Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neonatal hypoglycemia requires diagnostic consideration and urgent management to prevent recurrent hypoglycemia and avoid neurologic injury.
Neonatal metabolism in the first days of life reflects a transition from the passive glucose consumption of the fetus to the active regulation of glucose of the neonate.
Diagnosing the cause of hypoglycemia requires an evaluation of the hormonal and metabolic response to hypoglycemia.
Patients with hyperinsulinemic hypoglycemia should be assessed for diazoxide-responsiveness, and non-responsive patients should have an evaluation to determine if the process is due to focal or diffuse disease.
Diabetes diagnosed before 6 months of age is very likely to have a genetic cause.
Patients with neonatal diabetes due to pathogenic variants in the K ATP channel can be treated with oral sulfonylurea in place of insulin therapy.
Glucose is the primary metabolic fuel for the neonatal brain. Maintenance of normal glucose levels in the serum and across the blood-brain barrier is essential for normal neurologic function and development. Hypoglycemia in the neonate, therefore, requires thoughtful diagnostic evaluation and urgent treatment to prevent injury to the central nervous system (CNS). The mechanisms underlying neonatal hypoglycemia are best understood as inadequate hormonal and metabolic responses to hypoglycemia, occurring in the context of the necessary shift from fetal to neonatal glucose metabolism in the first days of life. These pathways and pathologies are the focus of the initial portion of this chapter.
In the latter portion of this chapter, we focus on hyperglycemia in neonates. Hyperglycemia most commonly occurs in the context of a physiologic stressor such as sepsis with cortisol and catecholamine release. Intravenous glucose infusion and exogenous glucocorticoid administration can also cause hyperglycemia. Rarely genetic causes of hyperglycemia result in transient neonatal diabetes mellitus (TNDM) or permanent neonatal diabetes mellitus (PNDM).
Fetal glucose supply is dependent on maternal plasma levels and its diffusion across the placenta. There is no evidence for the existence of fetal gluconeogenesis or a robust ability to adjust rapidly to maternal hypoglycemia. Once the placental link is interrupted, and glucose is no longer delivered continuously via the umbilical vein, the neonate must maintain normoglycemia and adequate cerebral glucose delivery despite minimal and sporadic enteral carbohydrate intake during the first 24 to 72 hours of