Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cranial sutures are patent at birth and serve as the major sites of expansion in the skull during early brain development. Typically 400 g at birth, the brain doubles in size by 6 months and triples by 2.5 years. Although it reaches 90% of its adult size by age 6, the brain progressively expands throughout childhood and adolescence, with total cerebral volume peaking by 14.5 years in males and 11.5 years in females. Synaptogenesis, myelination, and pruning occur this entire time to generate the full functionality of the brain.
Craniosynostosis is associated with premature fusion of the bone at the cranial sutures which results in interruption of normal patterns of skull growth and suture-specific craniofacial dysmorphology. Physiologic closure of the metopic suture may occur between 3 and 10 months of age, while the sagittal, coronal, and lambdoid sutures physiologically fuse in adulthood. Without surgical correction, the morphologic manifestations of craniosynostosis often progress with time.
Since the 1890s, surgery for craniosynostosis has been recommended to enhance brain growth and normalize skull shape. Craniosynostosis treatment had been proposed, at least in large part, to address a cosmetic defect. Initial studies based on Bayley Scales of Infant Development found that some children with nonsyndromic craniosynostosis obtained normal-range developmental scores and did not identify higher rates of cognitive or motor dysfunction in operated versus unoperated children.
A multitude of studies have shown since then, however, that despite normal global intelligence, children with craniosynostosis exhibit a variety of neurocognitive deficits. Boltshauser et al . showed that 30 individuals with untreated sagittal craniosynostosis between the ages of 2 and 25 exhibited deficits in attention, processing speed, learning, and memory when compared with their unaffected siblings. Magge et al . reported that even following strip craniectomy, sagittal synostosis portended a nine times higher risk of later learning disability, with a 45% incidence compared to the 5% expected in the general population. Starr and colleagues demonstrated that the infants in their mixed cohort of 209 with nonsyndromic craniosynostosis (NSC) had two times greater odds of experiencing any form of mental, psychomotor, or language delay compared to the general pediatric population. Shipster et al . found a 37% incidence of language impairment in their cohort of 76 with sagittal synostosis. Furthermore, Naran and colleagues found that abnormal language development occurred in 1 in 1.7 patients, and children with NSC required two to five times higher rates of speech therapy, compared to their typically developing counterparts, while Chieffo and colleagues found a 30% rate of speech delays.
The prevalence of neurocognitive deficits in NSC patients varies, but may be quite substantial, approaching 34%–50% of all patients. These deficits manifest as dysfunctions in higher-order processing, behavior, language, and visuospatial integration. Correction of craniosynostosis is necessary therefore to correct the abnormal appearance of the skull and optimize future neurocognitive development.
Neurologic dysfunction in NSC has been postulated to emerge from two distinct mechanisms. The first considers the shared regulatory influences of both the skull and the brain, which develop in concert due to coordination between bony and neural progenitor cells. Abnormalities intrinsic to both the skull and brain result in both abnormal suture fusion and neurocognitive deficits.
Alternatively, restriction of physiologic skull growth, and by consequence, brain growth, can impact on cognitive dysfunction, including the disruption and reorganization of functional neural networks, altered intracranial volume, and increased intracranial pressure. Neural plasticity and compensatory brain development, however, can ameliorate at least some of these potential outcomes.
Most likely, both intrinsic malformation as well as mechanical constraint contribute to the cognitive dysfunction seen in children with NSC. Questions remain as to which competing force exerts greater influence, and how surgery affects these forces.
The shared embryologic origin between the brain, meninges, and cranial bone suggest that the etiology of neurocognitive dysfunction and cranial suture pathology reflect a common biologic process. The neural crest, which gives rise to the frontal bone and the interparietal portion of the occipital bone, gives rise to the sagittal and metopic sutures, meninges of the forebrain and midbrain , in addition to the parenchymal forebrain and midbrain. Aberrant signaling in neural crest cells during migration and differentiation has been suggested as a possible mechanism for development of neural dysfunction.
