Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Bilirubin is one of three biologically active end-products of heme catabolism. Its clinical significance in the neonate relates to its propensity for deposition in the skin and mucous membranes, producing easily identifiable jaundice (French jaune , yellow) or icterus (Greek ikteros ). The yellow color, or the serum (or plasma) total bilirubin (TB) concentration at any point in time, represents the combined processes of bilirubin production and bilirubin elimination from the body, the latter process comprising bilirubin uptake into the hepatocyte, bilirubin conjugation, and excretion of the conjugated product. As long as these functions remain in balance, a moderate degree of jaundice may develop but should not endanger an otherwise healthy, nonhemolyzing infant. Imbalance between bilirubin production and its elimination may result in increasing jaundice or hyperbilirubinemia. In rare cases, the degree of bilirubin production relative to bilirubin elimination may be so great that bilirubin may deposit in the brain, where it may cause dysfunction in the form of acute bilirubin encephalopathy (ABE). Although some cases of ABE may be transient and reversible, chronic bilirubin encephalopathy with resultant permanent neuronal damage, a form of cerebral palsy known as kernicterus, may ensue. As many as 80% of otherwise healthy, term newborns develop some degree of elevated TB levels. In contrast, severe hyperbilirubinemia with its potentially devastating sequelae is rare. It is, therefore, important to distinguish between normal processes of bilirubin physiology from pathologic metabolism. Medical caretakers of newborns should possess a thorough understanding of normal bilirubin physiology as well as its abnormal metabolism and the potential complications of severe hyperbilirubinemia.
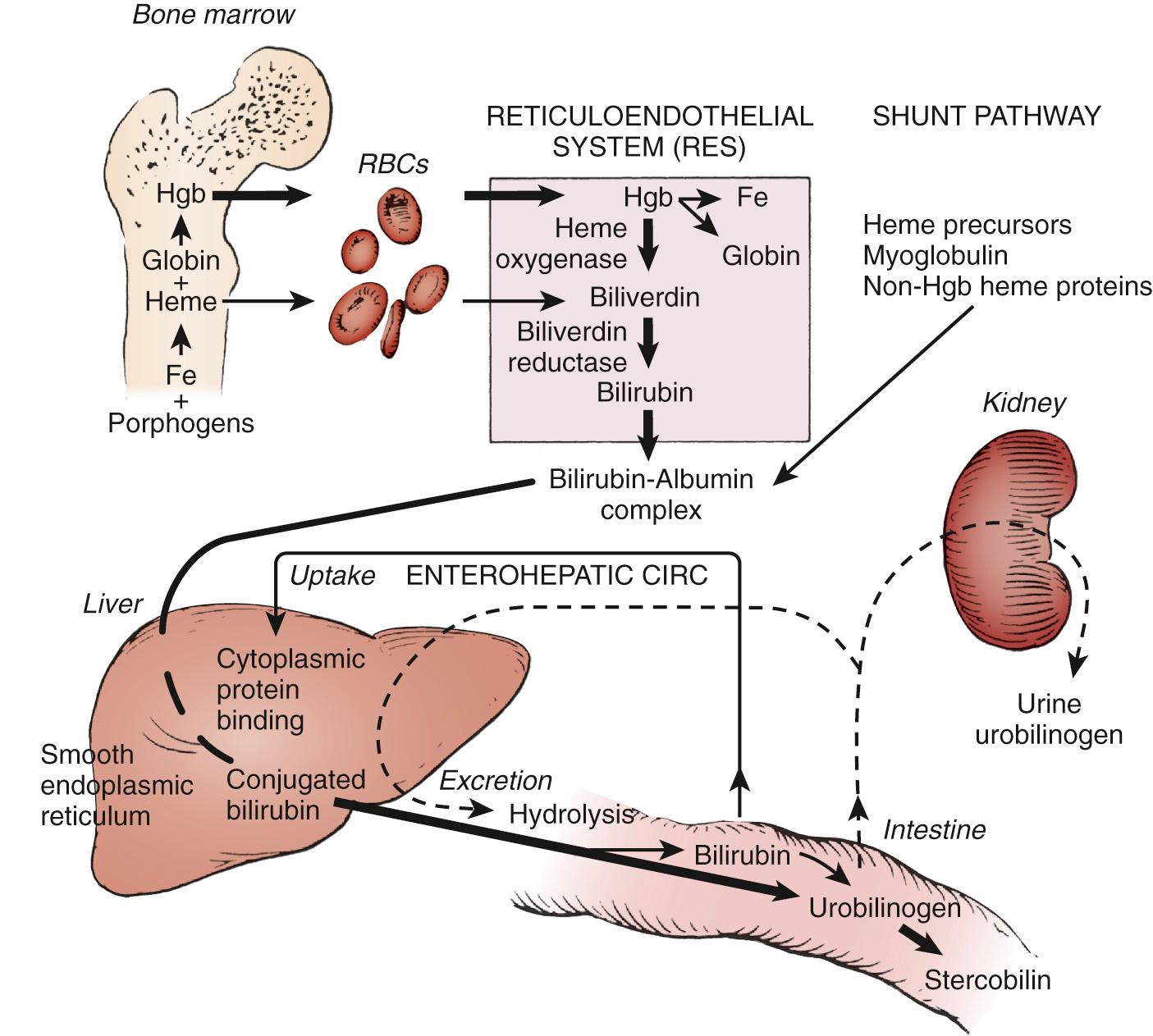
Throughout life, there is a continuum of bilirubin production and elimination from the body. Ongoing lysis of red blood cells (RBCs), whether physiologic or at increased rates (e.g., due to hemolysis), releases iron protoporphyrin (heme), the oxygen-carrying component of hemoglobin. Catalyzed by heme oxygenase (HO), heme is then converted to biliverdin and subsequently to bilirubin ( Fig. 91.1 ). This bilirubin in its unconjugated form is transported to the liver bound to albumin. In the hepatocyte, bilirubin is conjugated to glucuronic acid by the enzyme uridine diphosphoglucuronate (UDP)-glucuronosyltransferase 1A1 (UGT1A1). Water-soluble conjugated bilirubin can be now excreted into the bile from which it reaches the bowel and is ultimately eliminated from the body. This simplified overview of bilirubin biochemistry will be reviewed in greater detail later in this chapter.

Heme oxygenase-1 (HO-1), the inducible isoform of HO, is a membrane-bound enzyme found in cells of the liver and other organs that catalyzes the first step in the pathway by which heme is converted to biliverdin through oxidation of the former molecule's α-methene bridge carbon ( Fig. 91.2 ). HO-1 is inducible by its substrate heme. This rate-limiting step produces biliverdin; free iron (which can be reutilized for hemoglobin synthesis); and carbon monoxide (CO), which is excreted in the lungs in equimolar amounts. This process occurs in all nucleated cells except for mature, anucleated RBCs. Biliverdin is a blue-green water-soluble pigment that can be readily excreted by the liver and kidneys. In amphibians, reptiles, and certain avian species, the major pigmented end-product of heme catabolism is biliverdin. In mammals, however, biliverdin is converted to bilirubin by biliverdin reductase in the cytosol.

The degradation of 1 g of hemoglobin forms 34 mg of bilirubin. The isomeric form of bilirubin produced in this two-step process is IX-α ( Z,Z isomer), defining the relative positions of the four pyrrole rings and the hydrogen molecules on the two linking lateral carbons. This form of bilirubin is water-insoluble, owing to tertiary structural changes that internalize the keto and carboxy groups that would otherwise interact with water molecules. Intramolecular hydrogen bonding maintains this folded bilirubin structure.
Because bilirubin is a weak acid and is neither water soluble nor readily excreted at pH 7.4, to facilitate its excretion the molecule must be conjugated to mono- and diglucuronic acids by the specific hepatic enzyme isozyme (UGT1A1). The evolutionary advantage derived by mammalian species in the development of such an intricate energy-dependent system that first produces bilirubin from a water-soluble precursor and then converts it back to a water-soluble form for excretion is presently uncertain. The mammalian placenta is capable of removing unconjugated bilirubin but not biliverdin. Biliverdin accumulation in the mammalian fetus would presumably result in the accumulation of large amounts of potentially toxic metabolites. Evidence has shown that bilirubin and even CO may be biologically useful molecules. The inducibility of HO-1 would appear to indicate that bilirubin production is helpful to cells when they are stressed. Bilirubin is a potent antioxidant that readily binds to membrane lipids and is capable of limiting membrane damage by preventing peroxidative injury. Biologic evidence of potentially beneficial effects of moderate concentrations of bilirubin is tempered by the association of high levels of unconjugated bilirubin with neuronal dysfunction and necrosis. Although cells may be potential beneficiaries of small amounts of bilirubin, in greater circulating quantities, the same bilirubin molecule may be a causative factor of severe neuronal damage. The dilemma that faces the clinician is determining the desirable or “safe” level of bilirubin appropriate for a particular neonate.
The CO formed by heme degradation binds to hemoglobin to form carboxyhemoglobin (COHb) and is then transported in the circulation to the lung. Here, the CO separates from hemoglobin and is exhaled in breath. Although there are other potential endogenous and exogenous sources of CO, such as lipid peroxidation and photo-oxidation, the main source of endogenous CO is derived from heme catabolism. Therefore, quantitative estimation of its synthesis or excretion (in infants without significant lung disease or oxygen exposure) offers a reasonably accurate assessment of the rate of heme degradation from which the rate of bilirubin production can be derived. It is believed that other hemoproteins undergo the same degradative process.
