Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
By the end of this chapter the reader should:
Understand the scientific basis of diseases and conditions affecting the newborn, including the consequences of prematurity
Know about the acquired infections in newborn infants
Know about the physiology and principles of treatment of jaundice in the neonatal period
Understand the causes and mechanism and sequelae of brain injury in term infants
Know about the pathophysiology of respiratory disorders in term newborns and the principles of respiratory support
Know about the principles of nutrition and fluid and electrolyte management in the neonate
Most newborn infants are healthy or have minor, transient problems which should whenever possible be managed on the postnatal or transitional care wards to avoid separation from their mothers. However, 6–10% of infants are admitted to special care baby units and 1–2% need intensive care. There are 775,000 births per year in the UK, so 7750–15,500 babies require intensive care, with extremely preterm infants needing intensive, high dependency or special care for many weeks.
Perinatal and neonatal care in the UK are now organized in regional networks, with mothers or infants with significant medical problems referred to specialist tertiary centres. The advantages and disadvantages of this arrangement are listed in Box 11.1 . There are convincing data that good outcomes require sufficient throughput for staff to establish and maintain expertise in complex conditions. However, large, specialist units do not in themselves guarantee good outcomes; national and international comparisons of outcomes, for example of very-low-birth-weight infants, show marked variations between intensive care units. This highlights the importance of all units collecting and monitoring standardized outcome data and undertaking quality improvement programmes.
Brings together many specialists working as a team
Allows experience of rare conditions to be developed and maintained
Potentially provides better outcomes
Facilitates staff training and research
Minimizes cost by avoiding duplication
Parents and family have to travel further from home
Staff at other centres become de-skilled
Establishes a hierarchy of care
Good communication between all staff is more difficult because of geographical separation
Increased transfer of mothers and infants – specialist transport needs to be available
Parents may encounter more healthcare professionals, with teams at both local and tertiary centres
The main causes of morbidity and mortality of newborn infants are prematurity, infection, jaundice, respiratory and neurological problems, which will be described in this chapter. Whilst some congenital abnormalities will also be described, most are covered in the system chapters.
In the UK, 7% of births are preterm. The initial requirements for stabilizing the extremely preterm are listed in Table 11.1 . Preterm delivery may be:
Spontaneous onset of labour with intact membranes (40–45%)
Following preterm premature rupture of the membranes (PPROM) (25–30%)
For medical or fetal indications (30–35%).
| Airway, breathing | Respiratory support with clearing airway, oxygen, CPAP, high flow nasal therapy, mechanical ventilation as required Surfactant for preterm infants with respiratory distress |
| Circulation | Intravenous fluids to treat shock if required |
| Monitoring | Oxygen saturation – if preterm, keep at 91–95% (see Box 37.14 ) Heart rate, blood pressure, respiratory rate and temperature monitoring – peripheral and central Weight Urine output |
| Temperature control | Plastic bag and hat if extremely preterm at birth, radiant warmer or incubator, humidification |
| Venous and arterial lines | Peripheral intravenous line:
Umbilical venous catheter:
Arterial line:
Central venous line:
|
| Investigations | Full blood count, CRP, urea and electrolytes, blood glucose, blood gases Chest X-ray +/− abdominal X-ray if respiratory distress and for position of tracheal tube and central lines |
| Medication | Antibiotics – usually indicated Analgesia and sedation – as required Vitamin K – routine prophylaxis against haemorrhagic disease of the newborn |
| Parents | Time needs to be found to explain to parents and immediate relatives what is happening. If the mother cannot be with the baby, e.g. following caesarean section, photos or videos are reassuring. |
Most are idiopathic but causes include:
Intrauterine stretch – multiple gestation, polyhydramnios, uterine abnormality
Intrauterine bleeding – abruption, antepartum haemorrhage
Intrauterine infection – chorioamnionitis, bacterial vaginosis
PPROM
Fetal causes – IUGR, congenital malformations
Maternal medical conditions – pre-eclampsia, hypertension, chronic medical conditions
Cervical weakness
The epidemiological risk factors for preterm birth are poorly understood but include:
Previous preterm delivery
Maternal age – risk increased if <20 or >35 years old
Maternal nutrition – low BMI associated with spontaneous preterm birth, obesity because of increased risk of pre-eclampsia and diabetes mellitus
Ethnicity – increased in Black mothers (mothers from south-east Asia have increased incidence of low birth weight rather than preterm infants)
Multiple births – responsible for 15–20% of preterm births
Maternal infection – localized or generalized
Maternal stress, socio-economic deprivation
Maternal smoking, substance misuse.
Whenever possible, the aim is to deliver the infant at full term whilst ensuring the well-being of both mother and infant. The decision to deliver infants less than 28 weeks' gestation is especially difficult, and should involve the obstetrician, neonatologist and parents after detailed assessment of the risks to mother and infant.
Management known to improve neonatal outcome includes:
Antenatal steroids – reduces rate of respiratory distress syndrome, intraventricular haemorrhage and neonatal death (see Box 1.4 for details)
Magnesium sulphate – shown to reduce the risk of cerebral palsy in infants <32 weeks' gestation. Its mechanism of action is poorly understood.
Following PPROM, there is increased risk of neonatal morbidity and infection. It is associated with ascending maternal infection from the lower genital tract, with one third having positive amniotic cultures. Antibiotics are given to the mother to treat chorioamnionitis and reduce the risk of neonatal infection.
Tocolytics are often used to suppress contractions to allow time for antenatal corticosteroids or maternal transfer to a perinatal centre, but there is no clear evidence that they improve outcome.
The preterm infant at 23–25 weeks' gestation differs markedly in size, appearance and development from babies born at later gestations. Typical birth weight at 24 weeks is only 620 g for females, 700 g for males (50th centile). Their skin is red, thin and gelatinous, making them prone to high evaporative heat loss and is easily damaged, making it a potential portal for infection. They adopt an extended posture with uncoordinated movements, reflecting their early stage of neural development (see Chapter 28 , Neurology). Their eyelids may be fused or partially open, with infrequent eye movements, in contrast to the term infant who looks and follows faces. They are unlikely to breathe without respiratory support because of surfactant deficiency and lung immaturity. They are unable to coordinate sucking and will require nasogastric feeding, often augmented by parenteral nutrition; the ability to suck and coordinate swallowing usually only develops at 34–35 weeks. Babies born weighing less than 1.5 kg are at increased risk for a range of complications ( Table 11.2 ).
A baby is born at 25 weeks' gestation following a pregnancy complicated by preterm prolonged rupture of membranes. The child develops signs of respiratory distress syndrome (RDS). Regarding the pathophysiology of respiratory distress syndrome, which one of the following statements best describes the underlying problem. Select ONE answer only?
There is:
A decrease in pulmonary surfactant, a compliant chest wall and a higher surface tension at the alveolar surface
An absence of functional alveoli accompanied by an increase in the relative proportion of cartilage in the airways, which critically limits pulmonary oxygen exchange
An absence of pulmonary surfactant, a stiff chest wall and increased airways resistance
An increase in the relative protein content of surfactant which impairs surfactant recycling
Homogenous airways collapse with reduced airway resistance
A. A decrease in pulmonary surfactant, a compliant chest wall and a higher surface tension at the alveolar surface. See below for discussion.
| Condition | Complications and supportive therapy |
|---|---|
| Respiratory distress syndrome/lung immaturity – 70% | Surfactant therapy – 65% Conventional ventilation – 59% High-frequency ventilation – 26% Nasal CPAP – 73% Inhaled nitric oxide – 5% Air leaks/pneumothorax – 3% Bronchopulmonary dysplasia – 25% (O 2 therapy at 36 weeks) |
| Infection – 21% | Early-onset – 2% Late-onset – 13% |
| PDA (patent ductus arteriosus) – 29% | Medical treatment – 21% Surgical ligation – 5% |
| NEC (necrotizing enterocolitis) – 5% | Surgical treatment – 3% |
| Intraventricular haemorrhage – 19% PVL (cystic periventricular leukomalacia) – 3% |
Severe (Grade III/IV) – 8% |
| ROP (retinopathy of prematurity) | Severe – 6% Laser treatment – 3% |
Respiratory distress syndrome (RDS) is the most common lung problem that accompanies prematurity. It may lead to severe respiratory failure and death. It is caused by deficiency of surfactant, which leads to higher surface tension at the alveolar surface, difficulty in achieving adequate functional residual capacity and interferes with the normal exchange of respiratory gases. The incidence and severity of RDS is inversely proportional to gestational age because of the smaller number of functional alveoli with decreasing gestational age. The airways of the preterm infant are also incompletely formed and lack sufficient cartilage to remain patent. This contributes to collapse of lungs and increased airway resistance. The higher surface tension requires greater distending pressure to inflate the alveoli, according to Laplace's law: P = 2T/r, where P is the pressure, T is the surface tension and r is radius ( Figs 11.1 – 11.2 ). The chest wall of the preterm newborn is also more compliant than the lungs, thus tending to collapse when the infant attempts to increase negative intrathoracic pressure.
