Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Nearly all vascular interventions are prone to the development of neointimal hyperplasia because any manipulation of the arterial wall induces endothelial cell injury. Current enthusiasm for less invasive but more locally injurious percutaneous peripheral vascular therapies increases the importance of developing strategies designed to suppress neointimal hyperplasia after such interventions. Arterial injury initiates a cascade of inflammatory events that ultimately results in neointimal hyperplasia and limits the durability of these vascular interventions. Some vascular interventions are more prone to the development of neointimal hyperplasia; these include prosthetic bypass and arteriovenous grafts. However, percutaneous balloon angioplasty with or without stenting, endovascular atherectomy, vein bypass grafting, and endarterectomy are all susceptible to this process. Thus peripheral vascular interventions will remain temporarily effective and palliative rather than durable and potentially curative unless effective treatment of neointimal hyperplasia is achieved.
The classic description of neointimal hyperplasia resulting from the proliferation and migration of vascular smooth muscle cells (VSMCs) from the media to the intima, along with deposition of the extracellular matrix after endothelial cell injury, no longer accurately represents the pathophysiologic changes that occur during this injury process. Neointimal hyperplasia results from a complex cascade of inflammatory cellular events that involves all three layers of the arterial wall as well as circulating blood elements. Much has been learned about this process in the past decade that has changed the way neointimal hyperplasia is viewed and has opened up new strategies for therapeutic development. We now recognize that the adventitia as well as resident and circulating stem and progenitor cells play a large role in this process. In addition to the well-described role of cytokines and growth factors that are secreted from platelets and leukocytes at the site of injury, reactive oxygen species (ROS) and adiponectin, an adipokine produced from periadventitial adipose tissue, are intimately involved in the regulation of this process. This chapter summarizes the current concepts regarding the pathology, pathophysiology, and treatment strategies of neointimal hyperplasia.
Neointimal hyperplasia is the abnormal continued proliferation of cells and connective tissue elements that occurs at sites of arterial injury. During the first decade of the 20th century, Carrel and Guthrie noted that “within a few days after the operation, the stitches placed in making the anastomosis became covered with a glistening substance similar in appearance to the normal endothelium.” This early description of arterial healing most likely represents the normal response of an artery to injury. Neointimal hyperplasia, in contrast, is the result of an exaggeration of this response through the inability to control or the continued stimulation of this normal regenerative process.
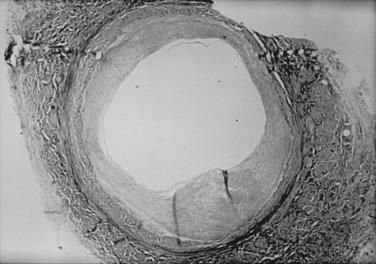
Lesions of neointimal hyperplasia are firm and homogeneous on gross examination. They look smooth, shiny, and subendothelial. These lesions were often referred to as myointimal hyperplasia, initially highlighting the thought that the medial VSMC is the origin of the proliferating tissue. However, we now recognize that multiple cell types contribute to the neointimal mass; therefore the term neointimal hyperplasia or intimal hyperplasia is more commonly used today. Assessment of neointimal lesions using intravascular ultrasound supports the notion that the majority (≈90%) of lesions are homogeneous in appearance. However, with advances in technology, such as intravascular optical coherence tomography, it appears that up to 40% of lesions may be heterogeneous in nature. Pathologic histologic examination of neointimal lesions reveals mainly stellate cells surrounded by a clear fibromyxomatous stroma and connective tissue. These stellate cells contain smooth muscle–like features and stain positively for actin and sulfated glycosaminoglycans. They appear to originate from several different sources, including the media, the adventitia (i.e., fibroblasts), the endothelium, and resident and circulating stem cells. Cellular proliferation begins within 24 hours of injury and is one of the hallmarks of neointimal hyperplasia. However, proliferation, migration, phenotypic differentiation, cellular infiltration, and extracellular matrix deposition all occur, resulting in wall thickening that continues to develop over several weeks ( Fig. 58.1 ).
