Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Muscle, the final component of the motor system, is the site of abnormalities with those essential clinical manifestations of hypotonia and weakness that unify the other disorders of the motor system (see Chapter 36 ). In this chapter, we deal with important myopathic disorders in the neonatal patient. In addition, certain restricted disorders of the motor system are reviewed.
Disorders of muscle account for a substantial proportion of infants affected with hypotonia and weakness. Although a relatively large number of myopathic disorders may cause manifestations in the neonatal period, only a few disorders account for most of the cases observed. Myotonic dystrophy is the most frequent (see subsequent sections). However, more of the myopathic disorders can be detected in the neonatal period if the physician’s index of suspicion is appropriately high. Detection is valuable for purposes of genetic counseling, determination of prognosis, and institution of therapy.
The major myopathic disorders of interest in newborns may be categorized into two broad groups: those in which the histology, although sometimes impressive, is not distinctive enough to be diagnostic and those in which the histology is distinctive enough to be diagnostic (with some qualifications) ( Box 37.1 ). In the following discussions, major emphasis is placed on the most common disorders.
Congenital myotonic dystrophy
Congenital muscular dystrophy
Facioscapulohumeral dystrophy
Polymyositis
Minimal change myopathy
Central core disease
Nemaline (rod body) myopathy
Myotubular (centronuclear) myopathy
Congenital fiber type disproportion
Multiminicore disease
Mitochondrial myopathies
COX deficiency
Mitochondrial DNA depletion
Metabolic myopathies
Glycogen disorders
Lipid disorders
Several disorders are characterized by neonatal hypotonia and weakness and by muscle histological findings that are sometimes impressive, although not distinctive enough to be diagnostic. This category includes the most frequently encountered examples of myopathic disease with clinical manifestations evident in the neonatal period. Myotonic dystrophy is the most common of all, although congenital muscular dystrophy (CMD) is not rare (see Box 37.1 ). Facioscapulohumeral dystrophy (FSHD) rarely manifests in the neonatal period but is important to recognize. Polymyositis is a rare but treatable muscle disorder in the newborn. Congenital myopathy with minimal or no change as determined by current laboratory investigation (i.e., minimal change myopathy) represents a shrinking group as diagnostic techniques improve.
Congenital myotonic dystrophy is an inherited disorder of muscle that exhibits numerous distinctive differences from myotonic dystrophy type 1 (DM1) of adult patients. The disorder may be associated with muscle weakness so severe that death results in the newborn period. The hallmark of congenital myotonic dystrophy is hypotonia, rather than myotonia, as observed in adult patients. The congenital form of myotonic dystrophy was first described clearly by Vanier in 1960.
The disorder is usually apparent in the first hours and days of life. Certain characteristics of the pregnancy often precede the neonatal disorder ( Table 37.1 ) and tend to be prominent in the most severely affected cases. Thus spontaneous abortion or premature birth with the associated increased risk of germinal matrix–intraventricular hemorrhage occurs in the most severely affected infants. Polyhydramnios is a common characteristic of the pregnancy (see Table 37.1 ). Indeed, this sign is almost invariable in the most severely affected infants and is thought to be caused by the disturbance in swallowing. Polyhydramnios in a mother with myotonic dystrophy is a fairly reliable indicator of serious involvement of the fetus. Abnormalities of labor, which may be either prolonged or abbreviated, have been attributed to maternal uterine muscle involvement.
| CLINICAL FEATURE | CASES EXHIBITING FEATURE (%) a |
|---|---|
| Reduced fetal movements | 68 |
| Polyhydramnios | 80 |
| Premature birth (<36 wk) | 52 |
| Facial diplegia | 100 |
| Feeding difficulties | 92 |
| Hypotonia | 100 |
| Muscle atrophy | 100 |
| Hyporeflexia or areflexia | 87 |
| Respiratory distress | 88 |
| Arthrogryposis | 82 |
| Edema | 54 |
| Elevated right hemidiaphragm | 49 |
| Transmission by mother | 100 b |
| Neonatal mortality | 41 |
| Intellectual disability in survivors | 100 |
b Paternal transmission has been documented, albeit very rarely.
