Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neuromuscular blocking agents (NMBAs) interrupt the usual transmission of nerve impulses at the neuromuscular junction (NMJ) by one of two mechanisms to interfere with neuromuscular transmission and produce paralysis of skeletal muscles. On the basis of differences in their mechanisms of action, these drugs can be classified as depolarizing NMBAs (which mimic the actions of acetylcholine [ACh] at the acetylcholine receptor [AChR]) and nondepolarizing NMBAs (which interfere with the actions of ACh by blocking binding sites on the AChR). Nondepolarizing NMBAs are further subdivided into long-, intermediate-, and short-acting drugs ( Table 11.1 ) based on their duration of action. Succinylcholine (SCh) is the only depolarizing NMBA that is used clinically.
| Mechanism of Action | Neuromuscular Blocking Agent | Duration of Action |
|---|---|---|
| Depolarizing | Succinylcholine | Ultrashort-acting |
| Nondepolarizing | Pancuronium | Long-acting |
| Vecuronium | Intermediate-acting | |
| Rocuronium | Intermediate-acting | |
| Atracurium | Intermediate-acting | |
| Cisatracurium | Intermediate-acting | |
| Mivacurium | Short-acting |
This chapter will describe the choice of NMBA, dosing of NMBAs, monitoring depth of neuromuscular blockade (NMB), and pharmacologic reversal of NMB.
NMBAs are used of NMBAs in the operating room to facilitate endotracheal intubation and optimize surgical conditions. They may also be administered in other clinical settings to facilitate emergent intubations, and to facilitate mechanical ventilation of intubated patients. NMBAs do not have analgesic or anesthetic effects and should not be used to render an inadequately anesthetized patient immobile. Patient airways must be secured and ventilation supported once an NMBA has been administered and this support continued until muscle strength has returned to baseline (a train-of-four ratio [TOFR] ≥0.9). Intraoperative evaluation of depth of NMB is monitored by the muscular response to stimulation of a peripheral nerve, and the results of monitoring are used to determine whether additional NMB is necessary and how to dose reversal agents.
The choice of NMBA is influenced by its speed of onset, duration of action, route of elimination, and associated adverse effects. The rapid onset and brief duration of skeletal muscle paralysis, characteristic of SCh-induced NMB, are useful when tracheal intubation is the only reason for administering an NMBA. Although SCh could be given intermittently for shorter procedures, doing so is associated with an increased risk of bradycardia, asystolic cardiac arrest, and prolonged duration of NMB. For intubation and maintenance of NMB, administration of short- or intermediate-acting nondepolarizing NMBAs may be appropriate. The choice of long-, intermediate-, or short-acting agent will depend on the duration of NMB that is necessary and the anticipated means of administration of the NMBA (intermittent boluses or an infusion). The choice of specific NMBA in each of these categories will also depend on availability of the NMBA, the presence of patient comorbidities, and plans for antagonism of residual NMB.
The NMJ consists of a prejunctional motor nerve ending separated from the highly folded postjunctional membrane of the skeletal muscle by a synaptic cleft ( Fig. 11.1 ). Nicotinic acetylcholine receptors (nAChRs) are located at prejunctional and postjunctional sites. Neuromuscular transmission is initiated by arrival of an impulse at the motor nerve terminal with an associated influx of calcium ions and subsequent release of the ligand ACh. ACh binds to AChRs (the ligand-gated channel) on the postjunctional muscle membrane, opening the AChR channel and causing a change in membrane permeability to ions through the cell membrane, principally potassium and sodium. This change in permeability and movement of ions causes a decrease in the transmembrane potential from about −90 mV to −45 mV (threshold potential), at which point a propagated action potential spreads over the surfaces of skeletal muscle fibers and leads to muscular contraction. ACh is rapidly hydrolyzed at the NMJ (within 15 ms) by the enzyme acetylcholinesterase (true cholinesterase) (AChE), so that it can no longer bind with the nAChR, restoring membrane permeability (repolarization) and preventing sustained depolarization.
