Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Imaging provides anatomic and functional information critical to the care of the high-risk neonate. An expanding selection of imaging tools offers safe and timely diagnostic procedures for infants both before and after birth. Advances such as fetal magnetic resonance imaging (MRI) and point-of-care ultrasound provide new opportunities to impact patient care earlier in the disease process. This chapter reviews imaging modalities key to the evaluation of the critically ill neonate, including both commonly performed radiographic and sonographic exams as well as more complex imaging techniques. Following an introduction to imaging by modality, the chapter will review the imaging approach to common medical and surgical conditions of the high-risk neonate.
Radiography of the chest and abdomen comprises the vast majority of imaging performed in the immediate postnatal period. Digital radiography, a widely available and relatively inexpensive diagnostic examination, can be performed at the bedside with minimal disruption to a fragile patient. Because a large number of portable radiographs may be performed in critically ill neonates, attention has been drawn to the potential cancer risk from cumulative doses of ionizing radiation from these exams. The radiation dose of a portable chest radiograph is approximately 0.02 mSv; this is a very small fraction of the 3 mSv of annual natural background radiation to which the average individual is exposed. Commonly performed fluoroscopy procedures in the newborn, such as an upper gastrointestinal series (UGI), contrast enema, or voiding cystourethrogram, can be performed at doses between 0.5 and 2.0 mSv. Computed tomography (CT) is also performed at low-radiation doses; most head and body CT scans can be performed at a dose less than 2 mSv. Current literature indicates that the risk of a future cancer from low-dose radiation (below 100 mSv for serial exams) is “too low to be detectable and likely nonexistent.” In the absence of data confirming a causal relationship between low-dose radiation from medical imaging and cancer, parents and providers should be reassured that the benefits of a medically necessary examination far exceed any potential future cancer risk. Current recommendations endorse limiting radiation exposure to doses that are “as low as reasonably achievable” by using patient size-based tube current and tube voltage, attention to patient positioning, artifact removal, and beam collimation.
Short scan times allow nearly all newborns to undergo CT scans of the brain and body without the need for sedation. Iodine-based intravenous contrast may be administered to evaluate for cardiovascular abnormalities, neoplasm, or infectious disease. In the absence of known renal failure, neonates show no increased risk for renal toxicity after receiving iodinated intravenous contrast material. A distinct disadvantage of CT imaging is the lack of portability. Because CT equipment requires room shielding and a relatively large footprint and is highly sensitive to small fluctuations in temperature and humidity, patients must travel to the radiology CT suite for imaging.
Following radiography, sonography is the most common imaging examination performed in the neonate. Ultrasound is particularly suitable for newborns because of its low cost, accessibility, portability, lack of ionizing radiation, and absence of known health risks. There is no need for patient sedation, and operators can easily capture both static and cine images that include characterization of flow dynamics with color and spectral Doppler. High-quality sonographic images are far more dependent on operator skill than any other imaging modality. Knowledge of both normal and abnormal anatomy, identification of optimal windows, and appropriate selection of ultrasound probes of varying frequencies are critical to obtaining diagnostic images. Small handheld ultrasound devices now offer rapid, real-time options for acute point-of-care diagnostic images performed by neonatologists. The role of lung ultrasound has increased in some centers where trained neonatologists use bedside sonography to diagnose respiratory distress syndrome, transient tachypnea of the newborn, neonatal pneumonia, pneumothorax, and atelectasis. Because sound waves travel poorly through air and calcified structures, sonographic evaluation is limited if obscured by gas-filled bowel, aerated lung, or overlying bone. Another disadvantage of ultrasound is the relatively low spatial resolution relative to CT and MRI.
MRI can provide vital detailed information that ultrasound and CT cannot—particularly in the diagnosis of traumatic or anoxic brain injury and congenital anomalies. Gadolinium-based intravenous contrast may be used without risk of renal toxicity in the absence of known renal failure. Despite scan times that may exceed 30 minutes, the majority of infants under 3 months of age can be scanned without sedation using the “feed and swaddle” technique. Because MR scanners require housing in a carefully shielded and monitored physical space, MR scanning in the most fragile infants is infrequent. New dedicated infant-sized MR scanners designed to be housed in the neonatal intensive care unit (NICU) may offer a solution to the risks of infection and injury while minimizing the disruption of care for critically ill neonates and their families.