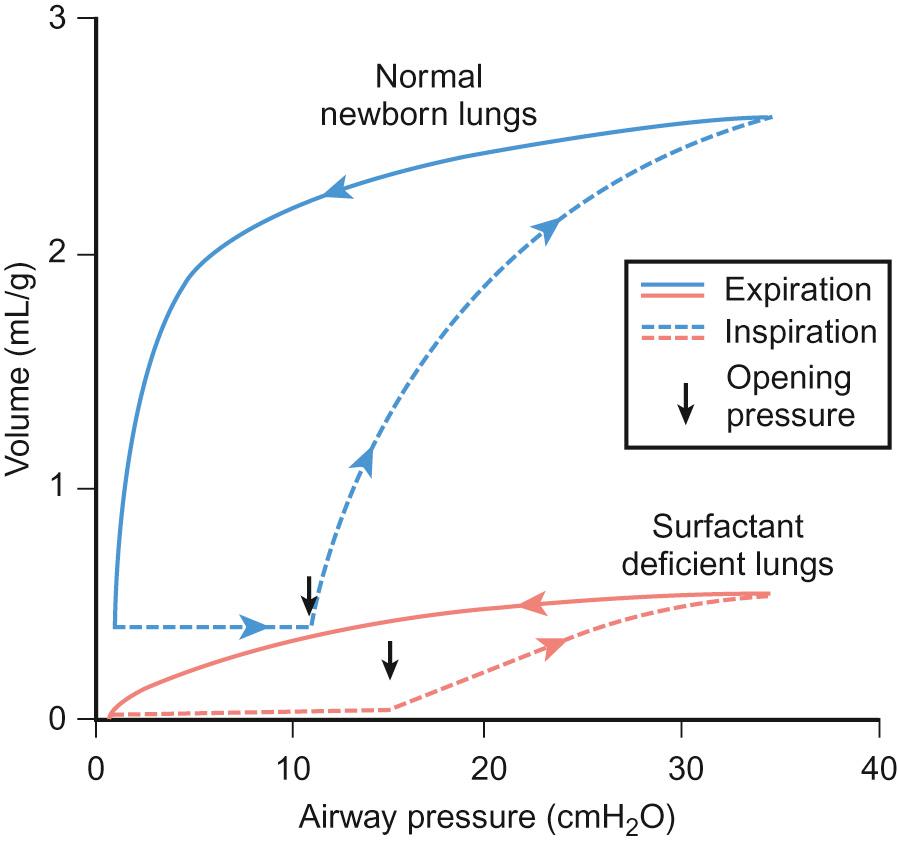
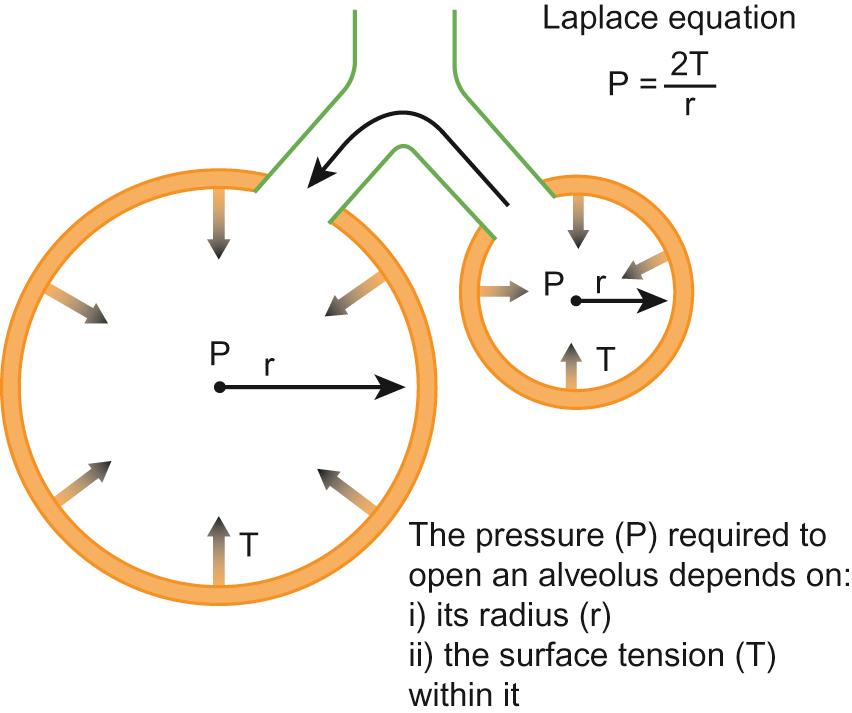
In summary, functional abnormalities which contribute to respiratory failure in preterm newborns include:
Decreased compliance
Increased resistance
Ventilation–perfusion imbalance
Impaired gas exchange
Increased work of breathing.
Pulmonary surfactant is synthesized and secreted into the alveolar spaces by type 2 epithelial cells. It is a complex suspension of several phospholipids (85%) and proteins (10%). Most of the phospholipids consist of phosphatidylcholine (PC), and one particular PC molecule, DPPC (dipalmitoyl phosphatidylcholine). The structure of DPPC is suited to form a stable monolayer generating the lower surface tension required to prevent alveolar collapse at end-expiration. Phospholipids alone do not exhibit all the biophysical properties of pulmonary surfactant. The contribution of low molecular weight SP-B and SP-C to both structural organization and functional durability is essential. The surfactant specific proteins SP-B and SP-C promote the rapid absorption of phospholipids at the air–liquid interface and account for the sustained low surface tension activity after dynamic compression. SP-B deficiency, inherited as an autosomal recessive condition, is lethal and results in fulminant respiratory failure. SP-C dramatically enhances the spread of phospholipids. Unlike SP-B deficiency, mutation in the SP-C gene presents later as chronic interstitial lung disease. The other specific proteins, such as SP-A and SP-D, are only marginally involved in the surface tension lowering ability of pulmonary surfactant but play an important role in the defence barrier against pathogenic organisms and in the recycling of surfactant.
The total surfactant lipid pool in preterm babies is less than 10 mg/kg compared to the surfactant lipid pool size in term infants of around 100 mg/kg. Furthermore, preterm infants with RDS have a lower per cent of saturated phosphatidylcholine species, phosphatidylglycerol, and surfactant-associated proteins in their pulmonary surfactant.
Exogenous surfactant preparations are either derived from animal sources (bovine and porcine) or can be prepared synthetically. Animal-derived surfactants consist of more than 80% phospholipids and specific proteins SP-B and SP-C, but not SP-D. The synthetic surfactants are composed mainly of DPPC. Unlike the older generation of synthetic surfactants that did not contain specific proteins, newer synthetic surfactants, such as lucinactant, do contain synthetic peptides whose spatial structure resembles one of the domains of SP-B and is clinically as effective as animal-derived surfactants, but is much less widely used than natural surfactants. Another newer synthetic surfactant is being developed which contains recombinant SP-C.
Administration of exogenous surfactant in a surfactant-deficient preterm newborn decreases the minimum pressure required to open the lungs, increases the functional residual capacity (FRC) and maximal lung volume, thus preventing lung collapse at low pressure and end-inspiration. It diminishes the work of breathing, stabilizes the respiratory tract, improves mucociliary transport, prevents oedema and contributes to lung defence against pathogens.
