Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Our work reported in this chapter is supported by the Health Research Council of New Zealand, the National Institutes of Health, the Lottery Health Board of New Zealand, the Auckland Medical Research Foundation, and the Neurological Foundation of New Zealand.
Moderate to severe hypoxic-ischemic encephalopathy (HIE) continues to be a significant cause of acute neurologic injury at birth, occurring in approximately 1 to 2 cases per 1000 term live births in the developed world. The risks are approximately 10-fold higher in the developing world. The possibility that hypothermia may alleviate asphyxial brain injury is a “dream revisited.” Early experimental studies, mainly in precocial animals such as kittens, demonstrated that hypothermia during postnatal hypoxia greatly extended the “time to last gasp” and improved neurologic recovery. This finding led to a series of small uncontrolled studies in the 1950s and 1960s in which infants who were not breathing spontaneously at 5 minutes after birth were immersed in cold water until respiration began and then allowed to rewarm spontaneously over many hours. Although outcomes were said to be better than for historical controls, this experimental approach was overtaken by two major developments: the introduction of active ventilation and resuscitation of infants exposed to asphyxia and the recognition that even mild hypothermia is associated with greater mortality in preterm newborns. Thus resuscitation guidelines for the newborn exposed to asphyxia have, until recently, simply emphasized keeping the newly born infant warm—that is to say, avoiding hypothermia.
The early experimental studies already noted focused entirely on the effects of cooling during severe hypoxia, which is now well established to be associated with potent dose-related long-lasting neuroprotection. The central clinical question is, of course, whether cooling after asphyxial or hypoxic-ischemic injury is beneficial. This chapter reviews the recent development and clinical translation of mild postresuscitation cooling, or therapeutic hypothermia, to improve outcomes.
The key advance was the clinical and experimental observation in term fetuses, newborns, and adults that injury to the brain is not a single “event” occurring at or just after an insult but rather an evolving process that leads to a significant proportion of cell death well after the end of acute hypoxia-ischemia. Using magnetic resonance spectroscopy, Wyatt and coworkers showed that infants with evidence of moderate to severe asphyxia often had normal cerebral oxidative metabolism shortly after birth, but many then went on to develop energy failure 6 to 15 hours later. Those infants who did not show transient recovery had a very high mortality, whereas in survivors the degree of secondary energy failure after 24 to 48 hours was closely associated with impaired neurodevelopmental outcome at 4 years of age. An identical pattern of initial recovery of cerebral oxidative metabolism followed by secondary energy failure was seen after hypoxia-ischemia in the piglet. Greater recovery of cerebral phosphocreatine levels after hypoxia-ischemia was associated with more favorable outcomes. In turn, the severity of subsequent secondary loss of oxidative metabolism was closely correlated with the severity of neuronal loss. This delay between insult and injury raised the tantalizing possibility that asphyxial cell death may be prevented even well after the insult.