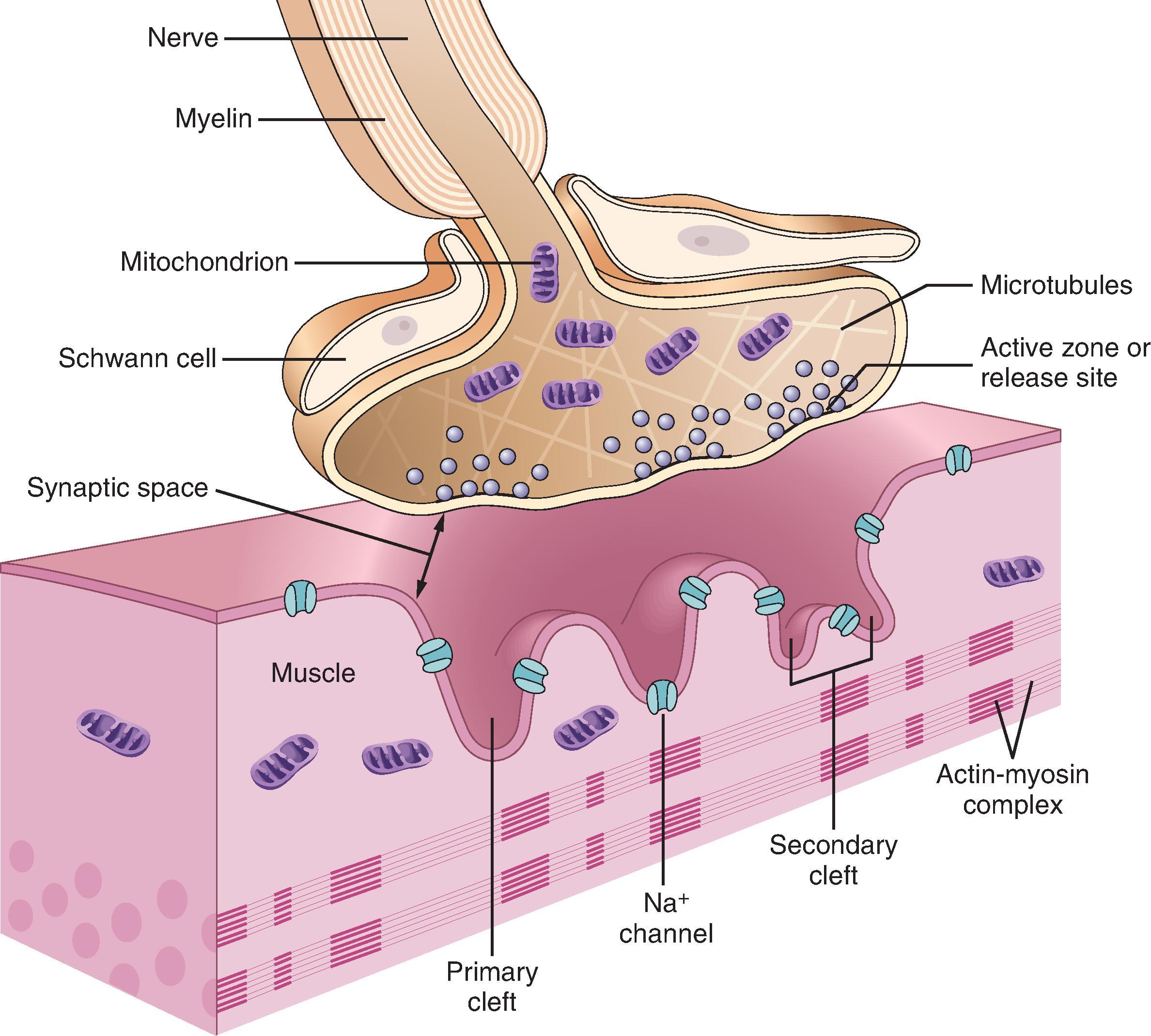
The release of ACh in response to neural stimulation is a calcium-dependent process, resulting in the release of 50 to 100 vesicles containing ACh. The connection between the impulse and depolarization and the rise in intracellular calcium is a voltage-gated calcium channel, which allows for intracellular movement of calcium in response to an electrical current. In response to the increase in intracellular calcium, the ACh-containing synaptic vesicles move to release sites on the nerve terminal membrane. The release of ACh from the synaptic vesicles is mediated by a series of proteins. The protein synapsin anchors the ACh vesicle to the release site of the nerve terminal through the formation of a ternary complex with syntaxin during depolarization and calcium entry. Assembly of the ternary complex forces the vesicle in close apposition to the nerve membrane at the active zone of the nerve terminal with release of its contents, ACh. The fusion is disassembled once the ACh has been released, and the vesicle is recycled.