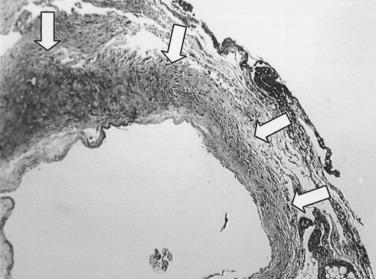
The classic description of the arterial injury response by both Clowes and Ross involves damage to the vascular endothelium and exposure of the underlying VSMCs to circulating blood elements. Exposure of the subendothelial arterial wall elements triggers the activation of a myriad of cellular and enzymatic events. The underlying internal elastic lamina and VSMCs are exposed to circulating blood elements. Platelets immediately aggregate and adhere to the site of injury. An inflammatory response follows, with the infiltration of neutrophils, macrophages, and leukocytes. Twenty-four hours after injury, under the influence of growth factors and cytokines, medial VSMCs convert from a contractile to a synthetic phenotype and begin to proliferate. VSMCs migrate to the neointima, where they continue to proliferate for up to 8 weeks. Endothelial cells concurrently regenerate through the stimulation of basic fibroblast growth factor (bFGF) within 24 hours after injury; this can continue for 6 to 10 weeks. Finally, transforming growth factor beta (TGF-β) stimulates extracellular matrix deposition. The culmination of these events results in the formation of neointimal hyperplasia ( Fig. 58.2 ).
The classic arterial injury response just described does not account for the role of the adventitia. However, the adventitia is now thought to be one of the main driving forces in the development of neointimal hyperplasia. The proliferative response after arterial injury has been characterized in rat and pig arteries. The proliferative response in the adventitia, compared with the intima and media, is much greater at almost all time points after arterial injury in both rat and pig animal models. Although 1.5- to 2-fold more proliferation was reported in the adventitia compared with the media in rat carotid arteries ( Table 58.1 ), nearly 7-fold more proliferation was reported in the adventitia than in the media in pig coronary arteries. On examination of early time points, proliferation was seen in the adventitia before the media as early as 4 hours after arterial injury (see Table 58.1 ).
| Time Point | Media | Adventitia |
|---|---|---|
| Noninjured | 0.2 | 0.1 |
| 4 h | 42 | 286 |
| 8 h | 37 | 510 |
| 12 h | 38 | 531 |
| 24 h | 369 | 582 |
| 48 h | 972 | 691 |
| 72 h | 930 (29%) | 1164 (53%) |
| 7 days | 576 (14%) | 1424 (41%) |
| 14 days | 420 (13%) | 810 (23%) |
| 30 days | 22 (0.4%) | 75 (2%) |
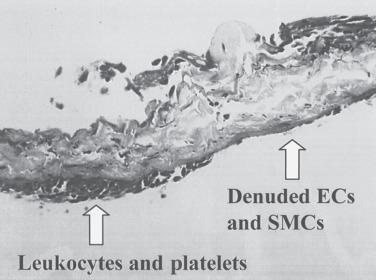
The development of neointimal hyperplasia in vein grafts is similar to neointimal hyperplasia after arterial injury but differs in that there are fewer medial cells that respond and there is an additional response to increased wall tension. Prominent features in rodent models of vein grafting include extensive early denudation of the endothelium and smooth muscle cell layers, attachment of leukocytes and platelets to the subendothelial matrix, and elaboration of cytokine and growth factor products by these cells. This results in the activation, migration, and proliferation of vein graft adventitial myofibroblasts to form a neointima. Pathologically, the trauma resulting from surgical manipulation and exposure of the vein graft to arterial pressure and flow results in nearly complete denudation of the endothelium and loss of the smooth muscle cell layers within the first 24 hours ( Fig. 58.3 ). Soon after denudation, attachment of leukocytes and platelets occurs. Macrophage infiltration dominates the early inflammatory changes observed with vein graft pathobiology. This prominent inflammatory response peaks during the first 2 weeks of vein graft adaptation, which exceeds the response observed in models of arterial injury ( Fig. 58.4 ). Stark and Hoch elegantly demonstrated that by inhibiting macrophage activity early in this inflammatory process, a significant reduction in neointimal hyperplasia was achieved.