life. Glucose homeostasis is accomplished in a manner generally similar to older children who are fasted: via secretion of the counter-regulatory hormones—namely cortisol, glucagon, growth hormone, and catecholamines—and their actions at target tissues, in combination with the suppression of insulin secretion. In concert, these hormonal changes regulate four different metabolic systems: glycogenolysis, gluconeogenesis, lipolysis, and ketogenesis ( Fig. 87.1 ). The result is to facilitate normoglycemia until carbohydrate intake and absorption occur on a more regular basis.
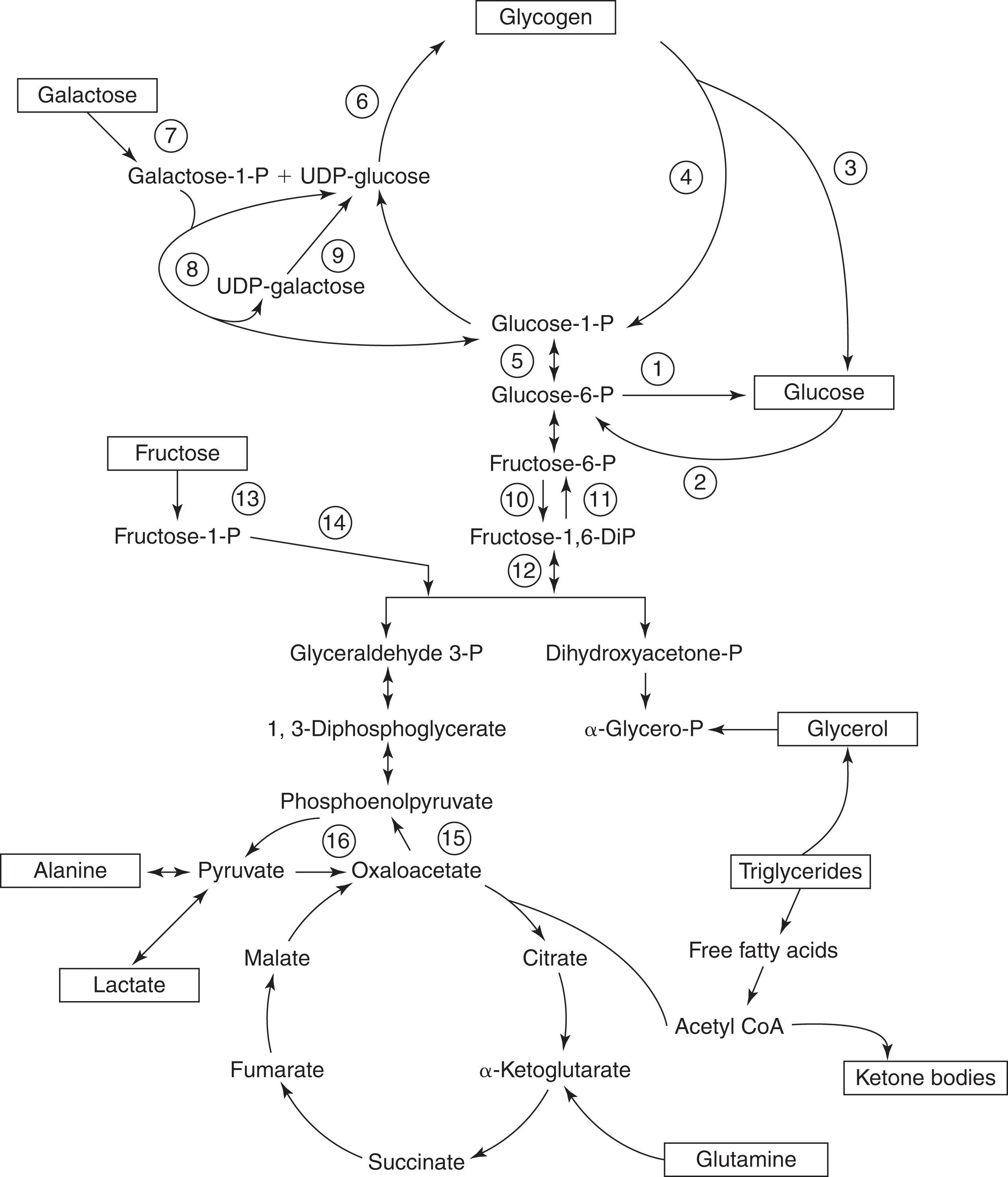
In response to decreased delivery of glucose in the first few hours of life, glucagon and catecholamine levels rapidly increase, and insulin falls. This combination shifts metabolic activity from anabolism to catabolism and induces enzymes necessary for glycogenolysis (glycogen phosphorylase) and gluconeogenesis (pyruvate carboxylase and phosphoenolpyruvate carboxykinase [PEPCK]). Glycogenolysis plays the largest role in meeting glucose needs during the first 24 hours (approximately 50%) and causes a depletion of glycogen stores from 50 mg/g of the liver at birth to <10 mg/g of the liver by 24 hours of life. Gluconeogenesis develops somewhat more slowly and is not fully active until 8 to 12 hours of life, providing only 20% to 30% of glucose needs in the first 24 hours.
Gluconeogenesis and lipolysis contribute to plasma glucose levels after 8 to 12 hours of life, with their role increasing as glycogen stores are depleted. Lipolysis produces glycerol, which can enter the gluconeogenic pathways, and free fatty acids can be oxidized directly by some organs, including the heart, kidney, and skeletal muscle, but long-chain fatty acids cannot cross the blood-brain barrier. Importantly, partial oxidation of fatty acids in the liver via ketogenesis produces ketones such as beta-hydroxybutyrate (BOHB) and acetoacetate, which the brain can metabolize. However, ketogenesis is impaired in the first 8 to 12 hours of life, coincident with the known transitional hypoglycemia of infancy discussed later. The importance of gluconeogenesis, lipolysis, and fatty acid metabolism is highlighted in breastfed infants as the macronutrient profile of colostrum favors protein and fatty acids, compared to the relative carbohydrate predominance of mature human milk.
Glucagon continues to rise gradually over the first few days of life, concurrent with the known, gradual increase and stabilization of glucose levels that normally occurs in infants by 48 to 72 hours of life.