Over the past 20 years, there have been numerous developments in fetal and perinatal management. Although ultrasound remains the screening modality of choice in the prenatal period, fetal MRI has become a valuable adjunct to evaluate the fetus requiring additional diagnostic imaging. Advantages of MRI such as high soft tissue contrast, large field of view, and high resolution can help exclude or confirm diagnoses suggested by ultrasound. Quantitative MRI analysis provides measurements of structural volumes in the fetus. In congenital diaphragmatic hernia (CDH), congenital pulmonary airway malformation (CPAM), and other processes associated with pulmonary hypoplasia, calculating the total lung volume is helpful to determine prognosis, assess for in utero fetoscopic endoluminal tracheal occlusion treatment, provide parental counseling, and plan a safe delivery. In cases of cervical masses or severe micrognathia, MRI can visualize the airway for patency and contribute to planning a safe delivery. Some centers perform ex utero intrapartum treatment (EXIT) procedures when there is high risk of airway obstruction or a repairable malformation that puts the infant at high risk of respiratory insufficiency at birth.
Fetal MRI has become integral to both identifying at-risk infants who may benefit from in utero intervention as well as preparing for optimal perinatal management.
Nuclear medicine contributes to the care of newborns with congenital anomalies, infection, and neoplasm. Radiopharmaceuticals, otherwise known as “tracers,” are nonallergenic and have shown no toxic effects. The radiation dose for most exams is between 0.6 to 5.5 mSv, in the range of CT imaging. Though most studies require that the patient remain still for a relatively long period of time, most neonates can be imaged without sedation. As with CT and ultrasound, these exams require patient transport to the nuclear medicine suite of the radiology department.
The chest radiograph is the most commonly performed imaging examination in the critically ill neonate. Portable chest radiographs are obtained using an anteroposterior (AP) projection in the supine infant. The arms should be extended away from the body or above the head and the torso immobilized. With proper positioning, the radiograph should demonstrate symmetry of the clavicles and ribs and a midline superior mediastinum ( Fig. 18.1 ). A rotation-distorted image may obscure or mimic cardiac and pulmonary pathology.
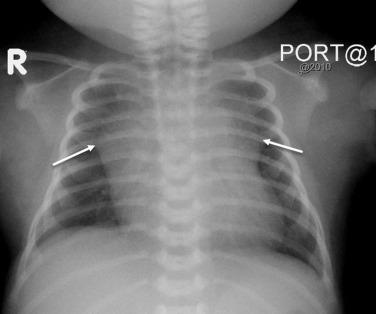
Familiarity with the normal appearance of the newborn chest radiograph improves recognition of pathologic changes. Normal lungs appear primarily radiolucent and symmetric in volume. The pulmonary vessels are seen as branching, linear shadows that taper in size as they extend from the hilum to the lung periphery. Normally collapsed, the pleural space is visualized only when it is distended from fluid, air, or pleural thickening. The heart borders should be distinct, and the diaphragm should be outlined clearly against aerated lung. The normal thymus is visible on the chest radiograph of most newborns. Extremely variable in size and shape, it is composed of two asymmetric lobes and may therefore have an asymmetric appearance in chest radiography. The “wavy” undulations of the lateral borders often silhouette a portion of the heart and can give the appearance of cardiomegaly.
Positioning of life support devices should be documented on all radiographs. The endotracheal tube (ETT) tip should overlie in the trachea between the medial ends of the clavicles and the carina. Intubation of the right mainstem bronchus is the most common site of tube malposition and may result in volume loss in the right upper lobe and left lung ( Fig. 18.2 ). The enteric tube should terminate in the stomach body, beyond the gastroesophageal junction.
