Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although athletes at all levels of participation may experience the same entrapment neuropathies as the general population, some sport-specific peripheral nerve factors must be considered with regard to the wrist and hand. Neuropathies may occur as a result of chronic repetitive motion or acute isolated trauma experienced during athletic activities. Just as with other pathologies that may have an effect on, or be affected by, sports participation, the type and timing of treatment will vary depending on severity and “sport season” considerations. Although “playing with pain” may be an acceptable choice in the presence of some repetitive traumatic injuries, hand and wrist neuropathies often necessitate careful and timely treatment to prevent irreversible damage. Although a multitude of potential upper extremity neuropathies exist, this chapter focuses on the ones that are most typical and/or cause the most concern involving the wrist and hand.
Wrist and hand neuropathies are more common with certain athletic activities. Median neuropathy at the wrist may result from activities that (1) apply prolonged direct compression, such as bicycling or wheelchair events, (2) elicit vibratory stimuli, such as motocross racing, or (3) require repetitive power gripping, such as in weightlifting and ice hockey. In addition to similar mechanisms of injury for the ulnar nerve at the wrist, secondary extrinsic causes of compression dominate, including a high prevalence of hook of hamate fractures as a result of using a stick in certain sports and ulnar artery aneurysm/thrombosis (hypothenar hammer syndrome) that occurs as a result of the repetitive blunt palmar trauma commonly experienced by baseball catchers and martial arts practitioners. The superficial sensory radial nerve is susceptible to either direct blunt trauma, which is most commonly associated with football, lacrosse, and ice hockey, or repetitive traction forces, which are often experienced by competitive rock climbers and powerlifters. Furthermore, the perpetual use of compressive wrist bands or athletic tape, which is frequently seen in athletes whose sport entails use of a racquet, may cause local irritation of the radial nerve at the wrist. The ulnar digital nerve of the thumb is vulnerable in bowlers, and the radial digital nerve of the index finger may be affected in tennis, racquetball, or squash players.
The symptoms of sports-related wrist and hand neuropathies may include subjective paresthesias and weakness in the affected nerve's distribution, loss of coordination, and a variable level of pain. Depending on the reported distribution of symptoms, further questioning should focus on potential areas of nerve compression or repetitive trauma precipitated by the athlete's activity requirements. Direct questioning of the athlete will also reveal if the symptoms worsen or improve in certain positions or occur while the athlete is sleeping. Determining the onset, frequency, and intensity of nerve symptoms may be helpful in deciding what type of an approach to take with further workup and treatment. The benefit or futility of prior treatment should be elicited. The practitioner should also remember that although symptoms and signs may be present in the wrist and hand, the true site of pathology may be more proximal, and therefore an inventory of proximally generated symptoms should be conducted.
As with most pathologies, the patient's history typically directs one to the diagnosis. It is important to avoid asking patients leading questions. Instead, allowing athletes to describe their own symptoms, exacerbating and remitting factors, and prior treatments often leads to the correct diagnosis and determines the course of further management.
The athlete's subjective complaints direct the focus of the physical examination. However, having a systematic approach to the upper extremity nerve exam is helpful. This includes visual inspection, a thorough sensory and a functional motor exam. Performing these tests on every patient allows the practitioner to pick up subtle clues on causation of the patient's symptoms.
When entering the examination room, it is important to visually assess the patient. During your history taking, is the patient demonstrating a posture of pain, such as slouching to one side and/or holding the extremity in a position of protection? Are there obvious differences in shoulder height or discrepancy in muscle bulk of the shoulders, arms, forearms, and hands? Unilateral atrophy is an important sign that a more proximal injury or lesion is present or there has been compensation because of a nerve compression/neuropathy that is distal. For example, if the patient lacks dexterity and has trouble gripping due to an ulnar neuropathy at the wrist, then there may be disuse atrophy of the forearm, which can be a late sign but visible on exam.