RDS is diagnosed using a composite of clinical features including gestational age, the presence of respiratory distress with impaired gas exchange and characteristic radiographic abnormalities.
This includes:
Prevention, using antenatal glucocorticoid (betamethasone) to induce endogenous surfactant formation
Exogenous surfactant replacement therapy (see below)
Artificial respiratory support either in the form of continuous distending pressure, such as CPAP, or intermittent positive airway pressure ventilation through a mechanical ventilator
Adjunctive measures include maintenance of adequate blood pressure, adequate oxygen-carrying capacity and physiological pH
Avoidance of complications such as air leaks and bronchopulmonary dysplasia (BPD).
The benefits of exogenous surfactant replacement therapy are well established, either given ‘prophylactically’ or as a ‘rescue treatment’. The clinical response to exogenous surfactant administration can be divided into three stages:
Acute treatment response (occurring within 10 minutes). This initial response results from the biophysical properties of surfactant and depends on rapid distribution of surfactant to distal lung areas. An improvement in oxygenation is usually the first clinical response to surfactant instillation.
The second stage involves sustained response to the initial surfactant dose (hours post administration). It results from improving lung mechanics and recycling of surfactant components from the air spaces into type 2 cells, where the lipids are, in part, diverted into lamellar bodies for re-secretion. In general, this cycling is more efficient in the preterm lungs, where recycling rates may be as high as 80–90%. This, however, does not guarantee that only one dose of surfactant will be effective. About 20–30% of infants receiving surfactant may still be receiving mechanical ventilation with FiO 2 of more than 30–40% several hours after the first dose and require re-treatment.
This comprises continued response to the initial surfactant dose and is attributed to the long half-life of surfactant components. The net balance of slow synthesis, secretion, metabolism and clearance of surfactant and its components allow the infant with RDS to accumulate a large amount of surfactant over many days.
Surfactant therapy in preterm infants with RDS reduces neonatal mortality and complications such as pneumothorax. However, none of the trials or meta-analysis have shown any benefit in terms of reducing the incidence of BPD (bronchopulmonary dysplasia).
Human breast milk is the optimal form of nutrition even for extremely preterm infants. Its gastrointestinal tolerance is better and the incidence of necrotizing enterocolitis and the risk of systemic infection are lower than in infants fed with infant formula. Even minimal volumes may help prime bacterial colonization of the gut.
Lack of success in producing sufficient breast milk is often a problem, which may be aggravated by physical separation of mother and baby, difficulty in maintaining milk supply over a long period, lack of motivation and inadequate support. Frequent expression, kangaroo care or skin-to-skin contact and relaxation tapes improve the volume and duration of breastfeeding. Breast pumping, massage prior to pumping and dopamine antagonists may improve production. Expressed donor milk (DBM), which has been pooled and pasteurized, is increasingly used when mother's milk is not available. If these options are not available then preterm infant formula can be given. It has been modified to meet the increased nutrient requirements of extremely preterm infants and results in faster weight, length and head circumference growth, reduced incidence of hyponatraemia, bone disease of prematurity, hypophosphataemia and hyperbilirubinaemia than breast milk. However, tolerance of feeds is poorer and risk of NEC (necrotizing enterocolitis) is increased.
Human milk fortifiers containing protein, energy, macrominerals, trace minerals and a range of vitamins are widely used to supplement expressed breast milk to meet the additional nutritional requirements. They provide short-term improvements in weight gain, linear and head growth, but evidence is lacking for long-term benefits. As they are based on cows' milk, their precise biological properties differ from that of human milk. More recently, ‘humanized’ milk fortifier and infant formula, produced from pooled donor breast milk, have been developed and appear to have a lower risk of necrotizing enterocolitis compared to preparations based on cows' milk.
Parenteral nutrition is required if adequate enteral feeding is not possible, as extremely preterm infants have very limited ability to withstand starvation due to their low protein, fat and carbohydrate stores. Further details about nutrition in preterm infants are covered in Chapter 13 , Nutrition.
The role of the ductus arteriosus in the fetus and its functional and anatomical closure after delivery are described in Chapter 10 , Perinatal medicine. Its patency is maintained by high blood flow, hypoxia and locally derived prostaglandin E2. In preterm infants, the ductal wall is thinner, the lumen is larger and postnatal constriction does not wholly obliterate the lumen. The incidence of functional PDA varies inversely with gestational age. Predisposing risk factors are respiratory distress syndrome, sepsis and fluid overload.
The left-to-right shunting of blood through the PDA results in increased pulmonary blood flow and higher venous return to the left atrium and left ventricle (high preload). This increased pulmonary blood flow can lead to pulmonary oedema, congestive cardiac failure or, less commonly, pulmonary haemorrhage and may increase the risk of bronchopulmonary dysplasia. A haemodynamically significant PDA decreases systemic blood flow and leads to hypotension (especially diastolic, resulting in wide pulse pressure), reduced gut and renal perfusion and metabolic acidosis. This in turn may lead to increased risk of complications such as necrotizing enterocolitis and intraventricular haemorrhage.
The clinical effects of PDA include tachypnoea, increased oxygen requirement, increased ventilatory requirement, extubation failure, apnoea, hepatomegaly from congestive heart failure and impaired weight gain. These are often accompanied by a systolic or pansystolic murmur at the left sternal edge (though this may be absent if the shunt is large), loud second heart sound, gallop rhythm, bounding pulses from wide pulse pressure and hepatomegaly from right heart failure.
Recent studies suggest that conservative management is often appropriate as in infants >1 kg birth weight, two thirds of PDA close spontaneously, and even if <1 kg, PDA closes in just over a third. Conservative treatment of restricted fluid early on in the first week has been shown to significantly decrease the risks of PDA. However, prolonged fluid restriction may worsen systemic hypoperfusion.
Pharmacological closure with indomethacin or ibuprofen works by decreasing the production of PGE 2 . However, indomethacin use is associated with more nephrotoxicity, NEC, gastrointestinal haemorrhage, platelet dysfunction and impaired cerebral blood flow. Ibuprofen, a non-selective cyclo-oxygenase inhibitor, is therefore currently recommended as first line medical treatment. Diuretics may worsen systemic hypoperfusion and increase the renal production of prostaglandins, which may promote ductal patency. Therefore, these should only be used in babies with heart failure. Surgical ligation may be indicated if medical intervention fails.
Which of the following statements are true (T) and which are false (F)?
A newborn term infant typically has IgG levels higher than its mother
At 24 weeks' gestation, a preterm infant has no maternal IgG
Breast milk is a significant additional source of IgG
Maternal antibodies attenuate infant responses to vaccination at birth
Vaccination accelerates the induction of the infant immune response
A. True; B. False; C. False; D. True; E. True.
See below for discussion.
Preterm infants are particularly vulnerable to early-onset and nosocomial (hospital-acquired) infection. This is partly due to lack of maternal IgG antibody transfer across the placenta (fetal IgG rises from approximately 10% of the maternal concentration at 17–22 weeks' gestation to 50% at 28–32 weeks' gestation; Fig. 11.3 ) and breach of natural defence barriers from invasive central and peripheral lines, catheters and tubes, including artificial ventilation. Transplacental transfer of antibodies is an active process that results in fetal IgG levels in excess of maternal levels by full term. Breast milk is another important source of passive immune protection for infants and is rich in IgA but contains little or no IgG.
