Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neonatal intensive care unit graduates are at risk of neurodevelopmental impairment (NDI).
NDI can occur in cognitive, motor, vision, hearing, or language domains and can seriously impair the child's social, academic, and behavioral functioning.
Periventricular white matter injury is the leading cause of long-term NDI, especially motor impairment in preterm infants.
Infants with hypoxic-ischemic encephalopathy, bronchopulmonary dysplasia, necrotizing enterocolitis, intraventricular hemorrhage, and sepsis are at high risk of NDI.
Neurodevelopmental impairment (NDI) is an important long-term complication for neonatal intensive care unit (NICU) graduates. Neonates admitted to the NICU are undergoing a critical period of neurologic development, and any insult to the brain could lead to detrimental neurodevelopmental outcomes. The improvement in high-risk neonates’ survival rates due to neonatal care advancements has highlighted the importance of studying long-term neurologic prognosis. NDI can occur in cognitive, motor, vision, hearing, or language domains and can seriously impair the child's social, academic, and behavioral functioning. To optimize outcomes, there should be a obligation to follow-up and ameliorate the neurodevelopmental outcomes of the NICU survivors.
This chapter discusses the epidemiology and pathophysiology of NDI in the context of five conditions frequently seen in the NICU. These are hypoxic-ischemic encephalopathy (HIE), bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), and sepsis. This review includes evidence from our own