Small amounts of ACh are released from nerve terminals without a nerve impulse. These result in miniature end-plate potentials (MEPPs). Although not large enough to cause a muscle depolarization, MEPPs are diminished by the administration of nondepolarizing NMBAs and increased by the administration of anticholinesterases.
Synchronized release of ACh from the synaptic vesicles of the nerve terminal in an amount adequate to generate an action potential occurs in response to an electrical impulse. The rapid release of ACh once an impulse arrives at the motor nerve terminal indicates that only those vesicles close to the membrane of the nerve terminal can participate in the process of exocytosis. With repetitive stimulation, vesicles are moved toward the motor nerve terminal for subsequent release, accounting for the post tetanic potentiation observed during NMB.
Not all of the released ACh binds to the AChR of the motor end plate. Most of it is hydrolyzed by AChE at the NMJ. The AChE is anchored to the basal lamina of the postjunctional membrane. Although firmly anchored to the motor end plate region, its catalytic sites have ready access to ACh in the junctional cleft. A different cholinesterase, butyrylcholinesterase, which metabolizes SCh, does not exist in the synaptic cleft of the NMJ.
Cholinergic receptors are found presynaptically, at the motor nerve terminal, and at the postsynaptic region of the NMJ. The prejunctional receptors are involved in the modulation of the release of ACh into the NMJ. Prejunctional nicotinic receptors are activated by ACh and are believed to function in a positive-feedback control system that serves to maintain the availability of ACh when demand for it is high. They are involved with the mobilization of ACh, but not the actual process of its release.
Presynaptic receptors, aided by calcium, facilitate replenishment of the motor nerve terminal. In addition to being stimulated by ACh, they are stimulated by SCh and neostigmine and depressed by small doses of nondepolarizing NMBAs. Inhibition of these presynaptic nAChRs explains the fade in response to high-frequency repetitive stimulation such as tetanic or even train-of-four (TOF) stimulation.
The postjunctional, mature AChR is an intrinsic membrane glycoprotein with five distinct subunits ( Fig. 11.2 ): two α, one β, one δ, and one ε. The α-subunits contain the major portion of the ACh receptor binding site. Each of the subunits contains four helical domains, M1 to M4, that traverse the cell membrane. The ion channel of each subunit has permeability that is equal to that of Na + and K + , allowing for the flow of ions across the cell membrane along their concentration gradients, with Na + entering and K + leaving the muscle cell. Calcium contributes ≈2.5% to the total permeability. This flow of ions is the basis of normal neuromuscular transmission. There are two distinct pockets for binding of ACh formed by the N and C termini of the protein. One occurs at the intersection of the α to ε subunits and the other at the intersection of the α to δ subunits. These binding sites have different affinities for different NMBAs. Occupation of one or both α-subunits by a nondepolarizing NMBA causes the ion channel to remain closed, in spite of the release of ACh from the prejunctional neuron so that the ion flow to produce depolarization cannot occur. SCh binds to the AChR binding sites and acts as an agonist, causing the ion channel to remain open (mimicking ACh) and resulting in prolonged depolarization. Its duration of action is longer than that of ACh because it remains in the NMJ available to bind to the AChR until it diffuses away from the NMJ, where it is metabolized by butyrylcholinesterase. SCh is not a metabolite for AChE.