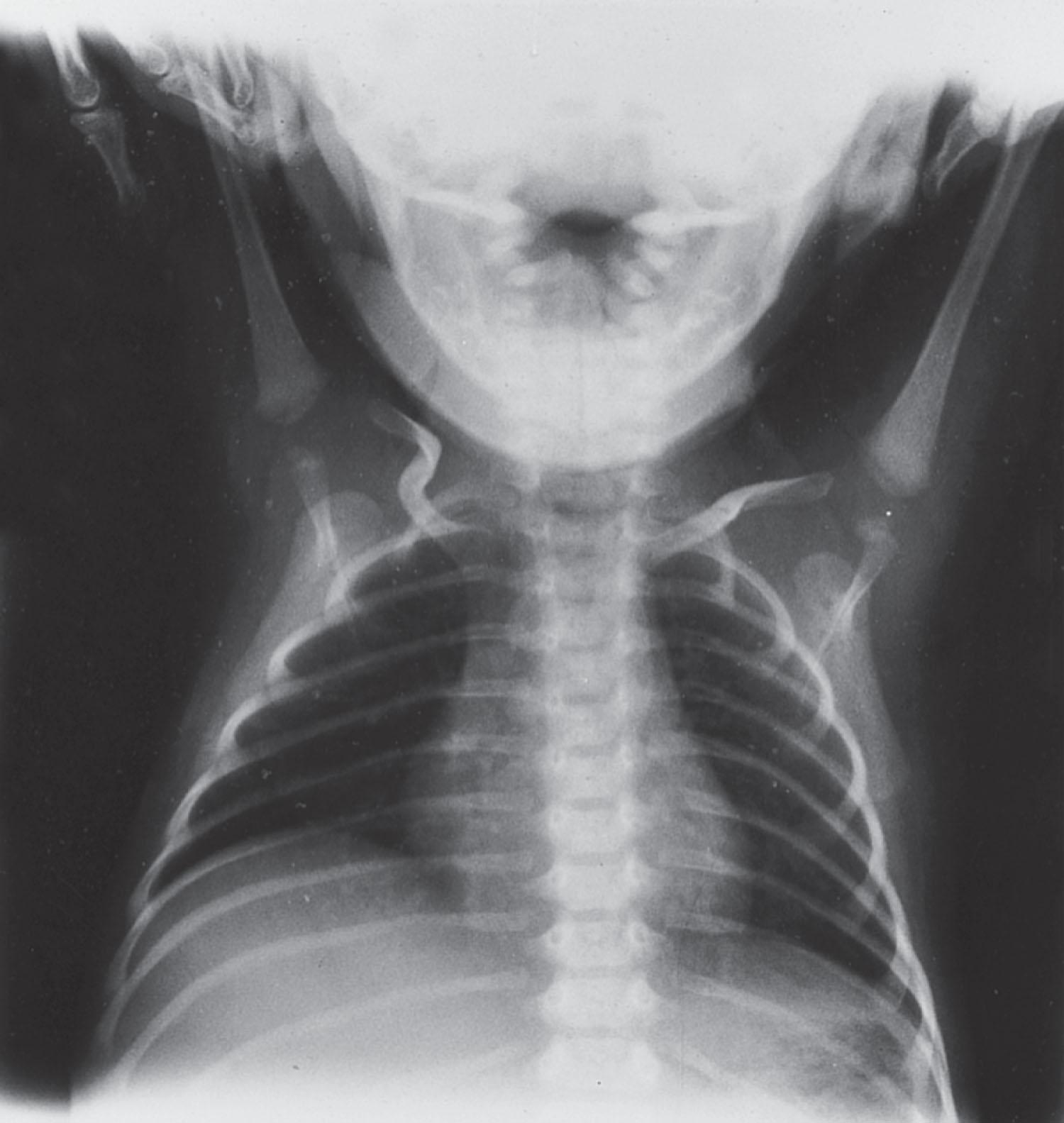
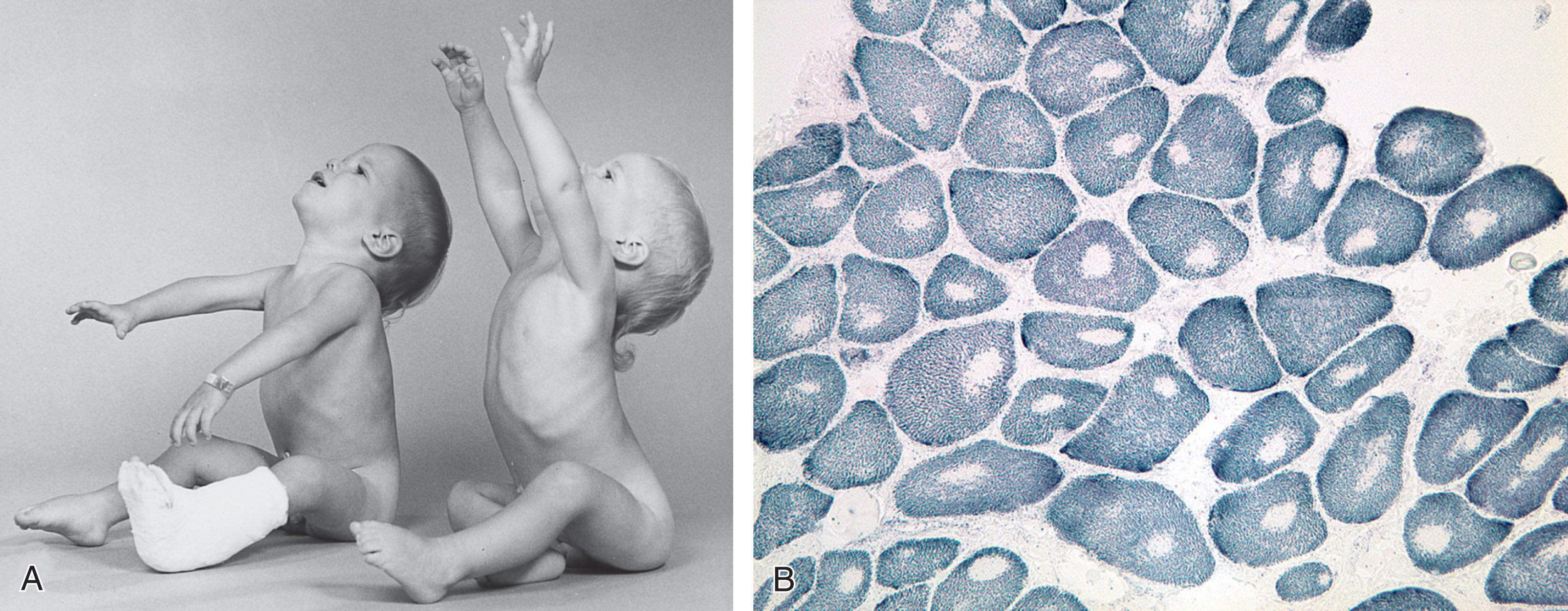
The clinical features in the neonatal period are usually marked and characteristic (see Table 37.1 ). In the well-established case, the most striking clinical features are facial diplegia, respiratory and feeding difficulties, arthrogryposis (especially of the lower extremities), and hypotonia. The facial diplegia imparts the characteristic tent-shaped appearance to the upper lip ( Fig. 37.1 ). The respiratory difficulties relate to weakness of respiratory muscles, impaired swallowing and handling of secretions, and occasionally diaphragmatic disturbance ( Fig. 37.2 ). At least 80% of patients require mechanical ventilation. The respiratory impairment may be so severe that the newborn infant fails to establish adequate ventilation and experiences an asphyxial episode that so dominates the clinical syndrome that the myopathic origin of the respiratory failure is overlooked. The feeding difficulties involve both sucking and swallowing and