Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neurocritical care is a discipline devoted to the application of critical care principles to seriously ill patients with acute neurological or neurosurgical conditions and has become one of the most rapidly growing subspecialties of neurology in recent years. Neurological-neurosurgical (or neuroscience) intensive care units (NICUs) are staffed by clinicians with solid knowledge of the principles of intensive care unit (ICU) management (mechanical ventilation, hemodynamic monitoring, nutrition, infection control and antibiotic prescription, general postoperative care, etc.) and specific interest in the treatment of acute neurological and neurosurgical diseases. In-depth knowledge of acute neurology is the sine qua non to mastery of the job.
Patients admitted to a NICU have central or peripheral nervous system dysfunction as a consequence of a primary neurological condition or as a complication of systemic illness. The most common diagnoses encountered in the NICU are acute ischemic strokes, intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), traumatic brain injury (TBI), brain tumors, elevated intracranial pressure (ICP; from any of the previous or other conditions), spinal cord injury, central nervous system (CNS) infections (meningitis, encephalitis, brain abscesses), status epilepticus, neuromuscular respiratory failure, and postoperative care (either after open neurosurgery or an endovascular procedure). Management of each of these conditions demands specific training that focuses on neurological recovery. Principles of general ICU care are applicable but must be adjusted accordingly.
The practice of neurology in the ICU demands specific clinical skills for timely and effective patient assessment. Since it is often impossible to gather direct history from the patient and the neurological examination must necessarily be more focused, attention to detail becomes crucial. Time for examination is very limited in neurological emergencies, and patients are often unconscious, sedated, acutely distressed, or confused and agitated. Physical findings may change rapidly, but a proficient physical examination remains central to determining diagnosis and prognosis in these critically ill patients.
The neurological examination for a NICU patient should always begin by defining the level and content of consciousness. Level of consciousness describes the patient’s degree of arousal or wakefulness. Scales are useful for facilitating communication and monitoring changes over serial examinations; the Glasgow Coma Scale (GCS) is the most widely used ( ). However, it loses accuracy in patients who are intubated or develop cerebral ptosis (inability or only partial ability to open the eyes [by contracting the frontalis muscle] because a brain lesion impairs control of eye-opening mechanisms) and fails to provide information on brainstem function and respiratory status. The FOUR Score addresses these shortcomings, has been validated in various patient populations, and merits consideration as an alternative ( ; Fig. 53.1 ). For patients with localized structural brain diseases, the National Institutes of Health (NIH) Stroke Scale may be used to grade and track focal neurological deficits.
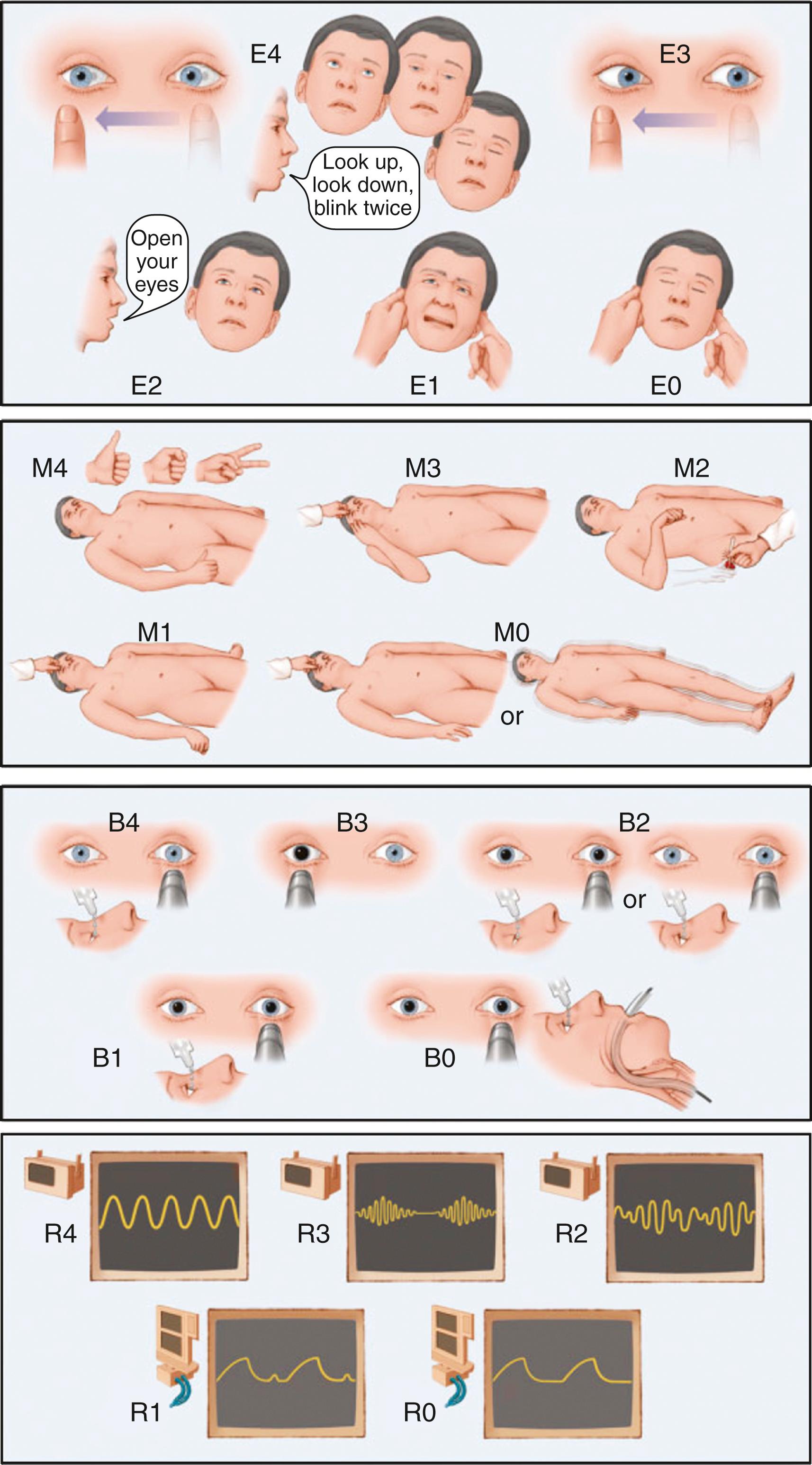