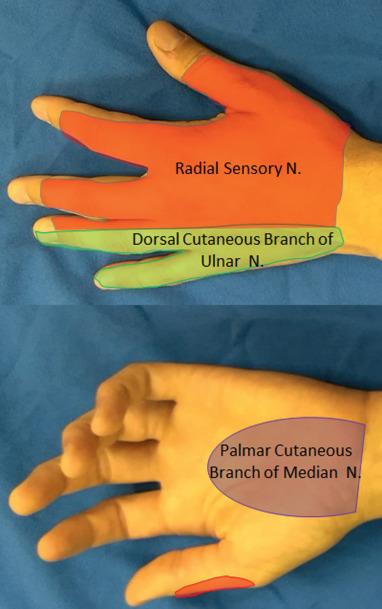
A complete sensory exam is important when evaluating for hand or wrist neuropathies. Rating light touch sensation in the distribution of the symptomatic nerve relative to the asymptomatic wrist and hand is fast, easy, and helpful. This determination of light touch sensation should include the dorsal sensory branch of the ulnar nerve, the palmar cutaneous branch of the median nerve, and the dorsal sensory branch of the radial nerve ( Fig. 75.1 ). Involvement of these terminal cutaneous nerve components is helpful in confirming the site of peripheral nerve compression or disease. For example, normal findings of a sensory examination over the dorsal ulnar hand in the setting of other ulnar nerve symptoms typically indicate ulnar nerve pathology in the wrist or hand rather than at the elbow. Altered sensation in the central palmar aspect of the hand indicates median nerve pathology proximal to the level of the carpal tunnel due to involvement of the palmar cutaneous branch of the median nerve. For digital neuropathies, such as bowler's thumb, altered sensation in the digital nerve is expected, and a palpable mass may be present.
Another fast and easily communicated way of assessing light touch sensation is the Strauch 10-10 test. The patient rates the level of light touch sensation in an injured area on a scale of 0 to 10, with 0 = no sensation, 10 = normal sensation, generally as compared with the unaffected contralateral hand (note: the original scale was 1 to 10, but we find patients better understand 0 = no sensation). More advanced sensory examinations, such as static and moving two-point discrimination, vibratory sense, and Semmes-Weinstein monofilament testing, provide good objective and standardized data points that are monitored for the progression or resolution of neuropathy.
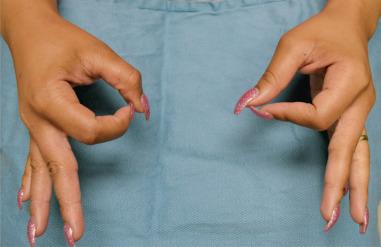
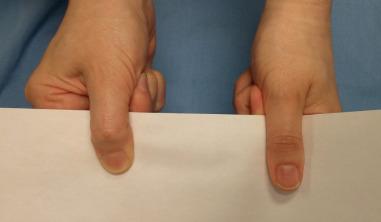
A thorough motor exam is important to perform in all patients with suspected neuropathy. A quick assessment of peripheral nerve motor function can be performed by having the athlete make the “OK” sign (median nerve), cross the index and long fingers (ulnar nerve), and extend the index finger and thumb (radial nerve). When making the OK sign, it is important for the patient to make a rounded circle with the thumb and index finger; flattening of the circle into a more oval shape is an abnormal result that indicates proximal involvement of the anterior interosseous nerve ( Fig. 75.2 ). Other nerve-specific maneuvers include having the athlete oppose the thumb to the base of the small finger (distal median and ulnar innervated thenar muscles), pinch a piece of paper between the thumb and clenched index finger (Froment sign: denervation or atrophy of ulnar innervated adductor pollicis requires the thumb to flex to pinch the paper) ( Fig. 75.3 ), and abduct the small finger against resistance (ulnar innervated abductor digiti minimi). Clawing of the ring and small fingers indicates significant ulnar neuropathy distal to the digital flexor muscles in the forearm. Formal tip-pinch, key-pinch, and grip strength measurements with dynamometers should be performed and provide objective results that are tracked over time.
Tinel testing along the course of the symptomatic peripheral nerve may help determine a specific site of chronic trauma or compressive neuropathy. It is usually helpful to initially tap lightly for this test to get a sense of the hypersensitivity/reactivity of the symptomatic nerve. The Finkelstein test (i.e., ulnar deviation of the athlete's wrist with the thumb in palm by the examiner) may cause dorsal radial sensory nerve symptoms. These symptoms should be differentiated from symptoms caused by de Quervain first extensor compartment tenosynovitis (i.e., pain at the radial styloid) by checking for the presence of nerve symptoms and a positive Tinel sign proximal to the radial styloid at the level where the brachioradialis (BR) and extensor carpi radialis longus (ECRL) meet and the dorsal radial sensory nerve exits.