are related particularly to weakness of the facial, masticatory, and pharyngeal musculature. (A role for myotonia of pharyngeal muscles is possible. ) A disturbance of gastric motility, a manifestation of the smooth muscle involvement in this disorder, may contribute in a major way to the feeding difficulties in some infants. Arthrogryposis ( Fig. 37.3 ) is almost invariable and usually involves at least the ankles, leading to clubfoot deformity. Indeed, in one large series of arthrogryposis multiplex congenita, 50% of the patients with cases related to muscle disease had congenital myotonic dystrophy. Hypotonia also is essentially invariable, accompanied by weakness and, in approximately 90% of the cases, by areflexia or marked hyporeflexia. Atrophy is usually obvious, especially after the first days of life, when edema, which is often excessive in these infants, subsides. Clinical myotonia, elicited by percussion of such muscles as the deltoid, has been detected as early as 3 hours of age, but it usually is not readily elicited until much later in infancy.
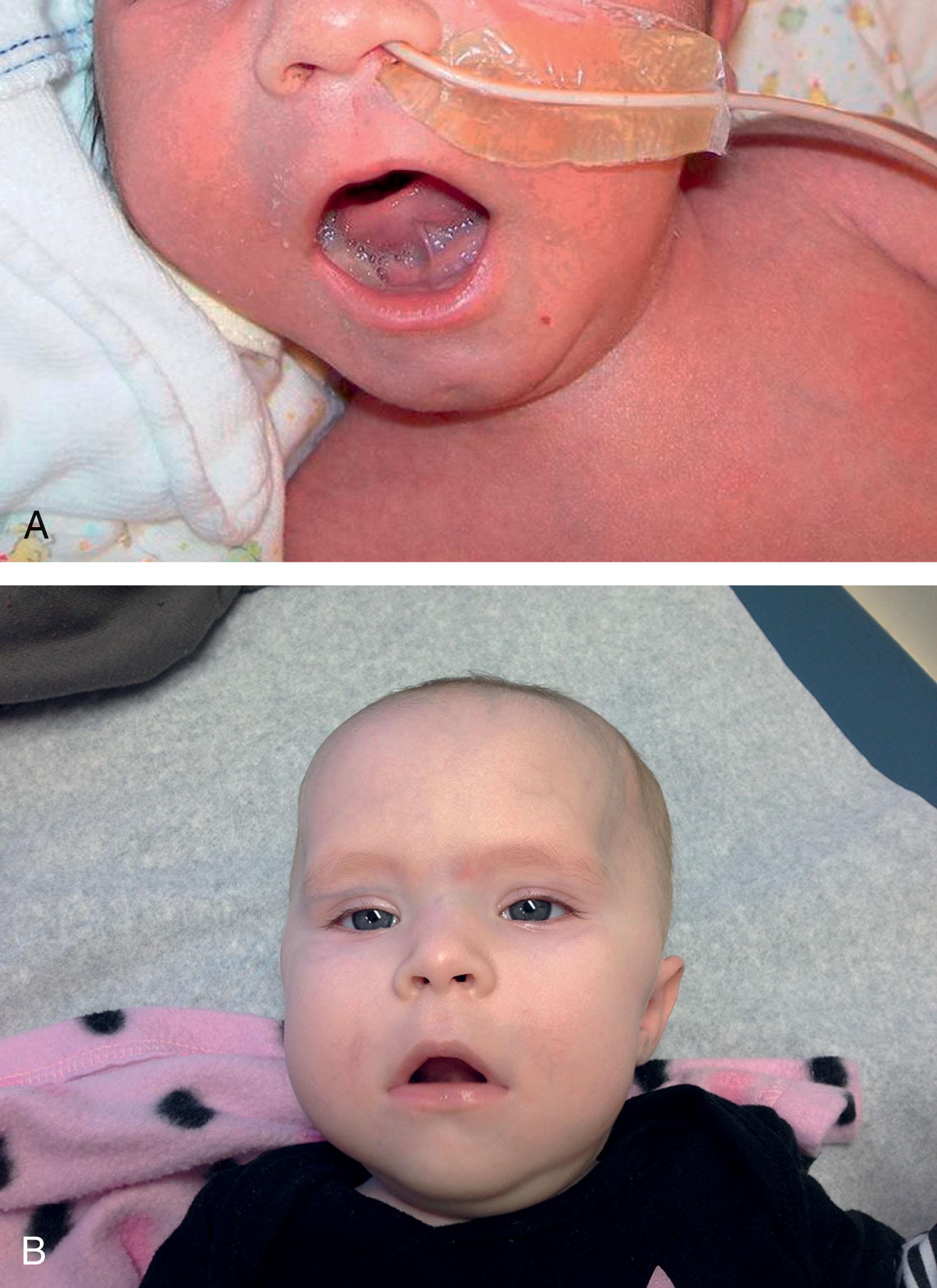
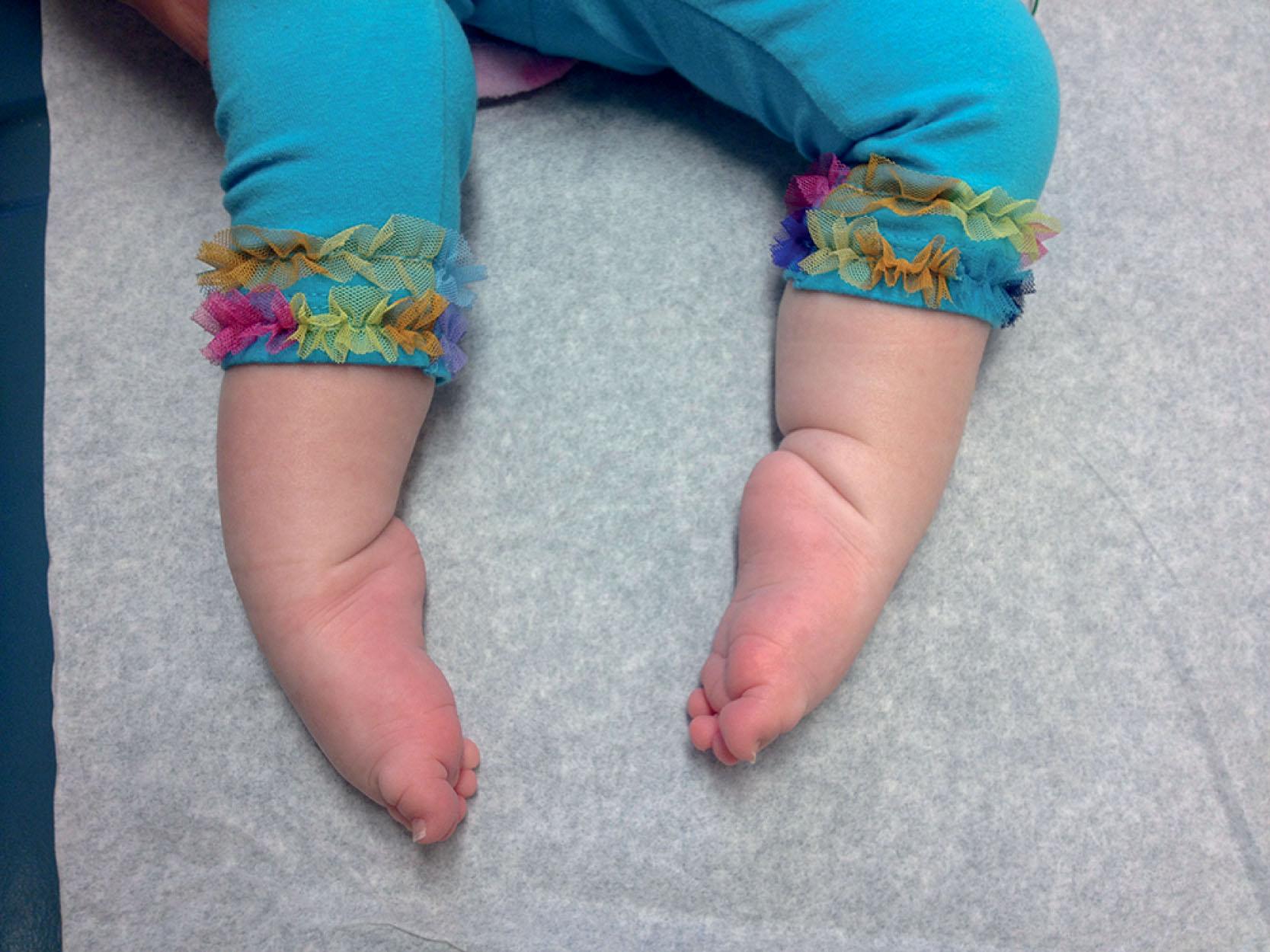
Less severely affected infants may go undetected in the newborn period. Facial weakness and hypotonia are the two most common manifestations in such patients.
The clinical course relates clearly to the severity of the disease. Neonatal mortality is generally approximately 15% to 20%, but it is as high as approximately 40% in severely affected patients. Feeding difficulties, which initially may require institution of tube feedings, usually improve, at least in reported survivors. However, approximately 20% of infants surviving to adolescence and young adulthood have major gastrointestinal symptoms (e.g., diarrhea and abdominal pain), presumably reflecting smooth muscle involvement. Moreover, the persistence of some degree of pharyngeal and palatal weakness leads to recurrent otitis media in approximately 25%, occasionally with accompanying hearing loss. Like the neonatal feeding difficulties, the