Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neuroblastoma is the most common solid extracranial malignancy of childhood and the most common malignant tumor in infants. The overall incidence of neuroblastoma is 1 per 100,000 children in the United States, thereby accounting for 7–10% of all malignancies diagnosed in patients younger than 15 years of age. Yet neuroblastoma is responsible for approximately 15% of all childhood cancer deaths. Neuroblastoma is a heterogeneous disease. Tumors can spontaneously regress or mature, or display a very aggressive, malignant phenotype. Because of these unique characteristics, neuroblastoma has been of great interest to both clinicians and basic science researchers. Progress in molecular and cellular biology in the past 40 years has contributed greatly to a better understanding of this disease. Unfortunately, this progress has not significantly altered the clinical outcome for patients with high-risk disease. Although the prognosis for these patients has improved somewhat in the past three decades, the long-term outcome remains very poor.
The etiology of neuroblastoma is currently unknown, and no environmental factors have been convincingly linked to its development. The disease generally occurs sporadically, but familial neuroblastoma occurs in about 2% of the cases. The substantial biologic and clinical heterogeneity is also observed in familial cases. The germline mutation associated with hereditary neuroblastoma has been identified: activating mutations in the tyrosine kinase domain of the anaplastic lymphoma kinase (ALK) oncogene on the short arm of chromosome 2 (2p23). These mutations can also be somatically acquired, with the prevalence of ALK activation in sporadic neuroblastoma being approximately 8%. The treatment of neuroblastoma reflects the heterogeneity of the disease, requires an understanding of the risk factors predictive of disease recurrence, and mandates a multidisciplinary approach. Although resection may be the only therapy required for patients at low risk for disease recurrence, with some low-risk tumors now simply being observed without any therapy, including surgical resection, the surgeon provides but one component of the modern multimodal treatment of children with high-risk disease. Oncologists, radiation therapists, and bone marrow transplantation (BMT) specialists are among the other important members of the pediatric oncology team. The therapy for patients with neuroblastoma, as for children with other malignancies, is generally driven by clinical research protocols. Many of these protocols are sponsored by the Children’s Oncology Group (COG), the New Approaches to Neuroblastoma Therapy (NANT) consortium, or the larger individual children’s hospitals.
Neuroblastoma is an embryonal tumor of the sympathetic nervous system. These tumors arise during fetal or early postnatal life from sympathetic cells (sympathogonia) derived from the neural crest. Therefore, tumors can originate anywhere along the path that neural crest cells migrate, including the adrenal medulla, paraspinal sympathetic ganglia, and sympathetic paraganglia such as the organ of Zuckerkandl. The German pathologist Rudolph Virchow is generally credited with being the first to describe the histologic appearance of what is now known as neuroblastoma in his 1864 article entitled, “Hyperplasia of the Pineal and Suprarenal Glands.” The first to use the term neuroblastoma was James Homer Wright in 1910, who described the classic appearance of rosettes of tumor cells around central neural fibrils and noted the similarity to the morphology of the fetal adrenal gland. He also noted the association between the common sites of tumor development and the pattern of migration of primitive neural cells.
As one of the small, round, blue cell tumors of infancy and childhood, neuroblastoma, particularly when undifferentiated, must be distinguished from other neoplasms in this group (Ewing sarcoma family of tumors [ESFTs], non-Hodgkin lymphoma, and rhabdomyosarcoma). Neuroblastoma can be distinguished histologically by the presence of neuritic processes (neuropil) and Homer Wright rosettes (neuroblasts surrounding eosinophilic neuropil). Scattered ganglion cells or immature chromaffin cells can also be seen. The histologic appearance of the tumor cells may vary from undifferentiated cells to fully mature ganglion cells. In addition, neuroblastomas have variable degrees of Schwannian cell stroma, reactive non-neoplastic tissue recruited by the tumor cells. This stroma is intermixed, to a greater or lesser degree, as wavy bundles and sheets of spindle cells, and produces antiproliferative and differentiation-inducing factors that are crucial to neuronal differentiation.
Neuroblastoma is characterized by several unique clinical behaviors, including the secretion of catecholamine products and the potential to regress or mature, either spontaneously or in response to treatment. Small nodules of primitive neuroblasts are routinely found in the developing adrenal gland, even during the early postnatal period. Beckwith and Perrin described microscopic nodules that they termed neuroblastoma in situ in the adrenals of infants undergoing autopsy following death from non-malignancy related causes. The incidence of this finding was more than 200-fold greater than the clinical incidence of neuroblastoma, which suggests that many neuroblastomas likely spontaneously regress or mature into lesions that never become clinically apparent. The process of involution is well described during embryonic life, especially in the developing central and peripheral nervous systems. Although initially thought to be mediated by the immune system, the process of involution may be the result of the withdrawal of neurotrophic maintenance factors such as nerve growth factor (NGF). Clinically apparent neuroblastoma can also regress or spontaneously mature, but the mechanism remains unknown.