Studies in animal models have also demonstrated the interplay between the brain, dura mater, and the rest of the bony vault. The dura mater, in particular, has been demonstrated as an intermediary in maintaining suture patency through signaling pathways (Fig. 24.4.1 ). Using rat and mouse models, Longaker and colleagues demonstrated the inhibition of normal suture fusion when the suture–dura interface was disrupted and altered.
Research surrounding the genetic underpinning of the brain–skull interaction and the concomitant neurocognitive dysfunction also continues to emerge. In syndromic forms of craniosynostosis, monogenic mutations occur in 15%–20% of patients, implicating over 50 genes, the most common being fibroblast growth factor receptors (FGFRs), homeobox protein MSX-2 (MSX2), Ephrin-B (EFNB), Twist-related protein 1 (TWIST), and Runt-related transcription factor 2 (RUNX2) related to osteoblastogenesis.
Mutations in FGR/FGFR 1–3 are causative for the most common forms of craniosynostosis syndromes, including Apert, Crouzon, Pfeiffer, and Muenke syndromes. FGF/FGFR signaling is critical for differentiation of mesenchymal and neuroectodermal cells. The first study to characterize the role of FGFR2 investigated the effect of adenoviral vectors that either enhanced or inhibited FGF expression on rat sutures. When FGF was inhibited, suture patency was found to be maintained, whereas in cases where FGF was overactivated, premature suture fusion was observed. The mechanism behind this relates to the premature differentiation of osteoblasts and osteoprogenitor cells along the cranial suture mesenchyme caused by the increased activation of the FGFR pathway. In addition to effects on suture patency, FGF/FGFR mutations have been noted to elicit pleiotropic neurologic effects. FGFR3, the responsible gene underlying Muenke syndrome, was found to regulate brain size and cortical thickness through its effects on progenitor cell proliferation and apoptosis. Outside of craniosynostosis, mutations in FGFR have been implicated in defective neuronal migration in Kallmann syndrome, and have also been associated with disturbed cognitive and social behavior in mice.