quality-improvement studies and an extensive literature review of the PubMed, Embase, and Scopus databases. To avoid bias in identifying studies, keywords were short-listed a priori from anecdotal experience and PubMed's Medical Subject Heading thesaurus. In this review, considerable overlap is demonstrated in the neurodevelopmental manifestations and neurologic abnormalities among neonatal conditions, which indicates multifactorial pathogenesis of NDI.
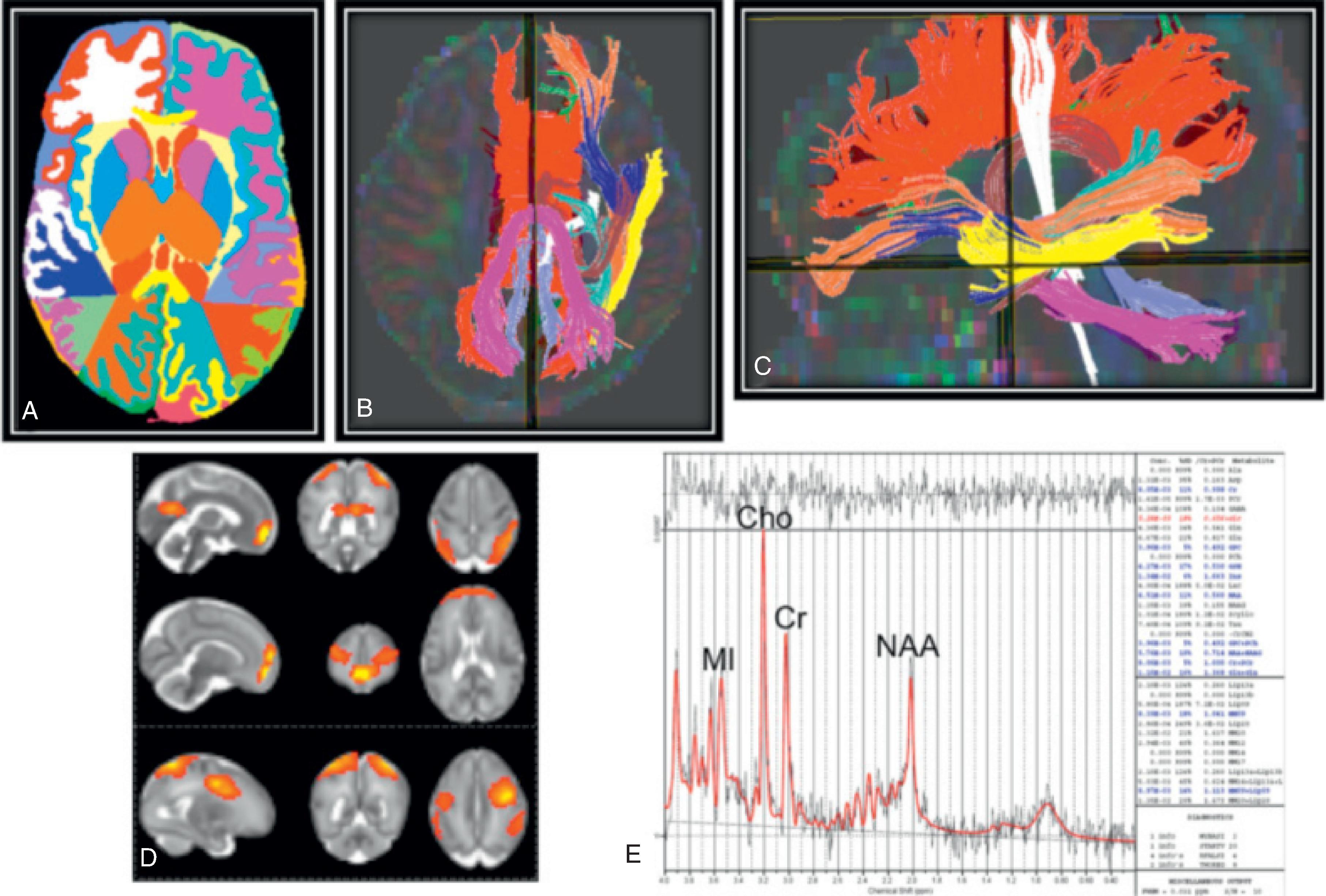
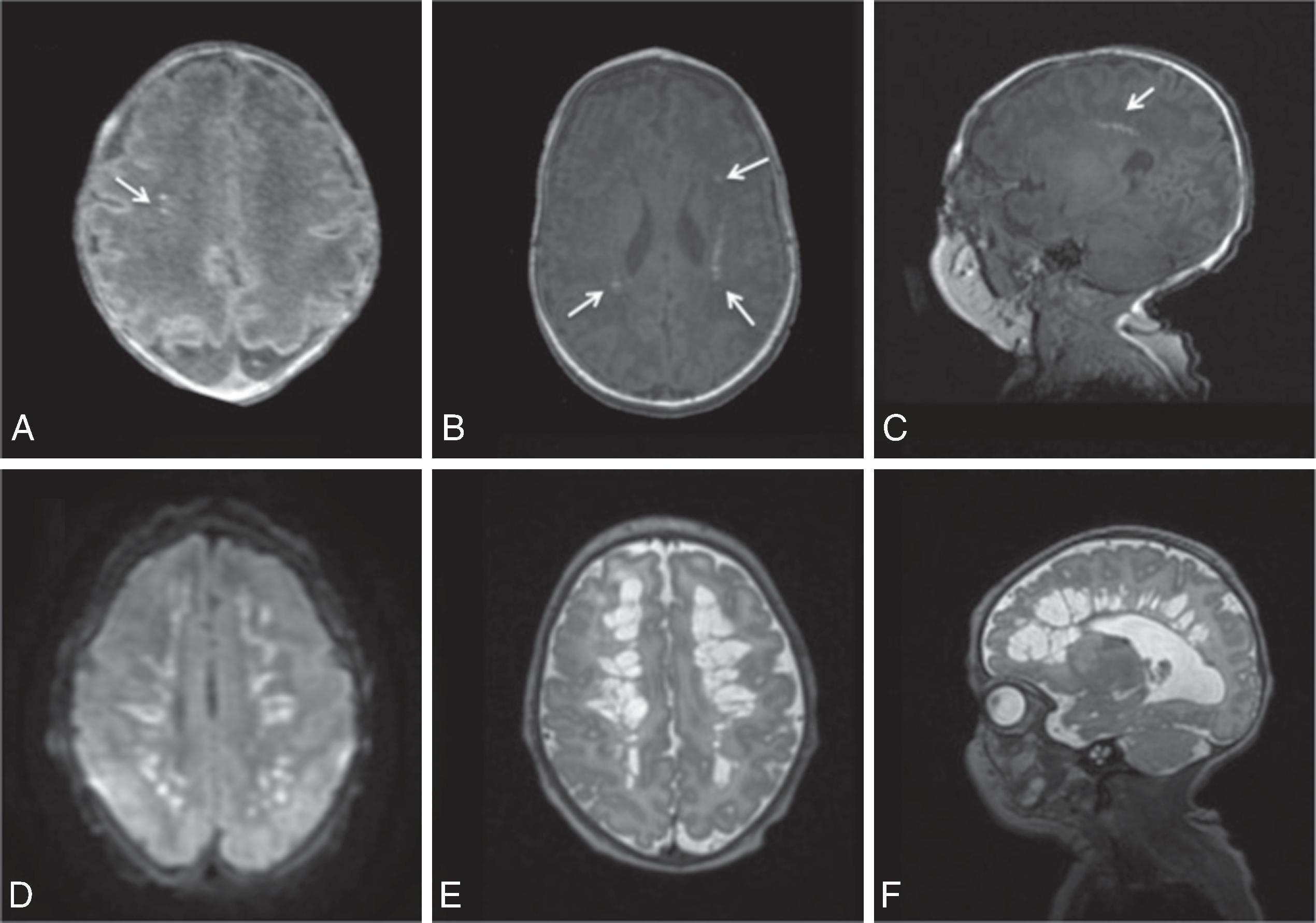
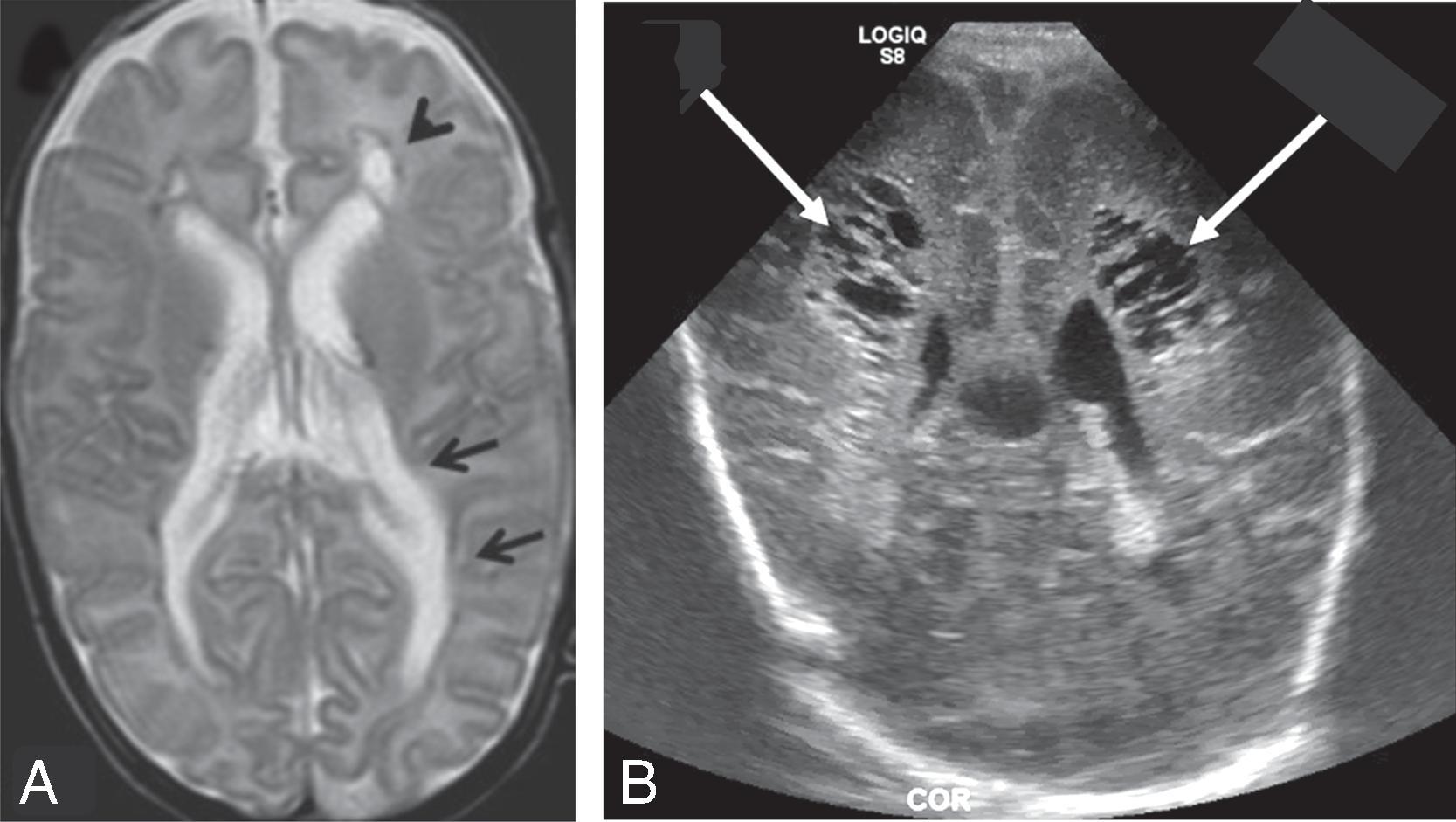
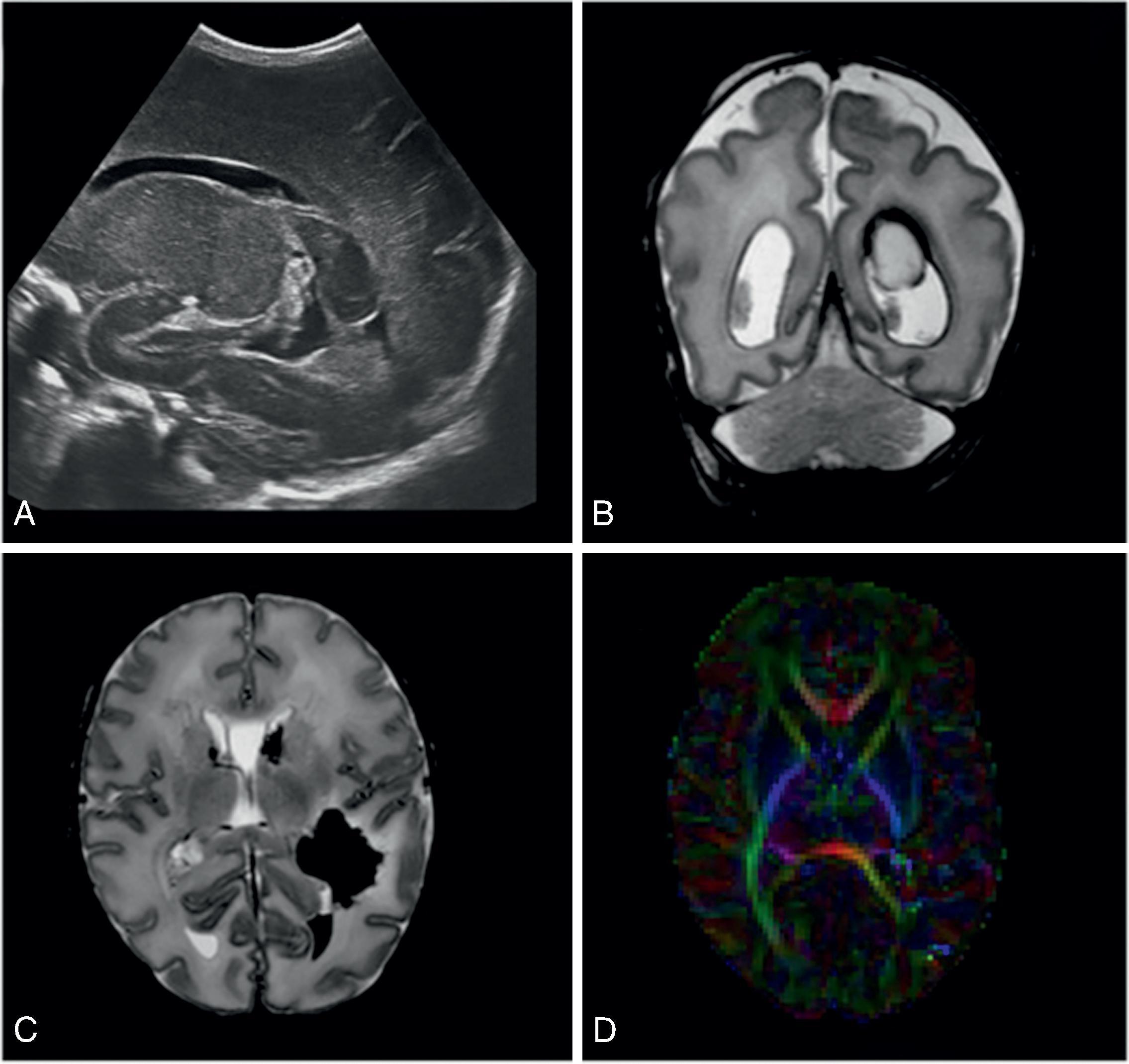
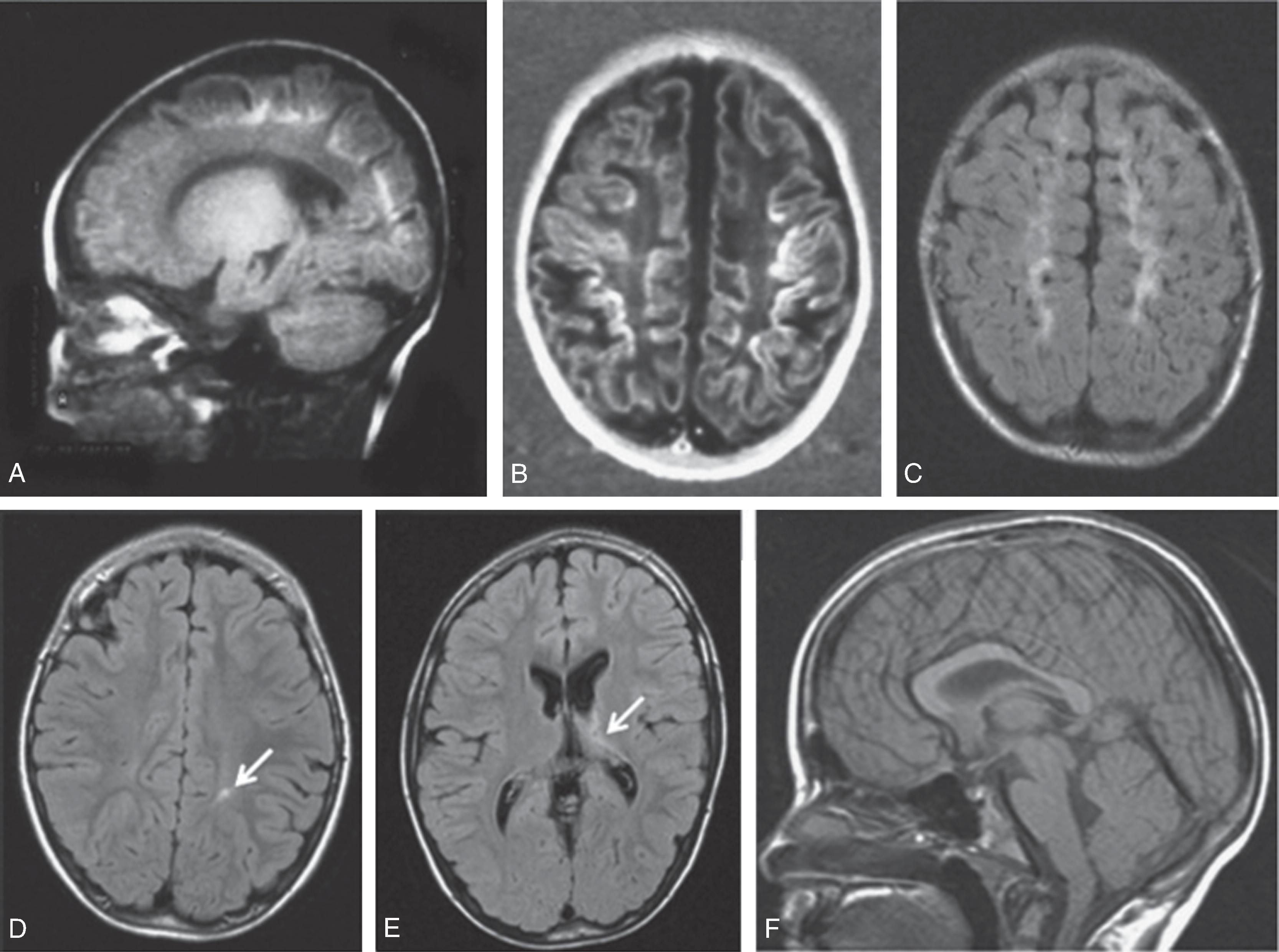
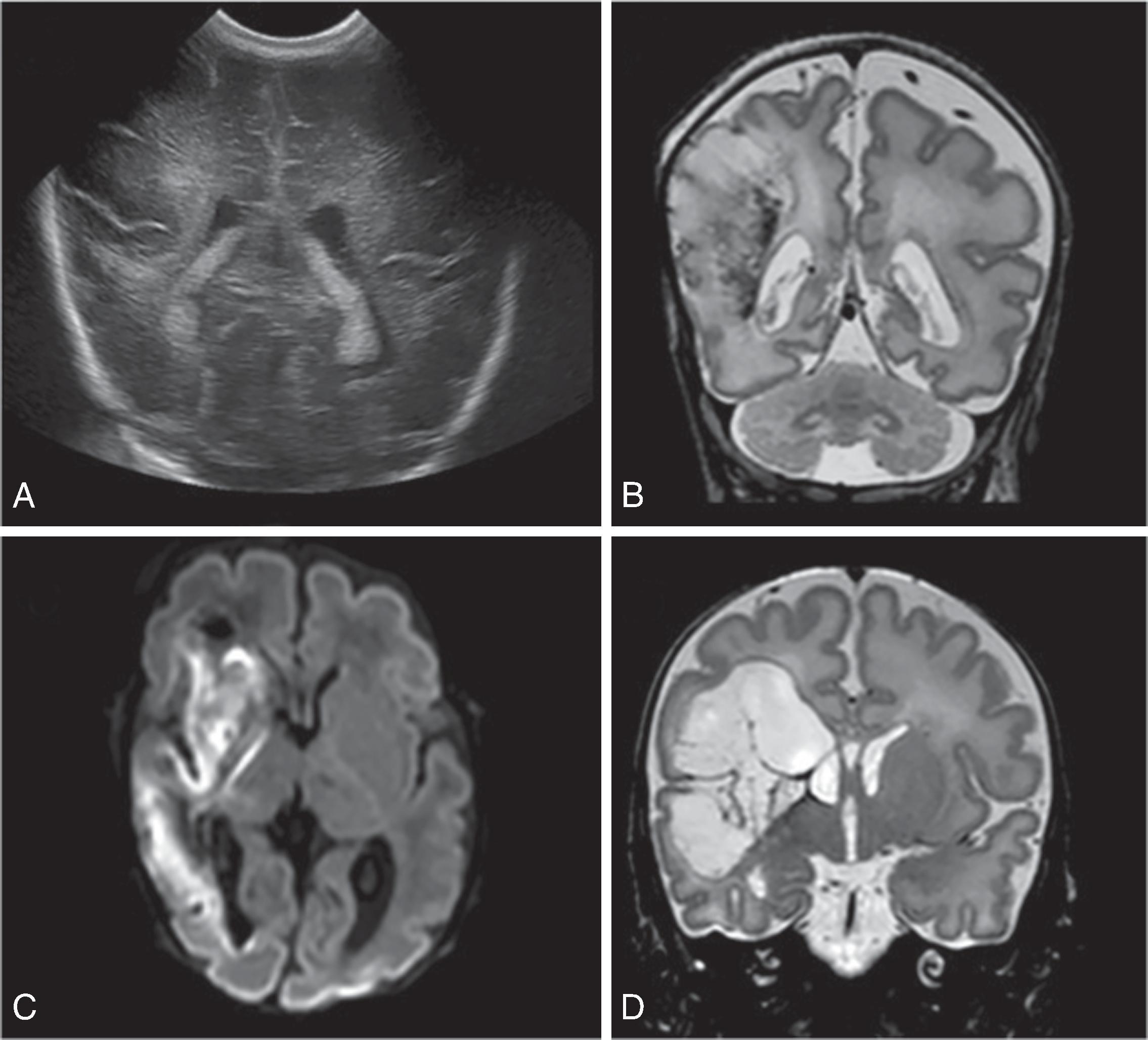