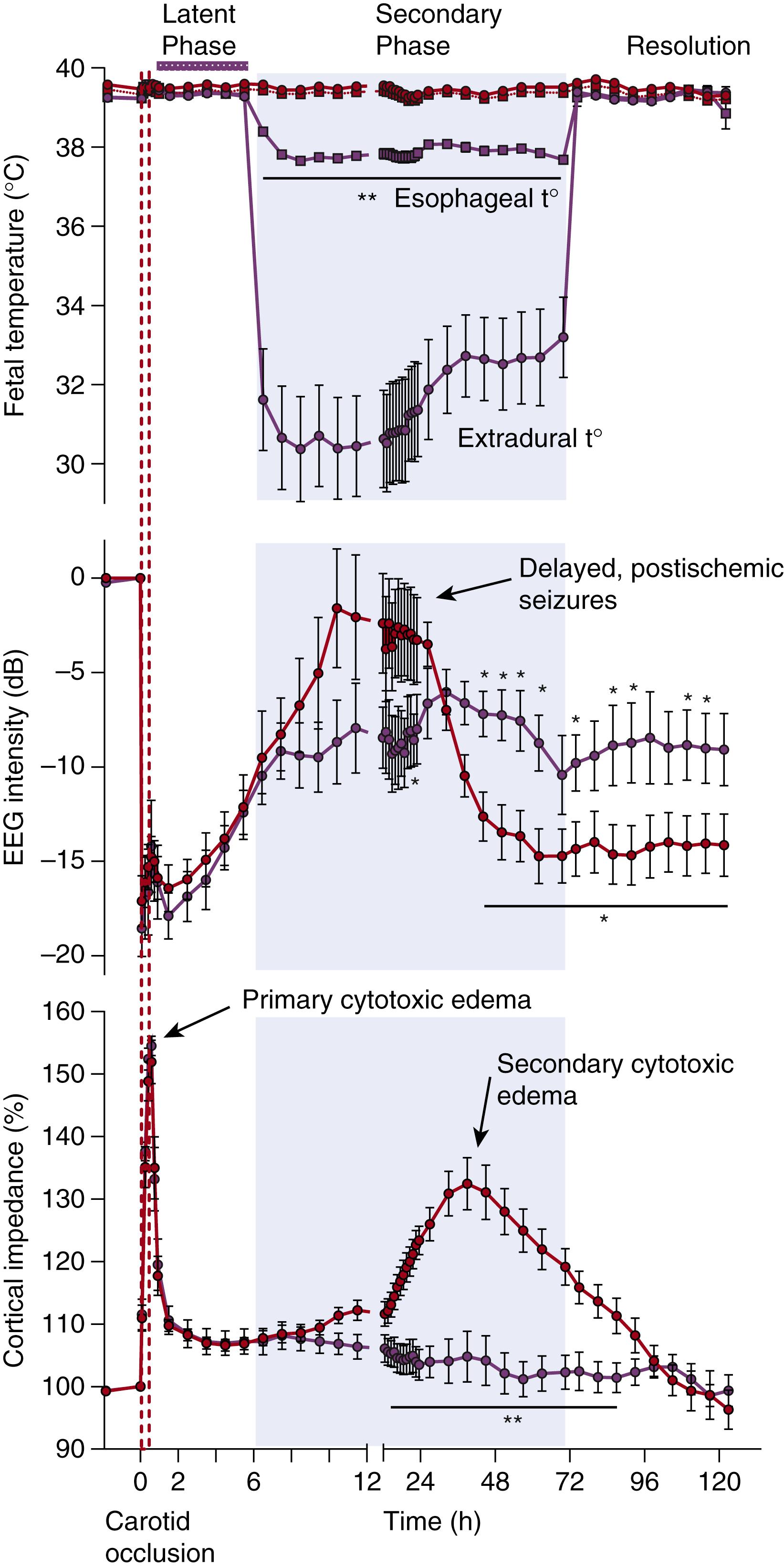
Distinct pathophysiologic phases of evolving injury may be distinguished, as illustrated in Fig. 134.1 . The actual period of hypoxia-ischemia is the primary phase of cell injury. During this phase, progressive hypoxic depolarization of cells leads to severe cytotoxic edema, with failure of reuptake, leading to the extracellular accumulation of excitatory amino acids ( excitotoxins ). Excessive levels of excitatory amino acids cause activation of their ion channels and promote further excessive entry of salt, water, and calcium into the cells. After the return of cerebral circulation during resuscitation, the initial hypoxia-induced cytotoxic edema may transiently resolve over approximately 30 to 60 minutes, with partial or even complete recovery of cerebral oxidative metabolism in a latent phase . This latent phase is characterized by continuing suppression of electroencephalographic (EEG) activity, with a delayed onset of cerebral hypoperfusion associated with suppressed cerebral metabolism. This is followed by a secondary phase of deterioration (∼6 to 15 hours later) that may extend over many days. At term gestation, this secondary phase is characterized by delayed onset seizures, secondary cytotoxic edema (see Fig. 134.1 ), extracellular accumulation of excitotoxins, progressive failure of cerebral oxidative energy metabolism, and ultimately neuronal and oligodendrocyte death.
The studies discussed in this chapter strongly suggest that it is the early recovery, or latent phase, that represents the key window of opportunity for intervention with therapeutic hypothermia.
Experimentally, the efficacy of neuroprotection with hypothermia is critically dependent on the timing of initiation of cooling, its depth, and its duration.
Brief hypothermia, for 1 to 2 hours, appears to be modestly neuroprotective provided that it is initiated immediately after the insult. For example, after 15 minutes of reversible ischemia in the piglet, mild hypothermia (a reduction of brain temperature of 2°C to 3°C from the normal basal temperature of 38°C in the piglet) for 1 to 3 hours significantly improved recovery and reduced neuronal loss 3 days later. , Critically, protection was lost if the brief interval of hypothermia was delayed by as little as 15 to 45 minutes after the primary insult. This extreme sensitivity to delay is consistent with the hypothesis that brief, resuscitative hypothermia can suppress damage secondary to oxygen free radical production during reperfusion. , Alternatively, this strategy may in effect represent intervention at the end of the primary phase, when cerebrovascular perfusion is being reestablished, cell function is just starting to recover, and levels of excitatory amino acids are still high.
Even if immediate cooling at birth was consistently effective, it is simply not possible at present to identify reliably the few infants requiring resuscitation who will go on to develop significant encephalopathy until some hours after birth.