respiratory difficulties subside over the ensuing weeks. However, approximately one-third of patients require mechanical ventilation for longer than 30 days. Even after cessation of mechanical ventilation, ventilatory reserve may be compromised in severely affected infants, and death secondary to respiratory complications in association with anesthesia or aspiration may occur later in infancy. Unlike the general improvement in neonatal feeding and in respiratory difficulties, facial diplegia usually becomes more obvious as “baby fat” disappears; ptosis often becomes apparent as well. Muscle weakness, which initially is generalized or more marked proximally, begins to assume the distal preponderance characteristic of the adult disease. However, in many patients, truncal and appendicular muscle strength improves gradually over time and thus they acquire the ability to sit independently, bear weight on their legs, and eventually walk ( Fig. 37.4 ). Clinical myotonia becomes elicitable later in infancy ( Fig. 37.5 ) and is present in the majority of patients after the age of 5 years. Cardiac muscle involvement, manifested by electrocardiographic abnormalities, also becomes more prominent later in infancy. However, in very severely affected newborns, cardiomyopathy may be apparent in the newborn period and may contribute to neonatal demise. In a series of 56 patients with congenital myotonic dystrophy and mean follow-up time of 8.0 (3.25–11.0) years, 54 underwent transthoracic echocardiography; no patients developed dilated cardiomyopathy or left ventricular systolic dysfunction. In this 56-patient cohort, there was high prevalence (87.8%) of progressive electrocardiographic abnormalities, mostly in the form of asymptomatic conduction diseases. Only 1 patient required pacemaker implantation; however, 4 patients died during follow-up (7.1%), including 3 who died suddenly with no clear explanation for their death as no autopsy was performed. Cataracts, baldness, gonadal atrophy, and marked facial atrophy develop later in adult life.