Vein graft neointimal changes persist and can progress despite reendothelialization. In contrast, arterial neointimal proliferation tends to halt its progression upon restitution of the endothelial cell layer. The ongoing neointimal thickening associated with vein graft neointimal hyperplasia is believed to be an adaptive response to arterial pressure and flow. Wall thickening is greater in regions of low shear (e.g., at the inner wall of a curve). Neointimal hyperplasia results in wall thickening, which decreases wall stress to more physiologic levels. These hyperplastic changes were first described by Carrel and Guthrie when artery and vein grafts were first examined histologically. They referred to this process as arterialization . We now known that this arterialization process involved phenotypic transformation of the vein cells to an arterial phenotype, as indicated by the loss of ephrin-B4. Wall thickening can be accentuated at valve cusps, perhaps because of local flow abnormalities; at the anastomoses, where shear and wall tension are greatest; and at sites of clamp injury. Hemodynamically significant lesions caused by intimal hyperplasia usually occur after the first month and are rare after a year. After the first year, vein grafts are prone to develop lesions similar to atherosclerosis.
In addition to the processes just described—which involve platelets, inflammatory cells, VSMCs, adventitial fibroblasts, and the extracellular matrix—there is now an understanding of the contribution of resident and circulating stem cells to the development of neointimal hyperplasia as well as the role of ROS in regulating many of these processes. Several mechanisms are likely responsible for stimulating these responses, including mechanical changes, such as turbulence and compliance mismatch, and complex interactions between these different cells. Thus a complex view has emerged that involves the synergistic action of several biologic pathways. The remainder of this section reviews, separately, the experimental basis for the hypothesized involvement of each of these systems.
A wide variety of hemodynamic and mechanical factors—including high- and low-flow velocities, high and low wall shear stress, and mechanical compliance mismatch—have been implicated in the formation of intimal hyperplasia. In general, low blood flow velocity tends to favor the development of neointimal hyperplasia. For example, in a canine carotid vein interposition model, segments with low-flow velocities developed significantly thicker intimal layers ; however, there are exceptions to this rule. In a high-flow renal artery–to–vena cava anastomosis, significant intimal thickening was documented by electron microscopic examination 3 months after formation. Similar intimal lesions have also been noted in arteriovenous fistulas (AVFs) constructed for hemodialysis access. This may be a result of the compliance mismatch noted between the arterial and venous systems. In a monkey iliac AVF model 6 months after construction, no increase in intimal thickening occurred on the experimental side. In that study, although flow rate and velocity were markedly increased on the side of the AVF, the calculated wall shear stress was equal on both sides. Equality of shear stress, despite a significant difference in flow velocities, was the result of a twofold increase in the diameter of the vessel lumen. The vessel wall regulates its diameter to produce a physiologic level of shear stress at the lumen. The endothelium regulates diameter by producing both vasoconstrictors such as endothelin and vasodilators such as nitric oxide (NO). These factors also affect VSMC proliferation and, when chronically present, induce remodeling of the vessel to maintain the new diameter without vasoactivity.
In other primate studies, arteriovenous flow was shown to inhibit neointimal thickening in highly porous polytetrafluoroethylene (PTFE) grafts. Return to normal flow causes neointimal thickening. In a rodent model of arterial injury, low flow caused increased intimal hyperplasia. These and many other studies demonstrate that intimal hyperplasia is increased by low shear and inhibited by high shear. Postmortem studies have shown that early atherosclerotic lesions occur more commonly in areas of low wall shear stress. As an example, in the carotid artery atherosclerosis starts at the outer wall of the bulb, where shear forces are low and oscillating.
Compliance mismatch is also an important hemodynamic factor in the production of anastomotic intimal hyperplasia. Compliance is the percentage of radial change per unit pressure and is a useful index of vessel wall distensibility to a pressure force. Experimental and clinical studies show that conduits with compliance values approaching that of the native artery have better patency. In one study, femoropopliteal autografts made from glutaraldehyde-treated carotid arteries had better patency when fixation produced a more compliant graft. However, other studies have shown that the creation of stiffer, less compliant grafts by treating arteries with glutaraldehyde results in markedly diminished patency rates. Textile and fabric prostheses are relatively noncompliant; this is another factor that contributes to their lower patency rates due to the aggressive formation of neointimal hyperplasia.