In 1984, Shimada et al. first developed an age-linked classification system of neuroblastic tumors based on tumor morphology in which neuroblastomas were divided into two prognostic subgroups: favorable histology and unfavorable histology. In 1999, the International Neuroblastoma Pathology Classification (INPC) was devised; it was then modified in 2003 and is an adaptation of the original Shimada system. The INPC is based mainly on morphologic changes associated with the maturational sequence of neuroblastic tumors. It remains an age-linked classification that depends on the differentiation grade of the neuroblasts, the cellular turnover index (mitosis-karyorrhexis index [MKI]), and the presence or absence of Schwannian stroma. The INPC classifies neuroblastic tumors into three morphologic categories: neuroblastoma, ganglioneuroblastoma, and ganglioneuroma ( Fig. 65.1 ).
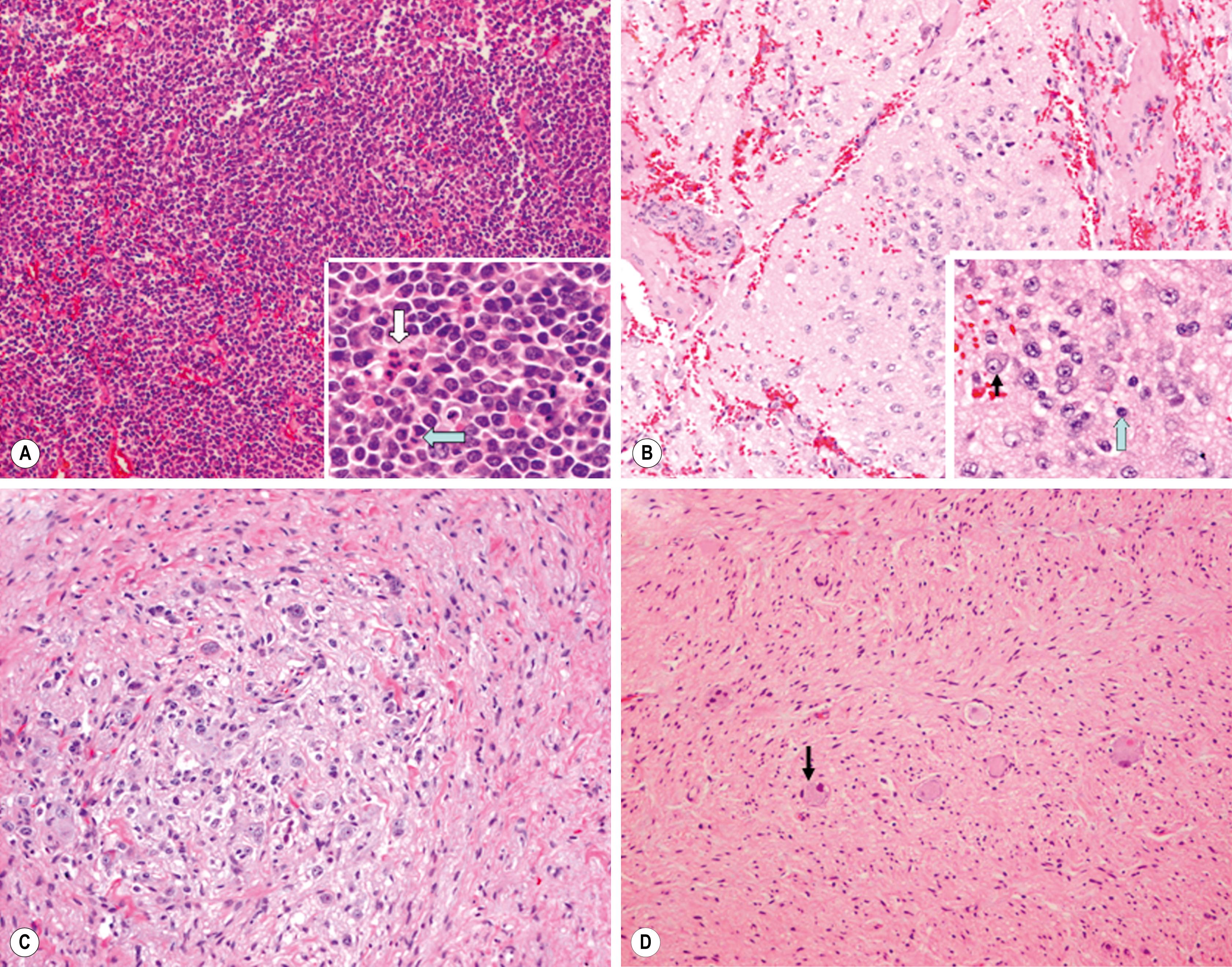
Neuroblastomas are, by definition, Schwannian stroma poor (<50% of the tumor tissue) and can be subtyped as undifferentiated, poorly differentiated, or differentiating. Undifferentiated tumors require supplemental diagnostic methods such as immunohistochemistry, electron microscopy, or cytogenetics to make the diagnosis of neuroblastoma. Moreover, neuropil is not present. In poorly differentiated tumors, <5% of tumor cells have features of differentiation, and neuropil is present. Differentiating tumors demonstrate >5% of tumor cells differentiating toward ganglion cells.
