Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Schneiderian papillomas
Squamous papilloma (nasal vestibule)
Minor salivary gland neoplasms
Lobular capillary hemangioma (pyogenic granuloma)
Sinonasal glomangiopericytoma (formerly sinonasal-type hemangiopericytoma)
Sinonasal tract meningioma
Ectopic pituitary adenoma
Solitary fibrous tumor
Benign peripheral nerve sheath tumor
Benign fibrous histiocytoma
Leiomyoma
Rhabdomyoma
Osteoma
Fibro-osseous lesions (ossifying fibroma, juvenile active ossifying fibroma)
Chondroma
Myxoma/fibromyxoma/chondromyxoid fibroma
Ossifying and nonossifying fibromyxoid tumor
Ameloblastoma
Others
Squamous cell carcinoma:
Keratinizing squamous cell carcinoma
Nonkeratinizing squamous cell carcinoma
Variants of squamous cell carcinoma (verrucous carcinoma, papillary squamous cell carcinoma, spindle cell squamous carcinoma, basaloid squamous cell carcinoma, lymphoepithelial carcinoma, adenosquamous carcinoma)
Sinonasal undifferentiated carcinoma
SMARCB1 (INI1)-deficient carcinoma
NUT midline carcinoma
HPV-associated adenoid cystic-like carcinoma
Adenocarcinoma:
Salivary gland types
Intestinal types
Nonsalivary, nonintestinal types
Olfactory neuroblastoma
Neuroendocrine carcinomas
Mucosal malignant melanoma
Non-Hodgkin malignant lymphomas
Ewing family of tumors
Undifferentiated pleomorphic sarcoma
Fibrosarcoma
Leiomyosarcoma
Malignant schwannoma
Angiosarcoma
Matrix-forming malignant tumors (osteosarcoma, chondrosarcoma)
Low-grade sarcoma with neural and myogenic differentiation (LGSSNMF)
Teratocarcinosarcoma
Secondary tumors
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| Maxillary Sinus | |
| T1 | Tumor limited to maxillary sinus mucosa with no erosion or destruction of bone |
| T2 | Tumor causing bone erosion or destruction, including extension into the hard palate and/or middle nasal meatus, except extension to posterior wall of maxillary sinus and pterygoid plates |
| T3 | Tumor invades any of the following: bone of the posterior wall of the maxillary sinus, subcutaneous tissues, floor or medial wall of orbit, pterygoid fossa, ethmoid sinuses |
| T4a | Moderately advanced local disease Tumor invades the anterior orbital contents, skin of the cheek, pterygoid plates, infratemporal fossa, cribriform plate, sphenoid or frontal sinuses |
| T4b | Very advanced local disease Tumor invades any of the following: orbital apex, dura, brain, middle cranial fossa, cranial nerves other than maxillary division of trigeminal nerve (V 2 ), nasopharynx, or clivus |
| Nasal Cavity and Ethmoid Sinuses | |
| T1 | Tumor restricted to any one subsite, with or without bony invasion |
| T2 | Tumor invading two subsites in a single region or extending to involve an adjacent region within the nasoethmoidal complex, with or without bony invasion |
| T3 | Tumor extends to invade the medial wall or floor of the orbit, maxillary sinus, palate, or cribriform plate |
| T4a | Moderately advanced local disease Tumor invades any of the following: anterior orbital contents, skin of the nose or cheek, minimal extension to anterior cranial fossa, pterygoid plates, sphenoid or ethmoid sinuses |
| T4b | Very advanced local disease Tumor invades any of the following: orbital apex, dura, brain, middle cranial fossa, cranial nerves other than maxillary division of trigeminal nerve (V 2 ), nasopharynx, or clivus |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node, 3 cm or less in greatest dimension |
| N2 | Metastasis in a single ipsilateral lymph node, more than 3 cm but not more than 6 cm in greatest dimension, or in multiple ipsilateral lymph nodes, none more than 6 cm in greatest dimension, or in bilateral or contralateral lymph nodes, none more than 6 cm in greatest dimension |
| N2a | Metastasis in a single ipsilateral lymph node, more than 3 cm but not more than 6 cm in greatest dimension |
| N2b | Metastasis in multiple ipsilateral lymph nodes none more than 6 cm in greatest dimension |
| N2c | Metastasis in bilateral or contralateral lymph nodes none more than 6 cm in greatest dimension |
| N3 | Metastasis in a lymph node more than 6 cm in greatest dimension |
| Distant Metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Clinical Stage | |
| Stage 0 | TisN0M0 |
| Stage I | T1N0M0 |
| Stage II | T2N0M0 |
| Stage III | T3N0M0 |
| T1N1M0 | |
| T2N1M0 | |
| T3N1M0 | |
| Stage IVA | T4aN0MO |
| T4aN1M0 | |
| T1N2M0 | |
| T2N2M0 | |
| T3N2M0 | |
| T4aN2M0 | |
| Stage IVB | T4b AnyN M0 |
| Any T N3M0 | |
| Stage IVC | Any T |
| Any N M1 | |
The sinonasal tract is the location for a wide variety of benign and malignant tumors.
The more common tumor types are of epithelial origin and include:
Benign neoplasms: Schneiderian papillomas
Malignant neoplasms: squamous cell carcinoma and variants thereof
Whether benign or malignant, neoplasms of the sinonasal tract often present with nasal obstruction, which may also be the clinical presentation for nonneoplastic lesions:
Presence of pain and/or manifestations of cranial nerve dysfunction is an indicator of a malignancy until proven otherwise.
Clinical information is paramount for the pathologist to evaluate any given case.
Carcinomas of the sinonasal tract account for less than 1% of all malignant neoplasms.
Carcinomas of the sinonasal tract account for approximately 3% of head and neck malignant neoplasms.
Based on the entire sinonasal tract, malignant neoplasms originate (in descending order of occurrence) from:
Maxillary sinus (60%)
Nasal cavity (20% to 30%)
Ethmoid sinus (10% to 15%)
Sphenoid and frontal sinuses (1%)
Based on the entire paranasal sinuses alone, malignant neoplasms originate (in descending order of occurrence) from:
Maxillary sinus (77%)
Ethmoid sinus (22%)
Sphenoid and frontal sinuses (1%)
Cause of sinonasal malignant neoplasms include:
Occupational exposure to wood dust is linked to the development of sinonasal adenocarcinomas, intestinal types, and to a lesser extent squamous cell carcinoma.
Occupational exposure to nickel refining and chromate pigment manufacture is linked to an increased risk of sinonasal carcinoma.
Epstein-Barr virus (EBV) present in association with some sinonasal tract neoplasms including:
NK/T-cell lymphoma
Lymphoepithelial-like carcinoma
Human papillomavirus (HPV), mainly high-risk types, may be present in association with some sinonasal tract neoplasms, including:
Schneiderian papilloma (include low- and high-risk HPV)
As many as 20% of sinonasal carcinomas may harbor transcriptionally active high-risk HPV:
Almost always nonkeratinizing squamous cell carcinoma (SCC)
Less commonly with other morphologic types including:
keratinizing SCC (de novo or in association with Schneiderian papilloma)
basaloid SCC
papillary SC
adenosquamous carcinoma
other morphologic types
Prognostic significance of transcriptionally active HPV in sinonasal SCC remains uncertain; unclear whether ameliorating prognostic effect as may occur in association with HPV-associated carcinomas of oropharynx also applicable to HPV-associated sinonasal carcinomas
Premalignant lesions:
Unlike other upper aerodigestive tract sites, in particular the oral cavity and larynx, isolated premalignant lesions of the sinonasal tract (i.e., high-grade intraepithelial dysplasia and carcinoma in situ) are rarely seen unless associated with another neoplasm:
Often seen in association with an invasive squamous cell carcinoma, including conventional keratinizing type, nonkeratinizing carcinoma, basaloid squamous cell carcinoma, others
May be seen in association with a carcinoma arising in association with a benign neoplasm (e.g., malignant transformation of Schneiderian papilloma)
Although an extremely rare occurrence, sinonasal high-grade intraepithelial dysplasia can be seen in association with non-neoplastic sinonasal tract lesions, such as inflammatory polyps.
Squamous cell carcinoma and variants thereof may develop from inverted and oncocytic types of Schneiderian papillomas:
HPV can be found in these types of Schneiderian papillomas, but there is no definitive link between the presence of HPV and the development of sinonasal squamous cell carcinoma.
No known association between the presence of squamous metaplasia of the sinonasal epithelium and the development of squamous cell carcinoma
Cytogenetics and molecular genetics:
For the majority of patients with head and neck squamous cell carcinoma and its variants, there are genetic alterations at the short arm of chromosome 9 regions.
Specific types of squamous cell carcinoma demonstrate distinctive molecular alterations.
These findings support the early involvement of these chromosomal loci in squamous epithelial carcinogenesis and support their temporal occurrence prior to the phenotypic diversion.
Not infrequently, sinonasal neoplasms are removed in pieces lacking orientation.
Partial maxillectomy:
Usually performed for tumors situated in the inferior portion of the maxilla (e.g., hard palate and alveolar recess)
The specimen includes a portion of the maxilla that may include the alveolar process, palate, and nasal turbinates.
For certain tumor types the incision may be extended to include the ethmoid complex and lateral nose.
Radical maxillectomy includes:
The entire maxillary wall, ethmoid labyrinth, with or without the orbital content
Anterior portion: anterior maxilla, anterolateral portion of the zygomatic process of the frontal bone, frontal processes of the maxilla, medial and inferior walls of the orbit:
Skin and soft tissue overlying the anterior maxilla may be removed.
Posterior margin: pterygoid muscle
If the orbit is resected, then the optic nerve and orbital content are the posterior margin.
Medial margin: transected hard palate and maxilla
Lateral margin: zygomatic arch
Superior margin:
If orbit removed, exposed orbital soft tissue
If orbit not removed, floor of orbit
For staging purposes the sites of inclusion include:
Maxillary sinus
The nasoethmoid complex, which includes:
Nasal cavity
Ethmoid sinuses
The nasal cavity is subdivided into four subsites:
Septum
Floor
Lateral wall
Vestibule
The maxillary sinus is divided into right and left.