In patients with altered consciousness, the results of one of these scales should be complemented with documentation of additional neurological features. A detailed description of movements of the eyes, gaze deviation, brainstem reflexes (pupillary light reactions, corneal, oculocephalic, oculovestibular, gag, cough), spontaneous movements and motor responses to pain, lateralizing signs, and breathing pattern must be recorded. In patients with delirium, the clinician must note the predominant behavioral abnormalities, degree of motor activity, and ability to interact with the environment. It is always important to dedicate special attention to any abnormal or adventitious movements, since seizures in critically ill patients may present with very subtle motor manifestations (e.g., nystagmoid eye movements). Fundoscopy may also offer valuable information and should be attempted; however, to avoid confounding future pupillary evaluations, mydriatic agents should not be administered. The reader is referred to Chapter 4, Chapter 5 for further information relative to clinical evaluation of comatose and delirious patients.
Another essential aspect of the examination in critically ill patients is evaluating neuromuscular respiratory weakness. Timely recognition of signs of impending neuromuscular respiratory failure may avoid potentially devastating complications. Among them, use of accessory muscles and paradoxical breathing pattern are most indicative of problems. Paradoxical breathing is defined as the loss of synchronicity in chest and abdominal movements during respiration (i.e., abnormal sinking of the abdomen during inspiration) and represents an unequivocal sign of diaphragmatic failure ( ).
It is important to integrate the information provided by the neurological examination with data from the general systemic examination, vital signs monitoring, and other physiological variables, including laboratory results. Alterations in heart rate, respiration, and blood pressure (BP), for example, often result from brain herniation.
Systemic monitoring in the NICU typically includes cardiac telemetry, frequent scheduled noninvasive BP measurements (by automatic cuff inflation) or continuous invasive arterial BP recording, pulse oximetry, and core body temperature. Continuous arterial BP monitoring is accomplished by inserting an indwelling cannula into a medium-caliber artery (e.g., radial arterial line). The invasiveness of the procedure is justified by the precise real-time information it provides. Continuous arterial BP monitoring is especially recommended in patients treated with induced hypertension (e.g., symptomatic vasospasm SAH), cases requiring very strict BP control to avoid hemorrhagic complications (e.g., ruptured arteriovenous malformations), patients with hypotension (e.g., shock), compromised cerebral perfusion pressure (CPP; e.g., TBI with raised ICP), or autonomic instability (e.g., Guillain-Barré syndrome [GBS]). Arterial lines provide the additional advantage of eliminating the need for repeated arterial punctures to measure arterial blood gases. However, although generally safe, placement of an arterial line may be complicated by local infection, leading to bacteremia, or thrombosis with a risk of digital ischemia. Careful attention to proper technique and adherence to strict sterile conditions during placement and manipulation of the catheter are mandatory ( ).
The most accurate method of measuring core body temperature is a pulmonary artery catheter thermistor, but since most patients in the NICU do not require pulmonary artery catheter insertion, bladder or rectal probes are most frequently used. Bladder and rectal probes correlate well with pulmonary artery catheter thermistor readings, but there is a lag in the detection of temperature changes by the probes. The site of temperature recording becomes particularly important in patients treated with cooling measures. Thus monitoring esophageal temperatures is recommended when certain intravascular cooling devices are being used.