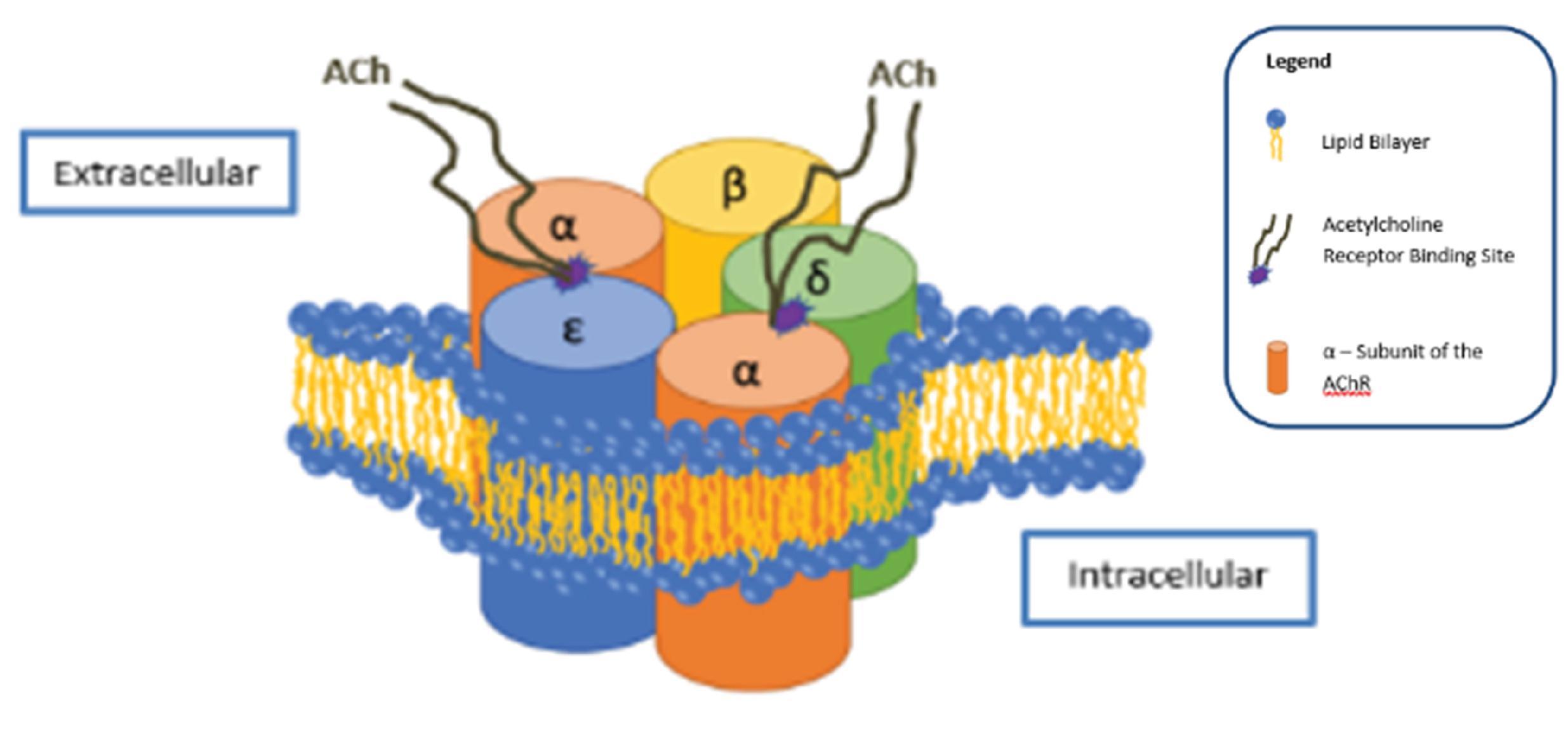
Nondepolarizing NMBAs may, in addition to competitively binding to the AChR, inhibit neuromuscular transmission by occluding the AChR channel preventing the normal flow of ions required for subsequent depolarization. NMB secondary to occlusion of channels is resistant to drug-enhanced antagonism with anticholinesterase drugs. The lipid environment around cholinergic receptors can be altered by volatile anesthetics, which change the properties of the ion channels. This may account in part for the augmentation of NMB by volatile anesthetics.