A limitation of Tinel testing is that it depends on the patient reporting whether symptoms are elicited. The scratch collapse test is a maneuver thought to be based upon an inhibitory spinal reflex such that when the site of compression is stimulated (i.e., scratched ), the patient is no longer able to sustain resisted shoulder external rotation. In brief, the patient sits with shoulders externally rotated, elbows bent to 90 degrees, hands outstretched with wrists neutral. The examiner applies bilateral adduction and internal rotation pressure to the patient's forearms. Next, the examiner scratches the compression point while the patient holds his or her arms steady. The examiner then applies pressure as before, and if the patient is unable to resist the force and collapses, it is considered a positive test indicative of nerve compression at that site. A hierarchical scratch collapse test can be done by “freezing out” compression points with ethyl chloride to unmask secondary compression points. Multiple prospective studies have examined this test for reliability in carpal tunnel syndrome and have demonstrated a relatively low sensitivity, 24% to 33%, and specificity ranged 60% to 61%. In contrast, Cheng et al. reported a significantly greater sensitivity of 64% and specificity of 99%. We generally find there is a significant learning curve with the test before it can be considered reliable by the practitioner and can be considered as a helpful component of a full and comprehensive evaluation.
Direct compression and/or flexion/extension tests along the course of the symptomatic nerve may also be helpful in localizing the site of compression. These tests are typically performed for 30 to 60 seconds or until symptoms occur, whichever comes first. The Phalen test for carpal tunnel/median nerve compression requires placing the wrist in full flexion and holding this for 60 seconds. For ulnar nerve symptoms at the wrist and hand, the practitioner should also consider a vascular examination because of possible associated ulnar artery aneurysm and/or thrombosis. This examination includes examining the athlete's nails for streaking (splinter hemorrhages) that could indicate thrombotic/embolic events and checking for ulcerations or skin breakdown due to ischemia, especially in the palmar fingertips. An Allen test can also be performed to determine to determine if there is a complete palmar arch.
As mentioned earlier, consideration of more proximal nerve disorders that could manifest symptoms distally in the hand and wrist is important. Symptoms and signs dictate whether the cervical spine, brachial plexus, thoracic outlet, arm, or proximal forearm should be scrutinized for the source of the problem. Cervical radiculopathy, thoracic outlet syndrome, and brachial plexopathy are not uncommon conditions in athletes who participate in contact sports.
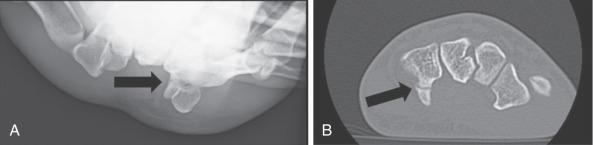
In general, radiographic and advanced imaging is not necessary for evaluation of hand and wrist neuropathies, although special circumstances may spur the examiner to pursue imaging. For example, ulnar nerve symptoms in an athlete with chronic trauma to the ulnar-palmar aspect of the hand may be a sign of a hamate fracture or vascular injury such as with hypothenar hammer syndrome ( Fig. 75.4 ). Plain radiographs are a recommended first step. Although the carpal tunnel view is an often-cited special view for assessing the hamate hook, an alternative view is much easier to perform reproducibly, especially with use of fluoroscopy. To achieve this view, start with the wrist in radial deviation and the thumb abducted, as if the patient were holding a cup. The wrist is then slightly supinated from a lateral x-ray view position to generate a slightly oblique view. In this position, the x-ray beam is directed between the thumb and finger metacarpals and the hamate hook is seen projecting palmarly. For athletes with symptoms and signs of proximal median nerve compression, elbow or humerus radiographs may reveal a supracondylar process. In the absence of a supracondylar process, magnetic resonance imaging (MRI) may be justified to evaluate for an occult forearm mass causing extrinsic compression on the median nerve.
Ultrasound (US) can also be used as an imaging modality to evaluate nerve compression, nerve ganglions, or masses causing compression of the nerve. It is inexpensive and is gaining acceptance as a diagnostic tool. Furthermore, US can also assess vascular abnormalities, such as aneurysms, pseudoaneurysms, and arteriovenous fistulas, thus providing significant information in a patient with the vague presentation of numbness or pain.