Central venous catheters allow monitoring of central venous pressure while also providing access for fluid and drug administration. They are, however, a frequent source of infection. Rigorous sterile techniques at the time of catheter insertion, cutaneous antisepsis with chlorhexidine (rather than povidone-iodine), topical application of anti-infective ointment or a chlorhexidine-impregnated dressing to the insertion site, and catheters with an anti-infective surface may reduce the risk of catheter-related bloodstream infection ( ). The role of pulmonary artery catheters in ICUs is shrinking as studies consistently demonstrate that their use is associated with higher rates of complications without improving patient outcome ( ; ; ). Newer devices for hemodynamic monitoring have become available. For instance, the Pulse index Continuous Cardiac Monitoring (PiCCO) system integrates static and dynamic hemodynamic data using a combination of transcardiopulmonary thermodilution and pulse contour analysis ( ); others, such as the Non-invasive Volume Management to Guide Clinical Decision Making (NICOM) device, use proprietary formulas to determine cardiac parameters (e.g., stroke volume, stroke volume variation, etc.) that correlate with thermodilution-obtained clinical information ( ). Although these devices are pathophysiologically sound, their value in improving patient care remains to be firmly established in critical care patients in general and neurocritical care patients in particular.
The neurological examination may lack sensitivity in critically ill patients who have depressed levels of consciousness due to brain disease or from the effect of sedative medications. Brain monitoring methods developed and refined over the past several decades may provide additional valuable information in these cases. These techniques offer real-time data, unlike imaging modalities that represent only “snapshots” of the patient’s condition at certain points in time. Therefore brain monitoring techniques are better suited to assess dynamic changes in the neurological status of critically ill patients.
Multiple brain monitoring methods are now available. They are most useful when they are applied in combination, a practice known as multimodality monitoring ( ) . It is important to be aware, however, that the endpoints of most studies validating the use of brain monitoring methods have been surrogate physiological measures rather than actual assessments of patients’ functional outcome. In fact, there is no class I evidence proving that the use of multimodality brain monitoring results in improved clinical outcomes. Currently the clinical application of brain monitoring techniques is restricted to large centers, especially those treating numerous TBI patients.
Methods for cerebral monitoring are divided into three main categories according to their spatial resolution: global, regional, and local brain monitoring ( Table 53.1 ). Global brain monitoring techniques measure ICP, CPP, electrical potentials, and venous oxygen saturation. Regional and local brain monitoring methods include cerebral blood flow (CBF), CBF velocities (BFVs), brain tissue metabolism, temperature, and oxygenation.
| Method | Spatial Resolution | Temporal Resolution | Purpose | Advantages | Disadvantages |
|---|---|---|---|---|---|
| ICP | Global | Continuous | Measure intracranial pressure |
|
|
| Jugular oximetry (SjvO 2 ) | Global | Continuous | Measure adequacy of hemispheric oxygenation |
|
|
| EEG | Global | Continuous | Monitoring electrical brain activity Detection of seizures |
|
|
| SSEP | Global | Continuous | Monitoring integrity of sensory pathways |
|
|
| Bedside Xe-133 CBF | Regional | Discontinuous | Measure hemispheric CBF |
|
|
| Laser Doppler flowmetry | Local | Continuous | Measure cortical CBF |
|
|
| Thermal diffusion flowmetry | Local | Continuous | Measure cortical CBF |
|
|
| TCD | Regional | Continuous | Measure CBF velocities |
|
|
| Brain tissue P o 2 | Local | Continuous | Measure cerebral oxygenation |
|
Invasive Susceptible to artifacts Monitors small volume of tissue |
| NIRS | Local | Continuous | Measure cerebral oxygenation |
|
|
| Microdialysis | Local | Discontinuous | Measure cerebral metabolism |
|
|
The intracranial space is occupied by three constituent compartments: the brain (accounting for 80%–90% of the intracranial volume), the blood, and the cerebrospinal fluid (CSF). Under normal conditions, local CNS pressure gradients are equilibrated if the craniospinal CSF circulation is patent. Because the skull is rigid, any expansion of one of these compartments must be compensated by a reduction in size of the others (a physiological principle known as the Monro-Kellie doctrine ) if ICP is to remain constant. If these compensations are insufficient, ICP rises. Small increases in intracranial volume can initially be accommodated with little or no effect on the ICP, but as more volume is added, intracranial compliance falls until it reaches a critical point beyond which a minimal increase in volume causes an exponential rise in ICP. This pressure-volume relationship is depicted in Fig. 53.2 . In other words, as long as CSF circulation is not obstructed and there remains a pressure gradient from the subarachnoid space to the dural venous sinuses, the initial physiological response to an increase in brain volume is a reduction in the CSF and venous blood volumes by shifting these fluids out of the intracranial space. Once these compensatory mechanisms are exhausted, the system becomes noncompliant and further increases in intracranial volume compromise arterial blood flow and eventually lead to the herniation of brain tissue.