The brain is the most metabolically active organ in the neonate, and its demand for glucose is proportional to brain weight. Glucose uptake and oxidation in the brain occurs via insulin-independent facilitated diffusion through glucose transporter (GLUT) channels and is dependent on arterial blood glucose concentration. An in vivo study using rats (which are believed to have GLUT channels with similar kinetics to humans) showed that the consumption of glucose in the brain outpaces its replacement via diffusion at an arterial concentration of 36 mg/dL. At this point, cerebral blood flow increases markedly to prevent severe CNS glucose depletion and neurologic sequelae. The relatively large size of the neonatal brain and its high metabolic demand are associated with a two- to threefold higher (per weight) hepatic glucose production compared to adults. Conditions that interfere with hepatic glucose production, therefore, place the infant at risk for hypoglycemia, some of which are discussed briefly in this chapter, and others are discussed in Chapter 29 .
In total, the combined counter-regulatory response and insulin suppression are similar to the starvation response that occurs in older children with two exceptions: (1) the additional complication that maternal factors and immaturity of the counter-regulatory response can interfere with glucose homeostasis in the neonatal period; and (2) the decrease in insulin production and release of glycogen stores is less robust than in older children. These latter factors may explain the “transitional hypoglycemia” seen in normal infants during the first 24 hours of life.
Plasma glucose values in the first hours of life are frequently lower than accepted thresholds for normoglycemia in older children, a phenomenon known as “transitional neonatal hypoglycemia.” Serial measurements of glucose in the first days of life in healthy, term appropriate for gestational age (AGA) infants demonstrate average values in the 50s to low 60s (mg/dL). If normal is defined as within two standard deviations from the mean, the lower limit of normal may be as low as the high 30s to low 40s in the first few hours of life. In support of this, Lubchenco and Bard showed that if feeding is delayed 3 to 6 hours from birth, approximately 10% of healthy, term AGA infants will have glucose <30 mg/dL.
A recent review of the data available on transitional hypoglycemia shows that it is characterized by relative hyperinsulinism as indicated by hypoketosis and preserved glycogen release in response to glucagon. An additional factor may be the time required for the enzymatic machinery of gluconeogenesis and glycogenolysis to become active in response to the rise in glucagon and catecholamine secretion after birth.
It is unknown whether the decline in glucose values or the relative hyperinsulinism seen with transitional hypoglycemia serves an adaptive function. The important diagnostic distinction is that this phase of hypoglycemia is transient. Just 2 of the 374 infants (0.5%) in the Lubchenco and Bard cohort had glucose <50 mg/dL prior to feeding on day 3 or 4 of life.
Neonates with hypoglycemia may have no detectable symptoms and may only be identified incidentally upon measurement of blood glucose levels or in the monitoring of a high-risk infant. When symptoms occur, they may be seen in a progression due to initial counterregulatory hormone responses (such as adrenergic hormones as well as cortisol and growth hormone), which result in symptoms due primarily to the autonomic system (autonomic, or “neurogenic” symptoms and signs). When deficient glucose supply to the brain occurs, neurological dysfunction is detectable and may be considered the symptoms of “neuroglycopenia” ( Box 87.1 ). However, these may be subtle and difficult to recognize clinically and may also be accompanied by other nonspecific symptoms such as apnea, cyanosis, temperature instability (especially hypothermia), and bradycardia.
It is difficult to define a consistent threshold in the neonate below which hypoglycemia produces the above symptoms, especially neuroglycopenia. One often-quoted study of 17 children showed changes in auditory evoked potentials below a whole blood glucose concentration of 47 mg/dL (2.6 mmol/L) but only included four neonates with hypoglycemia (aged 1 to 3 days). In those four neonates, three had no clinical signs at the time of the abnormal recorded evoked potentials, and one was reported to be drowsy. The challenge of defining a true threshold is underscored in these four infants, given that one of these was asymptomatic with normally evoked potentials at a whole blood glucose level of 1.9 mmol/L (34 mg/dL) on day 1 of life, while another infant was symptomatic at 2.5 mmol/L (45 mg/dL).
While symptomatic, prolonged hypoglycemia in neonates is a risk factor for cerebral injury and poorer neurodevelopmental outcomes, the lower limit of normoglycemia in asymptomatic infants has been difficult to elucidate. This is complicated by the pattern of transitional hypoglycemia mentioned above, during which plasma glucose values may drop to levels considered very low for older children. Maternal and neonatal conditions with a high risk for hypoglycemia are well-known, and it is a commonplace for newborn nurseries to have screening protocols for these infants ( Box 87.2 ). Such protocols commonly result in the treatment of asymptomatic infants based on point-of-care (POC) glucose values, with the limited evidence available to define the optimal threshold that minimizes overtreatment while still preventing neuroglycopenia and neurologic damage.
