pain-producing vasoactive and inflammatory substances from trigeminal nerve endings in the brain. Despite a theoretical fear of inducing serotonin syndrome (see Chapters 6 and 18 ) by prescribing a triptan to a patient who is taking a serotonin reuptake inhibitor, the number of such adverse reactions is miniscule.
A class of antiemetic agents, including granisetron (Kytril) and ondansetron (Zofran), are serotonin antagonists. By affecting 5HT 3 receptors in the medulla’s area postrema , one of the few areas of the brain unprotected by the blood-brain barrier, they reduce chemotherapy-induced nausea and vomiting. Similarly, second-generation antipsychotics typically act as serotonin antagonists (at 5HT 2A ) as well as D2 receptors.
Although increased serotonin activity is often therapeutic, excessive activity poses a danger. For example, combinations of medicines that simultaneously block serotonin reuptake and inhibit its metabolism lead to toxic serotonin concentrations and serotonin syndrome (see Chapters 6 and 18 ).
In another dangerous situation characterized by excessive serotonin activity, LSD (D-lysergic acid diethylamide), mescaline, and psilocybin induce hallucinations and euphoria by excessive stimulation of serotonin receptors. Similarly, “ecstasy” (methylenedioxymethamphetamine [MDMA]) sti-mulates dopaminergic activity while it excessively stimulates serotonin activity by provoking an outpouring from its presynaptic neuron. Ecstasy actually surpasses LSD in stimulating serotonin activity.
As with many other monoamine neurotransmitters, histamine is produced by the decarboxylation of an amino acid, histidine in this case. Similarly, VMAT2 probably packages histamine into presynaptic vesicles. However, histamine does not undergo reuptake.
Reflecting their input in regulating the circadian rhythm, histamine-producing neurons reside only in the posterior hypothalamus, which is adjacent to the optic chiasm. The histamine that these neurons release alerts people and spurs their appetite. Histamine generated in cells outside the nervous system participates in allergic reactions and in production of gastric acid.
Several atypical antipsychotic medications activate histamine receptors along with their other receptors. While that action may have beneficial effects, it stimulates the appetite and leads to weight gain. On the other hand, many medicines—some antipsychotics, antidepressants, diphenhydramine (an allergy-suppressant and hypnotic), and gastric acid suppressants—have antihistaminic side effects sufficient to cause undesired sleepiness without effectively suppressing appetite.
The combination of acetyl-coenzyme A and choline forms acetylcholine (ACh). Although ACh synthesis depends on the enzyme choline acetyltransferase (ChAT ), the rate-limiting factor is the concentration of choline. Subsequently, a form of VMAT2 aggregates ACh into presynaptic vesicles.
Unlike monoamines, ACh does not undergo reuptake. Instead, cholinesterase (the common shorthand for acetylcholinesterase) enzyme terminates its action within the synaptic cleft by hydrolyzing ACh back to acetyl-coenzyme A and choline.
In the CNS, most ACh tracts originate in the nucleus basalis of Meynert (located in the substantia innominata ) and adjacent nuclei situated in the basal forebrain (a rostral portion of the brainstem) ( Fig. 21.5 ). These nuclei send cholinergic projections throughout the cerebral cortex but particularly to the hippocampus, amygdala, and cortical association areas. In addition to its CNS functions, ACh serves as the neurotransmitter in the autonomic nervous system and at the neuromuscular junction (see Fig. 6.1 ).
ACh receptors fall into two categories, nicotinic and muscarinic . They differ in their anatomic distribution, effect (excitatory or inhibitory), and susceptibility to different blocking agents ( Table 21.3 ).
| Cerebral Cortex | Neuromuscular Junction | |
|---|---|---|
| Predominant acetylcholine receptors | Muscarinic | Nicotinic |
| Main action | Excitatory or inhibitory | Excitatory |
| Agents that block receptor | Atropine, scopolamine | Curare, α-bungarotoxin |
In the PNS, decreased neuromuscular junction ACh activity—from either impaired presynaptic ACh release or blockade of postsynaptic ACh receptors—leads to muscle paralysis. Conditions that interfere with neuromuscular junction ACh activity have different etiologies and induce distinct patterns of weakness. For example, in Lambert-Eaton syndrome, which is often a paraneoplastic disorder, antibodies against voltage-gated calcium channels impair the release of ACh from presynaptic neurons and cause weakness of limbs (see Chapters 6 and 19 ). Botulinum toxin also impairs ACh release from the presynaptic neuron. When ingested as a food poison, the toxin causes potentially fatal weakness of ocular, facial, limb, and respiratory muscles (botulism). When injected into affected muscles for treatment of focal dystonia, pharmacologic botulinum toxin inhibits overactive muscle contractions because it reduces ACh release from the presynaptic neuron at the neuromuscular junction (see Chapter 18 ).
Curare, many other poisons, and antibodies like those associated with myasthenia gravis, block ACh receptors at the postsynaptic neuron of the neuromuscular junction. The pattern of weakness in myasthenia gravis is distinctive: Patients have asymmetric paresis of the extraocular and facial muscles, but not the pupils. To overcome the ACh receptor blockade in myasthenia gravis, neurologists administer an anticholinesterase (the common contraction of anti-acetylcholinesterase), such as edrophonium, pyridostigmine, or physostigmine, to inhibit cholinesterase and thereby reduce the breakdown of ACh in the synaptic cleft (see Chapter 6 and Fig. 7.6 ). The ability of edrophonium to effectively but temporarily reverse myasthenia-induced paralysis led to the “Tensilon Test” (see Fig. 6.3 ) as a useful diagnostic tool, but its effect is too short-lived for it to be useful as a therapy. Neurologists prescribe pyridostigmine, a long-acting anticholinesterase, for therapeutic use in patients with myasthenia. The anticholinesterases used to treat myasthenia do not cross the blood-brain barrier and do not alter levels of ACh in the brain or effect cognitive function.
Ophthalmologists prescribe medicines to enhance ocular ACh activity as a treatment for glaucoma. For example, the common topical medication pilocarpine stimulates muscarinic receptors. That cholinergic effect constricts the pupils and thereby increases aqueous drainage that reduces intraocular pressure. Pilocarpine is sometimes absorbed into the general circulation and causes bradycardia and hypotension (see later, cholinergic crisis).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here