MRI is not commonly used in the evaluation of compression neuropathies but can have utility for evaluating mass lesions resulting in compression, when there may be a superimposed proximal or systemic neuropathy on a focal compression point, and in patients who cannot tolerate electrodiagnostic studies. Although a less common cause of compression neuropathy, schwannomas and other more rare tumors may present with compression symptoms, and any palpable masses should be evaluated with MRI for diagnosis and surgical planning. In cases such as bowler's thumb, MRI is the only modality that can preoperatively determine nodular neuroma formation versus perineurial fibrosis, which may present in a similar fashion.
As with any other athletic injury, decision-making is based on the nature and extent of the pathology and sport-specific considerations for the athlete. The patient's history of symptoms, presence of atrophy, and electrodiagnostic tests help to delineate the degree of nerve damage and the likelihood of success with different interventions. Although the diagnosis of carpal or cubital tunnel syndrome is considered “clinical,” we generally obtain neurodiagnostic tests including nerve conduction velocities (NCVs) and electromyography (EMG). NCV values show the integrity of the nerve: the speed of conduction reflects myelin integrity and nerve compression, whereas the amplitude reflects the number of conducting axons within the nerve. EMG directly samples muscle fibers, and the presence of motor unit potentials (MUPs) shows the connectivity of the spinal cord to the muscle in the form of MUPs. These tests confirm the diagnosis and severity and allow for accurate prediction of recovery because they can often differentiate ischemic versus myelin versus axonal injury ( Table 75.1 ). Ischemic changes are often dynamic and dependent on positioning; there is no break in the link between the spinal cord and muscle. Thus the EMG is normal, and similarly, NCV values are normal because the myelin is intact. With progressive compression, there is loss of myelin and a conduction block develops, reducing the NCV values, but the EMG remains normal. With axonal loss there is reduced amplitude in the NCV and loss of MUPs on EMG.
| Nerve Changes | Signs and Symptoms | Electrodiagnostics | Treatment Options | Recovery |
|---|---|---|---|---|
| Dynamic ischemic changes | Intermittent pain and paresthesias | NCVs: normal EMG: normal |
Night splinting, corticosteroid injection | Can expect full recovery |
| Demyelination injury | Pain and paresthesias, may be intermittent or constant; reduced nerve conduction velocities | NCVs: reduced velocities EMG: normal |
Operative decompression | Can expect full recovery in 3–4 months |
| Axonal injury | Constant pain and paresthesias, weakness and clumsiness with fine hand movements, appearance of muscle wasting | NCVs: reduced velocities EMG: fibrillations, chronic and severe may have loss of MUPS |
Operative decompression | Full or partial recovery at a rate of 1 in/month |
In the presence of recent symptoms or clear inciting actions and no slowing of nerve conduction, conservative and nonoperative treatment strategies are reasonable. When the symptoms have been present for longer and there are changes on nerve conduction studies and/or EMG, surgical intervention is most reasonable to prevent permanent sequelae. In cases of overt clawing or intrinsic wasting, a comprehensive work-up and surgical intervention, without delay, are paramount because there are already irreversible changes. However, in the context of the athlete, it is often helpful to present treatment options as they relate to the athlete's season, career, and remainder of his or her life, which helps the athlete to put the different choices into perspective.
Treatment options for the various peripheral nerve pathologies depend on the particular nerve involved, the severity of the involvement, and the type and timing of the patient's athletic activities. In general, wrist-neutral nighttime bracing is started and daytime and/or athletic-activity wrist-neutral bracing is attempted as much as possible. Note that most commercially available wrist braces are in mild wrist extension and must be flattened to neutral to be most helpful, because both wrist flexion and extension increase pressure in the carpal tunnel and can contribute to persistent symptoms. Although the use of oral antiinflammatory drugs along with bracing may be beneficial, using these drugs in isolation has not been shown to be effective. Modification of activities that worsen symptoms is helpful and may be facilitated by therapists and athletic trainers. There is also growing evidence that sleep position may predispose to carpal tunnel syndrome and that sleeping supine with wrists and elbows are extended may also reduce compressive symptoms. A 6- to 8-week trial of splinting and activity modification is reasonable before reassessing symptoms.