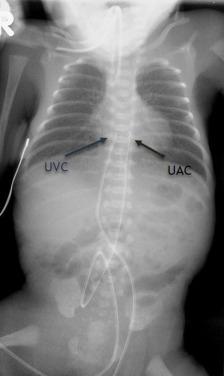
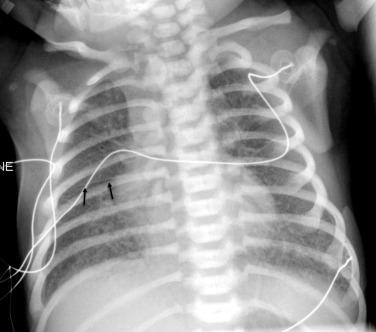
Umbilical catheters are easily identified by their characteristic paths ( Fig. 18.3 ). The umbilical venous catheter (UVC) courses superiorly and gradually posteriorly within the umbilical vein to intersect the left portal vein, continuing through the ductus venosus, left or middle hepatic vein, and then into the inferior vena cava (IVC). Its tip should be directed superiorly, ending at the right atrial (RA)/IVC junction. Malposition of the UVC within the liver is the most common complication and may result in portal vein thrombosis or portal hypertension. The umbilical arterial catheter (UAC) courses inferiorly to the junction of the umbilical artery and internal iliac artery before turning superiorly into the common iliac artery and aorta. The tip of the UAC should terminate below the ductus arteriosus and above or below the visceral arteries; in a “high position,” the tip ends between T6 and T10, and in a “low position,” the tip ends between L3 and L5.
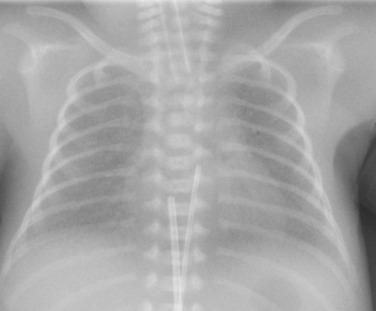
The position of percutaneously placed catheters, including peripheral inserted central venous catheters (PICC lines), central venous catheters, and extracorporeal membrane oxygenation (ECMO) catheters, must also be documented. The tip of an upper-extremity PICC line or central venous catheter should lie within the superior vena cava (SVC) or at the RA/SVC junction. A lower-extremity PICC line should end between the 9th and 12th ribs. An ECMO circuit may use a venous and an arterial catheter or a dual lumen veno-venous catheter. The venous catheter is inserted through the right internal jugular vein and ends in the RA. The arterial catheter is inserted into the common carotid artery and ends near the origin of the innominate artery.
Respiratory distress syndrome (RDS) from surfactant deficiency is the most common cause of respiratory distress and a leading cause of morbidity in the premature infant.
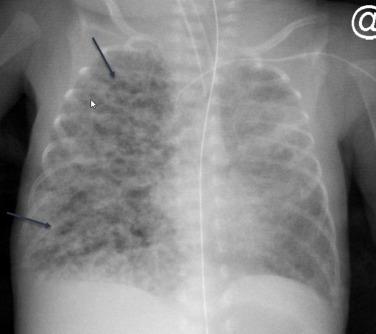
The typical radiographic appearance of RDS reflects generalized alveolar collapse and shows a finely granular or ground-glass pattern with diminished lung volumes. The severity of radiographic disease is variable and usually correlates with the severity of clinical disease. Mild radiographic disease is characterized by a finely granular pattern that allows visualization of normal vessels ( Fig. 18.4 ), whereas severe disease results in silhouetting of the heart borders and diaphragm. Air bronchograms may be seen with severe disease because of air in the bronchi visualized against a background of alveolar collapse. The distribution of disease is usually diffuse and symmetric; however, patchy or asymmetric disease may be seen. The radiographic changes associated with RDS are often seen immediately after birth but can also develop over the first 6 to 12 hours of life. The radiographic abnormalities related to uncomplicated RDS should resolve by the time the neonate is 3 to 4 days of age. New diffuse worsening of bilateral opacities in the lungs may be seen with pulmonary edema or pulmonary hemorrhage. The acute appearance of focal parenchymal opacity suggests atelectasis.