The pathway of bilirubin synthesis, transport, and metabolism is summarized in Fig. 91.3 . In the normal adult, bilirubin is derived primarily from the degradation of heme, which is released from senescing RBCs in the reticuloendothelial cells. Normally, about 20% of the bilirubin excreted into bile is derived from heme and other hemoproteins (mainly cytochromes, catalase). CO excretion in humans and more direct measurements in animals have demonstrated that, on the first day of life, bilirubin production is increased two to three times the rate of adults, to an estimated average of 8-10 mg/kg body weight per day. Bilirubin production decreases rapidly during the first 2 postnatal days. Several factors may explain this increased production in the newborn. The circulating RBC life span is shortened to 70-90 days compared with 120 days in the adult. Increased heme degradation arises from the very large pool of hematopoietic tissue, essential to intrauterine well-being, but ceases to function shortly after birth. An additional factor may possibly include an increased turnover of cytochromes. Another major contributor to the bilirubin pool in the neonate is an increase in bilirubin absorbed from the bowel as part of the enterohepatic circulation. This mechanism results from both reformation of unconjugated bilirubin from conjugated bilirubin in the bowel and enhanced absorption of unconjugated bilirubin by the intestinal mucosa back to the circulation (see Enterohepatic Absorption of Bilirubin ).
Unconjugated bilirubin is mostly insoluble in water at pH 7.4, with a solubility of less than 0.01 mg/dL, and when released into the circulation by the reticuloendothelial cells, it is rapidly bound to albumin. Each molecule of adult albumin is capable of binding at least two molecules of bilirubin; the first molecule is more tightly bound than the second. Additional binding sites with weaker affinities may also exist but are probably of little clinical importance. On average, 7-8 mg/dL of unconjugated bilirubin can be bound to each gram of albumin. Physiologically, newborns have a lower plasma-binding capacity for bilirubin compared with adults or older children. This occurs because of reduced neonatal albumin concentrations and reduced molar binding capacities. Binding of unconjugated bilirubin by albumin is believed to be of importance in determining bilirubin neurotoxicity, because the unbound bilirubin fraction is thought to be a more sensitive predictor of bilirubin-induced neurologic dysfunction (BIND) than the TB used clinically. However, except for Japan, there is currently no reliable and clinically available measurement to make the determination of unbound bilirubin concentrations a useful clinical tool in evaluating a newborn's risk for developing BIND or in the therapeutic decision-making process.
The TB (serum or plasma) concentration is the conventional clinical laboratory measurement of bilirubin and is the basis for decision making for the management of hyperbilirubinemia. Bilirubin exists in four different forms in circulation: (1) unconjugated bilirubin reversibly bound to albumin, which makes up the major portion; (2) a relatively minute, but potentially neurotoxic, fraction of unconjugated bilirubin not bound to albumin (known as free or unbound bilirubin); (3) conjugated bilirubin, comprising mainly mono- and diglucuronides, which have effluxed from the hepatocyte to the circulation and are readily excretable through the renal or biliary systems; and (4) conjugated bilirubin covalently bound to albumin, known as δ-bilirubin. Indirect, or unconjugated, bilirubin may increase in the serum or plasma in the presence of exaggerated hemolysis or diminished bilirubin glucuronidation. Conjugated or direct bilirubin will increase in association with excretory immaturity or with cholestatic diseases in which bilirubin is conjugated but its excretion is impaired. A similar effect may be seen following acute hemolytic episodes in which indirect bilirubin is conjugated, but the large amounts of conjugated bilirubin are unable to be excreted via the bile. δ-Bilirubin has a plasma disappearance rate similar to that of serum albumin ( Fig. 91.4 ).
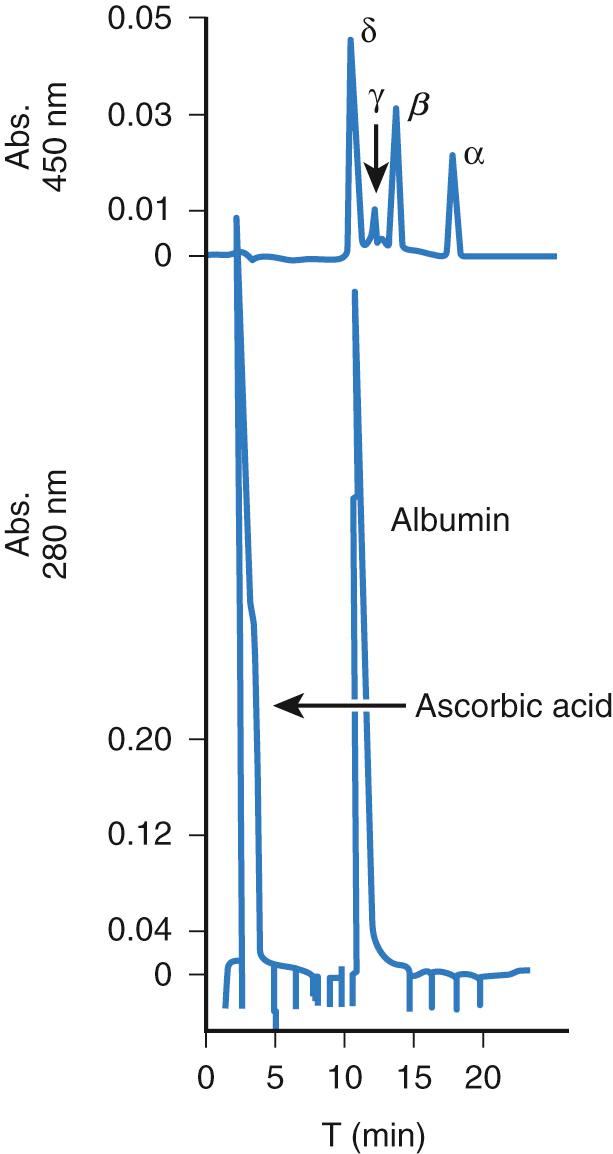
Conjugated bilirubin, but not δ-bilirubin, gives a “direct” reaction with standard diazo reagents; whereas, bound or unbound unconjugated bilirubin yields an “indirect” reaction. The terms indirect and direct bilirubin tend to be used interchangeably with unconjugated and conjugated bilirubin, respectively. However, the terms are not identical and measure differing bilirubin components. Direct bilirubin assays measure all conjugated bilirubin including mono- and diglucuronides as well as δ-bilirubin and some unconjugated bilirubin. Conjugated bilirubin implies measurement of mono- and diglucuronides only. Therefore, conjugated bilirubin determinations may be lower than direct measurements performed on an identical serum sample.
Conjugated bilirubin usually comprises a small fraction of the TB concentration. δ-Bilirubin can be measured only with newer techniques. It is found in detectable amounts in normal older neonates and children and in significantly increased concentrations in those with prolonged conjugated hyperbilirubinemia resulting from various liver disorders. However, it is virtually absent from the serum during the first 2 weeks of life.
Bilirubin dissociates from circulating albumin before entering the liver cell. The latter process occurs partly by a passive process of carrier-mediated diffusion and partly by mediation by organic anion transporter proteins. In the liver cell cytoplasm, the unconjugated bilirubin is bound to glutathione-S-transferase A, also known as ligandin, or with B-ligandin (Y protein). These are major intracellular transport proteins, and their bilirubin binding ability helps keep the potentially toxic unbound level low. Z protein, another hepatic cytoplasmic carrier, also binds bilirubin but with a lower affinity.
The equilibrium between the rates of bilirubin entry into the circulation, including de novo synthesis, enterohepatic circulation, and tissue shifts, and the bilirubin elimination process, including hepatic cell uptake and conjugation and excretion of bilirubin, determines the TB concentrations at any specific time. This concept is equally applicable under normal physiologic and pathologic circumstances alike.
A reduced capacity of net hepatic uptake of unconjugated bilirubin has been implicated in the development of physiologic jaundice. In the newborn monkey, deficiency of B-ligandin and reduced clearance of sulfobromophthalein were demonstrated in the first 3 days of life, the period during which this animal frequently has physiologic jaundice. Studies in the human indicate that a deficiency of bilirubin uptake is probably of less importance in the pathogenesis of unconjugated hyperbilirubinemia than an immaturity of the bilirubin conjugation system during the first 3 or 4 postnatal days. However, the relative contribution of uptake deficiency may be greater during the second week of life, when the rate of bilirubin conjugation increases and approaches that of normal adults.