To curb the effect of compliance mismatch, some have suggested imposing a short interposition of autogenous vein between the native artery and prosthetic grafts. These modifications include vein cuffs, vein patches, vein boots, and AVFs at the distal anastomosis ( Fig. 58.5 ). A prospective randomized trial to examine the efficacy of Miller vein cuffs in improving lower extremity PTFE graft patency showed that vein cuffs did not improve patency rates in bypasses to the above-knee popliteal artery. However, cuffed below-knee popliteal artery grafts had a 45% 3-year patency rate versus a 19% patency rate in uncuffed grafts ( P = .018). Similarly, the use of vein patches, such as those developed by Taylor and Linton, at the distal anastomosis yields better long-term patency rates versus prosthetic material alone. Although there have been no randomized prospective trials to date, retrospective reviews have demonstrated improved patency of vein-patched PTFE versus PTFE alone when used for below-knee bypasses. Taylor and colleagues reported a 5-year primary patency of 54% for 83 infrapopliteal PTFE grafts using his vein patch modification. Although there is no control arm to this study, previously reported patency rates with PTFE alone are significantly worse. A review of 145 patients who had undergone either above- or below-knee bypasses using autologous vein, unmodified PTFE, and Linton patch–modified PTFE found that above-knee bypasses had no statistical difference in 1- and 3-year patency rates between the three groups. However, the cumulative patency rates for 1, 3, and 5 years after below-knee bypasses were 93%, 75%, and 75% for the autologous vein group ( n = 30); 74%, 52%, and 42% for the unmodified PTFE group ( n = 37); and 93%, 93%, and 93% for the Linton patch–modified PTFE group ( n = 16), respectively. They speculated that the improved results over unmodified PTFE bypass grafts were due to improved compliance mismatch between the prosthetic graft and the targeted native artery. The study was limited by the small number of Linton patch–modified PTFE grafts used compared with the autologous vein and PTFE groups. Finally, a retrospective analysis of outcomes of patients with AVF-modified PTFE grafts versus PTFE alone to the infrapopliteal arteries showed that the 2-year patency rates were significantly better for grafts with the AVF modification (23% vs. 5% for the PTFE alone grafts, P < .05). The AVF, originally described by Ascer and colleagues to improve bypass graft outflow, is created by first mobilizing an adjacent vein and transecting it. The vein is then anastomosed to the artery in an end-to-side fashion. A venotomy is created near the AVF and the ePTFE is anastomosed, creating the AVF modification at the distal anastomosis (see Fig. 58.5 ). Despite these surgical improvements, patency rates remain poor, especially when the distal outflow is at or below the level of the popliteal artery, primarily as a result of the formation of neointimal hyperplasia.
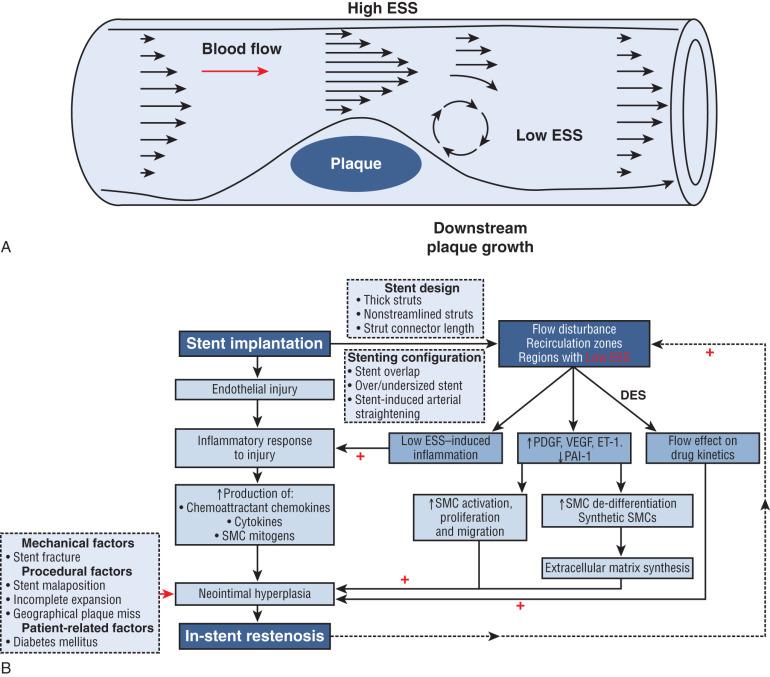
Endovascular interventions, similarly, are prone to neointimal hyperplasia. Despite antiplatelet therapy and drug-eluting stents designed to inhibit neointimal hyperplasia, in-stent stenosis exists because stenting alters the circumferential and shear stress on a vessel. In stented rabbit arteries, neointimal hyperplasia occurred most frequently in areas of low shear stress. Stent oversizing worsened neointimal hyperplasia, as was demonstrated in a porcine model with intentionally oversized stents and in human clinical studies examining percutaneous coronary interventions. Stenting in geometrically asymmetrical or stenotic arteries further alters shear stress on the endothelium and exacerbates the development of intimal hyperplasia ( Fig. 58.6 ).