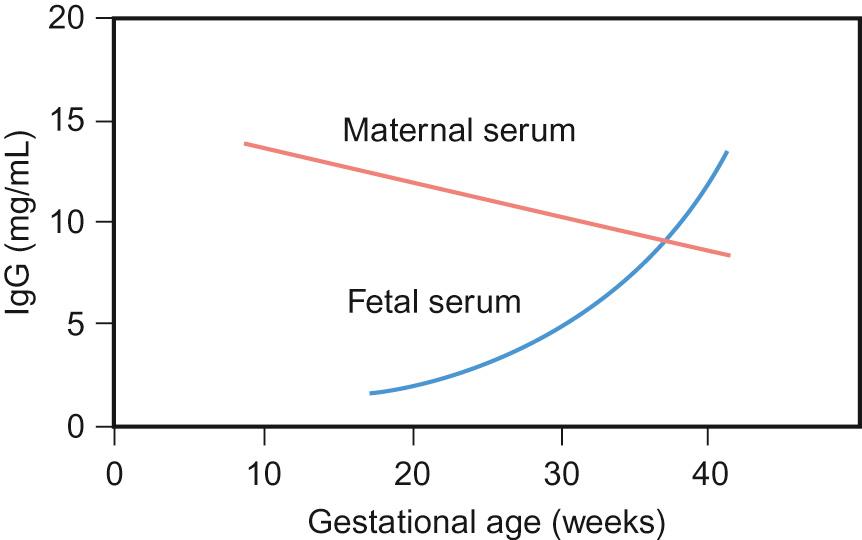
Following birth, a preterm infant should receive their immunizations at the same postnatal age as a term baby. Whilst immune response in very preterm infants may be suboptimal, these infants are also at increased risk of infection, so vaccination should not be delayed. Primary protection against infectious diseases at birth is provided mainly by maternal antibodies. However, these antibodies can hamper the humoral antibody response of the infant to vaccination, so the timing of vaccination should take their presence into consideration. The timing for childhood immunizations is based upon a complex risk–benefit analysis that takes account of this effect, which is why, for example, primary MMR vaccination is delayed until 12 months of age.
About 5% of all very-low-birthweight (VLBW) infants will develop necrotizing enterocolitis (NEC), with mortality ranging from 15–25%. It is a clinical diagnosis of abdominal distension and tenderness, bilious aspirates, bloody stools and intramural air (pneumatosis intestinalis) on abdominal X-ray. It may progress to peritonitis and bowel perforation.
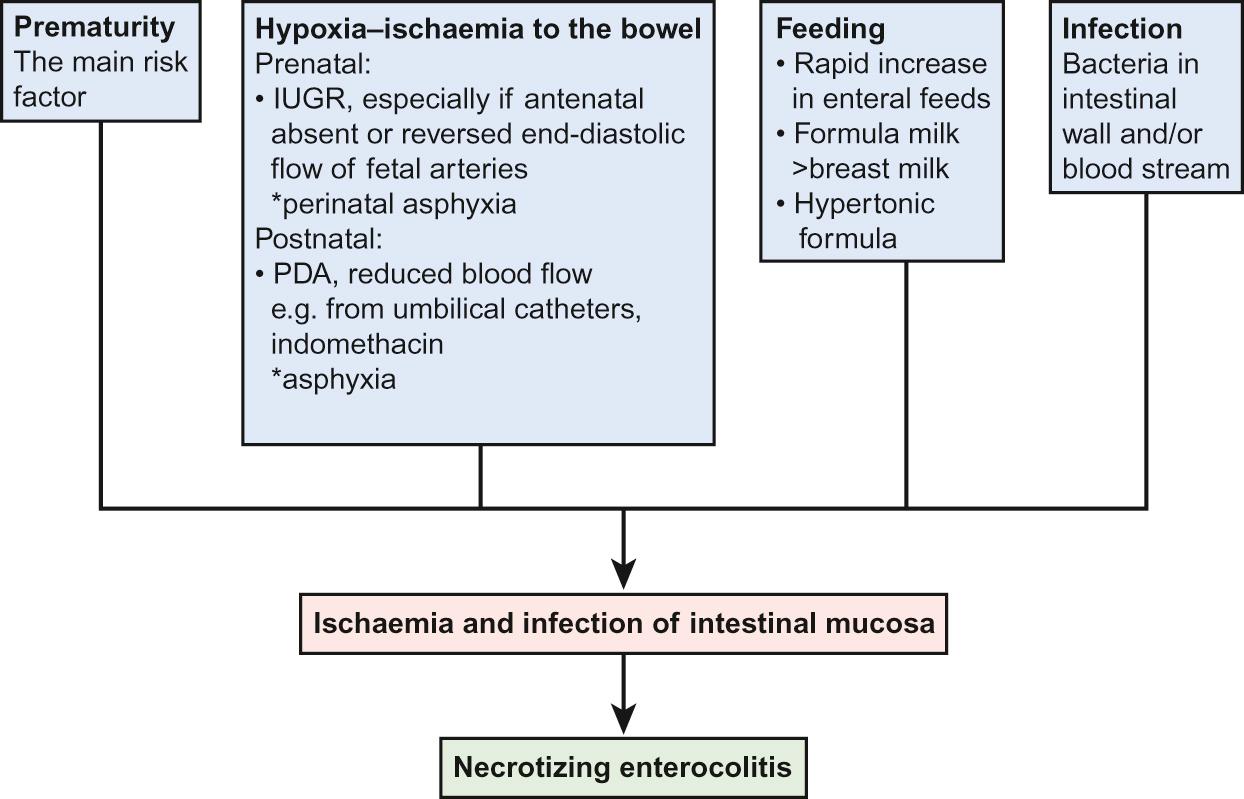
The exact cause is unknown but is probably multifactorial, including loss of bowel mucosal integrity leading to macromolecular absorption and bacterial translocation. There is significant deficiency of secretory IgA, with increased permeability to small and large molecules. Antenatal glucocorticoid therapy improves intestinal maturation and reduces permeability and hence offers a degree of protection. Infection may initiate mucosal injury leading to invasion of gas-producing bacteria, which can result in pneumatosis intestinalis. This gas in the bowel wall is mainly nitrogen and hydrogen. There may also be gas in the portal venous system. Histologically, there is necrosis of the mucosa with microthrombus formation, patchy mucosal ulceration, oedema and haemorrhage. Cytokines and inflammatory markers such as interleukins, tumour necrosis factor alpha (TNFα) and platelet activating factors (PAF) have an important role and enterocyte death may be induced due to imbalance in the pro- and anti-inflammatory mediators and increased pro-apoptotic protease activity. Any part of the small or large bowel may be affected, but the terminal ileum or sigmoid colon is usually involved. Risk factors for the development of NEC are shown in Figure 11.4 .
A recent proposal is that anaemia leads to compromise of the mesenteric blood flow causing intestinal hypoxia and mucosal injury. Transfusion-related reperfusion gut injury (TRAGI) of the hypoxaemic gut has been postulated to predispose anaemic preterm infants to NEC. Probiotics with or without prebiotics to help promote normal gut flora have been studied extensively; their benefit remains unproven.
Current management of NEC is to stop feeds and place a large bore naso/orogastric tube for intestinal decompression, start broad spectrum antibiotics and provide supportive care. Surgical intervention may be required for bowel perforation or failure of medical treatment, with peritoneal drainage at the bedside or resection of non-viable bowel and anastomosis or ileostomy or colostomy.
A. Extreme prematurity.
All are risk factors but extreme prematurity is of overriding importance.
Which of the following abnormalities in isolation confers the greatest risk of cerebral palsy in a preterm infant? Select ONE answer only.
Anterior cystic periventricular leukomalacia (PVL)
Grade III intraventricular haemorrhage (IVH)
Grade IV intraventricular haemorrhage (parenchymal)
Occipital cystic PVL
Ventricular dilatation
D. Occipital cystic PVL.
Whilst imaging alone cannot confidently exclude or determine the risk of cerebral palsy, there are several large cohort studies that report on the relative values of imaging abnormalities in predicting neurodisability. In particular, bilateral, occipital or parietal cystic periventricular leukomalacia confers a very high risk of cerebral palsy, with two thirds to three quarters developing cerebral palsy.