In order for bilirubin to be excreted into the bile, the nonpolar, water-insoluble unconjugated bilirubin must be converted to a more polar, water-soluble substance. The aim of this process is to alter the bilirubin molecule by solubilizing bilirubin IX-α. Bilirubin is presumed to be transported by hepatic ligandin from the liver cell plasma membrane to the endoplasmic reticulum, where the conjugating enzyme UGT1A1 is situated. Conjugation is a two-step enzymatic process in which each molecule of bilirubin is conjugated with two molecules of glucuronic acid. Glucuronic acid derives from activated uridine diphosphoglucuronic acid (UDPGA), itself synthesized by the soluble cytoplasmic enzyme uridine diphosphoglucose dehydrogenase from uridine diphosphoglucose, which is, in turn, synthesized from free glucose. The UGT1A1 enzyme first catalyzes the transfer of one glucuronic acid molecule from one of the two propionic acid side groups on one of the central pyrrole rings of bilirubin, in an ester linkage, to form bilirubin monoglucuronide. The physiologic reduction in enzyme activity in the newborn to less than 1% of normal may, therefore, result in unconjugated hyperbilirubinemia.
Although bilirubin monoglucuronide is water-soluble and capable of being excreted into bile without further alteration, about two-thirds of the total bilirubin excreted into bile in the adult human is in the form of a diglucuronide. The second step of the enzymatic conjugation process involves the esterification of a second glucuronide molecule to the now monoconjugate. This process is catalyzed primarily by the same UGT1A1 enzyme on the endoplasmic reticulum, although a second enzyme, UDP-glucuronate glucuronosyltransferase (transglucuronidase), located in the canalicular portion of the hepatocyte plasma cell membrane, may also play a role. The substrate for the canalicular transglucuronidation is believed to be bilirubin monoglucuronide. The enzyme transfers one molecule of glucuronic acid from one molecule of bilirubin monoglucuronide to another, resulting in the formation of one molecule of bilirubin diglucuronide. The latter molecule is excreted into the bile canaliculus; whereas, the remaining molecule of now unconjugated bilirubin is returned to the endoplasmic reticulum for subsequent reconjugation. In circumstances such as in severe chronic hemolysis, increased loads of bilirubin are delivered to the liver. Limited excretory ability may result in retention of conjugated bilirubin in the form of bilirubin monoglucuronide.
The result of the esterification is to disrupt the intramolecular hydrogen bonds, thereby opening the molecule and rendering the conjugated bilirubin water-soluble. The water-soluble form of bilirubin is excretable in the bowel. Water solubility also decreases the amount of bilirubin reabsorbed from the bowel, because hydrophilic agents do not pass through the intestinal wall easily.
In the normal adult, glucuronide conjugation accounts for the disposal of about 90% of all bilirubin. The remaining portion is converted to water-soluble substances by conjugation with substances other than glucuronic acid, or by oxidation, hydroxylation, or reduction. In humans, bilirubin forms a conjugate with glucose, xylose, possibly other carbohydrates, sulfates, and taurine. These non-glucuronide conjugates account for no more than 10% of the TB conjugates excreted in the bile.
A number of in vitro studies have demonstrated the existence of deficiencies in hepatic UGT1A1 activity in newborns of many species, including humans. In newborn rhesus monkeys, hepatic bilirubin conjugating capacity is extremely low during the first hours of life and functions at about 5% of adult capacity ( Fig. 91.5 ). However, by 24 hours of age, UGT1A1 activity increases sufficiently to process the bilirubin load presented to the liver, and the TB concentration begins to fall. In 1-day-old rats, the proportion of both xylose and glucose conjugates of bilirubin equals that of glucuronide conjugates. Total conjugating capacity increases to adult levels by the fourth day of life, when a mature pattern of glycoside distribution is present, with 75% of all conjugates being glucuronides. In human newborns, the monoglucuronide conjugate is the predominant bile pigment conjugate. UGT1A1 activity in term infants is about 1% of that of healthy adults (and even less in premature infants), and increases at an exponential rate until 3 months of age, when adult levels are reached. Non-glucuronide conjugates are insignificant in this period.
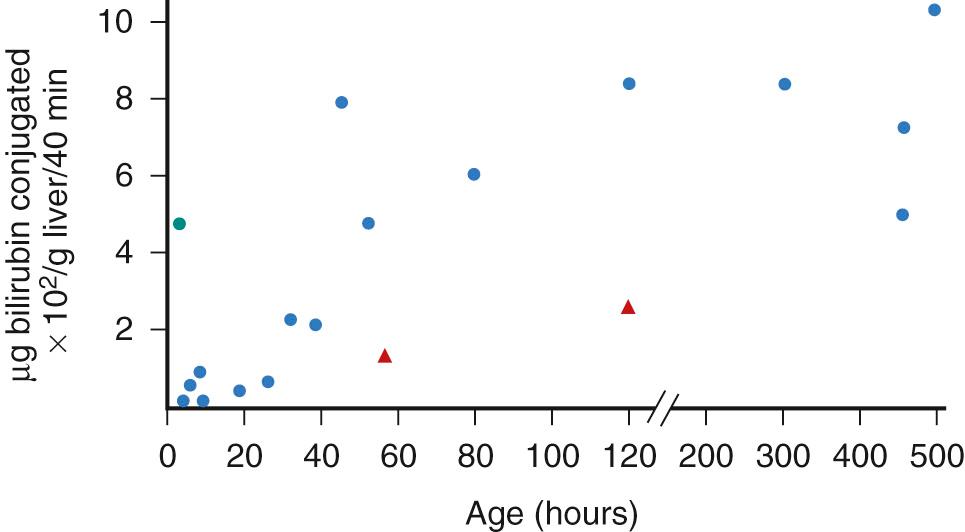
Excretion of the now polar, water-soluble bilirubin appears to be an energy-dependent concentrative process. The bilirubin conjugates are incorporated into mixed micelles along with bile acids, phospholipids, and cholesterol. The conjugates are excreted against a concentration gradient, and as a result, bile bilirubin concentration is about 100-fold that of the hepatocyte cytoplasm. Although the capacity for bilirubin excretion into bile is limited in newborn rhesus monkeys ( Fig. 91.6 ), excretory deficiency is not a rate-limiting factor in the overall hepatic elimination of bilirubin in the human newborn. In newborn babies, bilirubin uptake into the hepatocyte and the enzyme-mediated conjugation processes are the more restrictive steps and may result in a “bottleneck” effect. However, a large bilirubin pool requiring elimination, such as in hemolytic disease of the newborn, may overwhelm the excretory capacity with efflux of backed up conjugated bilirubin into the circulation. By contrast, in older humans and in the mature rhesus monkey and other mammals beyond the newborn period, hepatic excretion of conjugated bilirubin into bile predominates as the rate-limiting step in the presence of a large bilirubin load. At any age, in the presence of hepatic cell injury and biliary obstruction, hepatic excretory transport is the step most severely restricted, resulting in efflux of conjugated bilirubin from the hepatocyte to the serum with resultant conjugated hyperbilirubinemia. Thus, the hepatic excretory step may have the least reserve capacity of all the processes contributing to bilirubin elimination.
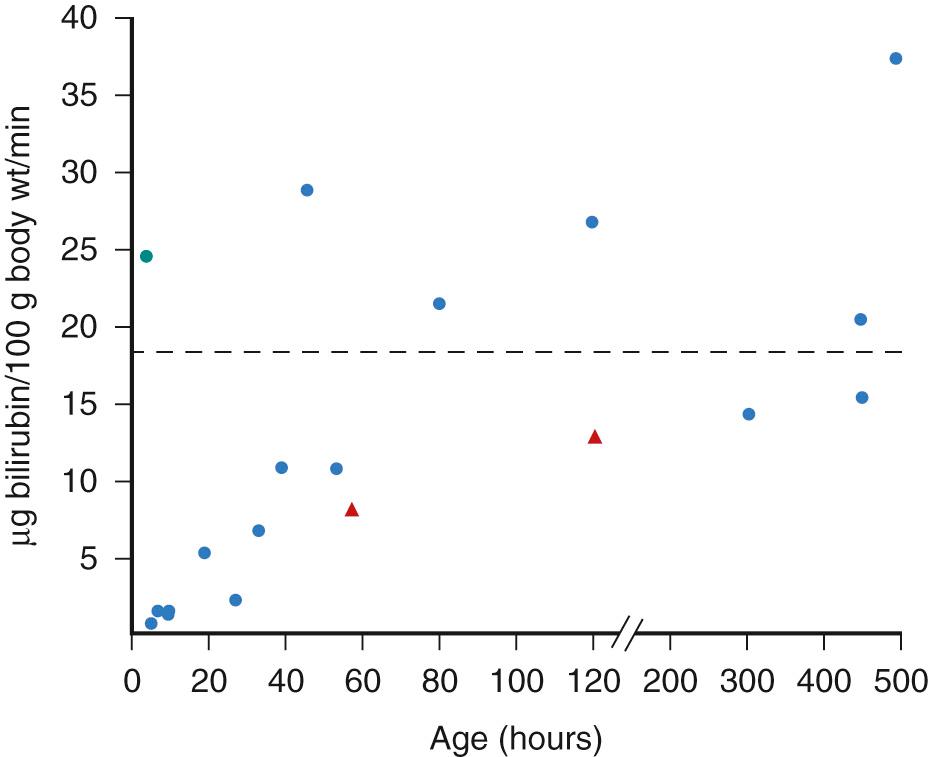
Conjugated bilirubin is not absorbed from the intestine. However, the mono- and diglucuronides of bilirubin are relatively unstable conjugates that are readily hydrolyzed to unconjugated bilirubin. Reverted unconjugated bilirubin may now be readily absorbed across the intestinal mucosa, contributing, through the enterohepatic circulation, to the circulating unconjugated bilirubin pool and again being presented to the liver for conjugation. Of importance in the mechanism of the enterohepatic circulation is the enteric mucosal enzyme β-glucuronidase, which is present in both term and premature neonates in high concentrations. Mild alkaline conditions present in the duodenum and jejunum contribute to the deconjugation process.
