Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Injury to other organ systems may have a direct or remote effect on the neurologic system. Hepatic, cardiac, pancreatic, and renal dysfunction can directly affect neurologic transmission and function through a variety of mechanisms. This chapter will examine what is currently understood about the immediate and long-term effects of critical illness on both the central and peripheral nervous systems. Methods to examine, prognosticate, and potentially treat these processes are important for all clinicians.
Septic encephalopathy is a nebulous term used to describe the encephalopathy encountered in the critically ill patient. It excludes patients that have direct neurologic injury, indirect effects of other failing organ systems, or exogenous toxins. Thus, it is a diagnosis of exclusion. The terminology itself is confusing since sepsis is common but is not necessary for the diagnosis. Historically defined as “altered brain function due to the presence of microorganisms or toxins in the blood,” this definition is not entirely accurate since bacteria are not often identified and exogenous substances are excluded from the definition. Sepsis-associated encephalopathy has now been generally accepted as the most appropriate terminology.
Sepsis is common and accounts for the majority of deaths in an intensive care unit. Mortality remains high despite recent advances in treatment. The encephalopathy associated with sepsis has an even worse prognosis. Studies report up to 71 percent of critically ill patients develop an encephalopathy. The presence and severity of the encephalopathy have significant impact on both immediate mortality and long-term cognitive capabilities.
A response to an infection is initiated when pattern-recognizing receptors on macrophages bind cellular components of microbial cell walls. Binding of these receptors initiates a signaling cascade, via the nuclear factor–κβ system, to release cytosolic factors which translocate to the nucleus. These translocated factors activate gene transcription for proinflammatory cytokines [tumor necrosis factor (TNF), and interleukin-1 (IL-1)], chemokines, intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1, and nitric oxide. These activated factors subsequently attract polymorphonuclear leukocytes (PMNs) to the site of the injury. PMNs release local substances which account for the changes seen with inflammation (erythema, edema, warmth).
Inflammation is then regulated through a balance between proinflammatory cytokines released by macrophages and anti-inflammatory factors released by PMNs. This balance typically will contain an infection locally. Sepsis occurs when the response to an infection generalizes beyond local boundaries to affect remote organ systems. The systemic inflammatory response syndrome (SIRS) reflects a similar process that is not initiated by an acute infection. Systemic release of proinflammatory cytokines, predominantly IL-1 and TNF, can become self-sustaining, leading to systemic inflammation, complement activation, and stimulation of both coagulation and fibrinolytic cascades. All of the above substances and features have been shown to be extensively involved in the development of sepsis and multiorgan failure in multiple animal models. Various single-nucleotide polymorphisms have been demonstrated to increase genetic susceptibility to sepsis.
Sepsis affects all organ systems primarily through its effect on the endothelium of vascular tissues. Sepsis induces both vasodilation (most likely through nitric oxide) and myocardial depression, leading to significant hypotension. Regional circulation is altered, affecting appropriate distribution to ischemic organs. Endothelial dysfunction leads to increased vascular permeability with subsequent tissue edema. Decreased functional capillaries at the microcirculatory level may be due to extrinsic compression from tissue edema or secondary to intravascular coagulation. Red cell deformability is decreased in sepsis, which further inhibits oxygen delivery.
Oxygen utilization at the mitochondrial level is directly affected by proinflammatory mediators which cause direct mitochondrial dysfunction, most likely through direct inhibition of respiratory chain enzymatic function. Oxidative stress damage is believed to account for an increase in mitochondrial breakdown products. Direct cell death may therefore be attributed to cellular inability to utilize oxygen. Secondary cell death through apoptosis is typically decreased in sepsis. The net effect however may be to worsen apoptosis as septic mediators and proinflammatory cytokines delay apoptosis in macrophages and neutrophils.
The endothelial and microvascular effect of sepsis affects all organs. Subsequent hypoxia from pulmonary fibrosis, uremia from renal failure, or alterations in amino acid metabolism from hepatic failure, all may contribute to the encephalopathy commonly encountered in septic patients. However, an encephalopathy can develop early in the course of sepsis or SIRS prior to the development of the effects of secondary organ failure. While the exact source and mechanism of how neurologic dysfunction occurs with inflammation is unknown, it is generally believed that septic mediators, primarily proinflammatory cytokines and complement factors, gain access to the brain neuropil leading to functional disruption of neurologic transmission and eventual cell damage and death.
How these factors gain access to the brain has been an area of considerable study. Animal experiments have supported a direct toxic effect of some mediators to the integrity of the blood–brain barrier (BBB). Neonatal kittens exposed to Escherichia coli lipopolysaccharide develop diffuse cerebral white matter disruption with noted extravasation of Evans blue dye throughout the brain. Similarly, horseradish peroxidase is transported retrograde into septic rat neuropil demonstrating a patchy disruption of the BBB. More recently, lipopolysaccharide exposures have been linked to direct transport of cytokines across the BBB. Inflammatory mediators may also gain direct access to the brain through the circumventricular organs located around the midline ventricular system.