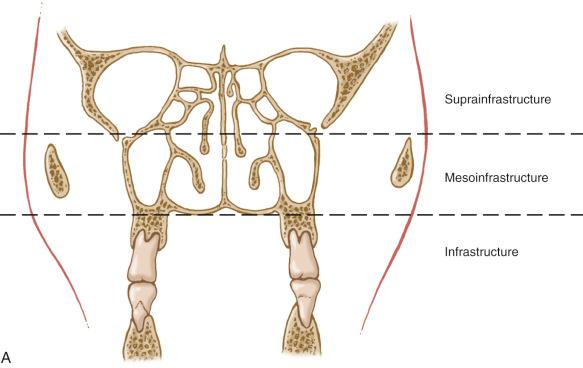
Ohngren line connecting the medial canthus of the eye to the angle of the mandible dividing the maxillary sinus into an anteroinferior portion (infrastructure) and superoposterior portion (suprastructure) structures (see Fig. 3-1 ):
Carcinomas of the infrastructure are associated with a good prognosis.
Carcinomas of the suprastructure are associated with a poor prognosis:
The poorer prognosis with carcinomas of the suprastructure reflects early access of these tumors to critical structures, including the eye, skull base, pterygoids, and infratemporal fossa.
Definition: Group of benign neoplasms arising from the sinonasal (Schneiderian) mucosa and composed of a squamous or columnar epithelial proliferation with associated mucous cells.
| Exophytic | Inverted | Oncocytic | |
|---|---|---|---|
| Percentage | 18% to 50% | 47% to 79% | 3% to 8% |
| Gender/Age | M > F; 20-50 yrs | M > F; 40-70 yrs | M = F; >50 yrs |
| Location | Nasal septum | Lateral nasal wall in region of middle turbinates with extension into sinuses (maxillary or ethmoid) | Lateral nasal wall and sinuses (maxillary or ethmoid) |
| Focality | Unilateral | Typically unilateral; rarely, bilateral (up to 10%); bilateral disease should prompt possibility of septal perforation from unilateral disease | Unilateral |
| Histology | Papillary fronds composed of a predominantly squamous (epidermoid) epithelium; mucocytes (goblet cells) and intraepithelial mucous cysts are present; delicate fibrovascular cores | Endophytic or “inverted” growth consisting of thickened squamous epithelium composed of squamous, transitional, and columnar cells (all three may be present in a given lesion) with admixed mucocytes (goblet cells) and intraepithelial mucous cysts; mixed chronic inflammatory cell infiltrate characteristically is seen within all layers of the surface epithelium | Multilayered epithelial proliferation composed of columnar cells with abundant eosinophilic and granular cytoplasm; outer surface of the epithelial proliferation may demonstrate cilia; intraepithelial mucous cysts, often containing polymorphonuclear leukocytes |
| Incidence of human papillomavirus | Approximately 50% positive; HPV 6 and 11 most common; rarely HPV 16 and 57b | Approximately 38% positive; HPV 6 and 11; less frequently HPV 16, 18; rarely HPV 57 | Typically absent |
| Treatment | Complete surgical excision | Complete surgical excision; may require lateral rhinotomy or medial maxillectomy with en bloc excision | Complete surgical excision; may require lateral rhinotomy or medial maxillectomy with en bloc excision |
| Prognosis | Good following complete surgical excision; will recur if incompletely resected | Good following complete surgical excision; will recur if incompletely resected | Good following complete surgical excision; will recur if incompletely resected |
| Incidence malignant transformation | Rare | Approximately 10% (range reported of 2% to 27%); most commonly SCC and NKSCC; less common types including VC, MEC, SCSC, adenocarcinoma; synchronous (61%) > metachronous (39%) | 4% to 17%; most commonly SCC; less common types include MEC, SCUNC, SNUC |
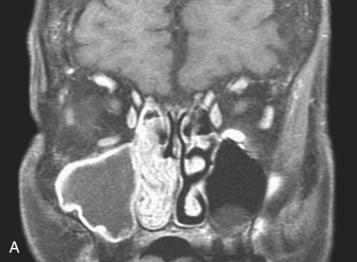
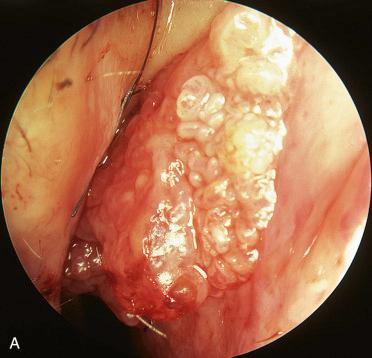
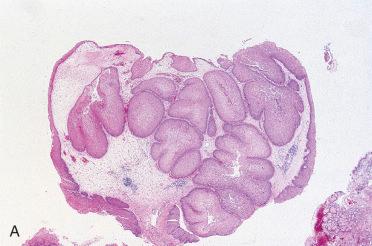
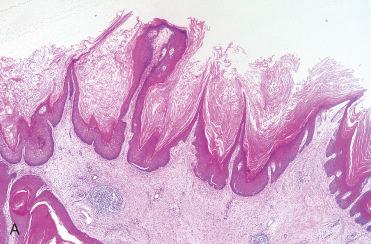
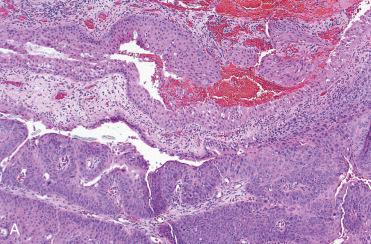
Synonym: Sinonasal-type papillomas
Ectodermally derived lining of the sinonasal tract, termed the Schneiderian membrane, may give rise to three morphologically distinct benign papillomas collectively referred to as Schneiderian or sinonasal-type papillomas. The three morphologic types are:
Inverted
Oncocytic (cylindric or columnar cell)
Exophytic (fungiform, septal) papillomas
Collectively, Schneiderian papillomas represent less than 5% of all sinonasal tract tumors.
As reported in the literature, among sinonasal-type papillomas, the exophytic (septal) papilloma is the most common type; however, practical experience indicates that the inverted type is the most common subtype; the oncocytic type is the least common.
In general, the sinonasal-type papillomas occur over a wide age range but are rare in children:
Inverted papillomas are most common in the fifth to eighth decades.
Oncocytic papillomas occur in a somewhat older age range (greater than 50 years) and are uncommon in patients younger than the fourth decade of life.
Exophytic (septal) papillomas tend to occur in a younger age group.
Localization:
Exophytic papillomas almost invariably are limited to the nasal septum.
Inverted papillomas occur along the lateral nasal wall (middle turbinate or ethmoid recesses) with secondary extension into the paranasal sinuses (maxillary and ethmoid, and less often sphenoid and frontal); less frequently, inverted papillomas may originate in a paranasal sinus.
Oncocytic papillomas also are most often seen along the lateral nasal wall but may originate within a paranasal sinus (maxillary or ethmoid).
Inverted and oncocytic subtypes rarely occur on the nasal septum.
Inverted papillomas may occur in a paranasal sinus without involvement of the nasal cavity.
Schneiderian papillomas have a tendency to spread along the mucosa into adjacent areas.
Although uncommon, the sinonasal-type (Schneiderian) papillomas may originate in the nonsinonasal tract sites, including the nasopharynx or the ear:
Typically in such situations there is no connection to the sinonasal tract and/or to a Schneiderian papilloma extending from the sinonasal tract to these locations.
In all likelihood such Schneiderian-type papillomas occurring in nonsinonasal tract sites arise from misplaced ectodermal-derived epithelial rests from the sinonasal tract.
Typically, Schneiderian papillomas are unilateral; bilateral papillomas, in particular the inverted subtype, may occur with reported incidence of up to 10%:
In the presence of bilaterality, clinical evaluation to exclude the possibility of extension from unilateral disease (i.e., septal perforation) should be undertaken.
Symptoms vary according to site of occurrence and include airway obstruction, epistaxis, asymptomatic mass, and pain.
Schneiderian papillomas may occur simultaneously with nasal inflammatory polyps or other lesion types including (but not limited to) sinonasal hamartomas.
Radiology: appearance varies with extent of disease:
A soft tissue density is seen early in the disease.
Opacification and mucosal thickening are present with more extensive disease.
Evidence of pressure erosion of bone may be seen.
Human papillomavirus (HPV) types 6/11, less often 16, and rarely other HPV types (e.g., HPV57), have been found in septal and inverted papillomas by various molecular biologic analyses (in situ hybridization and/or polymerase chain reaction):
Strong association identified between HPV and exophytic papillomas
Association between HPV and inverted papillomas less well defined:
Approximately 38% of inverted papillomas reported to be positive for HPV by molecular biologic evaluation
To date, molecular biologic analysis of oncocytic papillomas has not identified the presence of HPV.
Whether there is a cause and effect between the presence of HPV and the development of Schneiderian papillomas remains to be determined.
p16(INK4a) not considered a useful surrogate marker for HPV detection across the various types of Schneiderian papillomas.
HPV detection rates increase in inverted papillomas with epithelial dysplasia and malignant transformation (i.e., carcinoma) of inverted papillomas:
Increasing ratio of high-risk to low-risk HR HPV types as compared with “conventional” inverted papillomas (i.e., without dysplastic epithelium)
Epstein-Barr virus has not been found to be associated with Schneiderian papillomas.
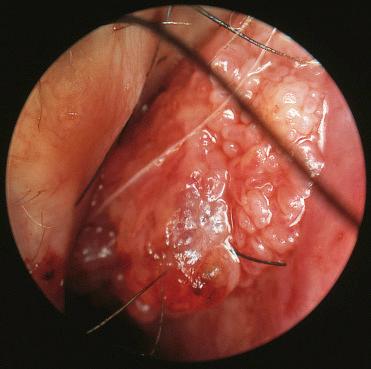
Large, bulky, translucent masses with a red to gray color, varying from firm to friable in consistency
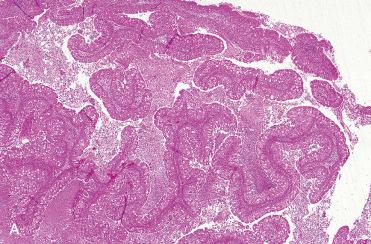
Endophytic or “inverted” growth pattern consisting of markedly thickened squamous epithelial proliferation growing downward into the underlying stroma:
Exophytic growth, in addition to endophytic growth, may be present.