Subsequent studies examined the strategy of suppressing secondary encephalopathic processes by maintaining hypothermia throughout the course of the secondary phase. Such extended periods of cooling of between 5 and 72 hours appear to be more consistently effective. For example, in a study of reversible middle cerebral artery occlusion in the adult rat, 21 hours but not 1 hour of mild hypothermia reduced the area of infarction after 48 hours of recovery. After severe global ischemia in the adult gerbil, 12 hours of mild hypothermia was ineffective, whereas extending the interval to 24 hours did afford protection.
Studies in newborn animals support these data. In unanesthetized infant rats subjected to moderate hypoxia-ischemia, mild hypothermia (2°C to 3°C reduction in brain temperature from 37°C in normothermic controls) for 72 hours from the end of hypoxia prevented cortical infarction, whereas 6 hours of cooling had only intermediate, nonsignificant results. In the same model, a greater reduction in body temperature (by 5°C) for 6 hours, starting immediately after the insult, gave significant neuroprotection as well as neurobehavioral improvement after both 1 and 6 weeks’ survival. Finally, in the anesthetized piglet exposed either to hypoxia with bilateral carotid ligation or to hypoxia with hypotension, 12 to 48 hours of moderate whole-body hypothermia or head cooling with mild systemic hypothermia (started immediately after hypoxia) prevented delayed energy failure, , reduced neuronal loss, and suppressed posthypoxic seizures.
A critical depth of cerebral hypothermia of between 32°C and 34°C appears to be required for effective neuronal rescue. In the fetal sheep cooled from 90 minutes after ischemia, substantial neuroprotection was seen only in fetuses that had a sustained fall of the extradural temperature to less than 34°C (normal temperature in the fetal sheep is 39.5°C). In the adult gerbil, cooling to a rectal temperature of 32°C (vs. 37°C in controls) was associated with greater behavioral and histologic neuroprotection than a temperature of 34°C. More recently, in 7-day-old rat pups that were cooled immediately after hypoxia-ischemia, cooling to 33.5°C for 5 hours was neuroprotective compared with 37°C, with no additional protection with cooling to deeper temperatures of 32°C, 30°C, 26°C, or 18°C. Similarly, in neonatal piglets, whole-body hypothermia with a reduction in body temperature of either 3.5°C or 5°C was associated with significant (and highly similar) overall neuroprotection after global cerebral ischemia, whereas a reduction of 8°C was associated with less neuroprotection.
In part this loss of neuroprotection is likely related to the adverse systemic effects of cooling, which increase below a core temperature of approximately 33°C to 34°C. In particular, after hypoxia-ischemia in newborn piglets, cooling to 30°C was associated with greater metabolic acidosis, increased blood glucose levels, and a greater risk of cardiac arrest and deaths compared with cooling to 33.5°C or 35°C. Similarly, in adult dogs, deep hypothermia (to a rectal temperature of 15°C) after cardiac arrest was detrimental, whereas mild hypothermia (34°C to 36°C), from 10 minutes until 12 hours after cardiac arrest was beneficial.
At present, the window of opportunity for any particular therapy can only be determined empirically. However, some general principles can be discerned. For example, initiation of neuronal degeneration is accelerated by more severe insults. DNA fragmentation in the hippocampus can be detected as early as 10 hours after a 60-minutes of hypoxia-ischemia in the rat, but in the same paradigm, after 15-minutes of hypoxia-ischemia, DNA fragmentation in the hippocampus is detectable only at 3 to 5 days. The appearance of DNA fragmentation and classic ischemic cell change represents only the terminal events of this cascade and is thus not a good guide to determining when cell death may still be reversible. In vitro studies have distinguished latent and active or execution phases during the process of programmed or apoptotic cell death. Whereas the active phase involves downstream factors that could induce DNA fragmentation and chromatin condensation within previously normal nuclei, the preceding latent phase is characterized by caspase activation (a large family of enzymes that mediate and amplify apoptosis) confined to the cytoplasm, without activation of downstream factors. These data suggest that it is the activation of intranuclear pathways of cell death that is the critical step corresponding to the transition from the latent to the execution phases of programmed neuronal death. Thus, in principle, it seems far more likely that intervention would be protective if it were applied during the initial, latent phase of programmed cell death rather than during the execution phase, even though the latter still precedes DNA fragmentation and cell death.