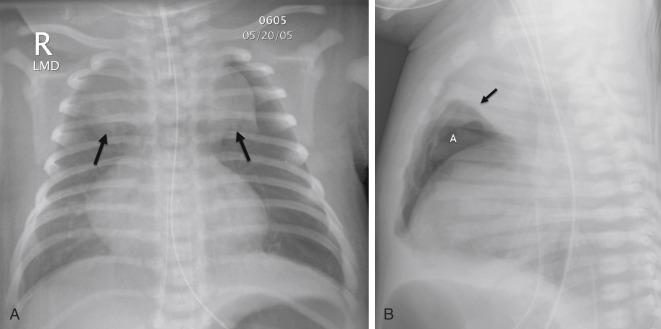
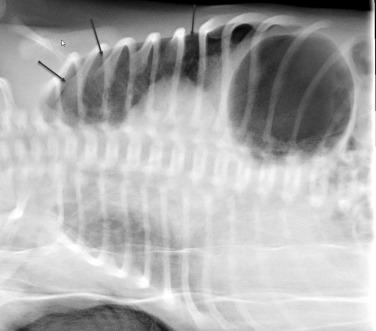
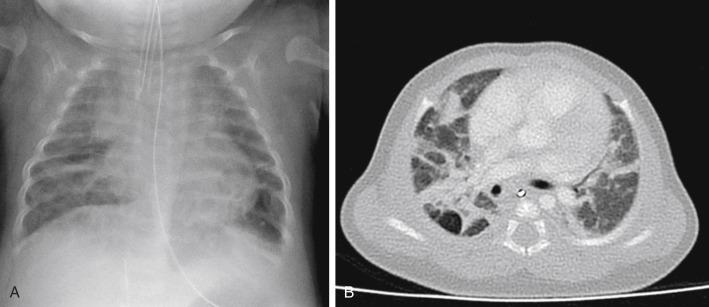
Complications can result from the high distending pressures of mechanical ventilation that may be required in the treatment of RDS. Alveolar rupture from overdistention may result in pulmonary interstitial emphysema (PIE). PIE may be diffuse or localized and appears as linear meandering lucencies from interstitial air coursing along the bronchovascular sheaths ( Fig. 18.5 ). Interstitial air may result in a larger focal air collection and create a discrete parenchymal pseudocyst. Interstitial air can dissect into the mediastinum or the pleural space, resulting in a pneumomediastinum or pneumothorax. Radiographic signs of pneumomediastinum include lateral displacement of the mediastinal pleura, a continuous diaphragm, and superior elevation of the thymus ( Fig. 18.6A,B ). Radiographic findings of pneumothorax include increased lucency, identification of the visceral pleural line, and increased sharpness of the adjacent mediastinal border or hemidiaphragm. Lateral decubitus or cross-table lateral radiographs can be useful in the detection of pneumothoraces ( Fig. 18.7 ). Large pneumothoraces can produce tension, resulting in contralateral shift of mediastinal structures and depression or eversion of the ipsilateral hemidiaphragm ( Fig. 18.8 ).
Neonatal pneumonias may be acquired in utero, during delivery, or after birth. Infection typically disseminates widely throughout the lungs because of incomplete formation of the interlobar fissures at this age. Diffuse granular or ground-glass opacities are often seen and may be indistinguishable from RDS. Alternatively, coarse nodularity or a streaky, hazy appearance of the lungs may be seen. The presence of pleural fluid should raise the suspicion of bacterial infection because effusions are uncommon in RDS or viral pneumonia.