Periventricular-intraventricular haemorrhage (PVH-IVH) is the most common neurological complication of preterm infants (the incidence is inversely related to gestational age) and is an important risk factor for neurodevelopmental impairment and death. It is caused by rupture of the fragile capillary network in the subependymal (also called germinal) matrix of the developing brain, which overlies the head of the caudate nucleus. The haemorrhage may be confined to the subependymal region (germinal matrix haemorrhage; GMH) or may extend into the body of the lateral ventricles (intraventricular haemorrhage) or involve the cerebral cortical parenchyma (parenchymal haemorrhage). The parenchymal lesion is not an extension of the haemorrhage as previously thought, but is a venous infarct related to obstruction to the venous drainage of the white matter. The parenchymal lesion subsequently undergoes cystic degeneration and by term there is a porencephalic cyst. Intraventricular haemorrhage is typically classified by grade (1–4) based on ultrasound appearances ( Box 11.2 ). Serial cranial ultrasound is used to detect intraventricular haemorrhage, porencephalic cysts and ventricular dilatation or hydrocephalus, a complication of intraventricular haemorrhage. The agreement between ultrasound and autopsy diagnosis has been reported to be >90%.
Haemorrhage
Grade I – isolated germinal matrix haemorrhage (GMH)
Grade II – intraventricular haemorrhage (GMH-IVH); <50% of ventricular area on parasagittal view
Grade III – intraventricular haemorrhage (GMH-IVH with dilatation); >50% of ventricular area on parasagittal view, usually distends lateral ventricle
Grade IV – haemorrhagic parenchymal infarct (parenchymal lesion); may evolve into a porencephalic cyst – single, large cyst
Cystic periventricular leukomalacia (PVL)
Periventricular white matter echodensity (PVE) – may evolve into periventricular or deep white matter cysts
Posthaemorrhagic ventricular dilatation/hydrocephalus
The pathogenesis is multifactorial but key factors are thought to be the immature germinal matrix capillary network, impaired cerebral autoregulation (failure to maintain cerebral blood flow within the normal limits in spite of wide fluctuations in blood pressure), and abnormal coagulation. This is summarized in Figure 11.5 .
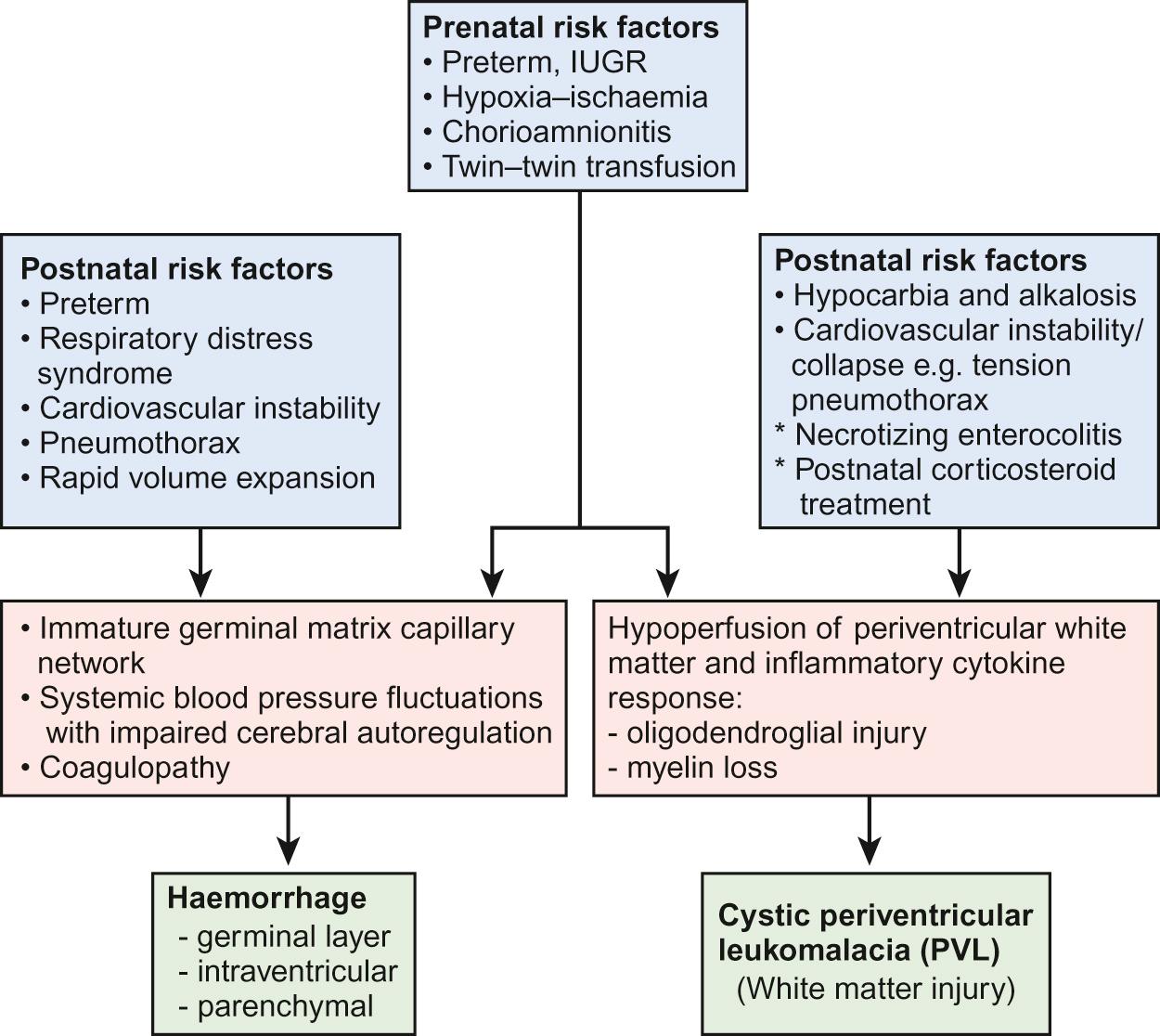
Preterm infants with GMH-IVH have a higher mortality compared to those without. Uncomplicated IVH (grades I and II) can also cause motor and cognitive sequelae, with about 9% risk of developing cerebral palsy. About a quarter of infants with grade III and half with grade IV develop cerebral palsy (CP) at two years of age.
Periventricular leukomalacia (PVL) is periventricular white matter injury, which usually results from a combination of ischaemia from hypoperfusion of the periventricular white matter and inflammation resulting in oligodendroglial injury and failure of myelination (see Fig. 11.5 ). In cystic PVL, there are focal macroscopic areas of necrosis in the periventricular white matter leading to small bilateral periventricular cysts which may be visible on cranial ultrasound from about two weeks of birth. The incidence of cystic PVL is 3% of very-low-birth-weight babies. It has a significant impact on neurodevelopmental outcome with a high incidence of diplegic CP, poor visual spatial skills and low IQ scores. More commonly, the focal lesions are microscopic in size and evolve to small glial scars, which are not usually detected on ultrasound but can be identified on MRI scan. It may well be responsible for some of the characteristic cognitive difficulties many extremely preterm babies have at school age, rather than cerebral palsy.
Preterm infants are prone to poor bone mineralization. This is caused by phosphate deficiency and results in reduced bone mineralization with widening and cupping of the wrists, knees and ribs on X-ray (as with rickets), a failure in linear growth and fractures, particularly of ribs and long bones.
Investigations show a low phosphate with calcium being either normal or elevated initially in conjunction with a markedly elevated alkaline phosphatase (a marker of bone turnover). It can be prevented by giving additional phosphate in parenteral nutrition and by giving oral phosphate or using preterm milk fortifier. Providing sufficient phosphate if on long-term parenteral nutrition can be difficult. Treatment is with oral phosphate and vitamin D supplements.
Retinopathy of prematurity (ROP) is an important cause of visual impairment and blindness in the preterm newborn (see Chapter 30 , Ophthalmology). The risk of its development should be minimized by avoiding hyperoxaemia (maintain oxygen saturation 91–95%), hypoxaemia and wide fluctuations in oxygen saturations.
Bronchopulmonary dysplasia (BPD) is defined clinically on the basis of clinical signs and dependence on ambient oxygen either at 28 days or more usually at 36 weeks post menstrual age. Although oxygen supplementation has routinely been used as a surrogate for assessing the severity of the underlying lung disease, this is subjective and it is not surprising that prevalence of BPD varies markedly between units and this variation has hampered epidemiological research. To rectify this, a stricter physiologic definition has been proposed in which infants receiving less than 30% supplemental oxygen are subjected to a stepwise 2% reduction of supplemental oxygen until they are breathing room air. The outcome of this allows the identification of:
No BPD – oxygen saturation >90% for 60 minutes in room air
BPD – if saturation <90% during the observation period
Babies undergoing an oxygen challenge test are monitored for apnoea, bradycardia and increased oxygen requirement. If any of these events occur and require treatment, this is considered a test failure and categorized as BPD. This method provides an objective assessment of the presence and severity of underlying lung disease. Using this physiological definition, the incidence of BPD is only about 10% of very-low-birthweight infants compared to 25% using the standard criteria.