Diabetes (gestational or pre-gestational)
Administration of drugs (β sympathomimetics [e.g., terbutaline, oral hypoglycemic agents])
Intrapartum dextrose infusion
Hypertension/preeclampsia
Prematurity
Intrauterine growth restriction
Hypoxia-ischemia
Large/small for gestational age
Sepsis
Hypothermia
Polycythemia
Presence of syndromic features (microphallus, midline defects, Beckwith-Wiedemann syndrome)
Multiple definitions of the ideal asymptomatic treatment threshold with regard to long-term neurologic outcomes have been proposed: 47 mg/dL, 45 mg/dL, 40 mg/dL, and 30 mg/dL. It is unlikely that a single threshold exists, as the point at which neurologic injury occurs is likely patient and situation-dependent and related to the availability of ketones and other substrates to the brain.
Recently, a prospective investigation into an appropriate glucose treatment threshold for infants was performed with a cohort of 404 infants of gestational age at least 35 weeks who were at risk for hypoglycemia (infant of mother with diabetes, birth <37 weeks gestational age, and birth weight <10th or >90th percentile). Infants also wore blinded continuous glucose monitoring (CGM) systems to allow the investigators to evaluate for outcomes related to subclinical hypoglycemia missed on the intermittent POC checks. Infants were treated to maintain a blood glucose concentration of at least 47 mg/dL for at least the first 48 hours of life. Neurodevelopmental outcomes were then assessed at 2 years of age using the Bayley Scales of Infant Development III and tests of executive and visual function. The authors found no association between hypoglycemic episodes and neurosensory impairment and concluded that neonatal hypoglycemia was not associated with the adverse neurologic outcomes when treatment was aimed at maintaining a blood concentration of 47 mg/dL in these high-risk infants. A follow-up study with the same cohort of patients aimed to assess higher cognitive function at age 4.5 years. While once again, no neurosensory impairment was seen on follow-up testing, an increased risk of poor executive and visual motor performance was seen. Additionally, the risk was highest in those with severe or recurrent hypoglycemia.
Recent Pediatric Endocrine Society (PES) guidelines make glucose threshold recommendations for infants that are at risk for hypoglycemia and without a known risk for permanent hypoglycemic disorders such as hyperinsulinism, hypopituitarism, or an inborn error of metabolism. According to the PES, such infants should have a treatment threshold of 50 mg/dL in the first 48 hours. Based on evidence that suggests average glucose values in normal infants older than 48 hours of age are no different than those of older children, the authors suggest that these infants should demonstrate an ability to maintain glucose >60 mg/dL during a 6 to 8 hours fast prior to discharge. The PES guidelines are designed to have high sensitivity to detect infants with a pathologic cause of persistent hypoglycemia and to detect these infants before they are discharged from the hospital. The American Academy of Pediatrics (AAP) issued guidelines in 2011 with a focus on the first 24 hours of life, acknowledging that transitional glucose levels in the first few hours of life can be as low as 30 mg/dL. The AAP guidelines recommend a target prefeed glucose of 40 mg/dL in the first 4 hours of life and 45 mg/dL from 4 to 24 hours of life. The differences between the two guidelines reflect the uncertainty regarding the significance of asymptomatic glucose levels <50 mg/dL in the first 48 hours of life, as well as the different weighting of the risks and benefits of continued screening and longer hospital stays in the context of the clinical reality that most term infants in the United States are discharged home between 24 and 48 hours of life.
For diagnostic workup of hypoglycemia after 48 hours of age, we recommend a glucose threshold of 50 mg/dL for collection of a “critical sample” to assess for the counterregulatory hormone response, insulin level, acidosis, and the presence of important metabolic substrates such as BOHB, lactate, serum amino acids, and free fatty acids ( Box 87.3 ). Most importantly, a plasma glucose level should be obtained simultaneously to allow for accurate interpretation of the critical sample, as the POC test result may be artificially low. It’s important that the plasma glucose sample reach the lab quickly because glucose in the plasma sample is lost through glycolysis at a rate of 5% to 7%/h. Higher rates of loss can occur with increased ambient temperature and in blood samples with high white blood cell counts.
| Critical Sample | Diagnostic Criteria for Hyperinsulinism |
|---|---|
|
Insulin’s role in maintaining normoglycemia is paramount. Insulin simultaneously lowers serum glucose concentration via glucose uptake through insulin-sensitive glucose transporters and represses the effects of counterregulatory hormones, whose primary function during hypoglycemia is to increase serum glucose values. It is therefore relevant to divide a discussion of the causes of hypoglycemia into those that are associated with appropriate suppression of insulin during hypoglycemia (normoinsulinemic hypoglycemia) and those that have inappropriate, elevated insulin levels at the time of hypoglycemia (hyperinsulinemic hypoglycemia) ( Box 87.4 ).