Epithelium varies in cellularity and is composed of squamous, transitional, and columnar cells (all three may be present in a given lesion) with admixed mucocytes (goblet cells) and intraepithelial mucous cysts.
Cells are generally bland in appearance with uniform nuclei and no piling up; however, pleomorphism and cytologic atypia may be present.
Mixed chronic inflammatory cell infiltrate characteristically is seen within all layers of the surface epithelium.
Epithelial component may demonstrate extensive clear cell features indicative of abundant glycogen content (diastase-sensitive, PAS-positive).
Mitotic figures may be seen in the basal and parabasal layers, but atypical mitotic figures are not seen.
Surface keratinization may be present.
Stromal component varies from myxoid to fibrous with admixed chronic inflammatory cells and variable vascularity.
Histochemistry:
Intraepithelial mucocytes show intracytoplasmic mucin-positive material (mucicarmine-positive; diastase-resistant, PAS-positive).
Sinonasal inflammatory polyps
Nonkeratinizing respiratory (“transitional”) carcinoma
Verrucous carcinoma
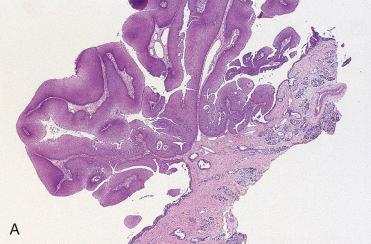
Septal papillomas are papillary, exophytic, verrucoid lesions with a pink to tan appearance and a firm to rubbery consistency; they are often attached to the mucosa by a narrow or broad-based stalk.
Papillary fronds are seen and are composed of a thick epithelium, which is predominantly squamous (epidermoid) and, less frequently, respiratory type.
Surface keratinization is uncommon.
Mucocytes (goblet cells) and intraepithelial mucous cysts are present.
Stromal component is composed of delicate fibrovascular cores.
Histochemistry:
Intraepithelial mucocytes will show intracytoplasmic mucin positive material (mucicarmine positive; diastase-resistant, PAS-positive).
Verruca vulgaris
Squamous papilloma:
In contrast to all of the sinonasal-type papillomas, squamous papilloma of the nasal vestibule does not have mucocytes as a part of the neoplastic proliferation.
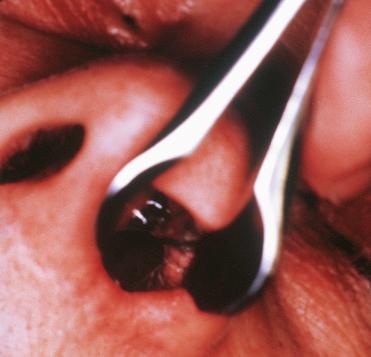
Oncocytic papillomas are dark red to brown, papillary, or polypoid lesions.
Multilayered epithelial proliferation composed of columnar cells with abundant eosinophilic and granular cytoplasm
Nuclei vary from vesicular to hyperchromatic; nucleoli are usually indistinct.
Outer surface of the epithelial proliferation may demonstrate cilia.
Intraepithelial mucin cysts, often containing polymorphonuclear leukocytes are seen; cysts are not identified in the submucosa.
Stromal component varies from myxoid to fibrous with admixed chronic inflammatory cells and variable vascularity.
Histochemistry:
Intraepithelial mucocytes will show intracytoplasmic mucin-positive material (mucicarmine-positive; diastase-resistant, PAS-positive).
Rhinosporidiosis
Low-grade papillary adenocarcinoma
Treatment for all sinonasal-type papillomas is complete surgical excision, including adjacent uninvolved mucosa; the latter is necessary because growth and extension along the mucosa results from the induction of squamous metaplasia in the adjacent sinonasal mucosa:
Adequate surgery includes a lateral rhinotomy or medial maxillectomy with en bloc excision.
Schneiderian papillomas of all histologic types will recur if incompletely resected; recurrence probably represents persistence of disease instead of multicentricity of the neoplasm.
In general, prognosis is good following complete surgical excision; however, if left unchecked, these neoplasms have the capability of continued growth with extension along the mucosal surface with destruction of bone and invasion of vital structures (e.g., central nervous system, others).
Adjuvant therapy (chemo- and radiotherapy) has not been demonstrated to be beneficial in sinonasal papilloma:
Radiation may prove beneficial in a select population of patients with unresectable tumors due to locally advanced disease.
Complications associated with Schneiderian papillomas include recurrence and malignant transformation:
Inverted papillomas and oncocytic papillomas can undergo malignant transformation.
Incidence of malignant transformation varies per subtype:
Malignant transformation reported for the inverted subtype ranges from 2% to 27% but is more likely around 11%.
Malignant transformation reported for the oncocytic subtype ranges from 4% to 17%.
Malignant transformation relative to exophytic (septal) papilloma rarely, if ever, occurs.
Majority of the malignancies occurring in association with Schneiderian papillomas are squamous cell carcinomas (keratinizing and nonkeratinizing), varying in appearance from well to poorly differentiated; less frequently, other carcinomas may occur, including verrucous carcinoma, mucoepidermoid carcinoma, spindle cell squamous carcinoma, small cell carcinoma, adenocarcinoma, and sinonasal undifferentiated carcinoma.
Carcinoma may occur synchronously or metachronously with the papilloma:
Metachronous carcinomas develop with a mean interval of 63 months (range 6 months to 13 years) from the onset of the papilloma to the development of the carcinoma.
Carcinomatous foci may be limited or extensive and may show epithelial dysplasia as well as carcinoma in situ or invasive carcinoma.
Evidence of a pre-existing papilloma may be present with obvious transition from benign papilloma to overt carcinoma; other possibilities include a tumor that is predominantly benign (papilloma) with only limited foci of malignancy or a tumor that is predominantly a carcinoma with very limited residual papilloma.
In some cases, there may be no residual evidence of a pre-existing benign tumor and only by history was the patient known to have had a previous benign sinonasal papilloma.
There are no reliable histologic features that predict which papillomas are likely to become malignant:
Papillomas with increased cellularity, pleomorphism, and increased mitotic activity do not necessarily become malignant.
Presence of moderate to severe intraepithelial dysplasia is a potential indicator of malignant transformation.
Dysplastic epithelium will have increased p16, p53, and Ki67 expression.
Surface keratinization and dyskeratosis have anecdotally been considered as possible predictors of malignant transformation.
Any sinonasal papilloma that shows moderate to severe intraepithelial dysplasia or has surface keratinization should prompt thorough histologic examination of all resected tissue to exclude the presence of malignancy.
There is no correlation between the number of recurrences and the development of carcinoma.
Treatment for malignant transformation of a sinonasal papilloma includes surgery and radiotherapy.
Prognosis for patients with malignant transformation varies:
In some patients the carcinomas are only locally invasive with favorable prognosis following treatment.
In other patients there may be extensive invasion with involvement of vital structures and/or metastatic disease; these patients generally have a poor clinical outcome irrespective of therapeutic intervention.
Squamous papillomas represent the most common benign neoplasms of the upper aerodigestive tract mucosa and are commonly seen in the oral cavity and larynx; less often, squamous papillomas occur in the nasopharynx and nasal vestibule.
For a more complete discussion on squamous papillomas see Section 2, Oral Cavity, and Section 5, Larynx.
Nasal vestibular squamous papillomas are of cutaneous origin.
In contrast to the sinonasal-type papillomas, squamous papillomas of the nasopharynx are endodermally derived.
Squamous papillomas are exophytic, warty, or cauliflower-like tumors ranging in size from a few millimeters up to 3.0 cm in greatest dimension.
Histologically, these tumors are composed of benign squamous epithelium arranged in multiple finger-like projections with prominent fibrovascular cores.
In contrast to the sinonasal-type papillomas, cutaneous squamous papillomas lack intraepithelial mucocytes.
In squamous papillomas the squamous epithelium is free of dysplastic change.
In general, these tumors lack surface keratin, but in any tumor there may be (hyper)keratosis, as well as para- and orthokeratosis:
Presence of surface keratin carries no additional risk for the development of carcinoma.
Surgical excision is the treatment of choice and is curative.
Recurrences occur infrequently and relate to inadequate excision.
Malignant transformation does not occur.
Benign salivary gland tumors of the sinonasal region are uncommon.
For a more complete discussion on benign salivary gland tumors see Section 6, Salivary Glands.
In general, minor salivary gland tumors occur most often in the nasal cavity and rarely in the paranasal sinuses.
Pleomorphic adenoma is the dominant histologic type seen; less often, monomorphic adenomas such as myoepithelioma and oncocytoma occur:
Pleomorphic adenomas of the sinonasal tract may be hypercellular, dominated by the presence of epithelial cells or myoepithelial cells or both cellular components.
Not infrequently, such neoplasms are myoepithelial-predominant pleomorphic adenomas.
There may be a limited amount of chondromyxoid stroma.
Pleomorphic adenomas tend to originate along the nasal septum (bony or cartilaginous component) more than any other site.
Although these tumors may arise from within the paranasal sinus, more often paranasal sinus involvement occurs secondary to extension from an intranasal lesion.
These tumors appear as polypoid or exophytic growths, usually covered by an intact mucosa, and vary in size from 1 to 7 cm.
As is true of all upper aerodigestive tract minor salivary gland tumors (benign or malignant), the pleomorphic adenomas are unencapsulated; however, in contrast to malignant minor salivary gland tumors, these tumors are relatively circumscribed without invasive growth; involvement of surface epithelium does not constitute invasion:
Diagnostic caution should be exercised when confronted with biopsy material that essentially includes lesional tissue with limited to absent surrounding tissues; in such biopsy material, although lesional cells appear bland, lacking cytomorphologic features of malignancy, there are histologic features shared by benign salivary gland neoplasms and malignant salivary gland neoplasms (e.g., growth patterns, cell types) such that differentiation may be predicated solely on the basis of the presence or absence of invasive growth.
In the above scenario, although the findings may suggest a diagnosis of a benign minor salivary gland neoplasm, a diagnosis of “minor salivary gland neoplasm, not further specified” or “minor salivary gland neoplasm, favor benign neoplasm” may be prudent, with the recommendation for conservative but complete surgical excision.