The underlying mechanism of NDI in preterm NICU infants could be related to anatomic and physiologic vascular abnormalities associated with the relative immaturity of the brain ( Fig. 96.1 ). Cerebral white matter is especially susceptible to prematurity-related vascular insults. Periventricular white matter injury (PWMI) such as that seen in the lesions in Figs. 96.2 and 96.3 is a leading cause of long-term NDI. , These lesions frequently lead to motor impairment. PWMI is attributed to cerebral white matter blood flow interference because of the immature vasculature; ischemic white matter is vulnerable to free radical–mediated injury to the oligodendrocyte progenitors. The severity of white matter abnormality in neonatal sepsis depends on the timing (postmenstrual age) of diagnosis, with more severe pathology in infants developing sepsis before 28 weeks. Figs. 96.4 and 96.5 show the evolution of white matter lesions over longer periods of time.
Oligodendrocyte progenitors are particularly susceptible to ischemia and inflammation in the process of preterm encephalopathy. The loss of lineage-specific progenitors and the consequent reduction in the number of mature oligodendrocytes can impair cerebral myelination. In animal models, cerebral hypoperfusion and hypoxia-ischemia have shown abnormalities in oligodendrocyte progenitor cells, periventricular white matter, and cortical gray matter. Along similar lines, excessive glutamate release during neonatal stress in the NICU could lead to excitotoxic damage to the oligodendrocyte progenitors and cause PWMI. In addition, cerebral maturation is impeded in NEC, as evidenced by a lower rate of increase of the N-acetyl cysteine–choline ratio in affected infants than in unaffected controls. Periventricular injuries could also be a consequence of moderate-severe IVH.