Extrajunctional receptors are different in structure than the postjunctional nAChRs. They retain the two α-subunits but have γ-unit replacing the ε-subunit. Additionally, whereas postjunctional receptors are confined to the area of the end plate of skeletal muscle that is opposite the prejunctional motor neurons (as a component of the motor end plate), extrajunctional receptors are present throughout skeletal muscles. Extrajunctional receptor synthesis is normally suppressed by neural activity. Prolonged inactivity, sepsis, skeletal muscle denervation, burn injury, or trauma may be associated with a proliferation of extrajunctional receptors. When activated, extrajunctional receptors stay open longer and permit more ions to flow across the muscle cell membrane, which in part explains the exaggerated hyperkalemic response when SCh is administered to patients with denervation or burn injury. Proliferation of these receptors also accounts for the resistance or tolerance to nondepolarizing NMBAs, which can be observed in patients with burns or prolonged immobilization. ,
NMBAs are quaternary ammonium compounds with at least one positively charged nitrogen atom ( Fig. 11.3 ) that will bind to one or both of the binding sites present on the postsynaptic cholinergic receptors. In addition, these compounds have structural similarities to the endogenous neurotransmitter ACh. For example, SCh is two molecules of ACh linked by methyl groups. The long, slender, flexible structure of ACh allows it to bind to and activate cholinergic receptors. Although the bulky rigid nondepolarizing NMBAs contain structural elements similar to ACh, they do not activate cholinergic receptors.
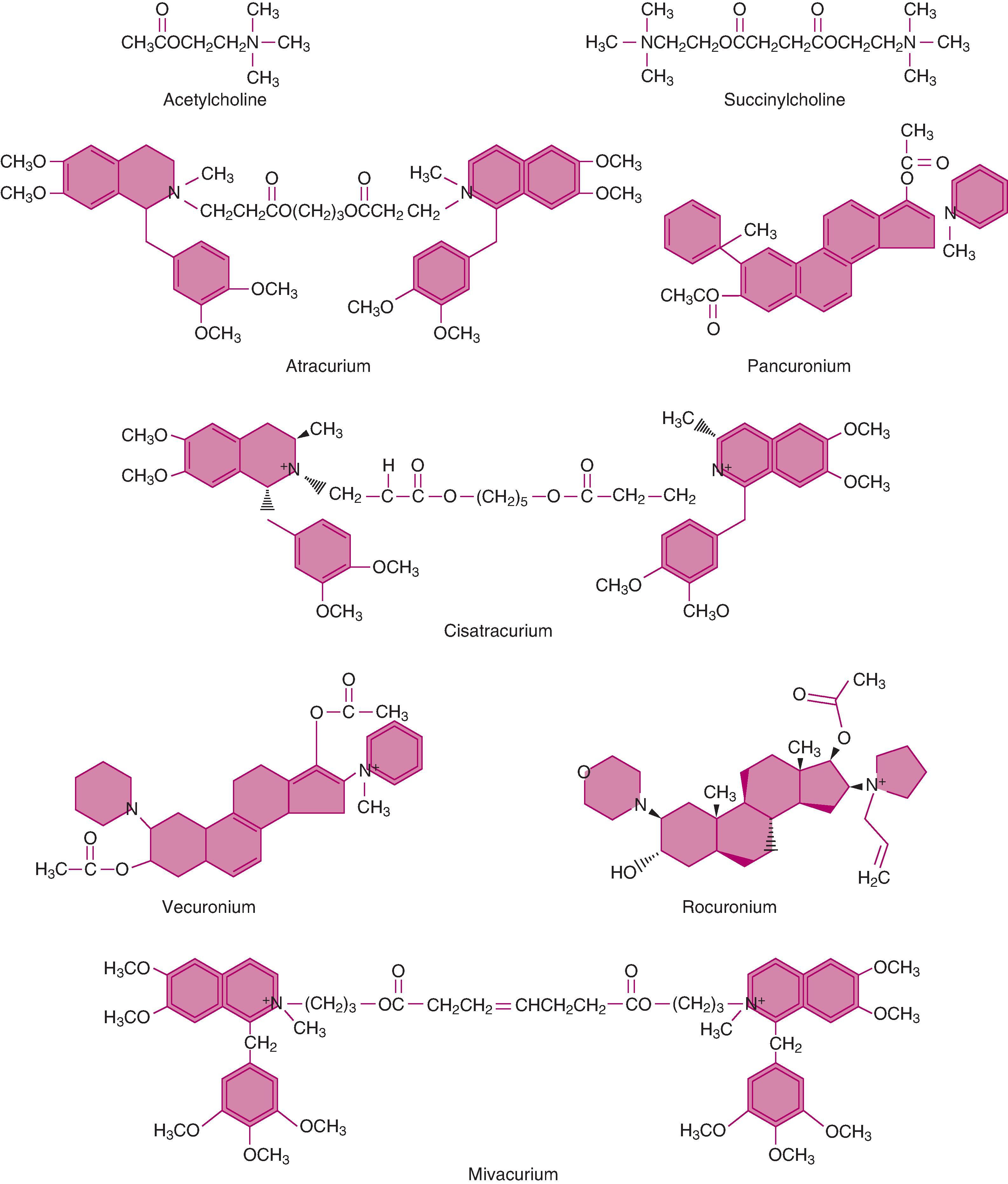