Transient:
Infants of diabetic mothers
Intrapartum dextrose infusion to mother
Stress in peripartum/postnatal period: trauma, asphyxia, hypothermia
Small for gestational age infants Permanent:
K ATP channel defects
Glutamate dehydrogenase (GLUD1)-activating mutation
Short-chain 3-hydroxyacyl-coenzyme A dehydrogenase (HADH or SCHAD) mutation
Glucokinase (GCK) activating mutation
HNF1A and HNF4A pathogenic variants
Uncoupling protein-2 (UCP2) pathogenic variants
Hexokinase-1 (HK1) pathogenic variants
Beckwith-Wiedemann syndrome (BWS)
Postfundoplication (dumping syndrome)
Hyperinsulinism in congenital disorders of glycosylation
β-cell adenoma—MEN1
Transient:
Developmental immaturity in adaptation to fasting: prematurity, SGA
Increased metabolic expenditure: sepsis, erythroblastosis fetalis, polycythemia
Maternal conditions: toxemia, administration of tocolytics (β sympathomimetics)
Permanent: Hypopituitarism
Primary adrenal insufficiency
Inborn errors of metabolism
Glycogen storage disease
Disorders of gluconeogenesis
Defects in fatty acid catabolism and ketogenesis
Organic acidurias
Galactosemia
Hereditary fructose intolerance
Infants born premature or small for gestational age (SGA) are at high risk for transient hypoglycemia due to immaturity of the metabolic pathways described above, exacerbated by inadequate stores of glycogen and triglycerides. The doubling of average fetal weight from 1700 g at 32 weeks to 3400 g at birth is largely due to the accrual of hepatic glycogen and adipose tissue fat stores, which then serve as an important reserve of substrates for energy metabolism in the first days of life. Hypoglycemia can also be caused by delayed maturation of enzymes necessary for gluconeogenesis. Premature infants can have markedly reduced glucose-6-phosphatase activity relative to term infants that may persist for months after birth. There is also a lack of glucose rise after administration of gluconeogenic precursors, which suggests the impaired activity of the enzymes of gluconeogenesis. For these reasons, SGA and premature infants should be screened for asymptomatic hypoglycemia and supported with IV dextrose or nasogastric feeding until their hypoglycemia resolves. It is important to note that hypoglycemia in these infants may be multifactorial, as hyperinsulinism may be a contributing factor as well (discussed later).
Deficiencies of cortisol, growth hormone (GH), or their combined deficiency in the neonatal period can cause hypoglycemia. Often these two deficiencies occur together in the context of hypopituitarism with adrenocorticotropic hormone (ACTH) hormone and GH deficiency. Infants with congenital hypopituitarism often have other signs of midline malformations such as a midline cleft palate, nystagmus (observed in optic-nerve hypoplasia; of note, however, is that nystagmus is usually not apparent until age 6 weeks, so it is not a distinguishing sign in the neonatal period), seizures (holoprosencephaly), direct hyperbilirubinemia (thyroid hormone deficiency), or micropenis and undescended testes in a male (gonadotropin deficiency). Brain MRI may reveal the underlying cause of hypopituitarism, which may range from severe midline malformations such as alobar holoprosencephaly to more subtle abnormalities such as an isolated ectopic posterior pituitary bright spot or a hypoplastic pituitary gland.
Biochemical evaluation of hypopituitarism requires careful consideration in the first months to a year of life. Conventional stimulation tests used to diagnose GH deficiency in older children have been used in infants, but normal responses are not well established. The stimulation test believed to be safest for use in infants is the glucagon stimulation test, which causes a rapid rise, then rapid fall in glucose, placing the infant at risk for hypoglycemia. Therefore, this testing should only be done under close monitoring, preferably in an ICU setting. As an alternative, taking into account normal physiological processes in the perinatal period, there is evidence that a single random GH measurement in the first week of life can adequately diagnose GH deficiency. Using a post-hoc defined threshold of 7 mcg/L, Binder et al. demonstrated a sensitivity and specificity of 100% and 98%, respectively, for the diagnosis of GH deficiency in the first week of life using a single random GH measurement.