Histologically, these tumors are identical to those of major salivary glands, including an admixture of ductular or tubular structures, spindle-shaped or plasmacytoid-appearing myoepithelial cells, and a chondromyxoid stroma.
There is a tendency for pleomorphic adenomas of the nasal cavity to be cellular, showing a predominant myoepithelial component:
Myoepithelial cells usually are in the form of plasmacytoid (hyaline cell) rather than spindle-shaped myoepithelial cells.
Given the presence of ductular or tubular structures and chondromyxoid stroma, these tumors would not be considered as myoepitheliomas.
Myoepithelial differentiation can be shown by immunoreactivity for cytokeratins as well as p63, p40, calponin, S100 protein, smooth muscle actin, glial fibrillary acidic protein, and vimentin.
Rarely, skeletal muscle differentiation can be seen.
Surgical excision is the preferred treatment for all types of benign minor salivary gland tumors.
Surgery is usually curative, with local recurrence seen in less than 10% of patients.
Definition: Benign polypoid form of capillary hemangioma occurring primarily on skin and mucous membranes.
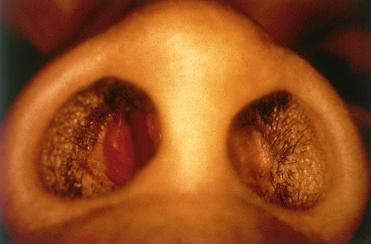
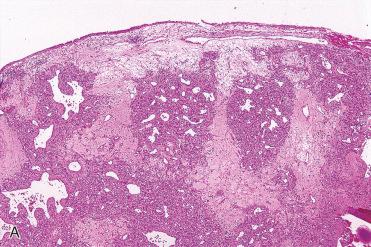
Synonyms: Pyogenic granuloma; pregnancy tumor; epulis gravidarum. The term pyogenic granuloma is a misnomer in that this lesion is neither an infectious process nor granulomatous.
No gender predilection; wide age range commonly seen in the fourth and fifth decades of life but uncommon under 16 years of age
Most often identified in the anterior portion of the nasal septum referred to as Little's area or Kisselbach's triangle; next most common sinonasal location is the tip of the turbinates.
Hemangiomas of the sinonasal tract tend to be mucosally based but also may arise from within the osseous components of this region (intraosseous hemangiomas).
Aside from the lobular capillary hemangioma, other types of hemangiomas of the sinonasal cavity and nasopharynx are rare.
Most common clinical complaint is epistaxis; an obstructive painless mass may be present.
Pathogenesis remains unclear:
A minority of cases may be associated with prior trauma.
LCH may occur in association with pregnancy and in association with oral contraceptive use, suggesting that hormonal factors may be involved.
A hormonal role is further supported by the regression of these tumors following parturition; however, immunohistochemical evaluation failed to identify estrogen or progesterone receptors in any of these tumors.
The mechanism for the regression of pregnancy-related pyogenic granuloma after parturition remains unclear; proposed mechanisms for regression include the absence of vascular endothelial growth factor (VEGF) and angiopoietin-2 (Ang-2), causing blood vessels to regress:
Amount of VEGF has been found to be high in the LCH in pregnancy and almost undetectable after parturition, suggesting that a lack of VEGF is associated with apoptosis of endothelial cells and regression of these tumors.
No role for Ang-2 alone in regression has been found.
Smooth, lobulated, polypoid red mass measuring up to 1.5 cm in diameter
Characterized by a submucosal vascular proliferation arranged in lobules or clusters composed of central capillaries and smaller ramifying tributaries:
Central capillaries vary in caliber, as well as in shape, and in more “mature” lesions may show a “staghorn” appearance.
Endothelial cell lining may be prominent and may display endothelial tufting, as well as mitoses, but atypical mitoses are not identified:
Designation of “active” LCH can be used in conjunction with those tumors showing an increase of mitotic activity, but these “active” lesions carry no additional risk of aggressive behavior or of transformation to an angiosarcoma.
There is no intercommunication of vascular spaces as seen in angiosarcomas nor is there true cytologic atypia or atypical mitoses.
Surrounding and intimately associated with the vascular component is granulation tissue and a mixed chronic inflammatory cell infiltrate.
Surface epithelium is often ulcerated with associated (fibrnioid) necrosis but may show squamous metaplasia with or without hyperplastic changes.
Immunohistochemistry:
Lesional cells are reactive for CD31, CD34, and Factor VIII–related antigen; androgen receptor (nuclear) reactivity may be present.
No immunoreactivity is present for glucose transporter 1 (GLUT1), human herpesvirus 8 (HHV-8).
Sinonasal-type hemangiopericytoma-like tumor:
Diffuse growth pattern composed of a single cell type and presence of perivascular hyalinization contrast to the findings seen in LCH
Angiofibroma
Infantile hemangiomas:
GLUT1 positive
See Section 6, Salivary Gland for more detailed discussion.
Angiosarcoma
Treatment includes conservative but complete surgical excision of the lesion.
Prognosis following excision is excellent.
Recurrences are relatively infrequent.
Cavernous hemangiomas occur less frequently in the upper respiratory tract when compared with the capillary hemangioma.
In general, cavernous hemangiomas have a similar clinical presentation to capillary hemangiomas but are more often identified in the turbinates, lateral nasal wall, or within bone (intraosseous) than in the nasal septum.
Similar to histology of cavernous hemangiomas of other sites, those of the sinonasal tract are composed of multiple, variably sized, dilated and thin-walled, endothelial cell-lined vascular spaces.
Surgical resection is curative.
Definition: Sinonasal tumor showing perivascular myoid differentiation, typically with indolent biologic behavior.
Synonyms: Sinonasal-type hemangioperciytoma; sinonasal glomus tumor
NOTES:
The classification of this sinonasal tumor has evolved from its designation for the last several decades as sinonasal hemangiopericytoma-like tumor, reflecting the uncertainty of its relationship to soft tissue hemangiopericytoma, to the current designation of sinonasal glomangiopericytoma, reflecting a perivascular myoid differentiation.
The current classification of soft tissue hemangiopericytoma incorporates it within the spectrum solitary fibrous tumor (SFT) based on the presence of NAB2-STAT6 gene fusion and STAT6 immunoreactivity.
Limited studies to date have shown that sinonasal hemangiopericytoma-like tumors are negative for NAB2-STAT6 gene fusion and/or STAT6 immunoreactivity, precluding classification within the spectrum of SFT and soft tissue hemangiopericytoma.
Genetic abnormalities seen in association with glomus tumor and pericytoma include miR143-NOTCH fusions and GLI1, respectively, but neither NOTCH or GLI reported in sinonasal hemangiopericytoma-like tumor.
Based on the limited genetic studies reported in the literature to date, it would appear that the sinonasal hemangiopericytoma-like tumor does not merit classification within the spectrum of SFT/soft tissue hemangiopericytoma or glomangiopericytoma but perhaps does merit the retention of the designation sinonasal hemangiopericytoma-like tumor, although its eventual classification based on more defining molecular findings may yet to be determined.
Represents less than 1% of all sinonasal tract tumors
No gender predilection; occurs over a wide age range but is most commonly seen in the sixth to seventh decades of life
Typically presents as a unilateral nasal mass with obstruction and epistaxis; extension into adjacent paranasal sinuses may occur, but isolated involvement to a paranasal sinus is uncommon.
Radiology:
Opacification of the involved sinus
Bone erosion due to pressure may be seen.
Arteriographic findings reveal a richly vascular neoplasm.
There are no known etiologic factors.
May rarely cause oncogenic osteomalacia (OO):
OO is a rare paraneoplastic syndrome of osteomalacia due to phosphate wasting.
Phosphaturic mesenchymal tumor (mixed connective tissue variant) extremely rare, distinctive tumor frequently associated with OO
Overexpression of fibroblast growth factor-23 (FGF-23), a phosphaturic hormone, shown in phosphaturic mesenchymal tumor
Phosphaturic mesenchymal tumor is a soft tissue tumor histologically characterized by:
Hemangiopericytoma-like areas and osteoclast-like giant cells with or without osseous and cartilaginous metaplasia
”Grungy” calcified matrix identified
Red to tan-gray, soft to firm polypoid mass of varying size
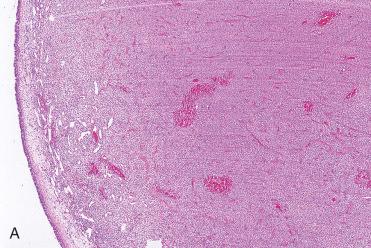
Submucosal, circumscribed, but unencapsulated cellular tumor with effacement of seromucous glands, although seromucous glands may be retained although surrounded by lesional cells.
Diffuse growth pattern composed of single cell type situated around endothelial-lined vascular spaces
Lesional cells are usually arrayed in short fascicles, and less often may show storiform, whorled, or even palisaded growth patterns with little intervening stromal collagen.
Lesional cells are usually uniform with round to oval nuclei, vesicular to hyperchromatic-appearing chromatin, and indistinct eosinophilic cytoplasm; occasionally, spindle-shaped cells are seen.
Mild nuclear pleomorphism and an occasional mitotic figures can be seen, but typically there is an absence of increased mitotic activity, atypical mitoses, and necrosis.
Vascular channels range from capillary size to large sinusoidal spaces that may have a “staghorn” or “antler-like” configuration.
A characteristic but not pathognomonic feature is the presence of perivascular hyalinization.
Cellular proliferation may compress and obscure blood vessels of smaller size.
Extravasated erythrocytes are often identified.
An inflammatory cell component usually including mast cells but also eosinophils is present scattered throughout the tumor.
Multinucleated (tumor) giant cells can be seen in a minority of cases.
Fibrosis or a myxoid stroma may be seen, especially in tumors undergoing degenerative change.
Heterologous metaplastic elements, including bone and cartilage, may occasionally be seen; lipomatous change may also be present.
Histochemistry:
Reticulin stain reveals a distinctive pattern characterized by envelopment of individual pericytes by reticulin fibers.