Its pathogenesis is multifactorial and includes:
Underdeveloped lungs due to prematurity
Initial injury to the lung due to primary disease process, e.g. RDS
Ventilator-induced lung injury mediated through barotrauma (high pressure)
Volutrauma (inappropriately high or low tidal delivery)
Oxygen toxicity
Inflammatory cascade
Inadequate nutrition
The pathophysiology and underlying pulmonary mechanics in infants with BPD includes reduction in lung compliance as well as increased airway resistance leading to increased work of breathing. Later, expiratory flow limitation may become more significant. Functional residual capacity may be reduced initially because of atelectasis but can increase in later stages from air trapping and hyperinflation. More recent findings in extremely premature infants reveal alveolar simplification, a reduction in the overall surface area for gas exchange, and failure of secondary alveolar crest to form normal alveoli (epithelial and endothelial cell growth abnormalities).
Once BPD has developed, treatment is only supportive. Most infants ultimately achieve normal lung function and thrive. They are at higher risk of death in the first year of life and long-term complications such as reactive airway disease, increased susceptibility to viral infection, particularly RSV (respiratory syncytial virus) infection, growth failure and neurodevelopmental abnormalities.
This includes survival to discharge from hospital, key morbidities and neurodevelopmental outcomes. With advances in perinatal medicine such as use of antenatal steroids, advanced ventilation techniques, good intensive care and decreased sepsis, the survival rate of extremely premature infants has increased markedly. However, they are at increased risk of medical problems at and following discharge and of neurodevelopmental problems. Medical problems at and following discharge include increased risk of:
Poor growth – at discharge, over 90% of VLBW infants are below the 10th centile for weight, length and head circumference. Many show some catch-up growth in the first 2–3 years
Pneumonia/wheezing/asthma
Bronchiolitis from RSV (respiratory syncytial virus) infection (hospitalization is reduced by giving RSV monoclonal antibody, palivizumab)
Bronchopulmonary dysplasia – may require supplemental oxygen therapy at home
Gastro-oesophageal reflux – especially with bronchopulmonary dysplasia
Complex nutritional and gastrointestinal disorders – following necrotizing enterocolitis or gastrointestinal surgery
Inguinal hernias – require surgical repair.
Readmission to hospital during the first year of life is increased approximately four-fold, mainly for respiratory disorders and surgical repair of inguinal hernias.
About 5–10% of VLBW infants develop cerebral palsy, but the most common impairments are learning difficulties. The prevalence of cognitive impairment and of other associated difficulties increases with decreasing gestational age at birth, and is greatest if born at very early gestational age (<26 weeks' gestation). It becomes increasingly evident when the individual child is compared to their peers at nursery or school. In addition, children may have difficulties with:
fine motor skills, e.g. threading beads
concentration, with short attention span
behaviour, especially attention deficit disorders
abstract reasoning, e.g. mathematics
processing several tasks simultaneously.
A small proportion also have hearing impairment, with 1–2% requiring amplification, or visual impairment, with 1% blind in both eyes. A greater proportion have refraction errors and squints and therefore require glasses.
Focusing on preterm babies born at 22–25 weeks' gestation, the most recent population-based outcome study from all those births in England in 2006 (EPICure 2) found that of 3133 deliveries, there were 2034 live births, 1686 neonatal admissions and 1041 discharges from hospital.
Survival rates of live-born infants were:
| 22 weeks' gestation | 2% |
| 23 weeks' gestation | 19% |
| 24 weeks' gestation | 40% |
| 25 weeks' gestation | 66% |
| 26 weeks' gestation | 77% |
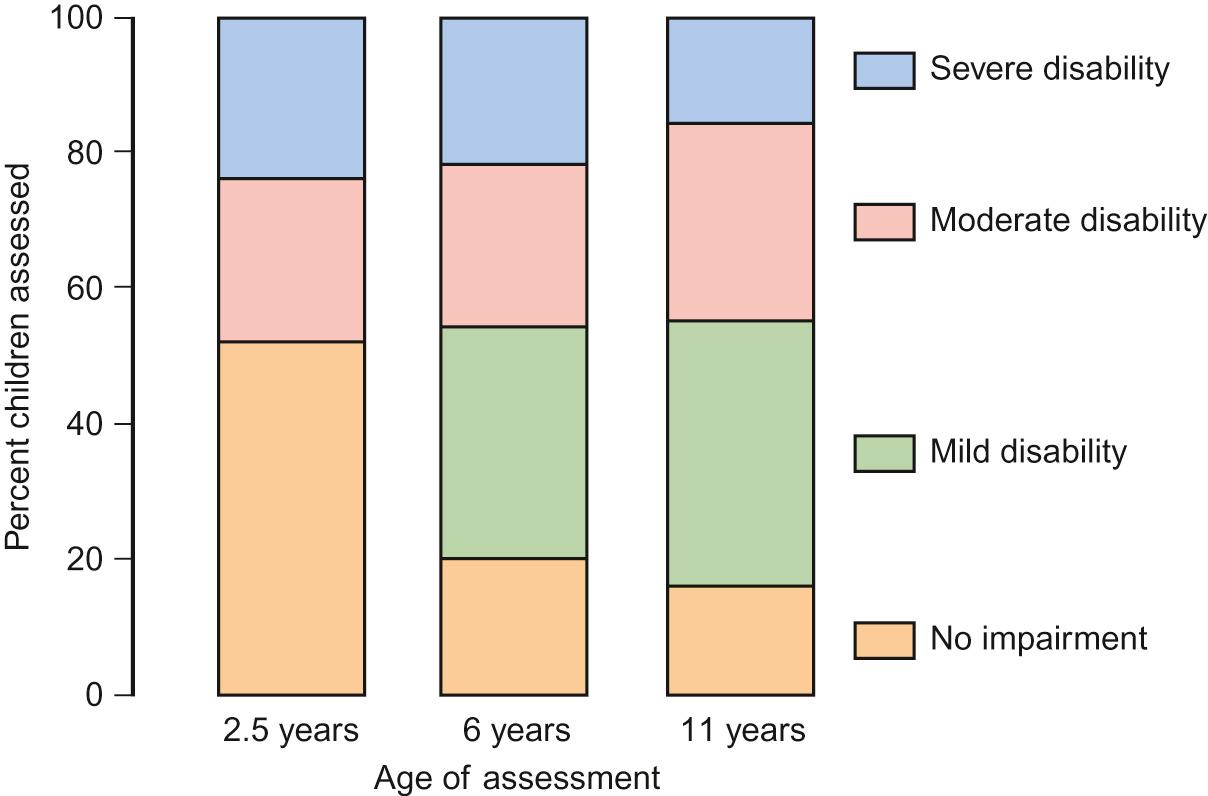
The evolution of disability up to 11 years of the babies born at 22–25 weeks in the UK in 1995 (EPICure 1) is shown in Figure 11.6 . This outcome data is important to inform debate about ethical decisions concerning babies born at gestational age at the limit of viability (see Chapter 35 , Ethics).
Extremely premature infants are born at an early stage of lung development, before the development of alveoli, and have deficient surfactant production for extrauterine life (see Chapter 17 , Respiratory medicine, for a description of the embryology). There are a number of factors that influence fetal lung growth or maturation, including physical (e.g. fetal respiration and fetal lung fluid), hormonal (e.g. glucocorticoids, prolactin and insulin), and local (cAMP, methylxanthines).
Airways are heterogeneous, conduct airflow, and do not participate in gas exchange. Stable pressure balance between collapsing forces and the dilator forces of supporting musculature help to maintain upper airway patency. Conducting airways of intrathoracic trachea (lower airway), however, do participate in respiratory gas exchange in portions of the terminal and respiratory bronchioles and alveolar ducts. Narrowing of the airways, from inflammation, excessive secretions or reactive airways, leads to increased resistance to airflow, thereby causing increased work of breathing.