Nondepolarizing NMBAs are either aminosteroid compounds (pancuronium, vecuronium, rocuronium) or benzylisoquinolinium compounds (atracurium, cisatracurium, mivacurium). Pancuronium is the bisquaternary aminosteroid NMBA most closely related to ACh structurally. The ACh-like fragments of pancuronium give the steroidal molecule its high degree of neuromuscular blocking activity. Vecuronium and rocuronium are monoquaternary analogs of pancuronium. Their different structures account for the differing means of elimination of the amino steroidal NMBAs and the benzylisoquinolinium NMBAs from the body ( Table 11.2 ).
| NMBA | Hepatic | Renal | Metabolism | |||||
| Unchanged, Biliary Excretion | Degradation | % | Means of Elimination | |||||
| Pancuronium | 5-10% | 10% | 80% | |||||
| Vecuronium | 40-75% | 20-30% | 15-25% | |||||
| Rocuronium | 50-70% | 10-20% | 10-25% | |||||
| Atracurium | 10-40% | 60-90% | Hofmann Elimination, Nonspecific Esterases | |||||
| Cisatracurium | 16% | 77% | Hofmann Elimination | |||||
| Mivacurium | <5% | 95-99% | Butyrylcholinesterase | |||||
SCh mimics the action of ACh and produces a sustained depolarization of the postjunctional membrane. Skeletal muscle paralysis occurs because a depolarized postjunctional membrane and inactivated sodium channels cannot respond to subsequent release of ACh. Depolarizing NMB is also referred to as phase I blockade . Phase II blockade is present when the postjunctional membrane has become repolarized but still does not respond normally to ACh (desensitization NMB). The mechanism of phase II blockade is unknown but may reflect the development of nonexcitable areas around the end plates that become repolarized but unable to promote the spread of impulses initiated by ACh. With the initial dose of SCh, subtle signs of a phase II blockade begin to appear (fade to tetanic stimulation). Phase II blockade, which resembles the blockade produced by nondepolarizing NMBAs, predominates when the intravenous dose of SCh exceeds 3 to 5 mg/kg.