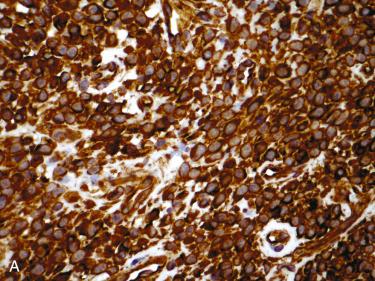
Immunohistochemistry:
Consistent immunoreactivity for vimentin and smooth muscle actin
Majority of cases stain for Factor XIIIa, muscle-specific actin, β-catenin (nuclear) and cyclin D1 (nuclear):
Nuclear expression of β-catenin and cyclin D1 represent sign of oncogenic activation and demonstrate that mutational activation of β-catenin and associated cyclin D1 overexpression may be central events in pathogeneisis.
Negative for STAT6
Typically negative for CD34, but in any given case CD34 staining may be present.
Variably reactivity for S100 protein
Typically do not stain for cytokeratins, CD31, Factor VIII–related antigen, desmin, neuron-specific enolase, EMA, CD68, bcl-2, CD99, TLE1, Fli1, and CD117
Electron microscopy:
Pericellular basal lamina, pinocytotic vesicles, intracytoplasmic (thin) filaments, dense bodies, and membranous attachment plaques
Cytogenetics and molecular biology:
Absence of NAB2-STAT6 gene fusion (this gene fusion reported in solitary fibrous tumors/soft tissue hemangiopericytoma)
Absence of miR143-NOTCH fusions (these gene fusions reported in glomus tumor)
Absence of GLI1 (this gene fusion seen in reported in pericytoma)
Lobular capillary hemangioma
Nasopharyngeal angiofibroma
Glomus tumor (glomangioma)
Solitary fibrous tumor (SFT):
In contrast to the solitary fibrous tumor, sinonasal hemangiopericytoma-like tumor lacks the presence of “ropey” keloidal-appearing collagen or amianthoid fibers and bcl-2 staining.
Sinonasal hemangiopericytoma-like tumor and SFT may show the presence of CD34 immunoreactivity; however, the extent of CD34 immunoreactivity varies:
In sinonasal hemangiopericytoma-like tumor CD34 staining if present is localized and not diffuse.
In solitary fibrous tumors CD34 tends to be diffuse and strong.
STAT6 immunoreactivity seen in SFT but not in sinonasal hemangiopericytoma-like tumor
NAB2-STAT6 gene fusion seen in SFT but not in sinonasal hemangiopericytoma-like tumor
Smooth muscle tumors (leiomyoma and leiomyosarcoma)
Benign and malignant fibrohistiocytic neoplasms
Synovial sarcoma
Mesenchymal chondrosarcoma
Surgery is the preferred treatment and should include complete excision to include tumor-free margins.
Considered radioresistant neoplasms
Indolent behavior with overall 5-year survival rates of greater than 90%
Local recurrence may occur in as high of 30% of cases and is likely due to inadequate surgical excision:
Recurrence can be anticipated over extended follow-up periods (1 to 2 decades).
Aggressively behaving sinonasal hemangiopericytoma-like tumors are uncommon and include tumors that are locally destructive or are metastatic.
Findings potentially linked to aggressive behavior include:
Large tumor size (greater than 5 cm)
Marked nuclear pleomorphism
Increased mitotic activity
Necrosis
Invasive growth (e.g., bone)
Proliferation index of greater than 10%
Metastatic tumor is uncommon; if it occurs, spread usually is to regional lymph nodes and lung and is preceded by (one or more) recurrent tumor.
Meningiomas are benign neoplasms of meningothelial cells representing from 13% to 18% of all intracranial tumors; occurrence outside the central nervous system is considered ectopic; ectopic meningiomas are divided into:
Primary ectopic meningiomas representing those tumors with no identifiable central nervous system connection
Secondary ectopic meningiomas representing those tumors with central nervous system connection
For a more complete discussion see Section 7, Ear and Temporal Bone.
Most common sites of occurrence of the ectopic meningiomas of the head and neck region include the middle ear and temporal bone and sinonasal cavity; less commonly, oral cavity and parotid gland.
Sinonasal tract meningiomas most often involve the nasal cavity, or a combination of nasal cavity and paranasal sinuses; less frequently, involvement may be isolated to the nasopharynx, frontal sinus, or sphenoid sinus.
In the sinonasal cavity, symptoms include nasal obstruction, epistaxis, headache, pain, visual disturbances, and facial deformity.
Tumors may erode the bones of the sinuses with involvement of surrounding soft tissues, the orbit, and occasionally the base of the skull.
Grossly, these tumors appear as a polypoid mass; often, the tumor is curetted out and received as fragments of solid, white tissue; a gritty consistency may be noted.
Among the histologic subtypes of meningioma, the meningotheliomatous type is the most common in the sinonasal cavity:
Histologic features include a lobular growth pattern with tumor nests separated by a variable amount of fibrous tissue.
Cells have a whorled arrangement and have round to oval or spindle-shaped nuclei with pale-staining cytoplasm and indistinct cell borders.
Characteristically, the nuclei have a punched-out or empty appearance resulting from intranuclear cytoplasmic inclusions.
Psammoma bodies, typical and numerous in intracranial meningothelial meningiomas, may be seen but are not as common in the ectopically located meningiomas.
Immunohistochemistry:
Reactivity with epithelial membrane antigen (EMA) and vimentin
Cytokeratins can be positive:
As a whole may be positive in 20% of cases
May approach 40% positivity in epithelial variants
No immunoreactivity with neuroendocrine markers (chromogranin and synaptophysin, others)
Surgical removal is the preferred treatment:
Complete surgical excision may be difficult to achieve, resulting in recurrence; recurrence rates range up to 30%.
Following the histologic diagnosis, it is essential to exclude spread from a primary intracranial neoplasm.
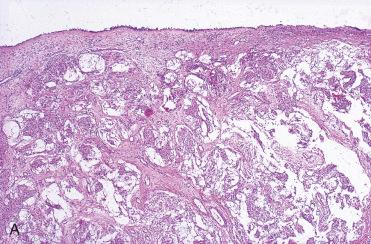
Definition: Pituitary adenoma that arises in upper aerodigestive tract mucosal sites from remnants of Rathke's pouch without any continuity/connection with the sella turcica.
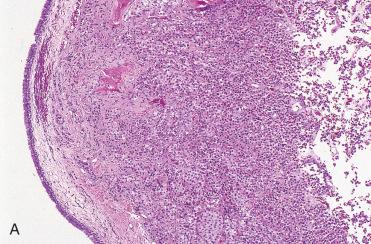
NOTE: Pituitary neoplasms originating from their usual origin in the sella turcica may occasionally extend into the sinonasal tract or nasopharynx and appear to present as a primary neoplasm of those regions. Therefore, prior to rendering a possible diagnosis of EPA, radiographic analysis is required to exclude derivation from the normally situated pituitary gland within the sella turcica.
No gender predilection; occurs in adults
Clinical presentation may include airway obstruction, chronic sinusitis, visual field defects, cerebrospinal fluid leakage, and endocrinopathic manifestations (e.g., Cushing's syndrome, hirsutism).
Most common ectopic sites of occurrence are the sphenoid sinus followed by the nasopharynx:
Other less common sites include the nasal cavity and ethmoid sinus; rarely, may involve the clivus.
Polypoid appearance
Submucosal epithelioid neoplastic proliferation with solid, organoid, and trabecular growth patterns:
Epithelioid cells have round nuclei with a dispersed nuclear chromatin pattern and granular eosinophilic–appearing cytoplasm.
Plasmacytoid-appearing cells may be present.
Mild to moderate nuclear pleomorphism may be identified.
Scattered mitotic figures may be present but atypical mitoses are not identified; necrosis is absent.
Gland-like spaces may be seen; absence of squamous differentiation
Calcifications and/or psammoma-like concretions may be identified.
Infiltrative growth can be identified, including in and around residual seromucous glands as well as into bone.
Immunohistochemistry:
Cytokeratin (AE1/AE3, CAM5.2, others) positive:
Tends to be diffuse and strong, and show paranuclear dot-like pattern
Neuroendocrine markers positive, including:
Chromogranin, synaptophysin, CD56, neuron-specific enolase
Vimentin and S100 protein positive:
S100 protein lacks peripheral sustentacular staining pattern seen in paragangliomas and olfactory neuroblastoma
Pituitary hormones, including growth hormone, adrenocorticotrophic hormone (ACTH), prolactin, thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH):
Reactivity with two or more hormones, so-called plurihormonal pituitary adenoma, may be seen in up to approximately 50% of cases.
Approximately one third of cases express a single hormone:
Prolactin most common
In up to approximately 20% reactivity with pituitary hormone markers may be absent:
Referred to as null cell pituitary adenoma.
Low proliferation rates (less than 5%) by Ki67 (MIB1) staining
Calretinin negative
p53 negative
Paraganglioma
Rarely if ever occurs in the sinonasal tract
Olfactory neuroblastoma (ONB)
ONBs predominantly arise in the superior aspect of the nasal cavity and rarely originate from the sphenoid sinus, the most common location for EPA.
Presence of diffuse and intense cytokeratin staining as seen in EPA essentially excludes a diagnosis of ONB, which is typically cytokeratin negative or at most only focally positive.
Neureondocrine carcinoma (e.g., carcinoid tumor):
Carcinoid tumor (well-differentiated neuroendocrine carcinoma) is so uncommon in the sinonasal tract as to be considered nonexistent.
Malignant epithelial neoplasms (e.g., SNUC, other)
| EPA | ONB | Carcinoid | Paraganglioma | |
|---|---|---|---|---|
| Location | Sphenoid sinus ≫ nasopharynx | Roof of nasal cavity | Uncommon in H&N; rarely occurs in SNT | Neck, ME; rarely if ever identified in SNT |
| Cytokeratins | Positive, diffuse/strong | Negative in majority of cases; may be positive in scattered lesional cells | Positive, diffuse/strong | Negative |
| NE markers | Positive | Positive | Positive | Positive |
| S100 | Positive in lesional cells but not at periphery of lobules | Positive at periphery of lobules as well as in lesional cells | Positive in lesional cells but not at periphery of lobules | Positive at periphery of lobules |
| Pituitary markers | Positive in majority of cases; may be negative | Negative | Negative | Negative |
| Calretinin | Negative | Positive | Negative | Negative |
| Ki67 | Low | Low: grades * 1, 2 High: grades * 3, 4 |
Low | Low |
Wide surgical resection is the preferred treatment:
Complete removal may prove curative without recurrent or progressive tumor, and with resolution of endocrinopathic effects associated with the tumor.