Intestinal inflammation could directly affect the severity of neuroinflammation ( Fig. 96.6 ). Immunogenic systemic inflammatory reaction with an increase in cytokines such as tumor necrosis factor might contribute to the pathogenesis of PWMI. Elevated plasma and cerebrospinal fluid interleukin-1 beta and tumor necrosis factor-alpha increase the risk for white matter injury in preterm neonates with early-onset sepsis. Biouss et al. reported that neonatal mice with NEC had reduced cortical girth, abnormal cell apoptosis, a decreased population of mature neurons and oligodendrocytes, and defective development of neural progenitor cells. Two other experimental models showed that animal neonates with NEC develop neuroinflammation associated with altered hippocampal gene expression, abnormal myelination, and cognitive deficits due to activation of microglial cells. ,
Furthermore, Niño et al. explained that inflamed intestinal tissue in NEC releases toll-like receptor 4 activators such as lipopolysaccharides that propagate neuroinflammation and promote oxidative stress; this, as a result, leads to reduced myelination in the hippocampus, midbrain, and corpus callosum. Haynes et al. hypothesized that free-radical oxidative and nitrative (due to nitric oxide) damage to oligodendrocyte progenitor cells contributes to PWMI. The studies described above indicate that early surgical removal of necrotic intestinal tissue in NEC could lower the severity of neuroinflammatory response in the brain, supporting a dose-related effect.