Additional factors that contribute to the prognostic distinction of stroma-poor neuroblastic tumors (neuroblastoma) as favorable or unfavorable subtypes include the MKI, which is defined as the number of tumor cells in mitosis or karyorrhexis per 5000 neuroblastic cells (i.e., low MKI, <100 cells; intermediate, 100–200 cells; high, >200 cells) and the patient’s age (<1.5 years, 1.5–5 years, >5 years) ( Table 65.1 ). It has been hypothesized that neuroblastic cells with maturational potential require a latent period before demonstrating histologic evidence of differentiation. Therefore, there is a certain allowance for mitotic and karyorrhectic activities of neuroblastic cells in tumors in infants and younger children.
| International Neuroblastoma Pathology Classification | Histologic Characteristics | Prognostic Group |
|---|---|---|
| Neuroblastoma | Schwannian stroma-poor | |
| <1.5 yrs | Poorly differentiated or differentiating & low or intermediate MKI tumor |
Favorable |
| 1.5-5 yrs | Differentiating & low MKI tumor | Favorable |
| <1.5 yrs | a) undifferentiated tumor a b) high MKI tumor |
Unfavorable |
| 1.5-5 yrs | a) undifferentiated or poorly differentiated tumor b) intermediate or high MKI tumor |
Unfavorable |
| ≥5 yrs | All tumors | Unfavorable |
| Ganglioneuroblastoma, intermixed | Schwannian stroma-rich | Favorable b |
| Ganglioneuroblastoma, nodular | Composite Schwannian stroma-rich/stroma-dominant and stroma-poor | Unfavorable b |
| Ganglioneuroma | Schwannian stroma-dominant | Favorable b |
| Maturing | ||
| Mature |
a Rare subtype, especially diagnosed in this age group. Further investigation and analysis required.
b Prognostic grouping for these tumor categories is not related to patient age.
The importance of this histopathologic classification was confirmed in a large, retrospective analysis reported by Shimada et al. The INPC classification of tumor histology provided independent prognostic information in which tumors of favorable histology had a 90.8% probability of 5-year event-free survival [EFS] compared with 31.2% EFS for tumors of unfavorable histology. More recently, the INPC classification was been shown to add independent prognostic information beyond the prognostic contribution of age. Therefore, histopathology remains in the current multifactorial risk stratification for certain patients with neuroblastoma. As the histopathologic pattern within a tumor can be heterogeneous, it is recommended that representative sections from at least 1 cm 3 of viable, non-necrotic tissue be analyzed to determine histopathologic classification. The prognostic value of assessing the histopathology of a neuroblastoma after chemotherapy or radiation therapy has not been validated.
Stroma-rich neuroblastic tumors are classified as either ganglioneuroblastomas or ganglioneuromas. Ganglioneuroblastomas contain cells that are transitioning toward differentiation but are not completely differentiated/mature. Also, less than 50% of the total volume is made up of neuroblastic cells. Ganglioneuroblastomas can be further divided into intermixed and nodular subtypes, depending on the distribution of the neuroblastic cells. The distinction is important because of the significantly worse prognosis associated with the latter subtype, in which the neuroblastic clones that comprise grossly distinct nodules appear to be responsible for the aggressive phenotype for this subtype. Ganglioneuromas contain either maturing or mature cells and lack any neuroblastomatous component. Most stroma-rich tumors (ganglioneuroblastoma, intermixed and ganglioneuroma, maturing subtype) are classified as favorable by the INPC. However, the pathologic/prognostic classification of the ganglioneuroblastoma, nodular subtype, is based on the morphologic evaluation of the neuroblastomatous nodule(s), and can, therefore, be unfavorable. Tumors that fit the criteria for ganglioneuroma, mature subtype, with abundant Schwannian stroma and fully mature ganglion cells, in the absence of neuroblasts, are considered benign, and are generally not considered for enrollment in protocols for neuroblastic tumors. Despite this, ganglioneuromas can be quite large and infiltrative, and attempts at removal can be associated with significant complications. In addition, survival does not seem to be influenced by extent of resection. Therefore, aggressive attempts at resection of ganglioneuromas are not recommended.