A study in adult rats demonstrates that enteric absorption of unconjugated bilirubin occurs predominantly in the duodenum and colon. The extent of absorption varies widely, depending on diet and caloric intake ( Fig. 91.7 ). Although quantitative estimates of the disposal of bilirubin have been performed only for adult humans, these data do indicate that about 25% of the TB excreted into the intestine is reabsorbed as unconjugated bilirubin. About 10% of the total is excreted in stool as unaltered bilirubin. The remaining pigment is converted to urobilinoids, most of which are excreted in stool, with a small portion being reabsorbed in the colon for subsequent excretion by both the liver and kidney.

Neonates have relatively high concentrations of unconjugated bilirubin in the intestine, which contribute to the enterohepatic circulation. Intestinal bilirubin is derived from increased bilirubin production, exaggerated hydrolysis of bilirubin glucuronide, and high concentrations of bilirubin found in meconium. The relative lack of bacterial flora in the newborn bowel to reduce bilirubin to urobilinogen further increases the intestinal bilirubin pool in comparison with that of the older child and adult. The increased hydrolysis of bilirubin conjugates in the newborn is enhanced by high mucosal β-glucuronidase activity and the excretion of predominantly monoglucuronide conjugates (in the newborn) rather than diglucuronides (in the adult). Oral administration of nonabsorbable bilirubin-binding substances, such as agar, activated charcoal, or a lipase inhibitor (e.g., Orlistat), may retain bilirubin in the bowel, thereby further increasing stool bilirubin content and reducing bilirubin reabsorption, thereby decreasing TB levels. Studies of intestinal bilirubin binding contribute to our understanding of the contribution of the enterohepatic circulation to unconjugated hyperbilirubinemia of the newborn.
HO-1 is the rate-limiting enzyme that catabolizes heme to biliverdin and then to bilirubin, with the simultaneous release of equimolar quantities of ferrous iron (Fe 3+ ) and CO. Polymorphisms of the HO-1 gene promoter region may modulate its transcriptional activity, with increased HO activity being associated with the overproduction of bilirubin.
The HO-1 gene promoter region has a polymorphic (GT) n repeat sequence with lengths ranging from 12-40 repeats. As can be seen in Fig. 91.8 , the (GT) n repeat distribution is bimodal with the main alleles at or around 23 and 30 repeat lengths. The number of (GT) n repeats can modulate the rate of transcriptional activity (hence, gene expression), with short sequences (less than 25) being associated with increased transcriptional activity compared with those with long (25 or greater) sequences.

The prevalence of the short (GT) n lengths is cardinal to the analyses of the gene's contribution to the pathophysiology of hyperbilirubinemia. Higher HO activity associated with short alleles should lead to increased heme catabolism and hyperbilirubinemia. Newborn studies, however, have shown contradictory results. Most published studies demonstrated no effect of HO-1 promoter polymorphisms on the TB levels. In two studies, however, from Taiwan and Japan, a modulating effect of short (GT) n repeats in increasing TB was reported. A potential role of short (GT) n repeats to exacerbate hyperbilirubinemia in the presence of hemolysis in which large amounts of released hemoglobin result in HO-1 induction has been suggested.
This process by which unconjugated bilirubin is taken up from the hepatic sinusoids and crosses the hepatocyte membrane to enter into that cell is facilitated by a carrier molecule, organic anion-transporting polypeptide-2 ( SLCO1B1 ). In humans, this carrier may play an important role in the metabolism of bilirubin and in the prevention of hyperbilirubinemia by facilitating the entry of bilirubin into hepatocytes. A mutation in the gene leading to an impaired maturation of the protein with reduced membrane localization and abolished transport function has been described, and a number of single-nucleotide polymorphisms (SNPs) have been identified, some of which are associated with an altered in vitro transport capability.
Perhaps one of the most important advances in our understanding of the genomics of bilirubin metabolism is the elucidation of the UGT1A1 gene encoding the bilirubin-conjugating enzyme, UGT1A1. It is becoming more apparent that the modulation of bilirubin metabolism and whether TB levels remain within physiologic or hyperbilirubinemic ranges lie within genetic control. Although not limited to the neonatal age group, Skierka et al. demonstrated that 146/181 neonatal and pediatric age group patients referred for hyperbilirubinemia had at least one heterozygous UGT1A1 variant, indicating that many cases of unconjugated hyperbilirubinemia could be attributed to variations at the UGT1A1 locus. Next is a short overview to allow the reader to comprehend mutations of this gene and interactions of these mutations with genetic or environmental factors in the mechanism of jaundice.
The UGT gene is a superfamily of genes whose function is to encode a biochemical reaction leading to the conjugation of glucuronic acid to certain target substrates to facilitate their elimination from the body. The UGT2 genes are located on chromosomes 4q13 and 4q28. The enzymes encoded by this family preferentially conjugate endobiotic substances, such as steroids and bile acids, and although of physiologic and pharmacologic importance, they are of little relevance to bilirubin metabolism. In contrast, the UGT1A1 gene isoform, which belongs to the UGT1 gene family, is of major importance to the conjugation and, therefore, elimination of bilirubin. This gene isoform has been mapped to chromosome 2q37. UGT1A1 was cloned by Ritter and associates in 1991. The gene consists of 4 common exons (exons 2, 3, 4, and 5) and 13 variable exons, of which only variable exon A1 is of any importance regarding bilirubin conjugation; the remaining exons play a role in the detoxification of a diverse range of chemical substances ( Fig. 91.9 ). The variable exon A1 functions in conjunction with common exons 2 to 5: in response to a specific signal, transcription processing splices mRNA from the variable exon to the common exons. This process provides a template for the synthesis of an individual enzyme isoform. Upstream of each variable exon is a regulatory noncoding promoter that contains a box sequence of thymidine-adenine (TA) repeat (TATAA) of nucleic acids. Mutations of variable exon A1, its promoter, or the common exons 2 to 5 may result in deficiencies of bilirubin conjugation. Single nucleotide polymorphisms of the noncoding promoter area affect bilirubin conjugation by diminishing expression of a normally structured enzyme, whereas mutations of the gene coding area may affect enzyme function by altering the structure of the enzyme molecule. Further information is supplied in the section on Conjugated Hyperbilirubinemia .
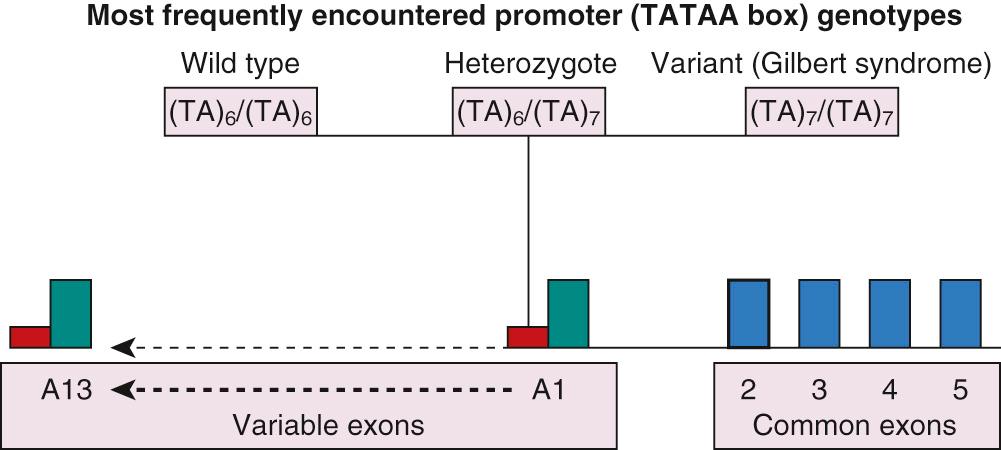
In a genome-wide study performed on adults in Sardinia, three loci were associated with the modulation of TB levels: UGT1A1, glucose-6-phosphate dehydrogenase (G6PD), and SLCO1B3, the latter a member of the SLC family implicated in bilirubin uptake into the hepatocyte. Pursuing this finding in relation to neonatal hyperbilirubinemia, Lin et al. studied the allele frequencies of mutations and polymorphisms of UGT1A1, G6PD, and SLCO1B1 in DNA samples, which were obtained from the DNA Polymorphism Discovery Resource of the National Human Genome Research Institute, and thought to be representative of the current US population. Although no clinical information was available for the individuals whose DNA was included in the sampling, a high rate of gene co-expression does suggest a potentially important role for genetic polymorphism co-inheritance in neonatal hyperbilirubinemia. Co-expression of genes with another, with mutations or polymorphisms, or with environmental factors may potentiate their role and exacerbate the pathophysiology of neonatal hyperbilirubinemia to a greater extent than each gene individually.