Thoracic and respiratory muscles which are involved in respiratory function include the diaphragm, intercostal and accessory muscles and abdominal muscles.
A 2-day-old male infant is rushed to the paediatric emergency department because his mother found him to have gasping respirations and looking pale and mottled. He has respiratory distress, a respiratory rate of 80 breaths/min, heart rate of 164 beats/min and oxygen saturation (right foot) of 70%. He is given oxygen. What would you do next?
Select ONE answer only:
Blood gas
Blood culture
Chest X-ray
ECG
Move the pulse oximeter probe to the right hand
E. Move the pulse oximeter probe to the right hand
A higher pre-ductal oxygen saturation would suggest critical congenital heart disease and giving prostaglandin to maintain ductal patency would be life-saving. (See Fig. 39.3 for details on oxygen saturation screening for detecting critical congenital heart disease.)
The clinical features of respiratory distress in newborn infants are tachypnoea (respiratory rate >60/min), nasal flaring, grunting, chest recession with variable cyanosis depending upon severity of illness. The differential diagnosis is wide ( Table 11.3 ).
| Common | Less common | Rare |
|---|---|---|
| Transient tachypnoea of the newborn Respiratory distress syndrome in preterm infants |
Pneumonia/sepsis Meconium aspiration Pneumothorax Congenital heart disease/heart failure Persistent pulmonary hypertension of the newborn (PPHN) Hypoxic–ischaemic encephalopathy |
Surfactant deficiency in term infants Congenital diaphragmatic hernia Tracheo-oesophageal fistula Pulmonary hypoplasia Pleural effusion (chylothorax) Milk aspiration Airway obstruction (e.g. choanal atresia) Lung anomalies (congenital pulmonary airway malformation – CPAM, lobar emphysema, pulmonary sequestration) Neuromuscular disorders Severe anaemia Metabolic acidosis (inborn error of metabolism) |
This is by far the most common cause of respiratory distress in term infants. It is more common following elective caesarean section. Although absence of thoracic squeezing of lung liquid from the chest during delivery is thought to be a factor, clearance of fetal lung fluid is largely dependent on reabsorption of alveolar fluid via sodium channels in the lung epithelium, which is influenced by the level of circulating catecholamines. The lower concentration of circulating catecholamines following elective delivery results in reduced absorption of lung liquid. At birth, the baby generates marked negative pressures (−60 cmH 2 O), which fill the lungs with air. With the first two or three breaths, much of the fetal lung fluid is expelled. This is enhanced by positive end expiratory pressure (PEEP), which is generated by the baby crying (against partially closed vocal cords). The remainder is absorbed into the pulmonary lymphatics and capillaries over the first 6–12 hours. Delay in the absorption of lung liquid may result in respiratory distress and for several days.
A male infant is born at 41 weeks' gestation in poor condition through meconium-stained amniotic fluid (MSAF). He is intubated and ventilated. He requires 90% oxygen and his oxygen saturation remains between 80% and 85%. Arterial blood gas shows a pH 7.31, PaO 2 3 Kpa, PaCO 2 4.5 Kpa and base deficit of 3. Which of the following is likely to be contributing to his hypoxia? Answer with true (T) or false (F).
Airway obstruction
Alveolar hypoventilation
Intrapulmonary shunt
Persistent pulmonary hypertension of the newborn
Systemic hypotension with R to L shunt through arterial duct
A. False; B. False; C. True; D. True; E. True.
A male infant is born at 41 weeks' gestation in poor condition through meconium-stained amniotic fluid (MSAF). He is managing in about 30% FiO 2 to maintain saturation between 90–95%. Arterial blood gas shows pH 7.18, PaO 2 9.5 Kpa, PaCO 2 11 Kpa. Which of the following are likely to contribute to his respiratory acidosis? Answer with true (T) or false (F).
Airway obstruction
Blocked endotracheal tube
Pneumothorax
R to L shunt through arterial duct
R to L shunt through foramen ovale
A. True; B. True; C. True; D. False; E. False.
See below for discussion.
Meconium stained amniotic fluid (MSAF) occurs in around 13% of all deliveries, but meconium aspiration syndrome (MAS) occurs only in only a small percentage of them. Aspiration most commonly occurs in utero and with thick MSAF consistency. Of affected infants, 30–60% require mechanical ventilation, 10–25% develop pneumothoraces and 2–5% die. Some 50–70% of infants with persistent pulmonary hypertension of the newborn have MAS as an underlying disorder.
The pathophysiology of MAS is complex, with proximal and distal airway obstruction and air trapping. There is also pulmonary parenchymal injury due to inflammatory cascade, which in turn can lead to surfactant inactivation. Those babies who suffered from chronic hypoxaemia before delivery may also have remodelling of their pulmonary vasculature leading to persistent pulmonary hypertension of the newborn (PPHN).
Of the various management strategies, few have been adequately evaluated. The mainstay is to provide adequate respiratory support to maintain oxygenation in the normal range, prevent air leaks using newer styles of ventilation including high frequency ventilation and treatment of PPHN with inhaled nitric oxide or extracorporeal membrane oxygen (ECMO). Most babies who survive have normal outcome unless MAS was a result of antenatal or intrapartum asphyxia. Such babies are at risk of subsequent neurodevelopmental delay.
This refers to a collection of gas outside the pulmonary space and includes pneumothorax, pneumomediastinum, pneumoperitoneum, and subcutaneous emphysema.
Several conditions increase the risk of pulmonary air leaks:
Respiratory distress syndrome (incidence 5–20%, the commonest cause)
Meconium aspiration syndrome (incidence 20–50%)
Congenital diaphragmatic hernia (14%)
Previous pneumothorax (risk of developing contralateral pneumothorax 45%)
Pulmonary hypoplasia
Pulmonary interstitial emphysema
Air leak syndrome arises via a common pathway that involves damage of the respiratory epithelium, which in turn allows air to enter the interstitial space, causing pulmonary interstitial emphysema. With continued high transpulmonary pressures, air dissects towards the visceral pleura and/or hilum by the peribronchial or perivascular space. Pneumothorax occurs when the pleural surface is ruptured resulting in the leakage of air into the pleural space. Acute pneumothorax is a serious complication causing collapse of the underlying lung, mediastinal shift and cardiovascular compromise due to reduced venous return and cardiac output. Diagnosis is made on suspicion from deterioration of clinical condition or increased oxygen requirement, clinical signs (reduced air entry, mediastinal shift), positive transillumination and chest radiograph. Needle aspiration can be used to treat a symptomatic pneumothorax. However, chest tube drainage (thoracostomy) is usually needed for continuous drainage of pneumothoraces that develop in infants receiving positive pressure ventilation, because the air leak may be persistent.
Under normal circumstances, pulmonary vascular resistance (PVR) falls rapidly after birth. If this does not occur, PPHN ensues and this leads to a variable degree of right-to-left shunt of blood through the foramen ovale and ductus arteriosus, which together with intrapulmonary shunting results in severe hypoxaemia. A similar clinical picture can arise from decreased systemic vascular resistance (SVR) or any condition in which the PVR : SVR ratio is more than one. PVR may be elevated as a result of an ‘appropriate’ response to an underlying acute pathological state (for example, pneumothorax or pneumonia), or as a result of structural abnormalities of the pulmonary vascular bed.
A number of non-structural (and therefore more reversible) factors may also impact on pulmonary vascular reactivity and pressure. Hypoxia, hypercarbia and acidosis cause vasoconstriction and elevate pulmonary artery pressure, and their presence may lead to failure of adaptation from fetal to neonatal (adult type) circulation. Conditions which cause increased pulmonary vascular resistance are of two types:
Where the pulmonary vascular morphology is normal, as seen in association with asphyxia, meconium aspiration syndrome, severe parenchymal lung disease, and sepsis/pneumonia
Increased pulmonary vascular resistance associated with morphologically abnormal pulmonary vasculature in association with pulmonary hypoplasia, congenital diaphragmatic hernia and congenital pulmonary airway malformations (CPAM). It may also occur in structurally abnormal heart disease, as for example in left ventricular outflow tract obstruction and anomalous pulmonary venous drainage.