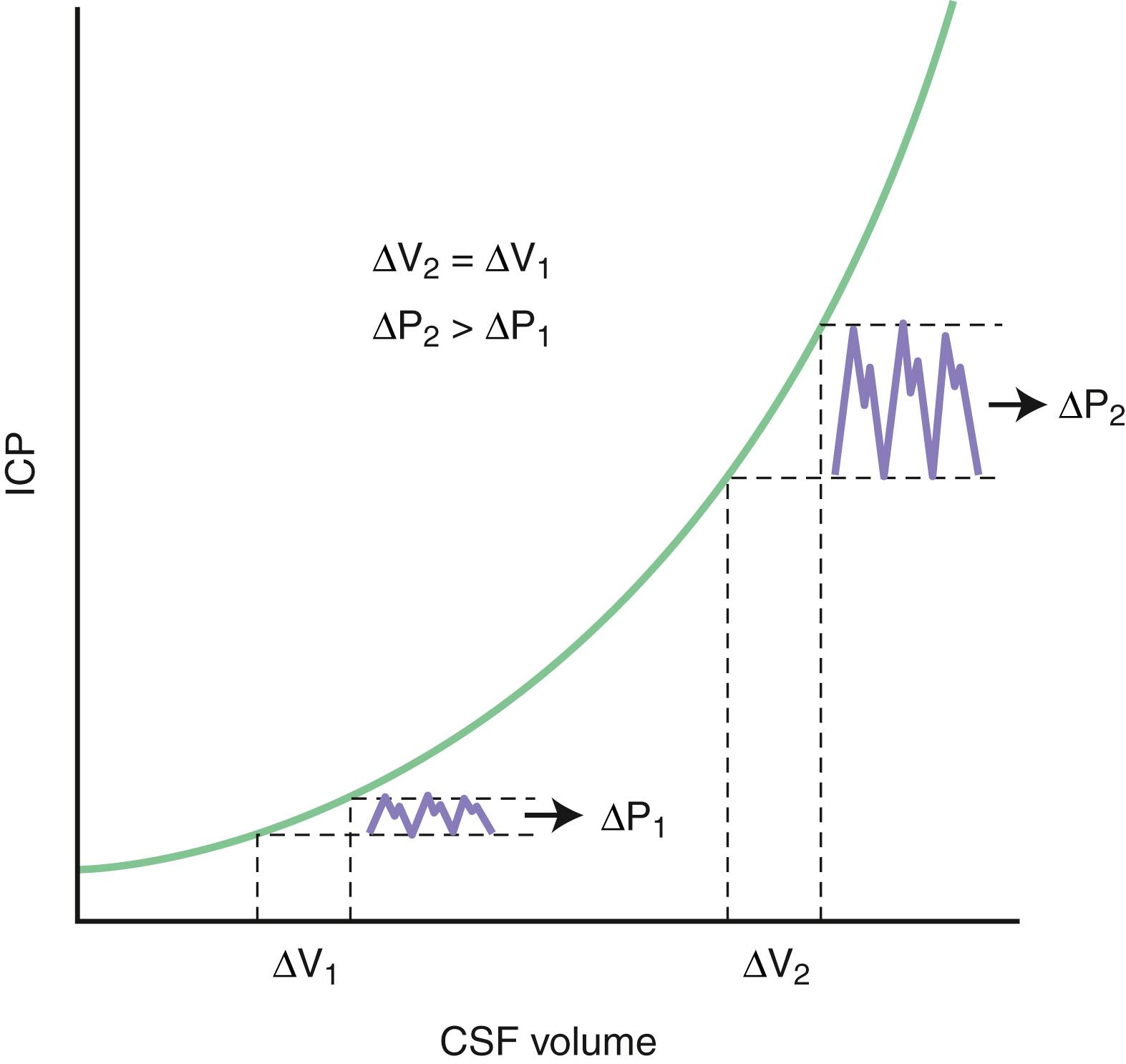
Normal ICP in a supine individual is less than 15 mm Hg when measured at the level of the foramen of Monro (typically referenced to the tragus). Levels exceeding 20–25 mm Hg define a generally accepted threshold of raised ICP, which deserves treatment. Knowing the actual ICP is a prerequisite to determining CPP, which is defined by the relationship between mean ICP and mean arterial pressure (MAP) as follows: CPP = MAP − ICP.
It has also been argued that the main purpose of ICP monitoring is maintenance of adequate CPP because the latter may be more related to secondary ischemic injury ( ). The relative importance of ICP and CPP as main targets of therapy remains a matter of debate.
ICP is pulsatile and the pressure waveforms provide useful information beyond numbers measured. ICP waveforms are made of three distinct components: heart pulse waves, respiratory waves, and slow vasogenic waves (Lundberg B waves), each with a characteristic frequency. The normal ICP waveform consists of three peaks ( Fig. 53.3 ). P 1 , the first and generally the tallest peak, is also known as the “percussion wave.” P 2 (the tidal wave) and P 3 (the dicrotic wave) are normally smaller peaks, and the notch between them corresponds to the dicrotic notch of the arterial waveform.