Advances in molecular biology research in the past four decades have resulted in an increased understanding of the genetic events in the pathogenesis and progression of many human malignancies, including those in children. Neuroblastoma, in particular, has served as a model for a molecular approach to treating patients with cancer, highlighting the utility of genetic analysis for diagnosis, risk stratification, and treatment planning. Segmental chromosome aberrations (SCAs) play a role in neuroblastomas, particularly those that result in the loss of tumor suppressors, gain of oncogenes or or gene amplification; as well as activating or inactivating mutations of relevant genes or their regulatory elements. The end result of alterations in these genetic elements, regardless of their specific mechanisms, is the disruption of the normal balance between cell proliferation and cell death.
Normal human cells contain two copies of each of 23 chromosomes; thus, a normal diploid cell has 46 chromosomes. The majority (55%) of primary neuroblastomas are triploid or “near-triploid/hyperdiploid” and contain between 58 and 80 chromosomes; the remainder (45%) are either near-diploid (35–57 chromosomes) or near-tetraploid (81–103 chromosomes). The DNA index of a tumor is the ratio of the number of chromosomes present to a diploid number of chromosomes (i.e., 46). Therefore, diploid cells have a DNA index of 1.0, whereas near-triploid cells have a DNA index ranging from 1.26 to 1.76. Neuroblastomas that are near-diploid or near-tetraploid usually have structural genetic abnormalities, most frequently chromosome 1p deletion and MYCN amplification. Near-triploid or hyperdiploid tumors are characterized by almost three complete haploid sets of chromosomes with few structural abnormalities. Importantly, patients with near-triploid tumors typically have favorable clinical and biologic prognostic factors and excellent survival rates, as compared with those patients who have near-diploid or near-tetraploid tumors. This association is most important for infants with advanced disease as the prognostic significance of tumor ploidy appears to be lost in patients older than 2 years. Currently, ploidy affects only the risk group assessment of very limited subgroups of patients with neuroblastoma.
Investigation of the molecular biology of neuroblastoma began with the cytogenetic characterization of tumor-derived cell lines. These studies showed the frequent presence of extrachromosomal double-minute chromatin bodies (DMs) and chromosomally integrated homogeneously staining regions (HSRs) characteristic of gene amplification ( Fig. 65.2 ). Since that time, it has been shown that the amplified region was derived from the distal short arm of chromosome 2 (2p24) and contained the MYCN proto-oncogene. MYCN encodes a 64-kDa nuclear phosphoprotein that forms a transcriptional complex by associating