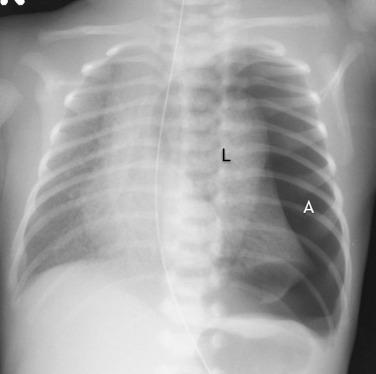
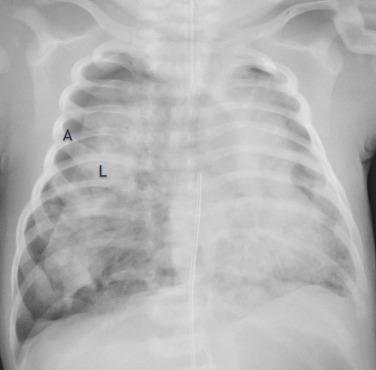
Transient tachypnea of the newborn (TTN) results from retained lung fluid that may be associated with precipitous delivery or cesarean section. Typically a clinical diagnosis, TTN has a varied and nonspecific radiographic appearance. The radiograph may appear normal or show streaky, perihilar opacities and hyperinflation. Small pleural effusions may also be seen ( Fig. 18.9 ). A normal heart size helps distinguish retained fluid from pulmonary edema or heart failure. The radiographic and clinical findings of TTN usually resolve within the first 24 to 48 hours of life.
Fetal aspiration of meconium causes obstruction of small airways with associated atelectasis and air trapping. Radiographic findings of meconium aspiration syndrome present in the first few hours of life and include coarse, patchy, or nodular opacities; segmental hyperinflation; and possible pleural effusion. The distribution of disease is bilateral and often asymmetric. Complications include chemical pneumonitis, surfactant inactivation, pulmonary hypertension, and air-leak phenomena such as pneumothorax and pneumomediastinum ( Fig. 18.10 ).
In intubated patients, the diagnosis of pulmonary hemorrhage is usually established by detecting blood in the endotracheal tube. The radiographic appearance of pulmonary hemorrhage is variable and nonspecific: small amounts of hemorrhage may not be visible, but more extensive hemorrhage results in focal or diffuse ground-glass opacities. Findings may mimic pneumonia or pulmonary edema. The radiographic changes from a single episode of pulmonary hemorrhage are usually transient, resolving within 24 to 48 hours. More recently, bedside ultrasound has been used to diagnose pulmonary hemorrhage.
Classic chest radiograph findings of bronchopulmonary dysplasia include: hyperinflation; asymmetric, coarse, and patchy opacities; and cystic emphysematous changes. CT findings range from near-normal to disordered lung architecture with hyperlucent areas, linear and subpleural opacities, and bullae typical of chronic lung disease ( Fig. 18.11A,B ).
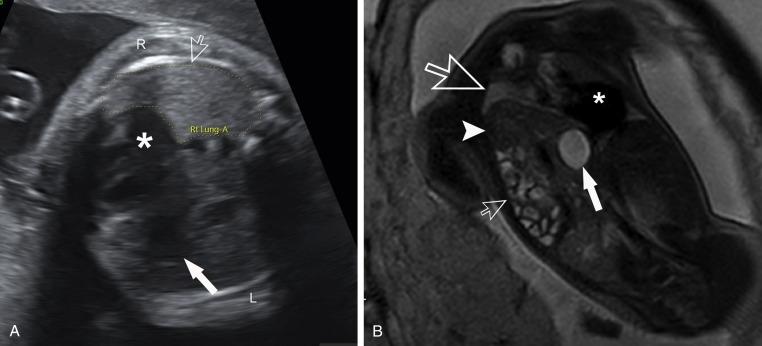
CDH is a complex, life-threatening lesion caused by defective fusion of the pleuroperitoneal folds during embryologic development. Bowel and solid organs may herniate through the foramen of Bochdalek into the hemithorax. Though more common on the left, hernias may occur on the right side or bilaterally. The role of prenatal imaging is instrumental in delivery and perinatal planning. Quantitative measurements of the lungs and thorax can help predict the risk of neonatal pulmonary insufficiency and need for EXIT procedure or immediate perinatal intubation. The size of the main right and left pulmonary arteries are assessed using the McGoon index to help predict the risk of postnatal pulmonary hypertension. MRI provides the most detailed assessment of the herniated organs and provides valuable prognostic information in patients where a lobe or the entire liver is in the thorax ( Fig. 18.12A,B ).