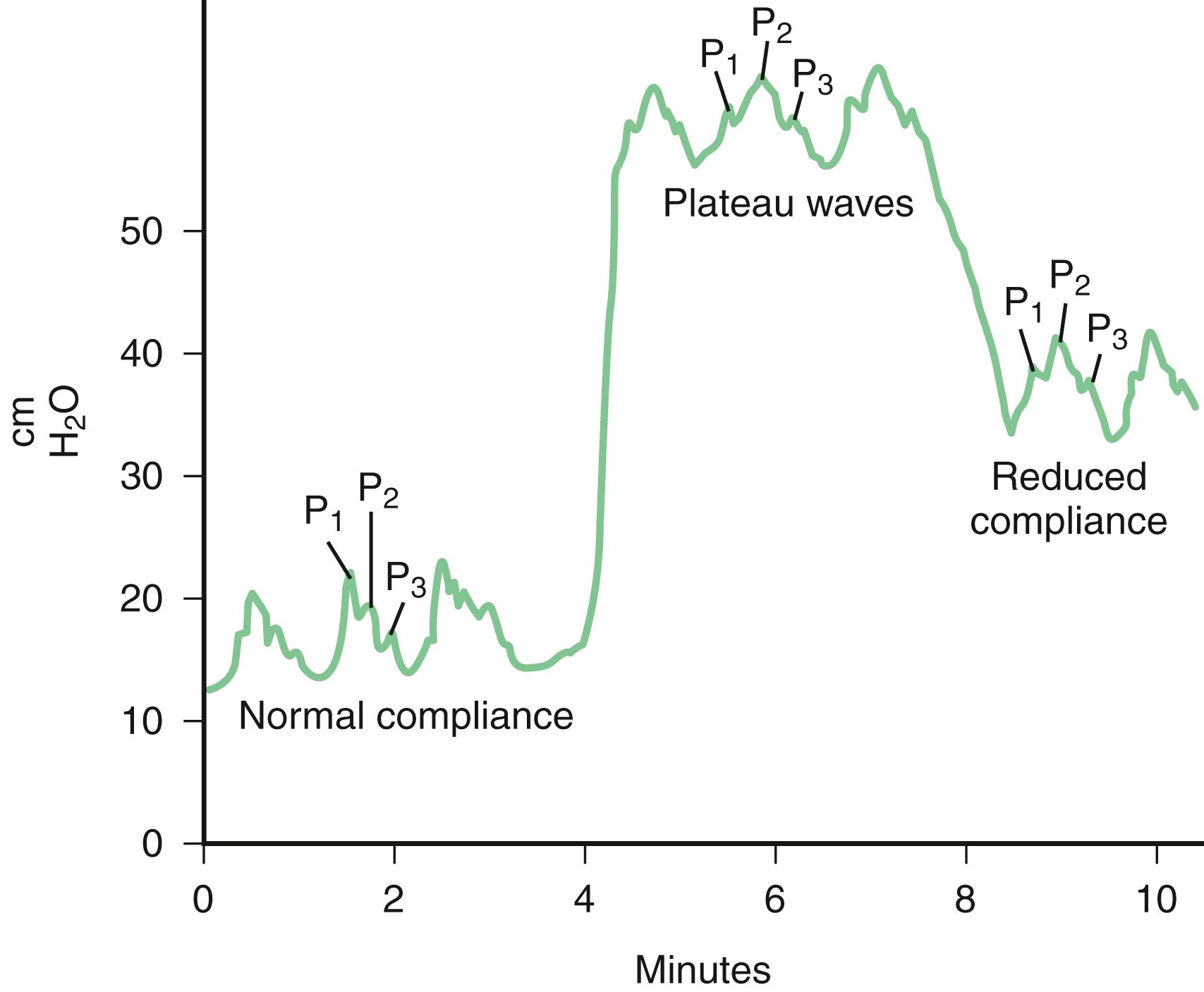
ICP can be monitored using intraparenchymal, intraventricular, epidural, or subdural devices. Intraventricular monitoring remains the gold standard because of its precision. It consists of a ventricular catheter connected to an external transducer that allows continuous ICP readings, as long as the catheter is clamped. Advantages of this technique are the feasibility of repetition measurements and that the measurement corresponds to the transmitted systolic BP. As ICP increases, P 2 and P 3 rise and eventually surpass P 1 . Ultimately, with continued elevation of ICP, the waveform loses distinct peaks and assumes a triangular morphology. Intracranial pathology leading to sustained elevations of ICP may produce plateau waves , also known as Lundberg (see Fig. 53.3 ). These waves reflect a sudden dramatic rise in ICP to levels of 40–100 mm Hg, often lasting 5–20 minutes. Plateau waves indicate critically low intracranial compliance leading to marked changes in ICP, even with very small variations in intracranial volume. Although their pathophysiology is not fully elucidated, plateau waves are thought to be generated by brief episodes of decreased CPP (often caused by systemic hypotension), leading to exaggerated cerebral vasodilation, increased blood volume, and increased ICP ( ). This further decreases CPP and contributes to a detrimental cycle unless broken by a sudden surge of hypertension (Cushing response) or another therapeutic intervention, such as hyperventilation, to cause cerebral arteriolar vasoconstriction.