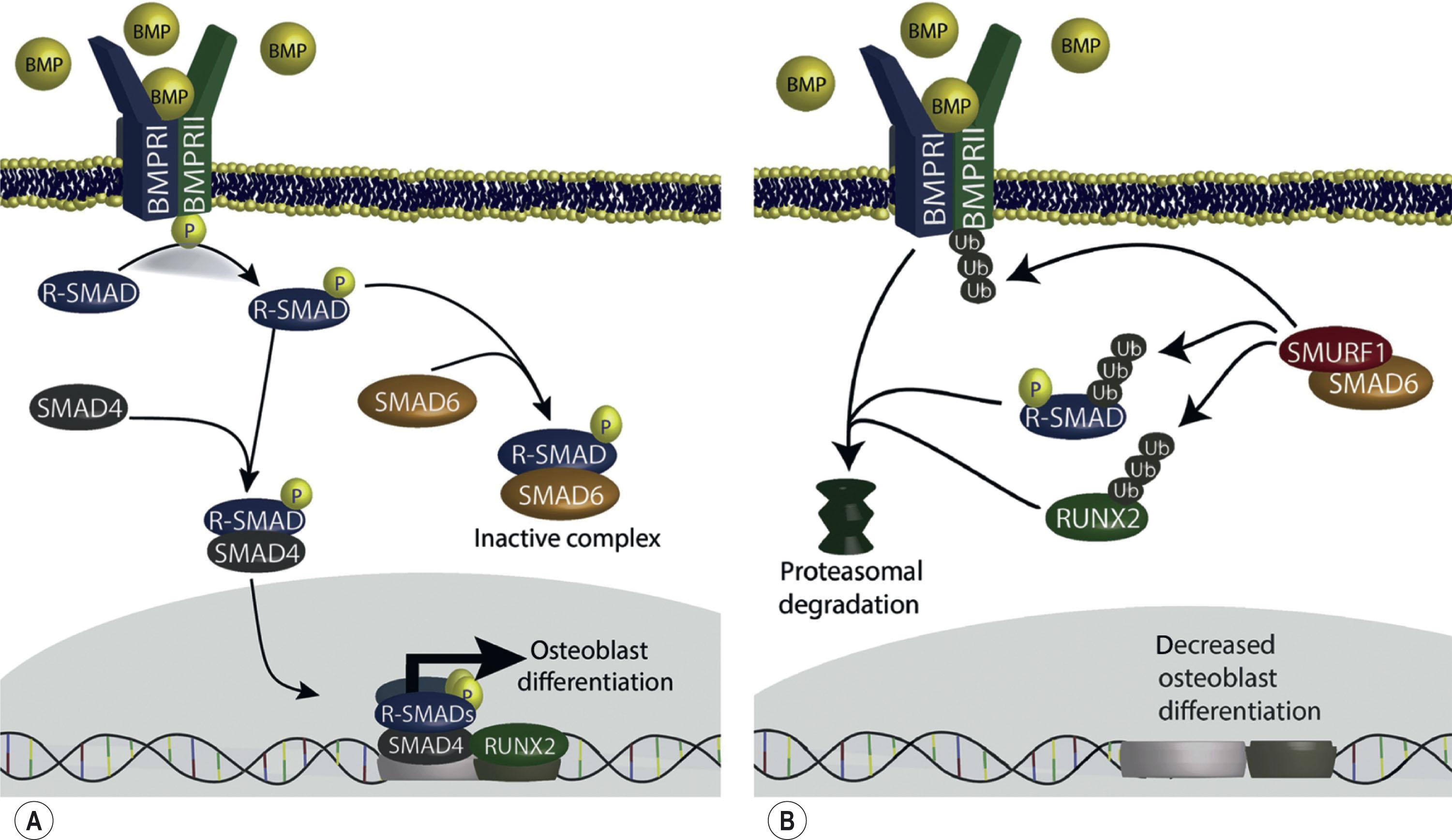
More recently, a role for a genetic influence on neurodevelopment has been suggested for nonsyndromic craniosynostosis (NSC) as well. The evidence for genetic determinants of NSC can be made based on familial analysis in first-degree relatives, and twin studies (60.9% vs. 5.3%). SMAD6 , an inhibitor of BMP, has been documented to underlie 7% of all midline craniosynostosis, and has also been associated with lower intelligence outcomes, including math, IQ, and motor coordination. Subsequent studies highlighting the role of de novo mutations in the BMP, Wnt, and FGF/MAPK pathways have also been identified in the pathogenesis of NSC, with de novo and transmitted mutations in these pathways being associated with at least 10% of NSC cases. This signaling cascade is in charge of regulation of not only bone, but also oligodendrocytes, which are involved in neuron myelination. The TCF12 gene, discovered in 2013, was found to be causative for craniosynostosis as well as increased risk for difficulties in social communication and other psychosocial issues.
In addition to mutations in specific genes, a study by Timberlake et al . established a role for damaging de novo mutations and transmitted loss of frequency (LOF) mutations in highly constrained genes as causal for neurodevelopmental delay in NSC. The overall observation was that those with high-risk genotypes had a 5.9-fold greater risk of having any delay that was still evident after 5 years of age (39.3% vs. 6.7%).
The Nobel Prize–winning work of Weisel and Hubel identified the role of external insults at critical times during development as responsible for irreversible neurologic deficits. Kittens visually deprived in one eye from birth, with one eyelid sutured closed alone, showed a specific time period of high susceptibility. Lid closure with resultant visual loss, for as little as 3 months immediately after birth, showed a dramatic decline in the number of lateral geniculate nucleus cells generated in the occipital region with blindness only in that eye ipsilateral to the closed eyelid.