Other potential pathogenic mechanisms contribute to NDI development in NICU infants; poor nutrition and decreased growth associated with a prolonged stay in a NICU and exposure to surgery and anesthetics could contribute to abnormal neurologic development. Ehrenkranz et al. demonstrated that growth velocity during NICU admission was inversely associated with cerebral palsy (CP) and NDI incidence in extremely low birth weight (ELBW) infants. Besides, poor nutrition and inadequate caloric intake in preterm infants can lead to poor head growth, representing poor neurologic development. It is important to note that antenatal steroids reduce the risk of NDI in extremely preterm infants partially by reducing the incidence of IVH and cystic periventricular leukomalacia. A summary of the most frequently seen magnetic resonance imaging (MRI) prognostic biomarkers is shown in Tables 96.1 and 96.2 .
| Most common cerebral quantitative measurements | (1) Regional brain diameter or volume on structural MRI; (2) fractional anisotropy and/or mean diffusivity on dMRI; (3) brain metabolites, most commonly N-acetylaspartate (NAA)/choline ratio on MRS |
| Brain regions most predictive of neurodevelopmental impairment (identified in three or more studies) | Corpus callosum; centrum semiovale; sensorimotor cortex; subcortical gray matter; posterior limb of the internal capsule; cerebellum |
| Predicted outcomes examined | Cerebral palsy; minor motor abnormalities; permanent hearing loss; cognitive deficits; working memory; executive function; psychological/behavioral abnormalities |
| Condition | Structural Abnormalities | Clinical Outcomes | References |
|---|---|---|---|
| Hypoxic-ischemic encephalopathy |
|
|
|
| Intraventricular hemorrhage |
|
|
|
| Bronchopulmonary dysplasia |
|
|
|
| Necrotizing enterocolitis |
|
|
|
| Neonatal sepsis |
|
|
MRI is better than cranial ultrasound at assessing cerebral parenchymal abnormalities. Cerebral MRI proves valuable in predicting neurodevelopmental outcomes (in motor, language, and cognition domains) in HIE infants; it had 95% sensitivity and 94% specificity in predicting neurodevelopment. There are 6.23 times higher odds for NDI in the form of delayed development if MRI and magnetic resonance spectroscopy findings suggest HIE in the first week of life. Cerebral MRI and magnetic resonance spectroscopy are validated biomarkers for predicting neurodevelopmental outcomes and evaluating the neurologic response to therapeutic hypothermia.
Doppler ultrasound of the anterior or middle cerebral artery can be helpful; increased diastolic blood flow is correlated with NDI. Annink et al. studied 10-year-old children with HIE and showed mammillary bodies and hippocampi structural defects on MRI and diffusion tensor imaging (DTI) scans associated with memory problems and cognitive deficits. An alteration or reversal of the white matter signal in the posterior limb of the internal capsule during the first week of life has a high sensitivity (92%) and a high positive predictive value (88%) for severe motor impairment at 2 years of age. Besides, basal ganglia-thalamic lesions in HIE have an 89% predictive accuracy for severe motor impairment. Other prominent injury sites are the cortical gray matter, brainstem, and cerebellar white matter, which are also associated with motor or cognitive NDI. Diffusion-weighted imaging can detect basal ganglia–thalami lesions in the first week of life, and a low apparent diffusion coefficient and high lactate/N-acetyl aspartate ratio can prognosticate poor neurologic outcomes. ,
HIE continues to be a major cause of NDI despite advancements in therapeutic hypothermia. HIE is one of the most common causes of CP; the other manifestations include epilepsy, global developmental delay, motor impairment, language delay, and cognitive impairment. , Neurologic prognosis depends on the severity of HIE; neonates with an initial cord blood pH <6.7 have a 90% risk for death or severe NDI at 18 months of age. In addition, Apgar scores of 0 to 3 at 5 minutes, a base deficit >20 mmol/L, decerebrate posture, lack of spontaneous activity, apnea, absence of oculocephalic reflexes, and refractory seizures increase the risk and severity of NDI. HIE is also associated with defects in visual function.
The hypothermia protocol in HIE states that therapeutic hypothermia should be initiated in neonates who are born at >36 weeks’ gestation and have evidence of HIE or moderate to severe encephalopathy. However, a systematic review by Conway et al. showed that 25% of infants with mild HIE had poor neurodevelopmental outcomes at 8 months or older. Therapeutic hypothermia can decrease the incidence of CP and developmental delay. Edmonds et al. showed that therapeutic hypothermia prevented NDI in 75.5% of neonates in their study sample; 12.1% of children developed minor neurologic signs and were at a higher risk for cognitive and behavioral defects.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here