ICP can be monitored using intraparenchymal, intraventricular, epidural, or subdural devices. Intraventricular monitoring remains the gold standard because of its precision. It consists of a ventricular catheter connected to an external transducer that allows continuous ICP readings as long as the catheter is clamped. Advantages of this technique are the feasibility of repetitive calibration to achieve accurate and reliable ICP measurements and allowing external drainage of CSF for the treatment of raised ICP. Hence, ventricular monitoring is indicated in patients with hydrocephalus and is often preferred in those with refractory intracranial hypertension. Major drawbacks are the difficulty of inserting a catheter in patients with brain edema and small ventricles, a higher risk of infection (the rate of ventriculitis is 3%–8% and it increases with duration of the ventriculostomy; ; ; ), risk of bleeding at the time of catheter placement (especially in patients with underlying coagulopathy or recent use of antithrombotics), and system malfunction (dampening of the waveform may be caused by apposition of the catheter tip against the ventricular wall or obstruction of the catheter by a blood clot or air bubble). Furthermore, ICP monitoring by ventricular catheters allows continuous monitoring of ICP only if the catheter is clamped. Spikes of raised ICP may go undetected by ventricular catheters that are open to drain. Risks may be minimized by careful placement of the catheter and maintenance of the system under strict sterile conditions, use of antibiotic prophylaxis (e.g., cefazolin 2 g every 8 hours from the time of catheter insertion until 24–48 hours after its removal, or use of antibiotic-impregnated catheters; ), and withdrawal of the catheter as soon as possible ( ). Exchange of the catheter every 5 days, although a common practice, does not appear to decrease the risk of infection ( ; ); in fact, repeated catheter insertions have been found to be associated with a higher risk of ventriculitis ( ).
Intraparenchymal fiberoptic monitors are also quite accurate. As compared with intraventricular catheters, the measurements provided by intraparenchymal monitors differ on average by ±2 to 5 mm Hg. Advantages of this monitoring system include simple and safe insertion technique, easy maintenance, continuous ICP measurements, relative lack of substantial drift (even after several days), and low risk of infection. Disadvantages include high cost; technical complications (e.g., breakage of the optical fiber); and, most importantly, inability to drain CSF. Epidural and subdural monitors are less reliable and therefore rarely used. ICP can be estimated using noninvasive techniques such as transcranial Doppler (TCD) to measure changes in arterial or venous blood flow velocity or analyze the pulse waveform, displacement of the tympanic membrane, or diameter of the optic nerve sheath by transorbital ultrasound or magnetic resonance imaging (MRI). Ultrasound technology is an attractive tool as there are essentially no adverse effects with this method, but its use can be substantially limited by technique and provider experience. These methods are not currently precise enough to be used clinically for ICP management decisions but may be useful in the future, particularly for screening and selecting patients for invasive monitoring.
Measuring ICP can provide additional useful information because it allows the calculation of secondary indices about cerebral physiology. For example, the cerebrovascular pressure reactivity index (PRx), a moving correlation coefficient between mean ICP and slow fluctuations in MAP ( ), reflects the ability of smooth muscle within cerebral arteriolar walls to react to changes in transmural pressure. An increase in pressure normally causes cerebral arterial and arteriolar vasoconstriction, which leads to decreased cerebral blood volume and thus decreased ICP. PRx estimates CBF autoregulation status (although it is not synonymous), and its value ranges from −1 to +1. Positive values indicate impaired autoregulation and have been correlated with clinical outcomes in TBI ( ). Another example of an ICP-derived index is RAP, the correlation between the amplitude of ICP to the mean pressure. RAP close to 0 indicates little or no change in the mean ICP in response to increases in volume, which indicates good pressure-volume compensatory reserve. When RAP is close to +1, the amplitude varies directly with ICP, suggesting low compensatory reserve and a shift to the right on the intracranial compliance curve ( ).
The previous Brain Trauma Foundation recommendations for ICP monitoring in patients with severe TBI, a GCS sum score below 9, and an abnormal computed tomography (CT) scan or a normal CT scan with two or more of the following criteria—age older than 40, unilateral or bilateral motor posturing, and systolic BP less than 90 mm Hg—have been less emphasized in the most current management guideline ( ) due to the lack of high-quality evidence to support them. A recent study in South America evaluated the benefit of ICP monitoring—where patients were treated based on their clinical examination and neuroimaging as compared with those with an ICP monitor to target a normal ICP measurement—and found no difference in clinical outcome ( ). The ideal patient who requires ICP monitoring following TBI has yet to be determined, and the decision to place an invasive ICP monitor is at the discretion of the treating neurosurgeon. In patients without TBI, some experts advocate monitoring ICP in comatose patients with a large intracranial mass lesion (hematoma, abscess, large infarctions, etc.) causing a radiologically documented tissue shift. Patients with SAH, ICH, or cerebellar ischemic or hemorrhagic strokes producing acute hydrocephalus typically have their ICP monitored once a ventriculostomy has been placed, although the catheter is often placed primarily for drainage purposes.