However, the exact factors that increase BBB permeability are unknown. Proinflammatory molecules are increased in patients with septic-associated encephalopathy and increase the permeability of endothelial cells through pinocytosis. The loss of BBB integrity leads to perimicrovascular edema with disruption of astrocytic end feet. Similar findings have been reported with complement activation and inflammatory cytokines. Evidence from murine models implicate TNF, anaphylaxin C5a, and the cytokines IL-6 and IL-1b as mediators of BBB disruption. ICAM is increased on microvessels in sepsis and mediates adherence and entry of leukocytes into the brain.
Once inflammatory mediators have gained access to the brain parenchyma, the results of these septic mediators are similar to their effects on other organs. Activated leukocytes generate free radicals that react with erythrocyte membranes causing increased swelling. This process can lead to cerebral hypoperfusion and may contribute to the encephalopathy. Inflammatory mediators in the brain as in other organs may impair oxygen extraction, utilization, or both at the mitochondrial level. Thus decreased cerebral blood flow in sepsis may be an expected response to decreased cerebral metabolism.
Cerebral ischemia itself, however, does appear to be present in sepsis. Dead neurons have been identified in septic pigs with maintained cerebral perfusion, and microvascular lesions are commonly found on imaging and pathology. Brain apoptosis in sepsis has been documented in exposure to lipopolysaccharide, presumably due to mitochondrial dysfunction. Postmortem studies have shown inducible nitric oxide synthase leadng to neural apoptosis in the hippocampus and cardiovascular centers. Neuronal damage is most evident in the highly metabolic neurons of the hippocampus.
An increased production of nitric oxide has also been postulated to contribute to the development of the encephalopathy. In several animal models, tumor necrosis factors increase the formation of intracellular nitric oxide. While the exact mechanism is unknown, several putative mechanisms involve the release of glutamate from both neurons and astrocytes, leading to excitotoxic death mediated through N -methyl- d -aspartate (NMDA) receptors; NMDA receptor blockade has limited the long-term cerebral effects of sepsis in animal models. Nitric oxide may also alter cerebral blood flow and metabolism and coupling as it is also an inhibitor of mitochondrial respiration. Neurotransmission is also impacted in septic-associated encephalopathy. Altered levels of amino acid neurotransmitters have been reported in the rat model of sepsis. Disrupted glutamate transmission appears to be particularly involved.
The parasympathetic and sympathetic nervous system also may play a role in the development of the encephalopathy. Parasympathetic activity may in part mediate the inflammatory response as increased vagal tone appears to decrease inflammation and improve mortality. Similarly, adrenergic activation appears to produce an anti-inflammatory effect which may be neuroprotective.
The encephalopathy associated with sepsis and inflammation may present acutely or subtly with a slow progression. Disorientation, impaired cognition, and inattentiveness can progress to agitation, delirium, stupor, and coma. Pupils are typically small and minimally reactive. Brainstem reflexes are generally maintained until late in the course. Focal signs are rare but can occur.
The diagnosis remains one of exclusion. The patient should be evaluated for meningitis and infectious or autoimmune/paraneoplastic encephalitides. Similarly, encephalopathies secondary to failure of other organ systems (hepatic, renal, pulmonary, pancreatic) must be evaluated and treated if present. Failure of clearance and metabolism of the sedative medications commonly employed to treat critically ill patients can be delayed in multiorgan failure, thus complicating the evaluation. Cerebral hypoperfusion from cardiac failure can also contribute to encephalopathy.