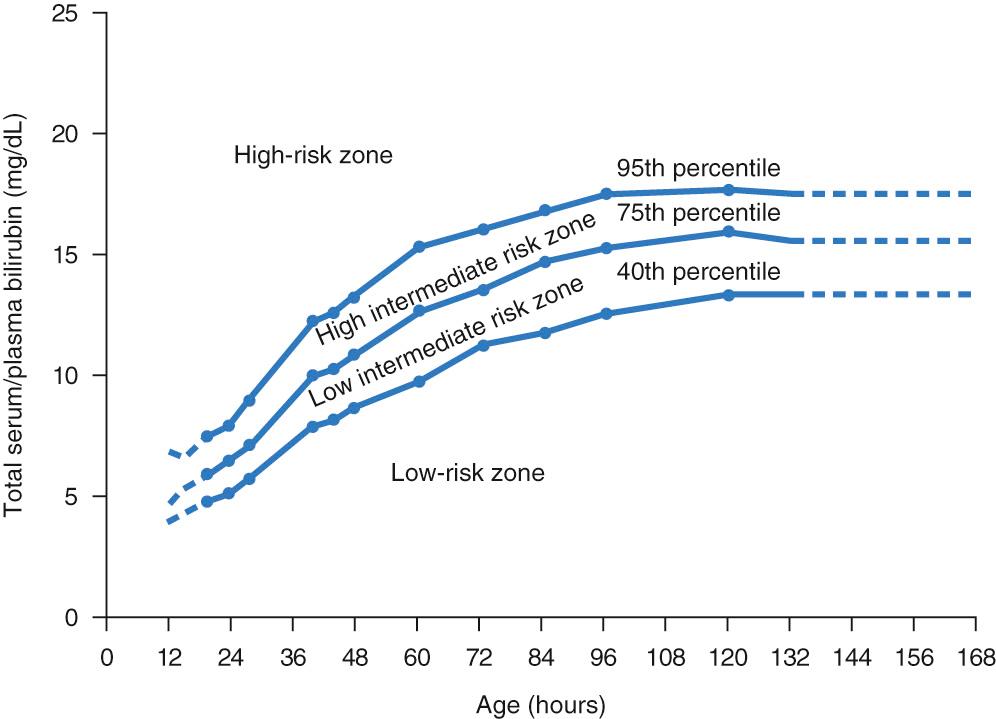
Elevations in unconjugated bilirubin occur ubiquitously in the human neonatal population. In a sense, this “normal” increase in TB levels is not true hyperbilirubinemia when compared with a reference group of all newborns. A more appropriate term that would add to our understanding of the phenomenon and distinguish the normal or physiologic state from the pathologic entity implied in the term hyperbilirubinemia may be physiologic bilirubinemia . Although the 40th percentile is spuriously elevated, the hour-specific TB nomogram ( Fig. 91.10 ), or more recently published transcutaneous bilirubin (TcB) nomograms, reflect the natural increase in TB during the first days of life, reaching a peak at about 5 days.
Unconjugated hyperbilirubinemia in the human, regardless of age, is defined as an indirect-reacting bilirubin concentration of 2.0 mg/dL (34 µmol/L) or greater, depending on the standard used in calibration of the reaction. Nearly all adults and older children normally have indirect-reacting bilirubin concentrations in circulation of less than 0.8 mg/dL (14 µmol/L) and δ-bilirubin of 0.2-0.3 mg/dL (3-5 µmol/L). Conjugated hyperbilirubinemia is defined as an elevation of the direct-reacting fraction in the van den Bergh diazo reaction of greater than 1.5 mg/dL (26 µmol/L) provided it comprises more than 10% of the TB concentration. The latter portion of the definition is added to guard against over-interpretation of direct reactions in newborns with markedly elevated indirect-reacting bilirubin concentrations, because up to 10% of the unconjugated pigment behaves as direct-reacting pigment in the van den Bergh–type methods.
Clinical situations in which the direct-reacting bilirubin concentration is equal to or close to the TB concentration are extremely rare, especially in the newborn period. In the neonate with conjugated hyperbilirubinemia, the hyperbilirubinemia is usually “mixed,” the elevated direct-reacting fraction accounting for 20%-70% of the total pigment. Thus, a neonate with mixed hyperbilirubinemia should be considered primarily to have conjugated hyperbilirubinemia. Except in cases of extreme hemolysis, such as in hemolytic disease of the newborn, pathology resulting from interference with hepatic cell excretion and bile transport, rather than from abnormalities of increased bilirubin production or deficient hepatic bilirubin uptake or conjugation, should be sought.
During the last stages of human gestation, the normal degradation of erythrocytes formed earlier in fetal life results in about a 150% increase in bilirubin production per unit of body weight compared with adults. The mammalian fetus of all species appears to be capable of degrading heme without limitation through the two enzymatic steps responsible for the formation of unconjugated bilirubin IX-α, CO, and biliverdin (see Fig. 91.2 ). However, notable species differences exist in the pattern of development of hepatic bilirubin conjugation. Marked deficiency in UGT activity is noted in rat, rabbit, guinea pig, sheep, dog, monkey, and human fetuses. At term, UGT activity in the rhesus monkey is only 1%-5% of that in the adult. In the human fetus, UGT activity is extremely low before 30 weeks of gestation at about 0.1% of adult activity and gradually increases to about 1% at term.
Diminished UGT activity is the central rate-limiting step that, in conjunction with additional processes, including increased bilirubin production, enhanced enterohepatic circulation, and diminished uptake into the hepatocyte, manifests as physiologic jaundice in monkeys and humans.
Significant hyperbilirubinemia is unusual in the human fetus, because the placenta transports unconjugated bilirubin from the fetus to the mother. Administration of radioactive unconjugated bilirubin into the fetal circulation of a dog, guinea pig, or monkey shows a rapid disappearance from the fetal side and recovery in the maternal bile. Even in states of severe intrauterine hemolysis from conditions such as Rh or other isoimmunizations, the degree of anemia by far exceeds the level of hyperbilirubinemia, and clinical jaundice is usually mild at birth. Indeed, intrauterine therapeutic interventions in this condition are indicated by fetal anemia rather than fetal hyperbilirubinemia. After delivery and separation of the placenta from the infant, increases in TB may be expected and may be excessive in the face of hemolytic disorders. By contrast, the placenta is barely permeable to conjugated bilirubin. Thus, in the absence of evidence of hemolytic disease, if clinical jaundice is present at birth, a conjugated hyperbilirubinemia caused by intrauterine hepatic pathology should be suspected.
A large amount of bilirubin is found in meconium, indicating appreciable activity of fetal hepatic bilirubin conjugation. A significant level of β-glucuronidase activity is found in meconium, suggesting that conjugated bilirubin in the fetal intestine can be hydrolyzed back to unconjugated bilirubin and then absorbed from the bowel into the portal circulation. This absorbed bilirubin may reenter the hepatocyte for subsequent reconjugation and re-excretion or may be transferred through the placenta into the maternal circulation. The efficiency of this process is protective to the fetus against severe hyperbilirubinemia, even when hemolysis is severe.
Conjugated hyperbilirubinemia in the mother, which may occur in hepatitis or recurrent jaundice of pregnancy, is not reflected in the cord blood. Severe hemolytic disease in the fetus results in small, but significant increases in amniotic fluid bilirubin concentrations. How bilirubin enters the amniotic fluid pool is not known, but suggestions have ranged from direct transfer across the placenta from the maternal circulation, to transudation of pigment across the amniotic membranes or cord vessels, to secretion of bilirubin in the pulmonary fluids flowing from the fetal lung into the fetal pharynx and oral cavity and then into the amniotic fluid. Although to a great extent replaced by noninvasive measurement of anterior cerebral artery flow as an index of fetal anemia, in recent decades, measurement of amniotic fluid bilirubin concentrations by spectrophotometry, combined with percutaneous umbilical blood sampling allowing for serial hematocrit determinations and fetal intravascular transfusions, resulted in markedly improved outcome for the now rare fetus and infant with Rh erythroblastosis (see Chapter 23 ).
In the full-term newborn, physiologic jaundice is characterized by a progressive rise in TB concentration from about 2 mg/dL (34 µmol/L) in cord blood to a mean peak of 5-6 mg/dL (86-103 µmol/L) between 48 and 120 hours of age in Caucasian and African-American infants, with most TB levels peaking at 72-96 hours of age, and 10-14 mg/dL (171-239 µmol/L) between 72 and 120 hours of age in Asian-American infants. This is followed by a rapid decline to about 3 mg/dL (51 µmol/L) by the 5th day of life ( Fig. 91.11 ) in Caucasian and African-American neonates and by the 7th-10th in Asian-American neonates. This early period of physiologic jaundice has been designated as phase 1 physiologic jaundice. During the period from the 5th-10th days of life in Caucasian and African-American infants, TB concentrations decline slowly, reaching the normal adult value of less than 2 mg/dL (34 µmol/L) by the end of that period. This late neonatal period of minimal, slowly declining hyperbilirubinemia has been designated as phase 2 physiologic jaundice. The epidemiology is dependent, in part, on the prevalence of breastfeeding in a population, because lower peak TB values will be found among predominantly formula-fed infants.