If there is no improvement after splinting or the presenting signs and symptoms are severe, surgical decompression is offered. Proceeding with surgery is often based on the patient's immediate, short-term, and long-term athletic schedule. For example, if it is off-season, the athlete may elect to proceed with surgical release as the most definitive treatment so he or she will not have persistent or worsening issues during the season. However, if the athlete is in the middle of a playoff run or a similar competitive scenario and taking time off for surgery is impossible, a corticosteroid injection, in addition to night splinting, is potentially helpful to improve symptoms over the course of several weeks to months.
For ulnar neuropathies of the wrist and hand, activity modification and intermittent bracing may be helpful. One difference in the treatment of ulnar neuropathy at the wrist involves the presence of motor involvement. Intrinsic muscle weakness or atrophy resulting from identifiable secondary pathology, such as a hamate hook fracture, ulnar artery thrombosis, or other mass, should prompt the surgeon to address the offending source of compression sooner rather than later. A 6- to 8-week period of observation and activity modification is reasonable if secondary pathology is absent. Situations typically occur in which a single acute trauma (e.g., a direct blow) or a few episodes of direct compression (e.g., from cycling) cause the ulnar nerve palsy. Steady clinical improvement warrants further observation without repeat neurodiagnostic tests, and many palsies resolve completely within this time frame with activity modification alone. Neurodiagnostic tests precede surgical intervention for cases that fail to resolve after the observation period . It is important to assess for any signs of ulnar deep motor branch compression in a patient with carpal tunnel syndrome because returning to the operating room to decompress the Guyon canal in an already operated field increases the difficulty of safe decompression due to scar tissue.
Although proximal radial neuropathy may cause both motor and sensory loss, involvement at the level of the wrist and hand is purely sensory in nature. Idiopathic neuropathy originating between the BR and ECRL is called Wartenberg syndrome, or cheiralgia paresthetica. The main goals of treatment for this condition are to improve symptoms and prevent worsening, which may lead to complex regional pain syndrome type II, also known as causalgia . The most common nonoperative treatment for radial neuropathy at the wrist is removal of any constrictive external devices, wraps, or bands. Avoiding power pronosupination and splinting may have a limited benefit; 37% of patients with radial sensory nerve compression can be managed successfully nonoperatively. For recalcitrant cases of radial sensory nerve disturbance at the wrist, an injection of a local anesthetic may be attempted, more for diagnostic than therapeutic purposes. If symptoms persist despite several (usually at least 6) months of conservative management or in the case of relief with diagnostic injection of a local anesthetic, surgical treatment can be offered. Surgical decompression of the radial sensory nerve is performed through a dorsoradial incision centered approximately 9 cm from the radial styloid. The radial sensory nerve is identified between the ECRL and BR, the tight fascia joining the BR and ECRL is released and full BR tenotomy is performed, if needed. Alternatively, the constricting portion of the BR and ECRL may be excised to ensure full nerve decompression.
Digital neuropathies that result from bowling, baseball, or racquet sports typically respond to nonoperative measures such as grip modification, padding, or other equipment changes. Several months of nonoperative treatment is warranted before considering surgery. Bowler's thumb is most notable and is due to repeat trauma to the ulnar aspect of the thumb and resultant subcutaneous and perineurial fibrosis. In severe cases this can develop into a true digital nerve neuroma. Rest and a plastic thumb guard are sufficient for most cases. Although it is uncommon to treat digital neuropathy surgically, procedures such as neurolysis and fibrotic synovectomy or nerve transposition have been described in cases of chronic compression and pain that have failed nonoperative management. For example, the ulnar digital nerve can be transposed dorsal to the adductor pollicis to prevent continued local mechanical trauma.
There are few medications that may reduce the severity of neurogenic pain and paresthesias. If neurogenic pain is severe, then a multidisciplinary approach must be taken. This includes the primary care physicians, pain management specialists, and the occupational therapists. Medications to treat nerve pain act centrally and have common adverse effects; thus it is rare that they are used to treat compression syndromes in the athlete.
Endoscopic carpal tunnel release has proven to be safe and effective, although not superior to the traditional open method. An earlier RTP is theoretically possible for most athletes and sports and is often cited as an early short-term advantage of this technique (extrapolating from return-to-work data). In terms of safety, median nerve injury is very rare with either technique, although the risk of nerve injury has been shown to be higher with endoscopic releases in one study. In the end, we recommend that the surgeon should do the technique with which they are most comfortable: that technique will be the most safe. At our institution we prefer the open technique. If there is any concern for ulnar nerve entrapment at the wrist, we also perform Guyon canal release.