with other nuclear proteins expressed in the developing nervous system and other tissues. Enforced expression of MYCN increases the rates of DNA synthesis and cell proliferation, and shortens the G 1 phase of the cell cycle. MYCN can also function as a classic dominant oncogene that cooperates with activated ras to transform normal cells. Targeted expression of MYCN in transgenic mice results in the development of neuroblastomas. This activity is potentiated when combined with mutations in ALK .
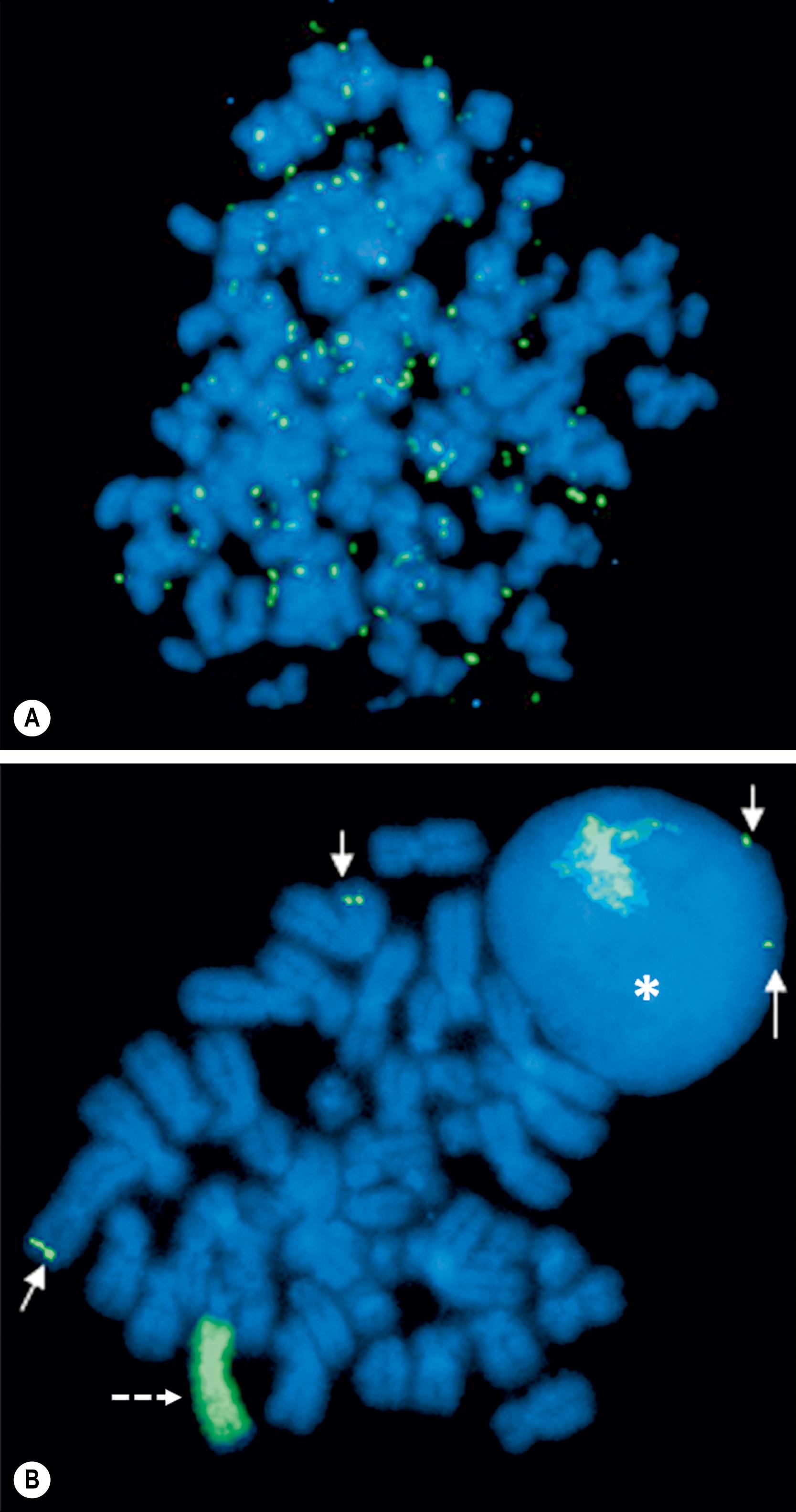
Overall, approximately 25% of primary neuroblastomas in children have MYCN amplification, with MYCN amplification being present in 40% with advanced disease but only 5–10% with low-stage disease. The copy number, which can range from 5- to 500-fold amplification, is usually consistent among primary and metastatic sites and at different times during tumor evolution and treatment. This finding suggests that MYCN amplification is an early event in the pathogenesis of neuroblastoma. Amplification of MYCN is associated with advanced stages of disease, rapid tumor progression, and poor outcome; therefore, it is a powerful prognostic indicator of tumor behavior. Amplification is most commonly assessed by fluorescence in situ hybridization (FISH), and current therapeutic neuroblastoma protocols have incorporated the presence or absence of MYCN amplification into their risk stratification schema.
Also noted on early karyotype analyses of neuroblastoma-derived cell lines were frequent deletions of the short arm of chromosome 1. Deletions of genetic material in tumors suggest the presence (and subsequent loss) of a tumor suppressor gene. Approximately 20–35% of primary neuroblastomas exhibit 1p deletion, as determined by FISH, with the smallest common region of loss located within region 1p36. About 70% of advanced-stage neuroblastomas have 1p deletions. Molecular studies have shown that there is a strong correlation between 1p deletion and MYCN amplification and other high-risk features such as age older than 1 year and advanced-stage disease. One study demonstrated that 1p deletions are independently associated with a worse outcome in patients with neuroblastoma.
Deletion of the long arm of chromosome 11 (11q) also appears to be common in neuroblastoma, being present in about 40% of cases. Unbalanced deletion of 11q (loss with either retention or gain of 11p material) is inversely related to MYCN amplification, yet is strongly associated with other high-risk features. Attiyeh et al., on behalf of the COG, showed in a large cohort of patients that unbalanced deletion of 11q and 1p36 was independently associated with a worse outcome in patients with neuroblastoma. Therefore, the duration of treatment for children with intermediate-risk neuroblastoma in a recent COG study was based, in part, on the 1p and 11q allelic status of the tumor.