Studies in the newborn rhesus monkey, an animal with a pattern of physiologic jaundice of the newborn that is similar to that in humans, show that phase 1 results from the combination of a six-fold postnatal increase in the load of bilirubin presented to the liver combined with a markedly diminished UGT activity. The presence of either of these factors alone would result in retention of unconjugated bilirubin to a lesser extent than when in combination. Hepatic uptake and excretion of bilirubin are also decreased during this period, although their function as rate-limiting steps in the transport of bilirubin from plasma into bile is dwarfed by the combination of increased bilirubin load to the liver and diminished conjugative capacity. The very large increase in bilirubin load appears to result from both increased de novo bilirubin synthesis and enteric reabsorption of unconjugated bilirubin. In the newborn monkey, the markedly increased load persists for 3-6 weeks, primarily because of enhanced intestinal bilirubin absorption. Similar data are not yet available for the human neonate.
In the human, UGT1A1 activity is extremely low in the fetal period. After birth, UGT1A1 activity increases at an exponential rate, reaching the adult level by 6-12 weeks of age ( Fig. 91.12 ). The early deficiency in enzyme activity may result from insufficient enzyme synthesis, inhibition of enzymatic activity by naturally occurring substances, deficient synthesis of the glucuronide donor UDPGA, or a combination of these factors. Phase 2 physiologic jaundice appears to result from an imbalance in which hepatic uptake of bilirubin remains diminished while an increased bilirubin load presented to the liver persists. Developmental deficiency of B-ligandin may contribute to deficient uptake of bilirubin.

Despite the development of physiologic jaundice of some degree in nearly every newborn, only half of all Caucasian and African-American term newborns become visibly jaundiced during the first 3 days of life. A greater proportion of exclusively breastfed infants can be expected to display some degree of jaundice. Cutaneous icterus in the newborn will not become evident until TB concentrations exceed 5-6 mg/dL (86-103 µmol/L). This situation contrasts with that of the older child and adult, in whom jaundice may be noticeable in the conjunctiva and skin at TB concentrations as low as 2 mg/dL (34 µmol/L). Variations in duration of hyperbilirubinemia, in skin color, and in perfusion may account for these differences. As the intensity of jaundice increases, clinical icterus progresses in a caudal direction. At lower levels of TB, only the head and conjunctiva may be affected, with the chest, abdomen, legs, and feet becoming jaundiced in parallel to increasing TB concentrations. Because routine daily TB determinations are not usually performed on full-term or even premature newborns, in the past, careful scrutiny of the nursery population several times a day by experienced personnel was essential to detect infants who were becoming jaundiced, as some of these may subsequently develop significant hyperbilirubinemia and require further TB testing. Visual assessment of jaundice, however, is largely subjective, inaccurate, and dependent on observer experience. More recent developments of TcB monitoring devices intended to measure the skin color objectively and noninvasively and convert this color reading to a bilirubin estimation may improve on the reliability of visual estimation. Daily noninvasive TcB determinations may enhance the predictive value of the technique by allowing the actual trajectory to be plotted against those of the hour-specific TB or TcB nomograms (see Transcutaneous Bilirubinometry ). This is especially important in predischarge assessment of newborns, especially those discharged before 72 hours of age.
Physiologic jaundice in premature neonates is more severe than in term neonates. In larger preterm infants, mean peak TB concentrations may reach 10-12 mg/dL (171-205 µmol/L) by the 5th day of life. This delay in reaching the maximal concentration compared with term neonates primarily reflects the delay in maturation of hepatic UGT1A1 activity. Because mean peak unconjugated bilirubin concentrations as low as 10-12 mg/dL (171-205 µmol/L) may be associated with ABE or kernicterus in certain high-risk, low birth weight neonates, all degrees of visible jaundice in premature neonates should be monitored closely and investigated fully. Small premature infants cared for in an intensive care nursery will rarely be allowed to reach the TB levels mentioned but will be treated with phototherapy at much lower levels. The natural peak TB level in small premature infants is, therefore, mainly unknown.
Despite lower UGT1A1 activity in premature neonates than in term neonates at birth, UGT1A1 activity increases rapidly, far exceeding the expected maturational rate noted in utero ( Fig. 91.13 ). This observation indicates that there are two components in the maturational process of hepatic UGT1A1: (1) chronologic maturation and (2) accelerated maturation related to birth. Nevertheless, normal TB concentrations in premature neonates may not be reached in many cases until the end of the first month of life.

Late preterm gestation (34 0/7 to 36 6/7 weeks) is an important risk factor for the development of severe neonatal hyperbilirubinemia and kernicterus. These infants are physiologically immature and have limited compensatory responses compared with term infants. They are at greater risk of morbidity and mortality than term counterparts. Among the infants registered in the voluntary US-based Kernicterus Registry, late preterm infants were disproportionately represented compared with term. At this point of gestation, hepatic conjugative capacity is still immature and may contribute to the greater prevalence, severity, and duration of neonatal jaundice in these infants. Additional risk factors increasing the incidence of severe hyperbilirubinemia in these infants include feeding with human breast milk, large-for-gestational-age status, male sex, G6PD deficiency, and others. Suck-swallow immaturity may also contribute to the risk of hyperbilirubinemia. These infants are at increased risk for readmission, primarily for hyperbilirubinemia. In a US survey, late prematurity increased the risk for neonatal hyperbilirubinemia more than fivefold. Scrupulous attention to screening for jaundice in the newborn nursery, adequate lactation support, parental education, and appropriate postdischarge follow-up should facilitate institution of treatment when clinically indicated.
Although within the definition of term, newborns born at the early end of term (37-38 weeks’ gestation) may be, as a result of relative immaturity, at higher risk for neonatal hyperbilirubinemia than full-term counterparts, and unexplained jaundice may be twofold greater. In a Canadian study, 37- and 38-week neonates had a higher risk of readmission for hyperbilirubinemia than those born ≥39 weeks. Similarly, in Utah, late-term and early-term neonates had higher rates of readmission for hyperbilirubinemia than those born at term.
Neonatal hyperbilirubinemia is the most common cause for readmission of otherwise healthy term infants. Many readmissions can be avoided by discharging the mother–infant dyad when both are ready to be discharged, when the mother has recovered sufficiently and is able to care for her newborn. Jaundice, if present, must be evaluated, and appropriate treatment or follow-up arranged according to the 2004 American Academy of Pediatrics (AAP) Practice Guideline.
Nearly all post-term neonates and about half of all term neonates who are small-for-gestational age may be expected to have little or no physiologic jaundice, with peak TB concentrations of less than 2.5 mg/dL (43 µmol/L). The mechanism for this acceleration of hepatic maturation is unknown. Similarly, neonates of mothers treated with phenobarbital, a drug known to stimulate hepatic UGT activity and the concentration of ligandin, and neonates of heroin users have less than the anticipated severity of physiologic jaundice. Other drugs, less well investigated, also may have similar “maturing” effects.
The severity of physiologic jaundice varies significantly among different ethnic populations. Mean maximal TB concentrations in Chinese, Japanese, Korean, Native-American, and other Asian term newborns are 10-14 mg/dL (171-239 µmol/L), about double those of the Caucasian and African-American populations. The incidence of bilirubin toxicity as defined by autopsy-proven kernicterus is also increased significantly in Asian newborns. There is no clinical evidence for increased hemolysis in Asian newborns to account for these dramatic differences, although some studies of CO production have suggested that bilirubin synthesis may be slightly increased compared with that in Caucasian or African-American neonates. A mutation (Gly71Arg) known as UGT1A1*6 in the gene for UGT frequently found in Japanese, Koreans, and Chinese, but rare in Caucasians, is associated with Gilbert syndrome in Asian populations and has been shown to be associated with an increased incidence of neonatal hyperbilirubinemia in these groups. In contrast, variation in the number of TA repeats in the promoter for the UGT1A1 gene are commonly encountered in Caucasians and are associated with Gilbert syndrome in that population group (see previous section Genetics of Diminished Bilirubin Conjugation ). The promoter polymorphism is rare in Asian communities. Thus, there is mounting evidence that the phenotypic variability in neonatal TB levels seen in different populations results in part from genotypic heterogeneity.
In contrast to Asians, the African-American race is often considered protective against hyperbilirubinemia, with a lower risk of developing TB levels greater than 20 mg/dL (342 µmol/L) than Caucasian infants. However, African-American infants are over-represented, relative to population statistics, in both the US-based Kernicterus Registry and a British and Irish series of severe hyperbilirubinemia. They also appear at greater risk of developing TB levels greater than 30 mg/dL (513 µmol/L) with resulting increased risk of kernicterus than Caucasian counterparts. This phenomenon may be explained in part by the high incidence of G6PD deficiency within this ethnic group.
Certain geographically distinct populations may demonstrate a markedly increased incidence of neonatal unconjugated hyperbilirubinemia without associated hemolysis. The most dramatic of these are from certain Greek islands, especially the islands of Lesbos and Rhodes. Although the incidence of G6PD deficiency in these populations is markedly increased compared with the remainder of the Greek population and the world, the incidence of hyperbilirubinemia was not directly correlated with the frequency of G6PD deficiency, suggesting interaction of additional icterogenic factors. Unless aggressively treated with phenobarbital prophylaxis, phototherapy, and/or exchange transfusion, the incidence of kernicterus was also much greater in the newborns from these Greek islands than in those of the mainland population.