Jugular bulb oximetry measures the oxygen saturation of venous blood returning from the brain (normal 50%–65%) by means of a fiberoptic catheter ( ). The main goal of jugular venous oxygen saturation (SjvO 2 ) monitoring is to provide a continuous measure of the changing balance between cerebral oxygen delivery and cerebral oxygen consumption. Simultaneous determination of SjvO 2 using the jugular bulb catheter and arterial oxygen saturation (SaO 2 ) allows for the calculation of the intracranial arteriovenous oxygen difference (AVDO 2 ; normal 24%–42%). Cerebral oxygen consumption can be calculated as the product of AVDO 2 and CBF. The cerebral oxygen extraction rate (O 2 ER) is derived from the ratio of cerebral oxygen consumption to cerebral oxygen delivery.
Jugular venous desaturations denote relative reductions of global cerebral oxygenation. SjvO 2 below 50% for 15 minutes or more is deemed indicative of ischemia. SjvO 2 monitoring has been mostly tested in patients with severe TBI. In these patients, jugular venous desaturations have previously been shown to correlate with the occurrence of secondary brain insults and poor outcome ( ; ). High SjvO 2 should not simply be equated with hyperemia; it may also be associated with poor outcome in comatose patients, possibly indicating lack of oxygen utilization after extensive neuronal death ( ). Favorable experience with jugular bulb oximetry has been reported in patients with SAH and ICH ( ), but interpreting SjvO 2 may be difficult in patients with severe unilateral hemispheric lesions. This technique is also used to monitor cerebral oxygenation during neurosurgical procedures.
Therapeutic interventions in response to information provided by jugular bulb oximetry have been proposed, including adjustment of the degree of hyperventilation, timing and intensity of osmotherapy, adjustment of MAP, and treatment of anemia ( ). There is no proof, however, that these interventions improve functional outcome; therefore jugular bulb oximetry is an uncommonly utilized monitoring method. As shown by the negative results observed in studies testing therapies guided by pulmonary artery catheters, the clinical value of aggressive interventions aimed at optimizing physiological parameters must be proven before we incorporate these into clinical practice.
Advantages of the jugular bulb catheter as a monitoring modality include the practicality of continuous bedside monitoring, the ability to confirm the oximeter reading by drawing blood through the catheter, and the numerous physiological parameters that can be derived from the SjvO 2 to ascertain cerebral oxygen balance. Disadvantages of the catheter include its susceptibility to positioning artifacts and the complications associated with catheter insertion, including carotid puncture, infection, accidental misplacement, and jugular thrombosis ( , ; ).
Continuous bedside electroencephalography (EEG) monitoring is based on four of its major neurobiological features ( ): (1) its close relationship to cerebral metabolic rate, (2) its sensitivity in detecting hypoxic-ischemic neuronal dysfunction at an early stage, (3) its obvious primacy as a monitor of seizure activity, and (4) its value in cerebral localization. Continuous EEG recording has been advocated as a valuable tool for monitoring critically ill neurosurgical and neurological patients.
Despite the fact that the technical aspects of EEG application in the NICU do not differ greatly from the standard routine EEG, some factors are relatively unique to the ICU setting. The main differences are the many sources of electrical artifact (ventilators, intravenous pumps, dialysis machines, suctioning equipment) and the patient’s inability to cooperate secondary to various degrees of encephalopathy. In addition, continuous bedside EEG monitoring requires EEG interpreters available to view the recording, frequently throughout the day, and specially trained nurses or technicians capable of recognizing meaningful changes in the tracing.
Status epilepticus is the most common indication for EEG monitoring because the clinical ascertainment of ongoing seizure activity is often obscured by the effect of sedatives and analgesic agents. The EEG is essential for monitoring the effects of treatment, especially when barbiturates or general anesthetics are administered to achieve a burst-suppression pattern. Detection of nonconvulsive seizures and nonconvulsive status epilepticus (NCSE) can be accomplished only by EEG monitoring. Timely diagnosis of NCSE is important because delayed recognition may be associated with increased mortality ( ).
Nonconvulsive seizures have been reported in up to one-third of unselected NICU patients, frequently involving the presence of NCSE ( ). Continuous EEG monitoring has documented nonconvulsive seizures after severe TBI, ischemic stroke, poor-grade SAH, ICH, encephalitis, and after termination of generalized convulsive status epilepticus ( ; ; , ). These events might exacerbate excitotoxic injury in vulnerable brains and have been associated with high mortality ( ). But although their prognostic value is fairly well established, the impact of aggressive treatment of nonconvulsive seizures on clinical outcome remains to be determined ( ), as seizures may represent severe brain injury associated with a poor outcome independent of the presence of electrographic abnormalities.
Continuous EEG monitoring has also been used as an aid for the early detection of ischemia in patients with SAH who are at high risk for vasospasm ( ; ), but there is not enough information to recommend continuous EEG for this indication. Intracortical EEG (based on the use of deep electrodes) may be substantially superior to scalp EEG for detecting changes related to secondary neurological insults in patients with various forms of acute brain injury ( ). Furthermore, recurrent cortical spreading depolarizations may exacerbate local brain hypoxia and cause a shift toward anaerobic metabolism in patients with TBI or SAH ( ; ), but the value of monitoring for these changes with intracortical EEG remains to be conclusively determined.