Proto-oncogene activation can also occur by point mutation, as occurs with the tyrosine kinase receptor, ALK, the gene for which resides on the short arm of chromosome 2 (2p23). Receptor tyrosine kinases (RTKs) are high-affinity cell surface receptors for many growth factors, cytokines, and hormones. When activated through ligand binding, these proteins mediate phosphorylation of tyrosine on target molecules or substrates, resulting in intracellular signaling and, ultimately, the regulation of normal cellular processes. Mutation of RTKs can lead to constitutive activation of the signaling pathway in the absence of ligand. Activating mutations of ALK have been shown to be the germline abnormality associated with hereditary neuroblastoma. These mutations can also be somatically acquired, as can amplification of the gene. Activated ALK has proven to be a targetable abnormality in neuroblastoma, with drugs such as crizotinib, an anti-ALK antibody, showing efficacy.
Further studies have identified loss-of-function mutations in the homeobox gene PHOXB2 on 4p13 that are also associated with familial neuroblastoma, particularly when occurring together with Hirschsprung disease and/or central hypoventilation.
More recently, inactivating mutations of ATRX, a transcriptional regulator that is part of a multiprotein complex that plays a role in regulating chromatin remodeling, nucleosome assembly, and telomere maintenance, have been found in neuroblastoma, particularly high-stage tumors in older patients. ATRX mutations appear to be loss-of-function mutations associated with an absence of the ATRX protein in the nucleus and with long telomeres. How these alterations lead to lengthened telomeres is uncertain. These results may provide a molecular marker and potential therapeutic target for neuroblastoma among adolescents and young adults. It may also delineate the subset of children with neuroblastoma who have a chronic but progressive clinical course when receiving standard therapeutic approaches and who may benefit from a different treatment strategy.
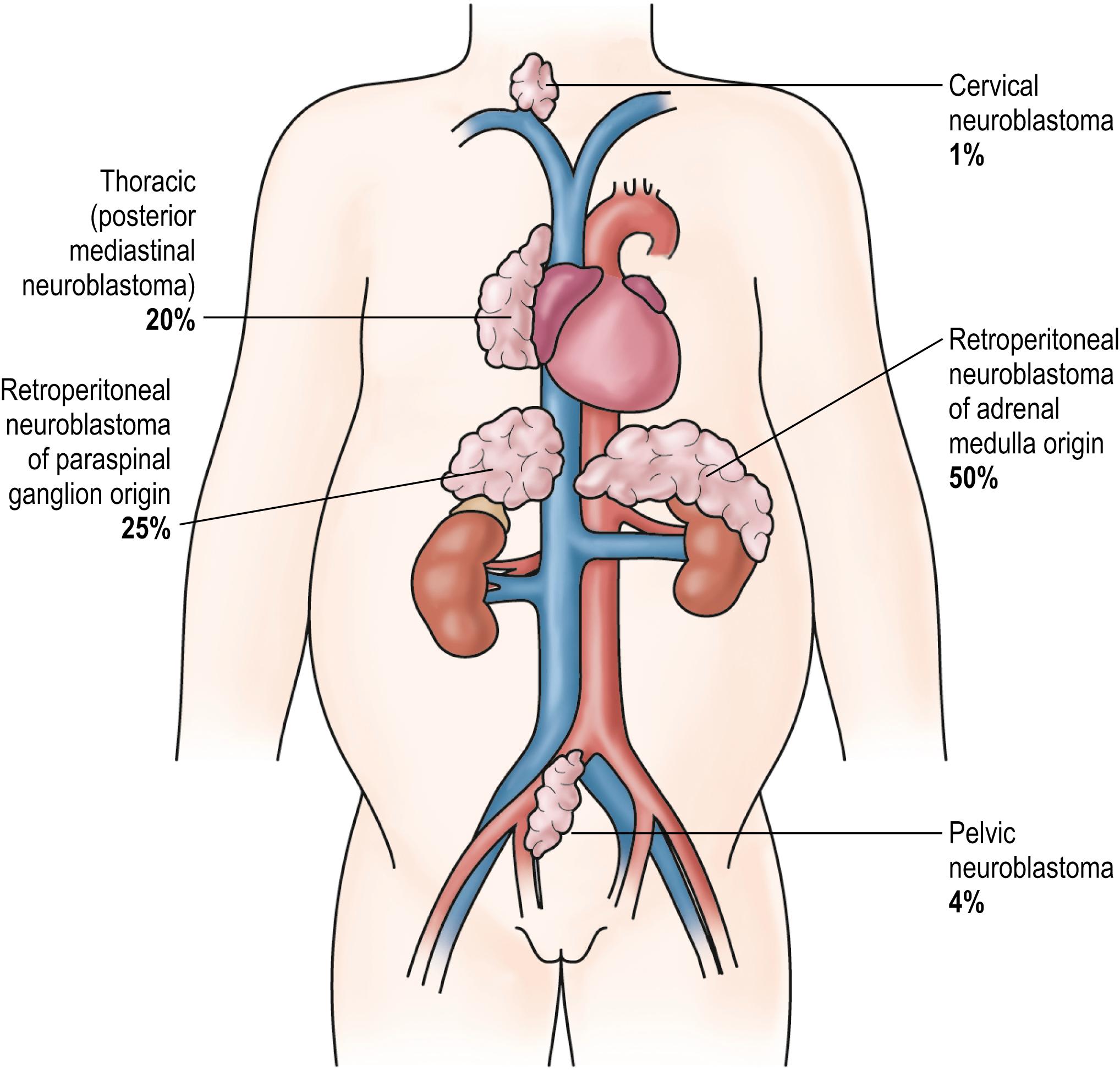
Patients with neuroblastoma usually present with signs and symptoms that reflect the primary site and extent of disease, although localized disease is often asymptomatic. As 75% of neuroblastoma occurs in the abdominal cavity, an abdominal mass detected on physical examination is a common clinical feature, as is the complaint of abdominal pain. Other primary sites of neuroblastoma include the posterior mediastinum (20%), the cervical region (1%), and the pelvis (4%) (organ of Zuckerkandel) ( Fig. 65.3 ). Respiratory distress or dysphagia may be a reflection of a thoracic tumor. Altered defecation or urination can be caused by mechanical compression from a pelvic tumor or by spinal cord compression from a paraspinal tumor. Spinal cord compression may also manifest as an altered gait. A tumor in the neck or upper thorax can produce Horner syndrome (ptosis, miosis, and anhydrosis), enophthalmos, and heterochromia of the iris. Acute cerebellar ataxia has also been observed, characterized by the dancing-eye syndrome, which includes opsoclonus, myoclonus, and chaotic nystagmus. Two-thirds of these cases occur in infants with mediastinal primary tumors. Additional signs and symptoms that reflect excessive catecholamine or vasoactive intestinal polypeptide (VIP) secretion include diarrhea, weight loss, and hypertension.
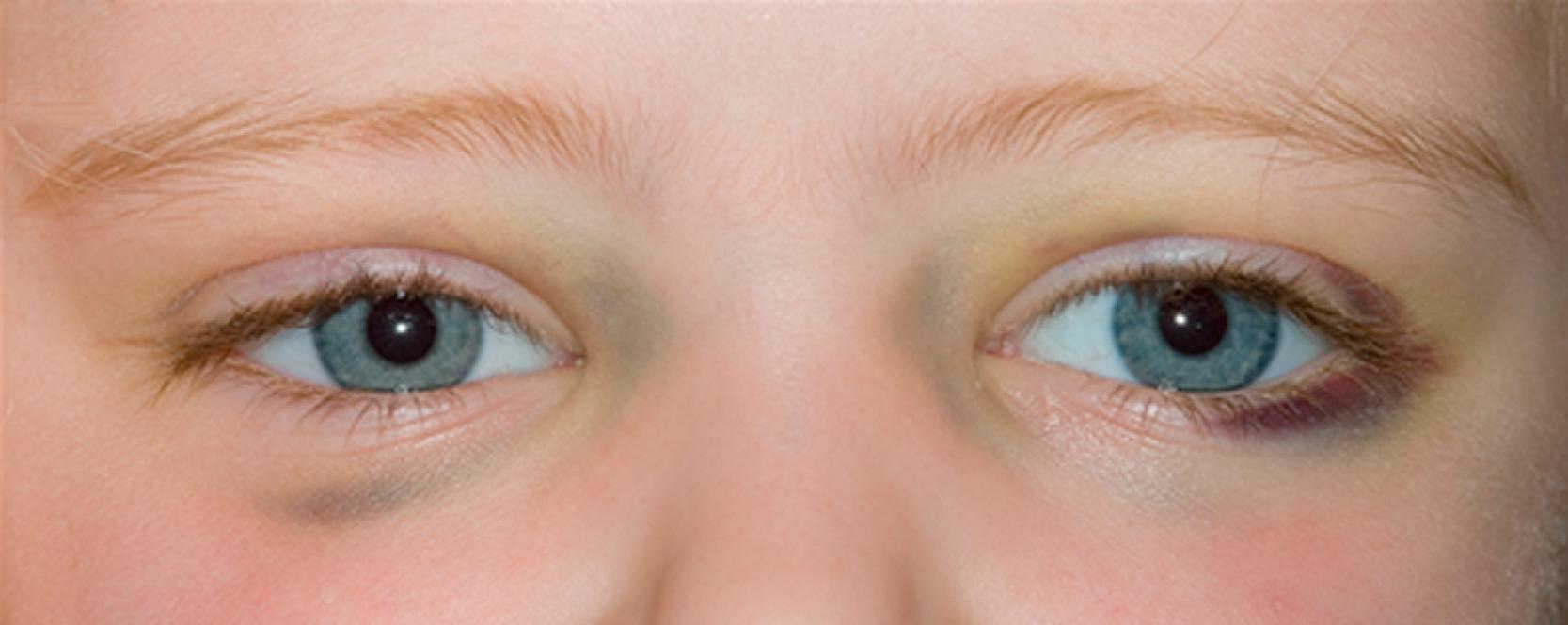
More than 40% of patients have metastatic disease at diagnosis. These patients are often quite ill and have systemic symptoms caused by widespread disease. Neuroblastoma in older patients has a pattern of metastatic disease in which metastases to the bone marrow, lymph nodes, and bone predominate. The frequency of involvement of distant sites is shown in Table 65.2 . These metastases may manifest as bone pain from cortical metastases or anemia from marrow infiltration. The brain, spinal cord, heart, and lungs are rare sites of metastases, except with end-stage disease. Metastatic disease also may be associated with darkened areas around the eyes, referred to as “raccoon eyes,” as a result of retroorbital venous plexus spread ( Fig. 65.4 ). This is an ominous physical sign, as is the presence of a limp in children without a history of head or extremity trauma. The diagnosis of neuroblastoma is generally made by histopathologic evaluation of the primary or metastatic tumor tissue, or by the demonstration of tumor cells in the bone marrow together with elevated levels of urinary catecholamines.
| Stage 4S (%) | Stage 4 <1 Year (%) | Stage 4≥1 Year (%) | Total (%) | |
|---|---|---|---|---|
| Bone marrow | 34.6 | 57.1 | 81.3 | 70.5 |
| Bone | 0 | 48.9 | 68.2 | 55.7 |
| Lymph node | 8.6 | 28.6 | 35.7 | 30.9 |
| Liver | 80.2 | 53.4 | 12.9 | 29.6 |
| Intracranial/orbit | 0 | 25.6 | 19.6 | 18.2 |
| Adrenal | 6.2 | 13.5 | 6.0 | 7.6 |
| Skin | 13.6 | 8.3 | 0.9 | 4.0 |
| Pleura | 0 | 4.5 | 3.7 | 3.4 |
| Lung | 0 | 2.3 | 4.1 | 3.2 |
| Peritoneum | 0 | 3.8 | 2.1 | 2.2 |