Platelets have long been known to play a central role in the reaction of a vessel wall to injury. Activation of platelets by the injured endothelium is a major factor in the development of intimal hyperplasia. Denudation of the arterial wall exposes the subendothelial matrix, which leads to adherence and subsequent activation of platelets. Platelet adherence requires the interaction of subendothelial collagen, a platelet membrane glycoprotein receptor (GP Ib), plasma von Willebrand factor, and fibronectin. After adherence, platelets undergo a morphologic change, stretching to cover the exposed surface. Activated platelets release adenosine diphosphate and activate the arachidonic acid pathway to release thromboxane A2. Both of these factors lead to platelet aggregation. The luminal surface quickly passivates by adsorption of plasma proteins, limiting the period of platelet aggregation to less than 24 hours. Recruitment of platelets requires the rapid expression of the platelet membrane receptor complexes GP IIb and GP IIIa, both of which promote platelet aggregation through the binding of circulating fibrinogen. Platelet adhesion and granule release also lead to a parallel acceleration of the coagulation cascade. This activation of clotting pathways, combined with high local concentrations of fibrinogen mediated by binding to the GP IIb/IIIa complex, creates a fibrin protein network that further stabilizes the aggregated platelet plug. Once platelet aggregation has been initiated by the pathways mentioned earlier, its continuation is actively inhibited by an intact endothelium. Thus a damaged endothelium not only initiates platelet activation but also impairs its inhibition.
Platelet-derived growth factor (PDGF) is secreted along with other granule constituents, including platelet factor 4, thromboglobulin, and thrombospondin. PDGF is a cationic protein with a molecular weight of 28 to 31 kDa. It comprises two subunits (α and β). Physiologically, PDGF functions as both a chemoattractant and a mitogen for VSMC and fibroblasts. Because it binds with high affinity to VSMC, some have suggested that PDGF may attract the VSMCs from the media into the intima, bind to them, and stimulate their proliferation. Interestingly, evidence has shown that the platelet may not be the only source of this protein. PDGF (α and β subunits) is produced by human umbilical vein and saphenous vein endothelial cells. In fact, a large increase in PDGF production can be measured in injured endothelial cells. Also, both α- and β-subunit messenger RNAs (mRNAs) have been noted in fresh endarterectomy specimens obtained during carotid surgery. VSMCs themselves also produce PDGF-like activity in response to arterial injury, and VSMCs from human atheromas contain mRNAs for the PDGF α subunit. Taken together, these findings may explain how proliferation continues after reendothelialization occurs. PDGF may be released by both platelets and endothelial cells, causing the activation and migration of VSMCs and myofibroblasts, which then secrete additional PDGF, leading to proliferation. Demonstrating the importance of PDGF to the arterial injury response, inhibition of PDGF using antibodies or antisense oligonucleotides in animal models of arterial injury has been shown to inhibit neointimal hyperplasia by 60% to 80%. Hence PDGF is a popular focus for the development of targeted therapy and nanotherapeutics designed to inhibit neointimal hyperplasia. From our understanding of platelet physiology during arterial injury came the clinical practice of pretreatment with antiplatelet agents prior to endovascular interventions as well as postprocedural antiplatelet therapy. Furthermore, in animal studies, technology is under development to create rouvastatin-loaded stents which accelerate endothelialization, and antiplatelet acetylcalicylic acid–eluting biodegradable stents with the potential to obviate the need for daily oral antiplatelet therapy to inhibit platelet aggregation and neointimal hyperplasia formation following endovascular interventions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here