Continuous EEG can also be useful for the recognition of nonconvulsive seizures and NCSE in patients with persistent coma of unknown cause ( ; ). EEG may also help in the evaluation of toxic and metabolic encephalopathy. In these cases, EEG serves to substantiate the diagnosis by showing diffusely slow low-amplitude activity and often triphasic waves, but does not distinguish between various causes of the condition. EEG can also be used as a confirmatory test of brain death ( ). After cardiac arrest, near-complete suppression, burst-suppression, nonreactive alpha or theta rhythms (alpha or theta coma), status epilepticus, and generalized periodic complexes are considered malignant patterns ( ; ). Although valuable for the prognostication of anoxic-ischemic encephalopathy, EEG data should not be interpreted in isolation in these patients ( ).
A position statement authored by the Critical Care Continuous EEG Task Force recommends continuous video EEG monitoring for many indications, including the detection of nonconvulsive seizures in patients with persistent encephalopathy and for the early detection of ischemia. The identified EEG abnormalities along the ictal-interictal continuum often seen on prolonged EEG monitoring have been shown to correlate with worse clinical outcome; however, as previously mentioned, the utility of treating these identified EEG abnormalities (secondary injury or a manifestation of the severity of the primary brain injury) is of unclear benefit ( ). As technology advances, quantitative EEG will likely become more routinely used in interpretation of critical care EEG.
A major focus in neurointensive care is to ensure that patients maintain adequate CBF. Unfortunately, CBF is not easily measured. Normal CBF in adult individuals ranges from 45 to 60 mL/100 g/min, and it is higher in the gray matter than in the white matter. Values below 10 mL/100 g/min are considered indicative of ischemia. Determinants of CBF include the status of brain metabolism, Pa co 2 , systemic BP, hematocrit, and cardiac output. Most of these determinants can be therapeutically manipulated by interventions such as the use of sedatives, changes in the ventilator setting, volume expansion, administration of vasoactive agents, blood transfusions, and inotropic medications. When information offered by CBF monitoring techniques is being interpreted, it is essential to understand the concept that CBF may be inappropriately low (i.e., metabolic demands exceed supply of blood flow, resulting in ischemia), appropriately low (i.e., metabolic demands are reduced and result in a coupled reduction in blood flow and oxygen consumption), inappropriately high (i.e., cerebral hyperemia), or appropriately high (i.e., situations of increased metabolic demand, such as seizures or fever). There are regional and local techniques for CBF monitoring. Regional modalities include (1) bedside xenon-133 intravenous injection technique, (2) stable xenon CT scan, (3) single-photon emission computed tomography (SPECT), (4) positron emission tomography (PET), (5) perfusion-weighted imaging by magnetic resonance imaging (PWI-MRI), and (6) CT perfusion scans.
The main disadvantage of most of these techniques is that they require transportation of the patient from the ICU to the location of the scanner. Consequently, they provide information about the status of CBF only at certain points in time, and CBF is a highly dynamic variable that may fluctuate extensively over time. The bedside xenon-133 technique is the only regional CBF monitoring modality that permits repeated testing in the NICU. However, it requires injection of small doses of the radioactive isotope. The xenon-CT technique involves transporting the patient to the CT scanner and administering nonradioactive xenon gas by inhalation. The inhaled gas can create a euphoric sensation, thus making this technique less desirable in agitated patients. SPECT, PET, MRI perfusion, and CT perfusion are valid options for assessing brain perfusion at a certain point in time. PET also allows measurement of the oxygen extraction fraction, which, when elevated, is a reliable indicator of hemodynamic failure and early ischemia. MRI scanning provides greater anatomical information and has the advantage of displaying areas of ischemia on diffusion-weighted imaging. CT perfusion is becoming increasingly available and offers quantifiable perfusion data. However, cumulative exposure to radiation limits the number of CT perfusion scans that can be safely performed for monitoring purposes.
Local CBF monitoring techniques include laser Doppler flowmetry and thermal diffusion flowmetry. Laser Doppler flowmetry is based on assessing the Doppler shift of low-power laser light captured by the moving red blood cells (red cell flux). It produces the continuous real-time flow output, which is linearly related to CBF, thus providing reliable information on local perfusion with excellent dynamic resolution. The main disadvantages of this technique, however, are its invasiveness (requires insertion of the probe via a burr hole), its susceptibility to movement artifact, its small sample volume (1–2 mm 3 ), and the qualitative nature of the information provided (this technique does not enable the quantification of CBF, and only relative changes can be assessed). Thermal diffusion flowmetry is used to estimate cortical blood flow by measuring changes in the temperature gradient between two thermistors within a probe applied to the cortex. Advantages include its simplicity and continuous measurement without using ionizing radiation. However, this technique can monitor only a 4- to 5-mm region of tissue, does not provide absolute measures of CBF, and has not been sufficiently standardized to be recommended for clinical practice.
TCD ultrasonography and various brain oxygenation monitoring techniques represent indirect measures of CBF monitoring.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here