| Other | 0 | 3.8 | 1.6 | 1.9 |
| Central nervous system | 0 | 0 | 0.9 | 0.6 |
Despite its lack of specificity, serum lactate dehydrogenase (LDH) can have great prognostic significance. High serum levels of LDH reflect high proliferative activity or a large tumor burden, and an LDH level higher than 1500 IU/L appears to be associated with a poor prognosis. Thus, LDH can be used to monitor disease activity or the response to therapy.
High levels of serum ferritin (>150 ng/mL) may also reflect a large tumor burden or rapid tumor progression. Elevated serum ferritin is often seen in advanced-stage neuroblastomas and indicates a poor prognosis. Levels often return to normal during clinical remission.
Neuroblastoma is characterized by the relatively unique capacity for secretion of catecholamine products, the metabolites of which can be detected in the urine of more than 90% of patients with neuroblastoma. Thus, a urine specimen is of clinical value in diagnosing neuroblastoma and determining the response to therapy. Documentation of elevated urinary catecholamines is required if the diagnosis of neuroblastoma is being made solely by the identification of neuroblasts in the bone marrow. Urinary levels of these two catabolites can also be used as markers of tumor progression or relapse and serve as a surrogate prognostic indicator. Random urine samples are preferable to 24-hour urine estimations for younger children.
Chest radiography can be a useful tool for demonstrating the presence of a posterior mediastinal mass, which in a child is usually a thoracic neuroblastoma. A Pediatric Oncology Group (POG) study demonstrated that a mediastinal mass was discovered on incidental chest radiographs in almost half of patients with thoracic neuroblastoma who had symptoms seemingly unrelated to their tumors. Abdominal radiography is less often the modality by which a neuroblastoma is discovered. However, as many as half of abdominal neuroblastomas are detectable as a mass with fine calcification.
Although ultrasonography (US) is the modality most often used during the initial assessment of a suspected abdominal mass, its sensitivity and accuracy are less than that of computed tomography (CT) or magnetic resonance imaging (MRI) for demonstrating a neuoblastoma. These latter modalities are generally used after screening with US to assist in generating a differential diagnosis and for further anatomic definition once the presence of a mass has been confirmed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here