Recurrence not uncommon especially for large tumors and/or incompletely excised tumors
Medical therapy, bromocriptine (dopamine agonist) may be employed to control endocrinopathic effects of the tumor.
Although benign, EPAs may be large and infiltrative, including into bone precluding surgical extirpation; in such cases radiation therapy can be used.
Rarely, malignant transformation may occur.
Definition: Solitary fibrous tumor (SFT) is a distinctive neoplasm composed of CD34-positive fibroblasts with hemangiopericytoma-like vascular pattern that are typically a (pleural) serosal-based proliferation but may occur in extrapleural sites.
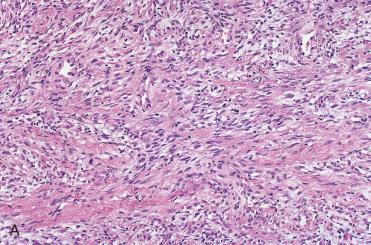
NOTE: Current soft tissue classification of tumors links solitary fibrous tumor and hemangiopericytoma.
SFTs are rare but do occur in the upper aerodigestive tract, primarily involving the nasal cavity and paranasal sinuses.
Patients with tumors in these sites present with nasal obstruction:
Usually, the symptoms have been present for an extended period of time (a year or more).
These tumors typically are polypoid.
Unencapsulated tumor composed of a variably cellular proliferation of bland spindle-shaped cells lacking any pattern of growth and associated with ropey, keloidal collagen bundles and associated thin-walled vascular spaces.
Vascular spaces may be focally prominent and often have the branching appearance as seen in hemangiopericytomas.
Rare mitoses may be seen, but the mitotic rate is not excessive; atypical mitoses are absent and necrosis is absent.
Histologic variants include:
Lipomatous SFT:
Rare variant that consists of histologically benign SFT admixed with areas of mature fat
SFT with giant cells (giant cell angiofibroma):
Giant cell–rich form of SFT
Immunohistochemistry:
Reactivity for CD34 (80% to 90%), CD99 (70%) and bcl-2 (30%), EMA (30%) and actin (20%):
CD34 reactivity tends to be diffuse and strong.
STAT6 immunoreactivity (nuclear)
Absence of S100 protein, desmin, and cytokeratins
Cytogenetics and molecular genetics:
Overexpression of NAB2-STAT6 gene fusion:
Establish NAB2-STAT6 as the defining driver mutation of SFT
Rearrangements of long arm of chromosome 12
Sinonasal hemangiopericytoma-like tumor
Smooth muscle tumors (leiomyoma)
Peripheral nerve sheath tumors
Fibrohistiocytic tumors
Fibromatosis
Nasopharyngeal angiofibroma
Given their tendency to be polypoid, solitary fibrous tumors of the sinonasal tract are amenable to complete surgical resection.
Complete surgical resection is usually curative:
Recurrences generally a function of incomplete excision
SFTs of the nasopharynx may be more difficult to completely excise; despite incomplete resection, these tumors are not generally associated with adverse biologic behavior at this anatomic location.
Malignant transformation of SFT may occur in particular for intrathoracic or soft tissue but rarely for those of head and neck sites; histologic features associated with malignancy include:
Increased cellularity, pleomorphism, and increased mitotic activity (>4/10 high-power fields)
This category of tumors includes benign schwannoma and neurofibroma.
For a more complete discussion of these tumor types see Section 2, Oral Cavity; 4, Neck; and 7, Ear and Temporal Bone.
Benign peripheral nerve sheath tumors of the head and neck are common, perhaps accounting for as many as 45% of all cases; benign peripheral nerve sheath tumors of the sinonasal tract (and nasopharynx) are uncommon, accounting for less than 4%.
In the sinonasal tract, schwannomas are substantially more common than neurofibromas.
Adults are most commonly affected, with no gender predilection.
Patients present with symptoms related to nasal obstruction and epistaxis:
Visual disturbances due to intracranial extension of the tumor may occur.
These tumors may cause pressure erosion of bone.
Nasopharyngeal involvement may result in unilateral serous otitis media.
There is no association with neurofibromatosis.
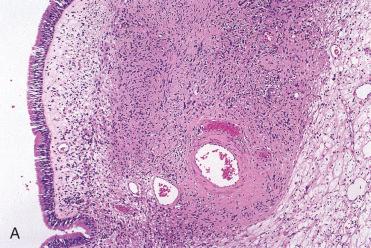
Unlike their soft tissue counterparts, benign schwannomas of the upper aerodigestive tract are unencapsulated although circumscribed.
Aside from the lack of encapsulation, the histologic features are similar to those described for benign peripheral nerve sheath tumors at other sites.
These tumors are submucosal, show variable cellularity, including increased cellularity, but significant pleomorphism is not seen.
Scattered mitotic figures may be present, but the mitotic rate is low and atypical mitoses are not present.
Immunohistochemistry:
Diffuse and intense S100 protein and SOX10 immunoreactivity (cytoplasmic and nuclear pattern) are present.
Cytokeratins, actins, desmin, CD34, and epithelial membrane antigen staining are absent.
Low proliferation rate less than 5% is seen by Ki67 (MIB1) staining.
Surgical resection is the preferred treatment and is curative.
Submucosal, circumscribed tumors composed of spindle-shaped cells with “wavy” or buckled, hyperchromatic nuclei, and indistinct cytoplasm
An associated collagenized and/or myxoid stromal component is present.
Neoplastic cells are S100 protein (and SOX10) positive but extent of staining is less than that seen in schwannomas.
Surgical resection is curative.
Definition: Benign neoplasm composed of an admixture of fibroblasts and histiocytic cells.
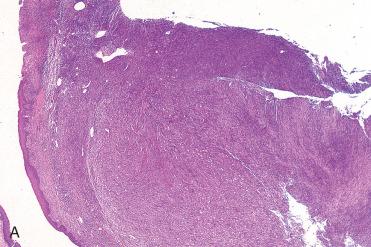
Synonyms: Sclerosing hemangioma; cutaneous-derived are called dermatofibroma
Relatively uncommon neoplasm in the head and neck accounting for less than 5% of all fibrous histiocytomas
More common in men than in women; occurs over a wide age range with a median age in the fifth decade of life
Excluding cutaneous sites, the most common location in the head and neck is the nasal cavity and paranasal sinuses; other more common sites of occurrence include neck, larynx, and trachea.
Symptoms vary according to site and include painless mass, nasal obstruction, epistaxis, pain, facial asymmetry, proptosis, loosening of teeth, hemoptysis, dyspnea, and stridor.
Polypoid or nodular, tan-white to yellow lesions varying in size
Submucosal lesion composed of an admixture of spindle-shaped cells (fibroblasts) and epithelioid cells (histiocytes) in a fascicular or storiform growth pattern
Multinucleated giant cells, an inflammatory cell infiltrate (lymphocytes, plasma cells, and eosinophils), and foam cells are seen scattered throughout the tumor.
Stroma varies and may consist of collagen production, myxoid change, and hyalinization.
Vascularity varies from relatively inconspicuous vessels to prominent vascular pattern with striking hyalinization.
Pleomorphism and mitoses may be seen, but these features in excess or the presence of abnormal mitotic figures suggest malignancy.
Histologic variants include:
Cellular fibrous histiocytoma:
Characterized by increased cellularity and more fascicular and less storiform growth pattern
Increased mitotic activity (mean 3 mitoses per 10 high-power fields) may be present.
Atypical fibrous histiocytoma:
Significantly greater nuclear atypia and mitotic activity in a lesion with background of classic fibrous histiocytoma
Immunohistochemical staining reflects presence of multiple cell types including reactivity for:
CD68 (macrophages)
S100 protein (Langerhan cells)
Vimentin
Desmin may occasionally be reactive.
No immunoreactivity for CD31, CD34, epithelial markers, endothelial cell markers
Cellular fibrous histiocytomas commonly express smooth muscle actin.
Nodular fasciitis
Fibromatosis (see Section 4, Neck, for more complete discussion)
Benign peripheral nerve sheath tumors (benign schwannoma, neurofibroma)
Leiomyoma
Solitary fibrous tumor
Dermatofibrosarcoma protuberans (DFSP):
See Section 7, Ear and Temporal Bone, for a more complete discussion.
Most frequent on the trunk and extremities
Histologically, DFSP infiltrates the dermis and subcutis and in its central aspect is composed of plump fibroblasts arranged in a distinct storiform pattern; cellular pleomorphism is mild and variable mitotic activity is seen.
CD34 immunoreactive
Wide local excision is the preferred treatment.
Undifferentiated pleomorphic sarcoma (formerly malignant fibrous histiocytoma)
Complete surgical excision is generally curative.
Definition: Benign tumor of smooth muscle.
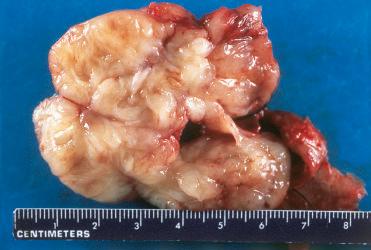
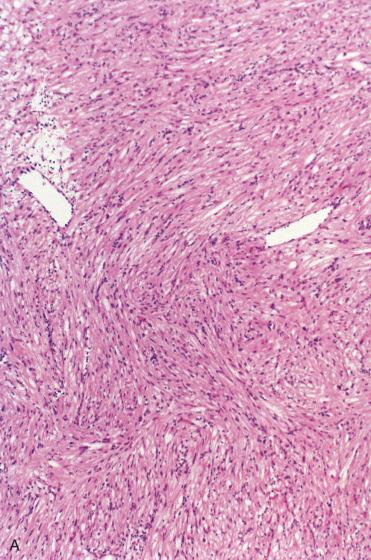
In general, one of the least common mesenchymal tumors in the head and neck area owing to the relative absence of smooth muscle in this region:
Smooth muscle found in association with blood vessels or cutaneous appendages represents origin for many head and neck leiomyomas
Leiomyoma arising from blood vessels termed vascular leiomyoma
More common in men than in women; may occur in all ages but generally is a tumor of adult life with a peak incidence in the sixth decade of life
Most common sites of occurrence in the head and neck are:
Skin and oral cavity (lips, tongue, and palate)
Other sites that may be affected include the sinonasal tract and larynx.