It has been speculated that the increased incidence of neonatal unconjugated hyperbilirubinemia in Asian and geographically identifiable populations may result either from environmental influences, such as the maternal ingestion of certain ethnically characteristic herbal medications or foods, or from a genetic predisposition to slower maturation of bilirubin metabolism and transport. Asian-origin infants born in the United States and Greek newborns born in Australia appear to be at similar risk for neonatal jaundice as natives of Asia and Greece, respectively, suggesting that geographic factors alone are not determinants. Differentiating the influence of drugs, foods, or traditional practices from that of genetic factors requires further investigation. Severe hyperbilirubinemia can result from hemolysis associated with sepsis or, if genetically vulnerable (e.g., in G6PD deficiency), exposure to chemicals (such as naphtha in mothballs) or pharmaceutical agents (such as antimalarials, sulfonamides, sulfones, antipyretics, and analgesics). In some societies with a high incidence of G6PD deficiency, application of henna to the skin or use of menthol-containing umbilical potions may precipitate severe hyperbilirubinemia and potentiate bilirubin encephalopathy. Even though some of these agents and stressors have received public attention, others represent generally unsuspected dangers, such as the intramuscular (IM) injection of vitamin K 3 (menadione) or the inhalation of paradichlorobenzene, which is used in moth repellents, air fresheners, and bathroom deodorizers. In addition, newborn exposure to a hemolytic agent, especially in the presence of G6PD deficiency, can occur transplacentally or through breast milk as in the case of maternal ingestion of fava beans, or directly by inhalation, ingestion, or injection.
Elevated concentrations of unconjugated bilirubin are of concern because of the danger of bilirubin encephalopathy or neuropathy associated with this fraction of bilirubin. Although there have been some reports of bilirubin encephalopathy associated with elevated levels of conjugated bilirubin, the role of conjugated hyperbilirubinemia in the mechanism of bilirubin encephalopathy is not clear. Most studies of kernicterus have related to the TB concentration, of which the conjugated fraction usually comprises only a small fraction. Elevated levels of conjugated bilirubin frequently indicate disease processes of hepatic origin. The following discussion, therefore, relates primarily to unconjugated, or indirect, hyperbilirubinemia and is followed by a section on conjugated hyperbilirubinemia .
The TB level at any point in time reflects a multiplicity of forces in delicate balance. Processes including bilirubin production, transport, uptake, conjugation, excretion, and reabsorption are not only interdependent, but are also influenced by tremendous physiologic flux present in this complex system in the first few days of the neonatal period. Examples of such changes include differences in the rate of heme catabolism and progressive maturation of the bilirubin conjugation system. Physiologically, the net result is an increase in TB levels up to about the fifth day of life, after which point TB values level off and then gradually decrease. Superimposed on these physiologic alterations of bilirubin metabolism may be specific disorders that may further exaggerate or prolong the normal pattern of an elevated TB level. These conditions may affect the entire spectrum of bilirubin metabolism and include disorders of bilirubin production as well as bilirubin conjugation and elimination.
Although increased bilirubin production could result from pathologic states in which degradation of nonhemoglobin heme (i.e., hemoproteins such as cytochromes, catalase) and erythrocyte hemoglobin precursor heme are increased, such disorders in fact have not been identified in the newborn period. The most common pathologic hemolytic causes of unconjugated hyperbilirubinemia in the newborn include isoimmune hemolytic disease, caused by blood group incompatibility between mother and fetus, and G6PD deficiency. Disorders associated with increased erythrocyte destruction are listed in Box 91.1 (see Chapter 23 ).
Rh incompatibility
ABO incompatibility
Other blood group incompatibilities
Glucose-6-phosphate dehydrogenase deficiency
Pyruvate kinase deficiency
Hexokinase deficiency
Congenital erythropoietic porphyria
Other biochemical defects
Hereditary spherocytosis
Hereditary elliptocytosis
Infantile pyknocytosis
Other
Bacterial
Viral
Protozoal
Subdural hematoma and cephalohematoma
Ecchymoses
Hemangiomas
Neonates who are acutely hemolyzing appear to be at a higher risk for developing bilirubin-induced brain damage compared with those without hemolysis. Indeed, the first association to be recognized between increasing TB levels and the risk for kernicterus was made in newborns with Rh isoimmunization. Some reports suggested that kernicterus in hyperbilirubinemic newborns with hemolytic disease may occur more frequently than that in their counterparts without evidence of hemolytic disease. Surveying the literature up to 1983, Watchko and Oski reinforced the concept that hyperbilirubinemia among neonates without hemolytic disease was less dangerous with regard to the development of kernicterus than in cases in which hemolysis was present. However, there are few data to substantiate this view. A study shedding some light on this question was performed by Ozmert and colleagues. In 102 children aged 8-13 years, indirect hyperbilirubinemia ranging from 17-48 mg/dL (291-821 µmol/L) associated with a positive direct Coombs' test (also known as the direct antiglobulin test [DAT]), presumed to reflect ongoing hemolysis, was associated with lower intelligence quotient (IQ) scores and a higher incidence of neurologic abnormalities. In these same children, the incidence of detected neurologic abnormalities increased as the time of exposure to high TB levels became more prolonged. Similarly, in Norway, Nilsen and coworkers found that of males born in the early 1960s who developed neonatal hyperbilirubinemia, those with a positive Coombs' test and hyperbilirubinemia for greater than 5 days had significantly lower IQ scores than average for that country.
In a reanalysis of the data from the Collaborative Perinatal Project, no relationship was found between maximum TB levels and IQ scores. However, in those children who had had a positive DAT, a TB of greater than 25 mg/dL (428 µmol/L) was associated with a decrease in IQ scores. A study of severe neonatal hyperbilirubinemia from Egypt reported the threshold TB level in identifying infants with bilirubin encephalopathy was lowered in those with identifiable risk factors associated with hemolysis, including Rh isoimmunization, ABO blood group incompatibility, and sepsis.
Although there is to date no hard evidence demonstrating higher levels of unbound bilirubin in hemolyzing neonates, many believe hemolysis to be a potential factor that increases the risk for bilirubin-related brain damage. Although a TB concentration of 20-24 mg/dL (342-410 µmol/L) may be associated with kernicterus in a neonate with Rh isoimmunization, a healthy, term infant without an obvious hemolytic condition will rarely be endangered by TB in this range. Conditions associated with hemolysis, including direct Coombs'-positive Rh and ABO immunization or other isoimmunizations and G6PD deficiency, may pose an increased threat to an otherwise healthy newborn. The Subcommittee on Hyperbilirubinemia of the AAP includes jaundice developing within the first 24 hours, blood group incompatibility with a positive DAT, and other known conditions including G6PD deficiency, all associated with increased hemolysis, as major risk factors for the development of severe hyperbilirubinemia. The AAP recommends initiating phototherapy or performing exchange transfusions at lower levels of TB in neonates with hemolytic conditions than in apparently nonhemolyzing counterparts. However, it is not proposed that a hyperbilirubinemic newborn without an obvious hemolytic condition will be unaffected by bilirubin encephalopathy. Patients with kernicterus have been reported in whom no evidence of hemolysis was evident. Crigler-Najjar syndrome, a condition not associated with increased hemolysis, is frequently complicated by bilirubin encephalopathy.
Absence of a defined diagnosis associated with hemolysis should not lead to a state of complacency or belief that hemolysis is not, in fact, taking place. Blood counts are notoriously unreliable indicators of hemolysis in newborns. Studies utilizing the endogenous production of CO, an accurate index of heme catabolism, have demonstrated increased hemolysis in many jaundiced newborns, even in the absence of evidence of a specific hemolytic disorder. The term nonhemolytic jaundice should be used cautiously so as not to unwittingly potentiate bilirubin neurotoxicity in a possibly hemolyzing infant in whom it could have been prevented.
Hemolytic conditions in the newborn are generally divided into two major etiologic groups: immune and nonimmune.
The hallmark of isoimmunization is a positive DAT (also known as the Coombs' test). This is indicative of a maternally produced antibody that has traversed the placenta and is now found within the fetus. The test is termed direct if the antiglobulin is adhered to the RBCs. An indirect test refers to the antibody being detected in the serum.
In past decades, Rh hemolytic disease was the most common cause of severe hemolytic hyperbilirubinemia and a frequent cause of kernicterus. However, maternal prophylaxis with high-titer anti-D immunoglobulin G (RhoGAM), combined with aggressive fetal surveillance and intrauterine blood transfusions, has greatly reduced the incidence and severity of this disease. Mothers sensitized before the development of immune serum prophylaxis are no longer commonly encountered in industrialized countries. Additional technologies used antenatally, which have led to improvement in outcome, include antenatal blood group genotyping by polymerase chain reaction (PCR) from fetal cells obtained by amniocentesis or even from maternal blood samples. Assessment of the degree of fetal anemia and determination of the need for intrauterine transfusion noninvasively by determining middle cerebral artery peak systolic velocity by the Doppler technique has reduced the need for invasive procedures.
Those without access to preventive treatment, immigrants from countries in which prophylaxis is not widely available, or those who did not receive prophylaxis following abortion or invasive procedures may continue to deliver affected infants. The problem continues to be rife in developing countries. Although the incidence of Rh negativity is lower in countries such as India, Nigeria, Pakistan, Kenya, and Thailand than in North America or in Europe because of their large populations, large numbers of women from these countries may be at risk. Zipursky and Paul estimate that in these low income countries more than 1 million women annually do not receive anti-D prophylaxis and that more than 100,000 children are born with Rh disease.