With respect to craniosynostosis, prolonged compression of the brain during critical periods of neurodevelopment may similarly result in delays in function and permanent deficits. Even small variations in neural organization, particularly at critical time periods, can lead to significant changes in cognitive function over time. Critical periods of neuron formation, related to neuronal migration, peak between gestational age (GA) weeks 12 to 20. Myelination begins in the brain stem at 29 weeks GA, and proceeds from inferior to superior and posterior to anterior. Synaptic refinement culminates at around GA weeks 24 to 28, while the peak period of synaptogenesis begins by GA week 34. GA weeks 39 to 44 mark the period during which formation of the brain’s functional networks begin. Conceivably, suture fusion, which occurs around GA weeks 15–16, could affect any or all of these neuro-ontogenic processes. Interestingly, synaptogenesis of the prefrontal cortex does not peak until age 8 months postnatally and continues through the second year of life. Thus, the synaptogenesis for higher-order processing, functions which are presumably affected in NSC, takes place later in development. This has important implications for the possible benefit that may be derived from craniosynostosis surgery depending on the age at intervention.
The physical restriction of growth imposed on the brain by the fused suture may alter other aspects of neurodevelopment. The abnormal shape of the cranium may result in an underlying brain with altered white matter tract length and skewed proportions of underlying gray and white matter. Local increases in pressure also have the potential to change regional blood flow and cortical metabolism. Conceivably, the compensatory changes that arise from premature suture fusion including abnormal gross brain structure, disorganization of functional networks, and alterations in metabolism and blood flow could thus contribute to the neurocognitive impairment seen in NSC.
Intracranial hypertension has been proposed as another plausible mechanism for the negative neurocognitive effects associated with craniosynostosis. Increased intracranial pressure in NSC has been reported to be 12%–14%, but some reports have been as high as 44%. Thompson et al . measured subdural intracranial pressure overnight and found a borderline or elevated intracranial pressure in 24% of sagittal cases. Both multisuture fusion and syndromic status have a greater potential for elevated intracranial pressure (ICP).
Renier and Marchac used bolt monitoring to record ICP in children with craniosynostosis to identify a correlation between ICP and neurocognitive outcome. One study measuring ICP recordings in 358 infants with sagittal craniosynostosis after surgical correction revealed a decrease from 74% to 7% in the 2–8 weeks following surgery. In that study, they found no correlation between ICP and developmental quotient (DQ) prior to treatment, with no improvement in full-scale intelligence quotient (IQ) in some groups of synostosis, compared to DQ after surgery. The conclusions that can be drawn from their initial study involving ICP are limited, however, as DQ and IQ do not measure the same outcomes and hence are not directly comparable as independent variables. Furthermore, DQ has also been shown to have only modest correlation with eventual neurocognitive outcome.
Subsequent studies by Arnaud et al . and Gewalli et al . did not find a link between elevated ICP and neurocognitive impairment. The invasiveness of ICP monitoring, however, has limited further investigation. Papilledema as a sign of elevated ICP has relatively low sensitivity in the pediatric population, and asymptomatic elevations in ICP may nevertheless have neurodevelopmental consequences. Moreover, ICP does not have normal reference ranges in children; it can vary depending on when it is recorded. However, the relatively low incidence of elevated ICP compared to the overall incidence of neurocognitive delay suggests that additional mechanisms may be at play.
A variety of tools have been employed to assess brain structure and function in infants with craniosynostosis. Computed tomography (CT) studies initially revealed changes in the brain shape mirroring the dysmorphia of the overlying skull. Advancements in imaging technology, however, have allowed for the identification of changes that occur in suture fusion with increased precision. Furthermore, the application of positron emission tomography (PET) scans, functional magnetic imaging, and event-related potentials (ERPs) have provided additional insight into functional consequences of craniosynostosis as well.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here