Within the sinonasal tract leiomyomas most often involve the turbinates.
Usually presents as a painless mass and nasal obstruction; other symptoms include dysphagia, voice changes, and pain.
Solitary, well-demarcated, sessile, tan-white submucosal lesion usually measuring less than 3 cm in diameter but may attain larger sizes
On cut section the tumor is homogeneous with a whorled appearance.
Localized to the submuosa, appearing delineated, and characterized by the presence of interlacing bundles or fascicles of cells composed of blunt-ended or “cigar-shaped” nuclei with abundant eosinophilic cytoplasm
Nuclear palisading and perinuclear vacuolization may be seen, but there is no significant pleomorphism or mitotic activity; the nuclei may appear epithelioid.
Neoplastic cells are seen in intimate association with vascular spaces (vascular leiomyoma).
Degenerative type changes, including stromal fibrosis and mucinous or myxoid alterations, may be present.
Hypercellular tumors, referred to as cellular leiomyoma, characterized by an absolute increase in cells, but lacking significant pleomorphism, mitotic activity, necrosis, or invasive growth may be identified.
SMTUMP is histologically characterized by increased cellularity, moderate nuclear pleomorphism, and the presence of no more than four mitoses per 10 high-power fields.
Locally, infiltrative growth (i.e., into bone) may occur in association with SMTUMP.
Histochemistry:
Cytoplasmic myofibrils can be demonstrated by special stains appearing red with Masson trichrome and blue with phosphotungstic acid-hematoxylin (PTAH).
Immunohistochemistry (for leiomyoma and SMTUMP):
Actin (smooth muscle and muscle-specific), vimentin, and desmin positive
S100 protein, CD34, CD31 negative
Proliferation rate for leiomyoma and SMTUMP as seen by Ki67 (MIB1) staining is low (less than or equal to 5%).
Electron microscopy:
Myofilaments, pinocytotic vesicles, investing basal laminae
Peripheral nerve sheath tumor (neurofibroma, schwannoma)
Solitary fibrous tumor
Complete surgical excision is curative.
Rarely recurs
Adult or fetal types of rhabdomyoma rarely occur in the sinonasal tract or nasopharynx.
See Section 5, Larynx, for a more complete discussion.
The cellular features of fetal rhabdomyomas show rhabdomyoblasts in different stages of differentiation, including spindle-shaped and strap cells; these findings may be worrisome for a diagnosis of rhabdomyosarcoma; however, in contrast to rhabdomyosarcoma, fetal rhabdomyomas tend to be circumscribed and lack nuclear atypia or mitotic activity.
Definition: Benign bone-forming tumors that are almost exclusively identified in the craniofacial skeleton.
Controversy exists as to whether the osteoma represents a true neoplasm or possibly represents the end-stage of fibro-osseous lesion.
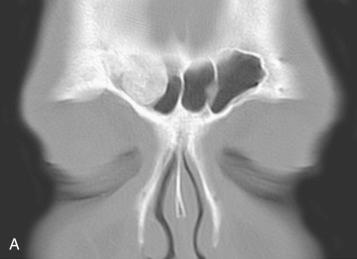
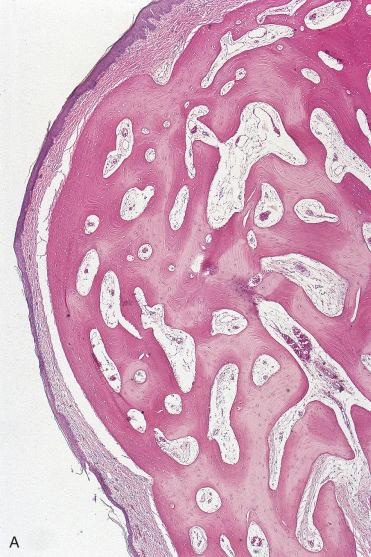
Sinonasal osteomas are more common in men and occur over a wide age range but are most often encountered in the second to fourth decades of life.
These tumors are usually asymptomatic and are found only by radiographic studies.
Symptoms associated with paranasal sinus osteomas include headaches, facial swelling or deformity, and ocular disturbances; meningitis secondary to cranial cavity extension and sinus or aural obstruction may occur.
Most common sites of involvement are the paranasal sinuses with the frontal and ethmoid sinuses most often involved (frontal > ethmoid > maxillary > sphenoid); other sites of involvement include:
Temporal bone (osseous external auditory canal), skull, mandible, maxilla
Radiology:
Sharply delineated radiopaque lesion confined to bone or protruding into a sinus
Cause linked to trauma, infection (sinusitis), and development
Sinonasal osteomas usually occur as a single lesion but may be associated with Gardner syndrome:
Autosomal dominant inheritance
Sentinel lesion for adenomatous polyposis coli (APC)
Characterized by intestinal (colorectal) polyposis, soft tissue lesions (fibromatosis, cutaneous epidermoid cysts, lipomas, leiomyomas), and multiple craniofacial osteomas
Osteomas are well circumscribed and are composed of dense, mature, predominantly lamellar bone sometimes rimmed by osteoblasts.
Interosseous spaces may be composed of fibrous, fibrovascular, or fatty tissue, and hematopoietic elements may be present.
Exostosis
Unless symptomatic, osteomas require no treatment.
Symptomatic osteomas require complete surgical excision, which is curative.
No known link to the development of osteosarcoma
Category of histologically similar–appearing benign fibro-osseous lesions that include ossifying fibroma and fibrous dysplasia
In the head and neck, occur most often in relation to gnathic (maxillary and mandibular) bones
For a more complete discussion on ossifying fibroma and fibrous dysplasia see Section 2, Oral Cavity.
Given its localization to the sinonasal tract, the psammomatoid ossifying fibroma is discussed here.
See Table 3-4 for a comparison of ossifying fibroma, psammomatoid ossifying fibroma, and fibrous dysplasia.
| POF | OF | FD | |
|---|---|---|---|
| Gender/age | F = M; younger age groups (first and second decades), but may occur in older individuals | F > M; third-fourth decades | F = M; first two decades of life |
| Location | Ethmoid sinus; supraorbital frontal region | No specific site of involvement | No specific site of involvement |
| Focality | Single site or involvement of multiple (contiguous) sites/sinuses | Single site | Monostotic (75%-80%); polyostotic (20%-25%) |
| Radiology | Lytic or mixed lytic/radiopaque osseous and/or soft tissue mass varying from well-demarcated to invasive with bone erosion | Well-circumscribed or sharply demarcated lesion with smooth contours | Poorly defined expansile osseous lesion with a thin intact cortex; predominantly fibrous lesions are radiolucent; predominantly osseous lesions are radiodense; lesions with an equal admixture of fibrous and osseous components have a ground-glass appearance |
| Histology | Bony spicules and distinctive mineralized or calcified “psammomatoid” bodies or ossicles admixed with a fibrous stroma; psammomatoid bodies vary from a few in number to a dense population of innumerable spheric bodies; osteoclasts are present within the ossicles, and osteoblasts can be seen along their peripheral aspects; the bony trabeculae vary in appearance and include odd shapes with a curvilinear pattern. The trabeculae are composed of lamellar bone with associated osteoclasts and osteoblastic rimming | Randomly distributed mature (lamellar) bone spicules rimmed by osteoblasts admixed with a fibrous stroma; central portions may be woven bone with lamellar bone at the periphery | Fibrous tissue component is nondescript and of variable cellularity; osseous component includes irregularly shaped trabeculae of osteoid and immature (woven) bone that is poorly oriented with misshapen bony trabeculae with odd geometric patterns including “ C ”-shaped or “ S ”-shaped configurations; the trabeculae typically lack osteoblastic rimming |
| Syndromes | No known association | No known association | Albright syndrome (1%-3%) |
| Treatment | Surgical resection | Surgical resection | Disease may stabilize at puberty and, in children, therapy should be delayed if possible until after puberty; surgical resection indicated in cases with compromise of function, progression of deformity, associated pathologic fracture(s), or the development of a malignancy |
| Prognosis | Good following complete excision; recurrence(s) often occur due to incomplete excision; may behave in an aggressive manner with local destruction and potential invasion into vital structures | Excellent | Good prognosis; recurrence rates are low and death due to extension into vital structures rarely occurs |
| Malignant transformation | Not known to occur | Not known to not occur | Malignant transformation (osteosarcoma) occurs in less than 1% |
Definition: Variant of ossifying fibroma that typically occurs in the sinonasal tract and potentially may behave aggressively with locally invasive and destructive capabilities.
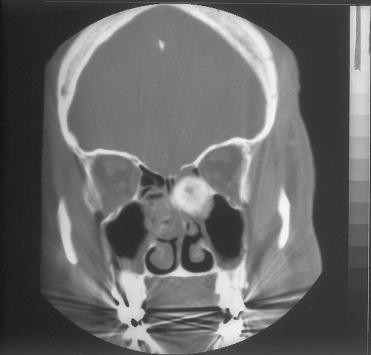
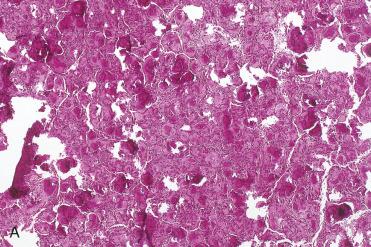
Synonyms: Cementifying or cemento-ossifying fibroma; juvenile active ossifying fibroma
No gender predilection; generally occurs in younger age groups (first and second decades) but can occur over a wide age range, including older individuals.
Presenting symptoms include facial swelling, nasal obstruction, pain, sinusitis, headache, and proptosis.
These lesions may occur in any area of the sinonasal tract but are most frequent in the ethmoid sinus and supraorbital frontal region (ethmoid > nasal cavity > maxillary sinus > frontal sinus); the orbit may also be involved.
There may be involvement of a single site or multiple sinuses.
Radiology:
Lytic or mixed lytic/radiopaque osseous and/or soft tissue mass varying from well-demarcated to invasive with bone erosion
Presenting symptoms include facial swelling, nasal obstruction, pain, sinusitis, headache, and proptosis.
The histology is that of a benign fibro-osseous proliferation composed of bony spicules and spherules admixed with a fibrous stroma.