The Rh blood group proteins are a highly antigenic group of proteins capable of causing severe isoimmunization with a high risk for fetal hydrops and death. Although several systems of nomenclature exist, the CDE system is most commonly used. These three loci each contain two major alleles (C,c; D,d; E,e) and several minor alleles. The D antigen may produce maternal sensitization with a fetomaternal hemorrhage as small as 0.1 mL. Whereas C and E alleles are relatively uncommon causes of isoimmunization, they can, on occasion, lead to severe hemolysis and hyperbilirubinemia. Indeed, Rh C disease may be as severe as Rh D isoimmunization. Furthermore, women with multiple RBC antibodies may develop significant hemolytic disease of the fetus and newborn to a greater extent than those with a single antibody, especially in the presence of anti-(Rh)D. The pathophysiology of this phenomenon may represent a more aggressive immune response in those with more than one RBC antibody. Rh disease in pregnancy is highly associated with both intrauterine hemolysis and severe hemolytic disease following delivery. Untreated, the condition can lead to intrauterine anemia and severe hydrops fetalis , with rapid postnatal evolvement of hyperbilirubinemia with the potential of kernicterus.
The immunization process may begin if an Rh-negative woman, usually D negative, is exposed to a D antigen. This usually occurs by ante- or intrapartum transplacental fetomaternal transfusion of fetal RBCs containing a D antigen, or by transfusion of Rh-positive RBCs during abortion, blood administration, or procedures including amniocentesis, chorionic villus sampling, or fetal blood sampling. Following exposure to the D antigen on the fetal RBCs, the mother's immune system responds by forming anti-D immunoglobulin G (IgG) antibodies. The IgG then crosses the placenta and adheres to fetal RBCs containing the D antigen. The subsequent antigen–antibody interaction leads to hemolysis and anemia. The immune response may become more severe and more rapid with progressive pregnancies. Resultant anemia causes bone marrow stimulation, with increased numbers of immature RBCs appearing in the circulation ( erythroblastosis ) and extramedullary hematopoiesis. Fetal hydrops , a condition characterized by generalized tissue edema and pleural, pericardial, and peritoneal effusions, may result from a combination of hypoproteinemia, tissue hypoxia, and capillary leak. Anemia with resultant poor myocardial function may further exacerbate the hydrops by causing congestive cardiac failure and venous congestion.
Elevated COHb levels detected in blood obtained by cordocentesis in affected fetuses of nonsmoking isoimmunized mothers confirm that destruction of erythrocytes begins in utero. However, the primary manifestation of the in utero hemolysis is that of anemia. Although large amounts of bilirubin are produced concomitantly, erythroblastotic infants are not severely icteric at birth. Concentrations of TB are usually kept below 5 mg/dL (86 µmol/L) by transfer of unconjugated bilirubin across the placenta. Jaundice may appear, however, shortly after delivery. Classically, in the initial stages, the TB is all indirect-reacting, although small amounts of conjugated bilirubin have been noted. After some days of excessive bilirubin load, the excretory system may become overwhelmed with efflux of conjugated bilirubin into the serum, and an increasing conjugated bilirubin fraction is not uncommonly seen. Hepatic conjugation may mature more rapidly than excretory function as a result of stimulation by chronic exposure to high concentrations of bilirubin in utero. Furthermore, hepatic excretory function may also be adversely affected by development of hepatic congestion secondary to heart failure and swelling caused by extramedullary hepatic hematopoiesis, anemia, and poor hepatic perfusion.
With the reduction of the incidence of Rh isoimmunization by immune prophylaxis, DAT-positive ABO incompatibility in industrialized countries with functional medical systems is now the single most prominent cause of immune hemolytic disease in the neonate. The clinical picture is usually milder than that of Rh disease, although infrequently severe hemolysis with hyperbilirubinemia may occur. In recent series of infants with either bilirubin encephalopathy/kernicterus or extreme hyperbilirubinemia reported from diverse countries including the United States, Canada, the United Kingdom, Ireland, Denmark, Switzerland, China, and Nigeria, in whom the etiology of the hyperbilirubinemia was determined, infants with blood group A or B born to group O mothers comprised 19% to 55%.
ABO blood group heterospecificity is the situation in which a blood group A or B infant is born to a group O mother, a setup occurring in about 12% of pregnancies. In some instances, women with blood group O have a high titer of naturally occurring anti-A or anti-B antibodies. High titers of anti-A or anti-B antibodies can sometimes be found in blood group O women even before their first pregnancy. This contrasts to Rh isoimmunization, in which immune sensitization occurs progressively with subsequent pregnancies. In contradistinction to blood group A or B individuals, in whom their respective anti-B or anti-A antibodies are IgM molecules with limited ability to cross the placenta, the respective antibodies of blood group O individuals are predominantly smaller IgG molecules and may cross the placenta. Attachment to corresponding fetal RBCs may follow, provided these cells have the A or B antigen. Extravascular hemolysis of the IgG-coated RBCs is thought to be mediated within the reticuloendothelial system by Fc-receptor-bearing cells. As with Rh isoimmunization, the immune process may commence in utero. However, unlike the Rh situation, there is little danger of severe hyperbilirubinemia, anemia, or hydrops in utero, and prenatal intervention is not indicated. Infants may sometimes be born with moderate anemia. After delivery, there is a potential danger of hyperbilirubinemia.
About one-third of blood group A or B neonates born to a blood group O mother will have a positive direct Coombs' test. Measurements of endogenous formation of CO, reflective of heme catabolism, have demonstrated, overall, an increased rate of heme catabolism in affected infants compared with controls. In one study, those infants who developed hyperbilirubinemia (TB >95th percentile on the Bhutani nomogram) had even higher COHb values than the already high values of those who were nonhyperbilirubinemic. Strength of DAT may also be predictive: ABO-heterospecific neonates with ++ DAT had a higher incidence of hyperbilirubinemia and higher COHbc values than those with ± or + DAT. Not all DAT-positive neonates, however, develop severe hyperbilirubinemia. In one study, only 20% of DAT-positive neonates actually developed TB levels greater than 12.8 mg/dL (219 µmol/L); whereas, in another study, only 19.6% required phototherapy. Despite this apparent clinical mildness, newborns with severe hyperbilirubinemia of early onset who do not respond to phototherapy and require intravenous immune globulin (IVIG) therapy or exchange transfusion are occasionally encountered. In contrast to the above-mentioned studies, a study from Israel found that 52% of 164 DAT-positive, ABO-incompatible newborns developed a TB >95th percentile, many of these within the first 24 hours. At the extreme end of the spectrum, as already mentioned, kernicterus has been described. ABO blood group incompatibility with a negative DAT, not usually predictive of hemolysis or hyperbilirubinemia, may sometimes cause early and rapidly progressing jaundice, reminiscent of DAT-positive hemolytic disease.
Paucity of A and B antigenic sites on neonatal RBCs or weak expression of these antigens in neonates compared with adults may explain, in part, absence of clinical disease in many DAT-positive newborns. A or B antigenic sites situated in sites other than the RBC may bind with transplacentally acquired antibodies, limiting their availability to the RBC.
Because many ABO-incompatible, direct Coombs'-positive neonates have no evidence of ongoing hemolysis and do not develop early jaundice or hyperbilirubinemia, ABO heterospecificity with a positive DAT does not necessarily indicate ABO hemolytic disease. Some or all of the following criteria are necessary to support the diagnosis of ABO hemolytic disease:
Indirect hyperbilirubinemia, especially during the first 24 hours of life
Mother with blood group O; infant with blood group A or B
Spherocytosis on blood smear
Increased reticulocyte count
Evidence of hemolysis based on increased endogenous production of CO as assessed using end-tidal CO measurements, corrected for ambient CO (ETCOc) levels
In DAT-negative, ABO-heterospecific newborns, an interaction with a polymorphism for the (TA) 7 sequence in the promoter of the gene encoding UGT1A1, significantly increases the incidence of TB of at least 15 mg/dL (257 µmol/L) compared with controls and has been described.
It is essential to closely observe any newborn born to a blood group O mother and to perform a TcB or TB measurement at the first appearance of jaundice. Routine blood group and DAT determination on umbilical cord blood is an option, which may allow for additional risk determination.
More than 50 RBC antigens may cause hemolytic disease of the newborn. The most important of these with regard to prenatal hemolysis include anti-C, anti-Kell, and anti-E, although others may also infrequently be problematic. Alloimmunization caused by these autobodies can sometimes cause severe hemolytic disease of the fetus requiring prenatal intervention. Fetal surveillance protocols and clinical strategies developed for RhD alloimmunization are useful in monitoring all alloimmunized pregnancies. Similarly, the postnatal management should be based on the principles outlined in the management of the RhD-immunized newborn (see Therapy for Unconjugated Hyperbilirubinemia ). Anti-Kell isoimmunization warrants special mention because fetal anemia, rather than hyperbilirubinemia, often predominates the clinical picture. This may be due to erythropoietic suppression in addition to a hemolytic process.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here