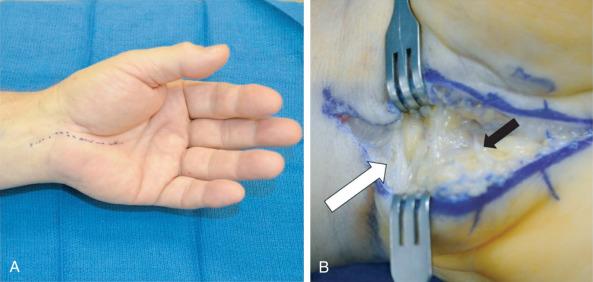
Induction of anesthesia according to the surgeon's preference and sterile skin preparation and draping of the patient's upper extremity are performed. A tourniquet can be placed on the proximal forearm if desired. A 3-cm linear incision is marked slightly ulnar and parallel to the thenar skin crease extending from the wrist crease distally ( Fig. 75.5A ). The incision is made ulnar to the thenar crease to protect the course of the palmar cutaneous branch of the median nerve and to avoid a possible transligamentous thenar branch. A Bruner-style incision is marked across the distal wrist crease to be used if necessary to fully release the antebrachial fascia (see Fig. 75.5B ). The palmar fascia is opened longitudinally in line with the skin incision. Dissection proceeds to expose the transverse carpal ligament and the distal border of the release is visualized, which forms a “V” from confluence of the thenar and hypothenar musculature. The transverse carpal ligament is completely released from the distal volar wrist crease to the “V.” The fat pad protecting the superficial palmar arch and common digital nerve branches is visualized and protected. To ensure complete release the antebrachial fascia, it is helpful for the surgeon to move to the end of the hand table. Under direct visualization, the fascia can be released in a controlled manner. This fascia can be surprisingly tight in muscular forearms of athletes and may extend well proximal to the volar wrist crease. Do not hesitate to extend the incision proximal across the wrist crease if needed to avoid an incomplete release.
If a Guyon canal release is also indicated in the patient, then this release is approached prior to the carpal tunnel release. The palmaris brevis and palmar aponeurosis are divided, and ulnar sensory branches (one large branch is commonly encountered 2.5 cm distal to the wrist crease and 1 cm ulnar to the thenar crease) are carefully avoided. The neurovascular bundle is identified and swept ulnarly with a blunt retractor. The hook of hamate is palpated and the leading edge of the hypothenar musculature is identified. The motor branch of the ulnar nerve is not visualized until the fascia of the hypothenar muscle is released. Decompression of the motor branch can be achieved with release of the fascia of the hypothenar musculature. Once released, the dissection of the soft tissue is carried radially and the transverse carpal ligament is identified and released as described previously.
Once a complete release has been confirmed with direct visualization, the tourniquet (if used) is released and hemostasis is achieved. The choice regarding the type of suture and technique to be used for skin closure is made by the surgeon, but horizontal mattress eversion of the skin edges with 4-0 nylon is standard. A bulky dressing is applied, and the patient is encouraged to move their fingers often.
After carpal tunnel release, finger motion is encouraged immediately but patients are instructed to not lift anything heavier than a pen or piece of paper for 2 weeks. The dressing is removed in 5 days, and the patients are allowed to shower. The skin is usually healed by 2 weeks after surgery, and if range of motion is full or nearly full, the athlete can start gradually increasing their activities as permitted by their level of pain and the condition of their soft tissues. Strengthening exercises and full-weight bearing is usually initiated by 4 weeks. Pain, swelling, and other postoperative symptoms usually wax and wane depending on activities performed and dictate how rapidly and aggressively the athlete can progress. Once the skin is healed, athletes may return to play (RTP) if time is of the essence and if it seems physically possible for them to compete at a high level. This decision typically depends on their pain tolerance, strength, and overall coordination. Otherwise, if early RTP is less critical, a more measured approach may involve a gradual return to athletic participation once strength is 50% to 75% of the unaffected contralateral side, which is a good postoperative rule of thumb for other nerve-related procedures involving the wrist and hand as well. Formal therapy is recommended for athletes because they may benefit from work with a therapist and/or an athletic trainer to facilitate an earlier RTP.