Most distinctive component is the presence of mineralized or calcified “psammomatoid” bodies or ossicles:
These ossicles vary from a few in number to a dense population of innumerable spheric bodies.
Ossicles are demarcated with a central blue to black appearance surrounded by a pink-appearing rim and with concentric laminations.
Ossicles vary from small with a round to oval shape to being larger and irregularly shaped and are present within the bony trabeculae as well as within the adjacent cellular stroma.
Osteoclasts are present within the ossicles, and osteoblasts can be seen along their peripheral aspects.
Bony trabeculae vary in appearance and include odd shapes with a curvilinear pattern:
Trabeculae are composed of lamellar bone with associated osteoclasts and osteoblastic rimming.
Transition zones between the spherical ossicles and bony trabeculae can be seen.
Nonosseous component includes a cellular stroma with a fascicular to storiform growth composed of round to polyhedral to spindle-shaped cells with prominent basophilic nuclei and inapparent cytoplasmic borders:
Mitotic figures can be seen, but mitotic activity is not prominent and atypical mitoses are not present.
Cellular pleomorphism may be present, but anaplasia and necrosis are not identified.
Giant cells can be seen among the psammomatoid ossicles or scattered throughout the nonosseous stromal component.
Osteoid formation may be focally present.
Trabecular (juvenile) ossifying fibroma:
Considered to be clinicopathologically distinct from psammomatoid ossifying fibroma
Less common than psammomatoid ossifying fibroma
Reported to occur in more narrow age range (2 to 12 years) and younger mean age (8.5 to 12 years) than psammomatoid ossifying fibroma (3 months to 72 years and 16 to 33 years)
Predominantly affects the jaws
Histologically composed of trabeculae of fibrillary osteoid and woven bone
Aggressive growth occurs in some—but not all—cases of both types.
Complete surgical excision is the preferred treatment.
Prognosis is good following complete excision but, if margins are involved, recurrences often occur, and the tumors may behave in an aggressive manner with local destruction and potential invasion into vital structures.
Definition: Benign neoplasm of cartilage.
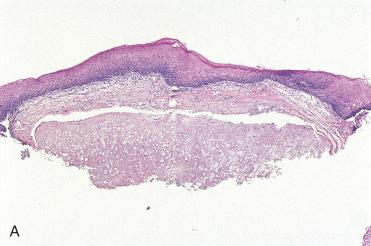
Chondromas of the sinonasal tract and nasopharynx are rare.
The most frequent sites of occurrence include the nasal septum and the nasopharynx.
Radiology:
Sinus opacification or a circumscribed radiolucent lesion can be seen by radiographic studies.
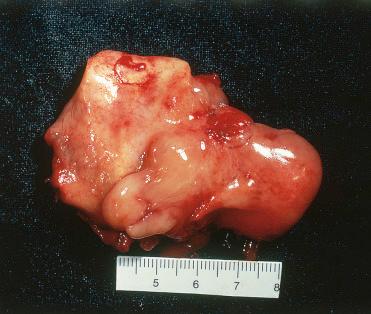
Sinonasal chondromas appear as a polypoid, firm, smooth-surfaced nodule measuring usually from 0.5 to 2.0 cm and rarely greater than 3.0 cm.
Histologically, these are lobulated tumors composed of chondrocytes recapitulating the normal histology of cartilage.
Cellular pleomorphism, binucleate chondrocytes, or increased mitotic activity is not present.
Conservative but complete surgical excision is the preferred treatment.
Recurrences are uncommon.
Definition: Myxomas and fibromyxomas are benign neoplasms of uncertain histogenesis with a characteristic histologic appearance often behaving in an aggressive (infiltrating) manner. When a relatively greater amount of collagen is present, the term fibromyxoma (or myxofibroma) is used.
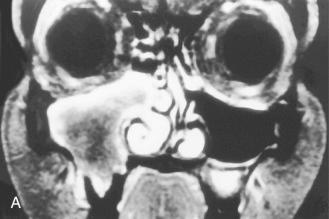
In the head and neck two forms of myxomas/fibromyxomas are identified:
Facial skeletal–derived
Soft tissue–derived
No gender predilection; occurs over a wide age range but most frequently seen in the second and third decades of life
In general, this is a tumor of the jaw bones and is uncommon in extragnathic locations:
The mandible (posterior and condylar regions) is affected more often than the maxilla (zygomatic process and alveolar bone).
Extragnathic tumors are uncommon and primarily involve the sinonasal tract; specifically, the maxillary sinus (antrum) is most often involved with secondary extension into the nasal cavity; extension to orbit and cranial cavity may occur.
Presentation usually is as a painless swelling of the affected area.
Radiology:
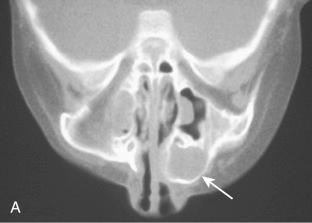
Unilocular or multilocular radiolucency with a “honeycomb” or “soap-bubble” appearance
May vary from being delineated or circumscribed to expansile and infiltrative
Localization to the jaw bones has led to the belief that these tumors take origin from the primordial odontogenic mesenchyme or from an osteogenic embryonic connective tissue; in the sinonasal tract, these tumors appear to be of osseous derivation.
Primarily a tumor involving the extremities
No gender predilection; seen over a wide age range and is not specific to any decade of life
In the head and neck, common sites include the paraoral soft tissues, pharynx, larynx, parotid gland, tonsil, and ear:
Myxomas of the ear are associated with Carney complex.
See Section 7, Ear and Temporal Bone, for a more detailed discussion.
Presentation is that of an asymptomatic mass.
Association between myxomas and fibrous dysplasia has been noted:
Referred to as Mazabraud syndrome
Multiple myxomas are present and tend to be intramuscular in localization.
Majority of patients have polyostotic type of fibrous dysplasia.
Patients also may suffer from McCune-Albright syndrome.
Carney complex:
Syndrome of myxomas, spotty pigmentation, and endocrine overactivity
Autosomal dominant inheritance
Include cardiac myxomas, as well as myxomas of other sites (e.g., intraoral, ear)
Death due to cardiac myxoma may occur in up to 25% of patients with this complex.
Pigmented nodular adrenocortical hyperplasia, Cushing syndrome, and acromegaly also occur in this complex.
Delineated but unencapsulated multinodular, rubbery to firm, tan-yellow to gray-white lesion with a gelatinous appearance
Histology is the same irrespective of the setting in which it occurs.
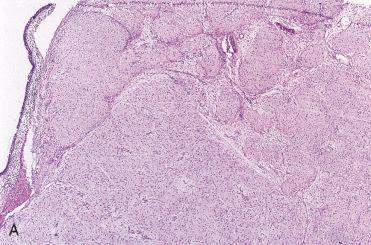
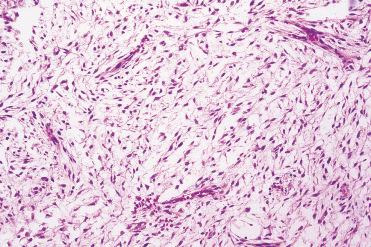
These tumors show a scant, loosely cellular proliferation consisting of spindle-shaped or stellate-appearing cells embedded in an abundant mucinous stroma.
Nuclei are small and hyperchromatic; cellular pleomorphism, mitotic figures, and necrosis are absent.
The amount of collagenous fibrillary material varies between cases and, depending on the extent, its presence may confer designation “fibromyxoma.”
Periphery of the tumor appears circumscribed, but local infiltration with replacement of bone can be seen.
A vascular component is present but is limited in extent:
Absence of the delicate plexiform capillary vascular network, a finding that can be seen in various sarcomas
In intraoral location, odontogenic epithelium may or may not be seen.
Mucinous stroma stains positively for acid mucopolysaccharides.
Immunohistochemistry:
Vimentin positive
Negative for MUC4, β-catenin, CD34
Sinonasal inflammatory polyps
Dental papillae (for intraoral lesions)
Vocal cord polyps (for laryngeal lesions)
Peripheral nerve sheath tumors
Low-grade fibromyxoid sarcoma
Sarcomas with myxoid component (e.g., myxofibrosarcoma, liposarcoma, rhabdomyosarcoma, others)
Cartilaginous tumors:
A separate and distinct benign tumor of cartilaginous origin that may involve the craniofacial and paranasal sinus bones is the chondromyxoid fibroma ( Fig. 3-33 ):
More common in men than in women; most common in the second to third decades of life
Mandible is the most common site (symphysis or molar-retromolar area).
Symptoms include pain, loosening of the teeth, difficulties in opening the jaws, and headaches.
Radiographic appearance varies, depending on the involved bone, from round or oval; well-demarcated, radiolucent lesion usually measuring less than 5 cm in long bones to large, irregularly outlined lesions in flat bones.
Histology is characterized by a lobular appearance with lobules separated by vascularized connective tissue and a zonal arrangement of the cellular component: center of the lobules are predominantly myxoid with scattered stellate-appearing cells; periphery of the lobules are more cellular, composed of cells with round to oval to spindle-shaped nuclei, an eosinophilic to amphophilic cytoplasm often with multipolar, stellate extensions
FGF-23 expression reported in some cases of chondromyxoid fibroma (as well as aneurysmal bone cyst)
With time, the myxoid areas may become fibrotic; presence of cartilage varies but never exceeds 75% of the total tumor volume; osteoclastic giant cells and calcifications may be seen.
Surgery (en bloc resection) is the preferred treatment; local recurrences may occur if incompletely excised (curettage); malignant transformation in the absence of prior irradiation rarely occurs.
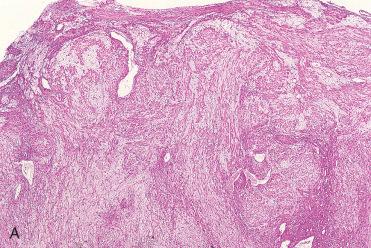
Conservative but wide local excision is the preferred treatment.
These tumors tend to be slow-growing and usually follow a benign course but may have the potential for local destruction following inadequate excision.
Recurrence or metastasis does not occur:
Metastasis from a presumptive sinonasal myxoma or fibromyxoma should seriously place that diagnosis in doubt and probably represents a myxoid variant of a sarcoma (liposarcoma, myxofibrosarcoma, or rhabdomyosarcoma).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here