Similarly, optimal testing to diagnose adrenal insufficiency in the neonatal period is a matter of debate. Glucagon stimulation testing can be used to simultaneously assess for GH and ACTH deficiency but carries the risk of hypoglycemia mentioned above. The conventional ACTH stimulation test using Cortrosyn has been used in infants. However, the appropriate dose of 125 mcg, 1 mcg, or 15 mcg/kg has not been well established, and endocrinologist preference varies. Importantly, infants with ACTH deficiency will not develop classical salt-wasting with hyponatremia and hypokalemia due to intact aldosterone production and secretion, which is regulated not by the pituitary but by the renin-angiotensin system. Hyponatremia may occur but is less severe relative to primary adrenal insufficiency and is likely due to reduced free water clearance, for which both cortisol and thyroid hormone play a role.
An important consideration in patients with hypopituitarism is that thyroid hormone should not be replaced until adrenal insufficiency has been either treated or ruled out, as thyroid hormone replacement can precipitate an adrenal crisis if done in the context of untreated adrenal insufficiency.
Isolated ACTH deficiency is very rare and is associated with pathogenic variants in the genes responsible for the production and/or modification of the proopiomelanocortin (POMC) precursor polypeptide. The TBX19 gene regulates transcription of the POMC/ACTH gene in corticotrophs, and deficiency has been associated with adrenal insufficiency in infants. A low estriol level on the prenatal triple-marker screen may be a predictor of TBX19 pathogenic variant and ACTH deficiency in general. Allelic pathogenic variants of the POMC gene itself affect all POMC peptides, including α-melanocyte-stimulating hormone (α-MSH), resulting in red hair and fair skin, in addition to ACTH deficiency, with severe obesity developing within the first few months of life. Pathogenic variants in prohormone convertase 1/3 (PCSK1) result in impaired cleavage of ACTH from POMC, as well as several gut hormones. Deficiency results in severe congenital diarrhea and failure to thrive, and a high risk of ACTH deficiency as well as panhypopituitarism, including central diabetes insipidus.
Cortisol deficiency in infants is most commonly due to pathology of the adrenal gland (primary adrenal insufficiency) caused by congenital adrenal hyperplasia (CAH). The most common form of CAH, 21-hydroxylase deficiency (incidence 1/10,000 to 1/15,000 annually), results in ambiguous, virilized genitalia in an XX infant driven by overproduction of adrenal androgens synthesized from precursors upstream of the enzyme block. However, in an XY infant, the presentation of CAH due to 21-hydroxylase is more subtle and may present only with hyperpigmentation of the skin, particularly of the scrotum. The classic presentation of hyponatremia, hyperkalemia and severe dehydration due to the cortisol and aldosterone deficiency of primary adrenal insufficiency often do not develop until 7 to 10 days of life, so their absence in a newborn with hypoglycemia does not rule out CAH. Newborn screening for the 21-hydroxylase deficiency form of CAH is now widespread in the U.S., and thus a diagnosis is often suspected early due to elevated 17-hydroxyprogesterone (17-OHP) levels on newborn bloodspot samples. Where hypoglycemia occurs in an infant, especially with any of the features mentioned above of salt wasting or ambiguous genitalia, confirmation of a normal 17OHP should be sought. Treatment is with hydrocortisone and fludrocortisone for glucocorticoid and mineralocorticoid replacement, respectively, along with sodium chloride supplementation due to the low salt content of breastmilk and most formulas.
More rare forms of CAH include 11-beta hydroxylase deficiency (1/100,000 or 1/5000 in Jews of Moroccan ancestry), and the very rare 3-beta hydroxysteroid dehydrogenase (HSD) deficiency. Similar to 21-hydroxylase pathogenic variants, 46 XX infants with 11-beta hydroxylase deficiency resulting in hypoglycemia will have ambiguous genitalia. They may also have hypertension due to excessive production of deoxycorticosterone, a potent mineralocorticoid located upstream of the 11-beta hydroxylase enzyme block. 3-beta HSD deficiency results in undervirilized male genitalia due to deficient testosterone synthesis but may be associated with virilized female genitalia due to elevated DHEA levels. Lipoid CAH is associated with pathogenic variants in the STAR gene, which result in loss of function in the steroidogenic acute regulatory protein (StAR), which impairs the transfer of cholesterol into the mitochondria. This disrupts the necessary first step of steroid synthesis, the conversion of cholesterol to pregnenolone. These patients have cortisol deficiency, almost always have aldosterone deficiency, and XY infants can have phenotypically female external genitalia due to impaired androgen synthesis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here