Differential diagnosis of persistent hypoxaemia in the term/near term infant includes:
Primary lung disease
Cyanotic congenital heart disease
PPHN, with or without lung disease.
Echocardiography is helpful in confirming the presence of PPHN and identifying congenital heart disease. Treatment includes general supportive measures, mechanical ventilation and pharmacotherapy including pulmonary vasodilators, mainly inhaled nitric oxide. If there is an inadequate responsive to these measures, extracorporeal membrane oxygenation (ECMO) has been shown to significantly reduce death without causing any increase in adverse neurological outcome in later life.
Congenital anomalies in the lung can be categorized as malformations in:
The tracheo-bronchial tree
Distal lung parenchyma
Abnormalities in the pulmonary arterial and venous trees and the lymphatics
Most pulmonary malformations arise during the embryonic and the pseudoglandular stages of lung development. However, acute lung injury in the neonatal period may alter subsequent alveolar and airway growth and development. The most common pulmonary and extra pulmonary malformations are congenital diaphragmatic hernia (CDH), congenital pulmonary airway malformations (CPAM), tracheo-oesophageal fistula (TOF), bronchopulmonary sequestration (BPS) and congenital hydrothorax.
Congenital diaphragmatic hernia (CDH) occurs in 1 in 2000–3000 births. In roughly two thirds, it is an isolated lesion. The remainder are complex and it can be part of a syndrome including chromosomal anomalies. It results from failure of normal development of the diaphragm during first trimester and has different types:
Posterior lateral defect, more often on the left than right
Anterior or central portion defect
Complete absence of diaphragm
Eventration, which is not a true hernia but results from failure of muscle development in the primitive diaphragm.
Diaphragmatic hernia produces a spectrum of pathology from very mild pulmonary hypoplasia (causing minimal clinical compromise) to severe (incompatible with life).
The compression of the fetal lungs results in lung hypoplasia, especially in the ipsilateral lung. In severe cases, there may be compromised cardiac function. Cardiorespiratory function is further compromised by gaseous distension of the intrathoracic gut following delivery. This is a particular hazard if babies are given bag-valve-mask ventilation.
Pulmonary hypoplasia (including abnormalities of the pulmonary vasculature) and poor oxygenation following delivery commonly result in severe persistent pulmonary hypertension of the newborn (PPHN). In mild cases, the clinical presentation may be delayed.
This may comprise:
Fetal surgery – fetal endoscopic tracheal occlusion (FETO), which is reserved for severe CDH to promote lung growth and limit pulmonary hypoplasia. It may not alter the lung parenchyma and pulmonary arterioles qualitatively. Using fetal tracheoscopy, a balloon is inserted (ideally at 26–28 weeks) and the occlusion is reversed in utero at 34 weeks. Overall fetal surgery for CDH offers little benefit, and its use should be limited to clinical trials.
At delivery, if diagnosed antenatally:
Face mask ventilation is avoided as it distends the gastrointestinal tract
Intubation and artificial ventilation is instituted as soon as possible
Continuous drainage (decompression) of gas from abdomen is started
Factors that could precipitate PPHN and lung injury are minimized
Adequate ventilatory support is provided
Pulmonary vasodilators are given if PPHN is present
Surfactant – there is no evidence to support its recommendation except in premature infants with coexisting surfactant deficiency
Surgical repair is clearly essential but should be performed when the baby is stable
ECMO (extracorporeal membrane oxygenation) is able to provide stability and control PPHN but its benefit in CDH is unclear.
This occurs in 1 in 3000–4500 live births and results from failure of the process of separation of the primitive foregut into the respiratory and gastrointestinal tract at 3–6 weeks of gestation ( Fig. 11.7 ). It is usually found in combination with various forms of oesophageal atresia, the most common combination being oesophageal atresia with distal tracheo-oesophageal fistula (about 91%). Tracheo-oesophageal fistula without oesophageal atresia (H-type fistula) is extremely rare and usually presents after the neonatal period.
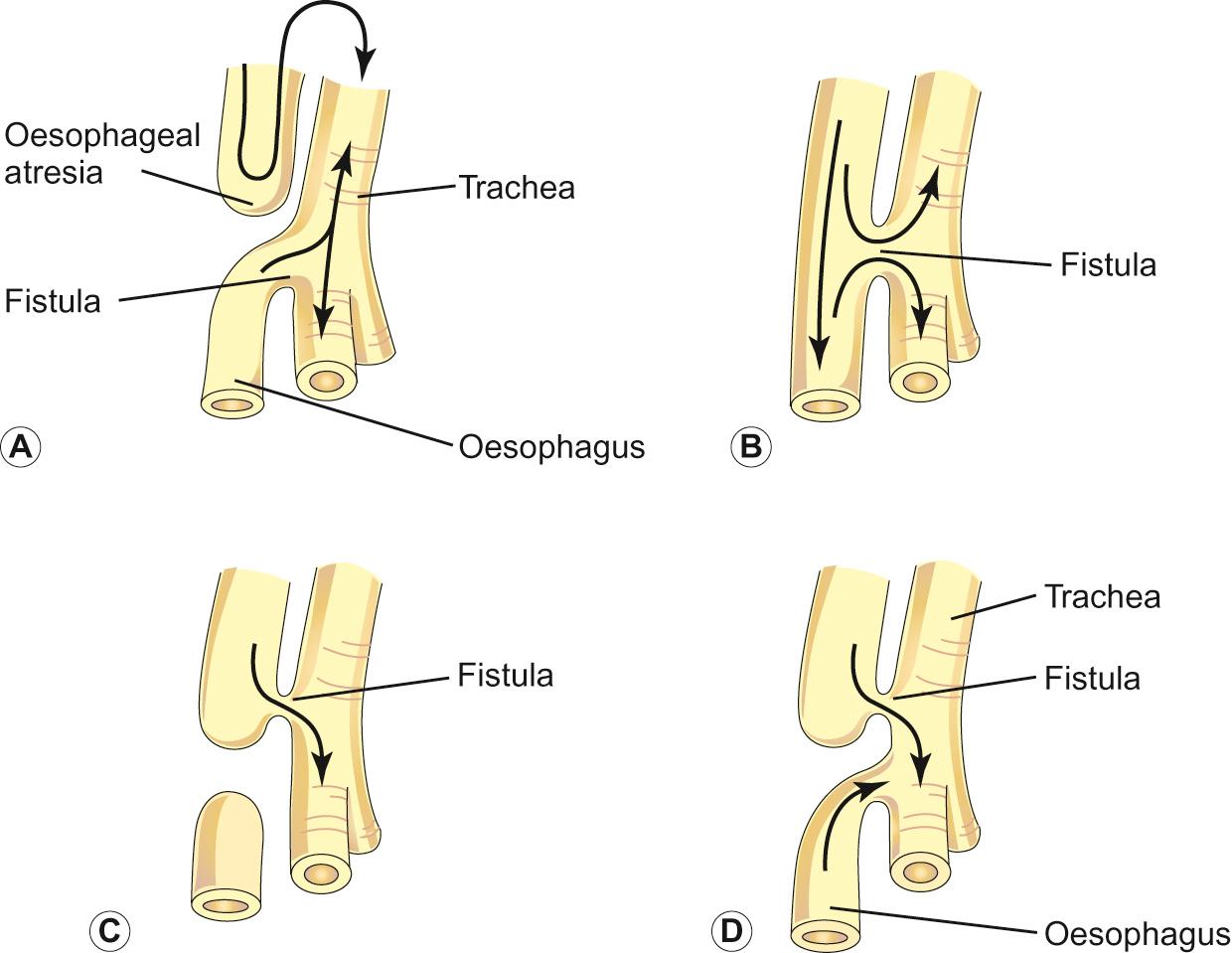
Absence of softening in the cartilaginous rings causes the trachea to collapse on expiration. There is a reduction in the cartilage to soft tissue ratio. The anomaly may be segmental or diffuse.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here