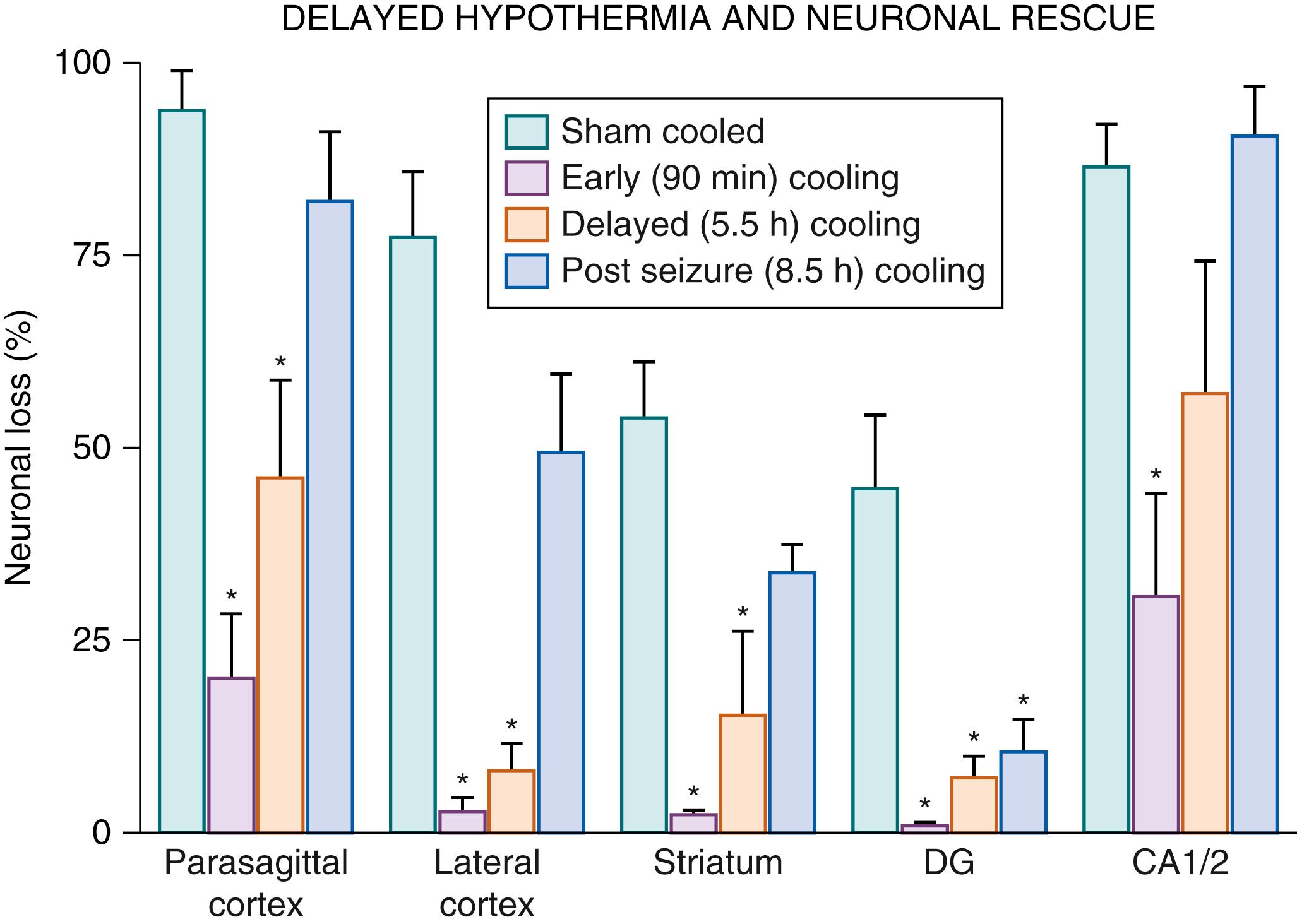
Systematic in vivo studies support that the latent phase defines the window of opportunity for treatment with therapeutic hypothermia. In near-term fetal sheep, moderate hypothermia induced 90 minutes after reperfusion (i.e., in the early latent phase) and continued until 72 hours after ischemia prevented secondary cytotoxic edema and improved EEG recovery. There was a concomitant substantial reduction in parasagittal cortical infarction and improvement in neuronal loss scores in all regions. When the start of hypothermia was delayed until just before the onset of secondary seizures in this paradigm, 5.5 hours after reperfusion, only partial protection was seen ( Fig. 134.2 ). With further delay (until after seizures were established, 8.5 hours after reperfusion), there was no electrophysiologic or overall histologic protection with cooling. Similarly, in 7-day-old rat pups, mild (33.5°C) hypothermia for 5 hours was protective after a “moderate” (90-minute) duration of hypoxia-ischemia, such that immediate induction of hypothermia after moderate hypoxia-ischemia significantly reduced the area of cortical infarction, with partial protection after 3 hours and loss of protection after 6 hours of delay. It is important to note that in that study, immediate hypothermia did not improve outcomes after very prolonged hypoxia-ischemia (150 minutes), illustrating that the window of opportunity for therapeutic hypothermia is dependent on the severity of hypoxia-ischemia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here