SCh is the only depolarizing NMBA used clinically. Its mechanism of action and its relatively rapid metabolism ( Fig. 11.4 ) are responsible for its unique pharmacodynamic properties, which include both a rapid onset and an ultrashort duration of action. Typically, doses of 0.5 to 1.5 mg/kg are administered intravenously to produce a rapid onset of skeletal muscle paralysis (30 to 60 seconds) that lasts 5 to 10 minutes. These pharmacodynamic characteristics are useful in a compound that is administered to facilitate tracheal intubation. SCh has been used clinically for more than 60 years, and despite consistent industrial efforts, no NMBA has been developed that has these pharmacodynamic properties. Although an intravenous dose of 0.5 mg/kg may be adequate, larger doses are commonly administered to facilitate tracheal intubation. If a subparalyzing dose of a nondepolarizing NMBA (pretreatment with 5% to 10% ED95—the dose that, on average, will cause 95% suppression of muscle response to neural stimulation) is administered 2 to 4 minutes before administration of SCh to blunt fasciculations, the dose of SCh should be increased by about 70%. It is important to remember that the administration of even small, precurarizing or defasciculating doses of NMBAs can cause symptomatic muscle weakness and put patients at risk of difficulty breathing or swallowing.
The sustained depolarization produced by the initial administration of SCh is initially manifested as transient generalized skeletal muscle contractions known as fasciculations . The sustained opening of sodium channels produced by SCh is associated with leakage of potassium from the interior of cells sufficient to increase plasma concentrations of potassium by about 0.1 to 0.4 mEq/L in a healthy patient. In patients with denervation, burns, or trauma, where there is likely to be proliferation of extrajunctional nAChRs and damaged muscle membranes, many more channels will leak potassium, causing hyperkalemia.
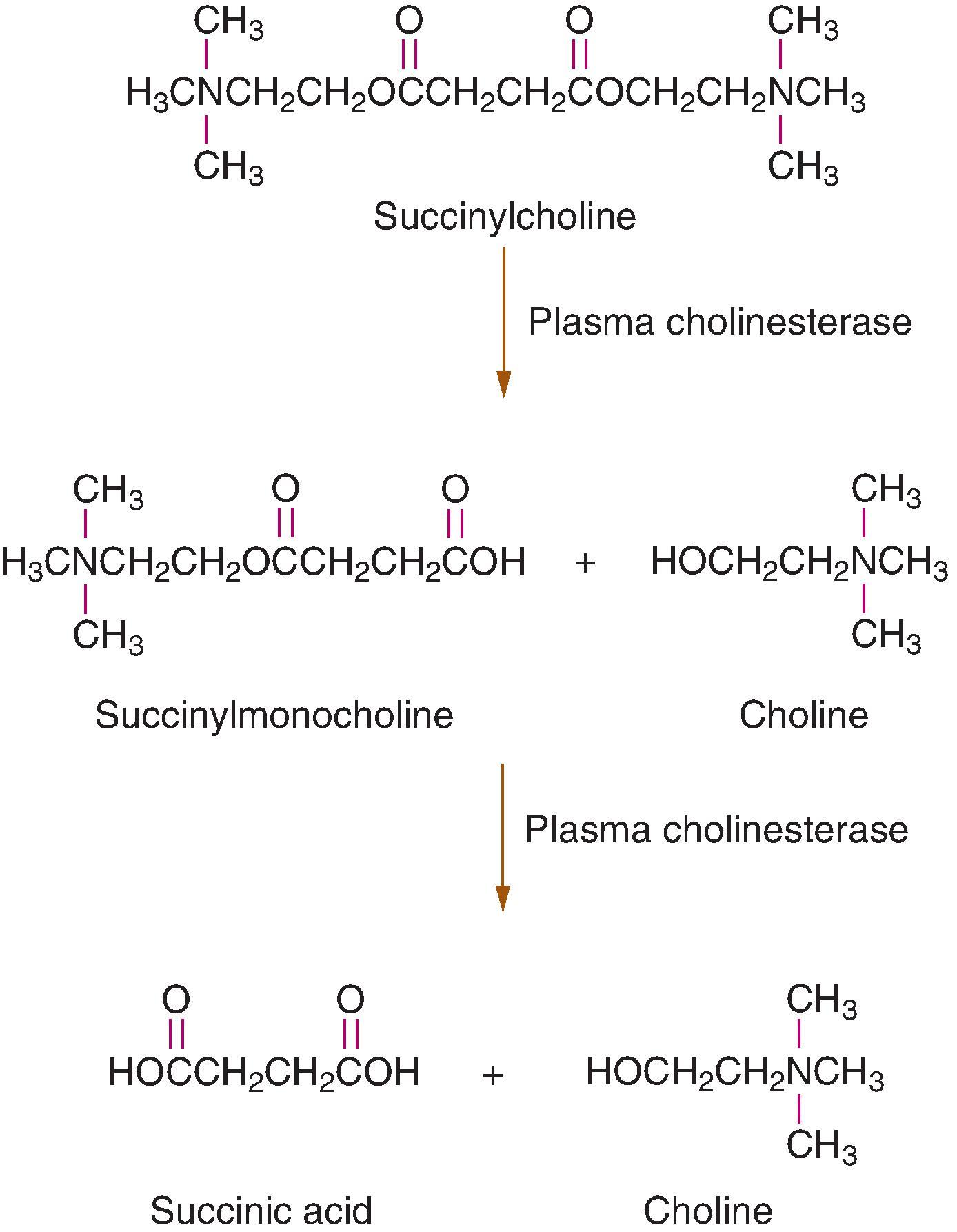
Hydrolysis of SCh to inactive metabolites is accomplished by plasma cholinesterase (pseudocholinesterase, butyrylcholinesterase), which is produced in the liver. Plasma cholinesterase has an enormous capacity to hydrolyze SCh at a rate rapid enough that only a small fraction of the original intravenous dose of SCh reaches the NMJ. Because plasma cholinesterase is not present at the NMJ, the NMB produced by SCh is terminated by its diffusion from the NMJ into plasma. Therefore plasma cholinesterase influences the duration of action of SCh by controlling the amount of SCh that is hydrolyzed