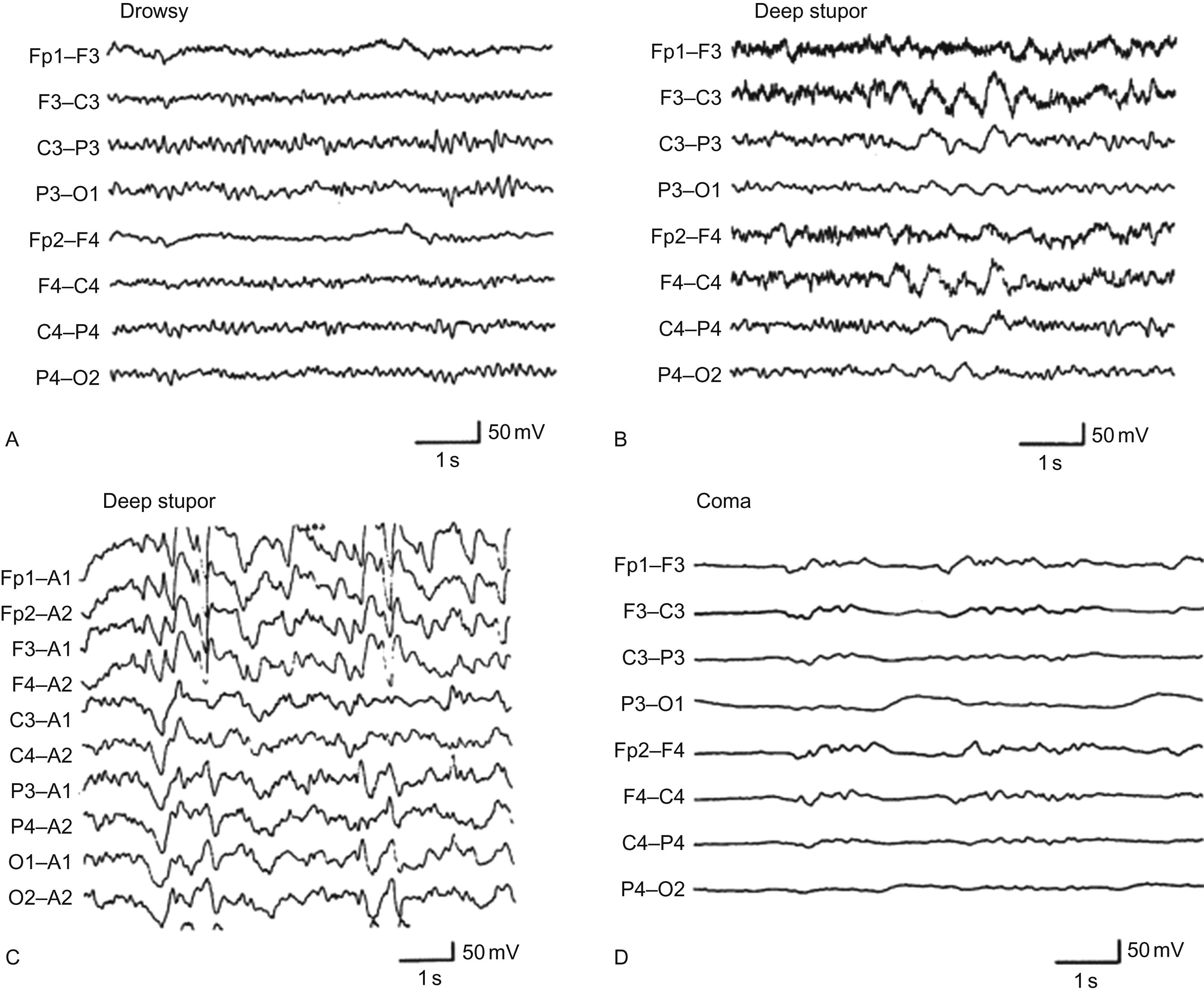
Serum levels of neuron-specific enolase and S-100B are markers of neuronal and astrocytic damage. Both are increased with septic encephalopathy and correlate with worse outcome. The electroencephalogram (EEG) can be normal but becomes slower, progressing from theta to delta activity and finally burst suppression as the encephalopathy worsens ( Fig. 56-1 ). Mortality similarly correlates with worse EEG findings. Nonconvulsive status epilepticus can occur and is probably under-recognized. Continuous EEG monitoring may be beneficial to demonstrate seizures and guide management. Magnetic resonance imaging (MRI) is sensitive to the changes that can occur during sepsis; microhemorrhages and small ischemic lesions are detected in approximately 10 percent of cases; white matter changes are common; and cortical atrophy is noted in long-term cases. Somatosensory evoked potentials, functional MRI, and positron emission tomography require more study as to their use in evaluating the encephalopathy associated with infection.
The long-term neurologic effects of sepsis have come under increasing scrutiny. Mild to moderate neurocognitive deficits have been reported consistently in septic patients and can be persistent. While recovery is possible in younger patients who are less severely affected, global cognitive impairment has been reported in 17 percent of patients evaluated 3 years postevent. Short-term memory appears to be particularly vulnerable. Analyses from long-term epidemiologic studies suggest that early dementia is twice as likely in patients who have an episode of sepsis.
There are no specific treatments for the encephalopathy associated with sepsis and inflammation. Early recognition and treatment of the underlying illness is the most effective therapy.
The multitude of inflammatory mediators that are believed to be involved in the development of the encephalopathy makes development of specific targets of treatment difficult. Inhibitors of nitric oxide synthase have been disappointing in animal studies. Inhibitors of glutamate, antioxidants, and complement factors have shown promise, but their use in humans remains a future direction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here