At birth, fluid-filled bowel appears as nonspecific opacity over the ipsilateral hemithorax. Within the first 24 to 48 hours of life, air fills bowel loops in the thorax, and a paucity of bowel loops is apparent in the abdomen. The ipsilateral lung is almost universally hypoplastic, and there is usually contralateral mediastinal shift, resulting in contralateral lung hypoplasia ( Fig. 18.13 ).
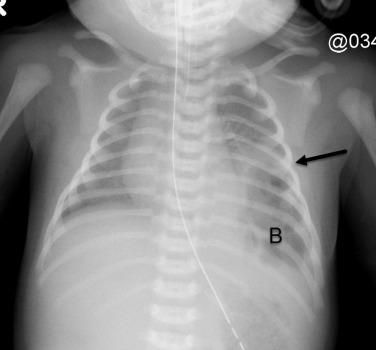
CPAMs are a group of congenital, hamartomatous cystic and noncystic lung masses characterized by overgrowth of the primary bronchioles and a proximal communication with a defective bronchial tree. Bronchopulmonary sequestration is a lesion containing bronchial and pulmonary tissue without communicating with the tracheobronchial tree or pulmonary arteries. They can be extralobar (extrapleural) or intralobar (intrapleural), and either supradiaphragmatic or infradiaphragmatic. These lesions will appear mostly solid in imaging, and the systemic feeding vessel is frequently identified on prenatal ultrasound and MRI ( Fig. 18.14A,B ).
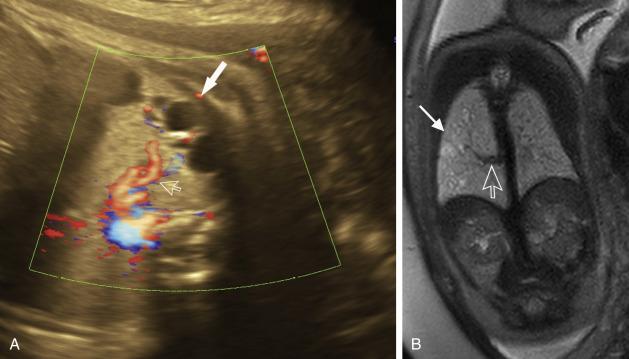
Prenatal sonography and fetal MR classifies CPAMs primarily based on the presence of macrocysts, microcysts, or solid tissue ( Fig. 18.15A,B ) . The CPAM volume ratio is calculated to predict the risk of hydrops in the setting of the pulmonary lesion; prenatal ultrasound and fetal MRI provide similar metrics. MRI can confirm or exclude the presence of a systemic vessel arising from the aorta in the setting of a “hybrid” lesion.
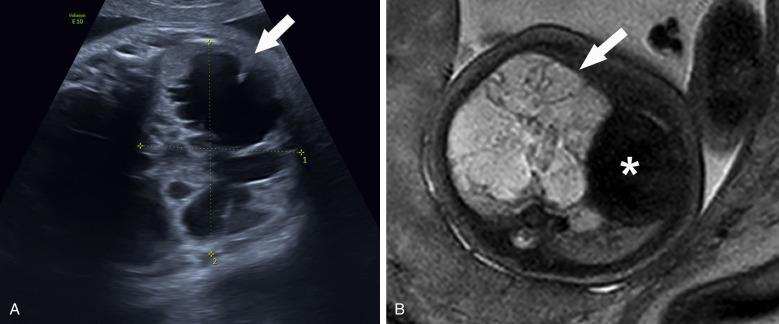
At birth, chest radiography may show a range of findings from a large single or multiple air-filled cystic structures to solid lesions that resemble consolidation. CT angiography (CTA) provides the most detailed anatomic evaluation of the lung parenchymal and vascular anatomy of these lesions in the postnatal period.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here