before it reaches the NMJ. Liver disease must be severe before decreases in the synthesis of plasma cholinesterase are sufficient to prolong the effects of SCh. Anticholinesterases, as used in the treatment of myasthenia gravis, and certain chemotherapeutic drugs (nitrogen mustard, cyclophosphamide) may decrease plasma cholinesterase activity enough that prolonged skeletal muscle paralysis follows the administration of SCh.
Atypical plasma cholinesterase lacks the ability to hydrolyze ester bonds in drugs such as SCh and mivacurium. The presence of this atypical enzyme is often recognized only after an otherwise healthy patient experiences prolonged skeletal muscle paralysis (>1 hour) after the administration of a conventional dose of SCh or mivacurium. Subsequent determination of the dibucaine number permits diagnosis of the presence of atypical plasma cholinesterase. Dibucaine is an amide local anesthetic that inhibits normal plasma cholinesterase activity by about 80%, whereas the activity of atypical enzyme is inhibited by only 20% ( Table 11.3 ). The dibucaine number reflects the quality of plasma cholinesterase (ability to metabolize SCh and mivacurium) and not the quantity of enzyme that is circulating in plasma. For example, decreases in plasma cholinesterase activity because of liver disease, advanced age, pregnancy, or treatment with an anticholinesterase are often associated with a normal dibucaine number.
| Variants of Plasma Cholinesterase | Incidence | Dibucaine Number (% inhibition of enzyme activity) | Duration of SCh-Induced Neuromuscular Blockade (min) |
|---|---|---|---|
| Homozygous, typical | Normal | 70–80 | 5–10 |
| Heterozygous | 1/480 | 50–60 | 20 |
| Homozygous, atypical | 1/3200 | 20–30 | 60–180 |
In spite of its utility in facilitating tracheal intubation, SCh has many adverse effects ( Box 11.1 ). Adverse side effects after the administration of SCh are numerous and may limit or even contraindicate the use of this NMBA in certain patients. SCh should not be given to patients more than 24 hours after major burns, trauma, and extensive denervation of skeletal muscles because it may cause acute hyperkalemia and cardiac arrest. When administered to boys with unrecognized muscular dystrophy, SCh has resulted in acute hyperkalemia and cardiac arrest. Because of this, the U.S. Food and Drug Administration (FDA) issued a warning against the use of SCh in children, except for emergency control of the airway. When administered with volatile anesthetics in susceptible patients it can trigger malignant hyperthermia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here