If the diagnosis of carpal tunnel syndrome is correct and the surgical release is performed, postoperative results should be consistently satisfactory. Most authors report improvement for more than 90% of patients. However, the degree to which patients improve is harder to predict. If the athlete experienced nocturnal symptoms or positional aggravation, these symptoms often improve first and potentially immediately. Daytime pain and paresthesias improve on a less predictable timetable and may never fully resolve. The patient's strength is usually the last thing to improve, and full recovery of strength is usually incomplete. Any preoperative thenar atrophy will likely remain visible. Results for ulnar, radial, and digital nerve procedures at the wrist and hand for athletes are less predictable unless a secondary extrinsic inciting cause is eliminated by the surgery. One of the main reasons for this current unpredictability is the relative paucity of outcomes published for these non–carpal tunnel syndrome neuropathies, especially when they are idiopathic. Most publications concerning these nerve syndromes are small case series or case reports for particular causes of the peripheral nerve condition.
Fortunately, major complications after carpal tunnel release are relatively uncommon. However, iatrogenic injuries to the median and ulnar nerves, ulnar artery/superficial arch, and digital flexor tendons have been reported for all techniques described in the literature. The surgeon should use the utmost caution during the procedure, to avoid these devastating complications. A more common complication is persistent or recurrent carpal tunnel syndrome. Persistent carpal tunnel syndrome is defined as having unchanged or worse symptoms after surgery, in contrast to patients who have steady improvement as part of the natural history of the postoperative course. True recurrence is defined as having had a significant interval of symptom relief after surgery followed by a return of symptoms. Recurrence, when it does happen, usually does not occur until the late postoperative period (often years after surgery). Recurrence or persistence rates vary between 1% and 10% over the lifetime of the patient after carpal tunnel release.
Severe and acute worsening of median nerve symptoms after carpal tunnel release may be due to direct median nerve injury, hematoma, or infection. If these early causes of median nerve compromise are evident, an urgent return to the operating room is strongly recommended.
Infection after carpal tunnel release is rare, and routine administration of preoperative antibiotics is unnecessary. Administration of preoperative antibiotics for clean hand procedures generally also can be considered for patients who smoke or have diabetes and for procedures that may last longer than 2 hours. However, even for these potential indications for use of preoperative antibiotics in higher risk patients, it is currently unknown if antibiotic use actually helps to prevent infection. Postoperative infections are treated with oral antibiotics and local wound care if they are superficial and with operative débridement if they are deep or nonresponsive to nonoperative care. Wound dehiscence occurs more frequently than true wound infection and heals reliably with moist to dry dressing changes and time. Patients often experience “pillar pain” along the radial and ulnar borders of the carpal canal for several weeks to months after surgery, which is usually considered a consequence, as opposed to a complication, of the procedure.
Nerve compression in the hand and wrist is not a novel concept in the athlete, but there are a few newer diagnostic and therapeutic tools to consider. With regards to diagnosis, nerve US is gaining ground. Multiple studies have compared cross-sectional area of the median nerve with nerve conduction studies to accurately diagnose carpal tunnel syndrome. The use of US may emerge as a more valuable diagnostic tool in this arena and perhaps even replacing electrodiagnostic testing. Magnetic resonance neurography may also become more clinically available as an adjuvant study in the work-up of nerve-related pathology.
With regards to therapeutic advances, surgical techniques and intraoperative adjunctive therapies continue to be developed and evaluated. As previously stated, comparison of endoscopic and open techniques has validated the efficacy and safety of both procedures, future studies will need to determine cost efficacy. Recent work has shown that endoscopic procedures are slightly more expensive in total cost, but full analysis of the sources of extra cost is forthcoming. As an adjunct to the standard techniques, electrical stimulation is showing promise. In a preliminary randomized controlled clinical study, 1 hour of electrical stimulation following carpal tunnel release surgery resulted in accelerated motor and sensory conduction speed and improved thenar musculature reinnervation versus carpal tunnel release surgery only controls. However, these improvements did not correlate to improved clinical functional outcome measures, including Semmes-Weinstein monofilament testing, Purdue Pegboard Test, and Levine Carpal Tunnel Syndrome Questionnaire.
Early recognition and response to symptomatology will likely remain the best safeguard to prevent permanent and often debilitating neurologic deterioration regardless of the cause. Future studies should also help to delineate the most cost-effective and time-effective evaluation and management options for compressive neuropathies in the general population and among athletes or other patients who require or seek the most rapid recuperation with the highest function possible.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here