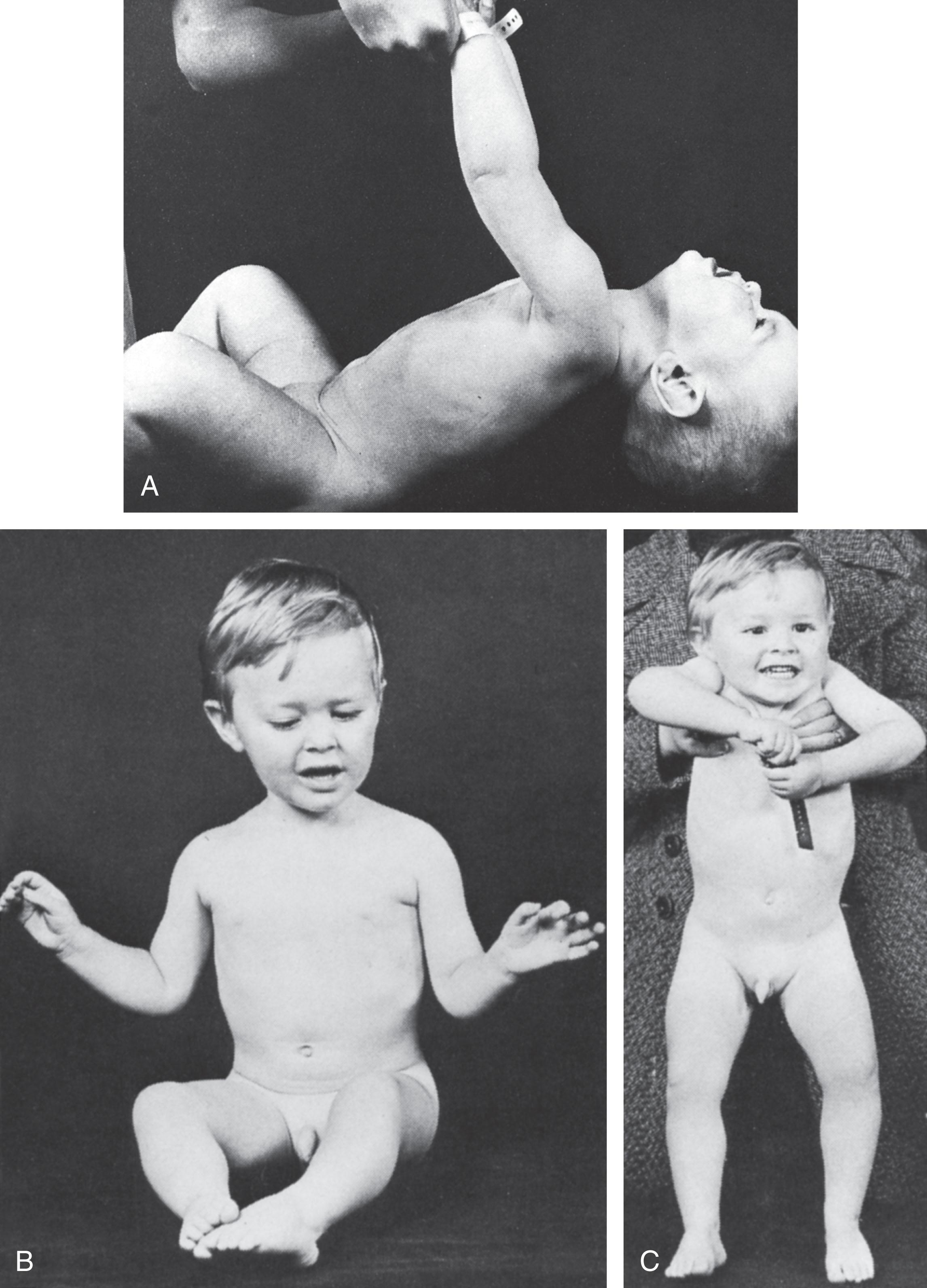
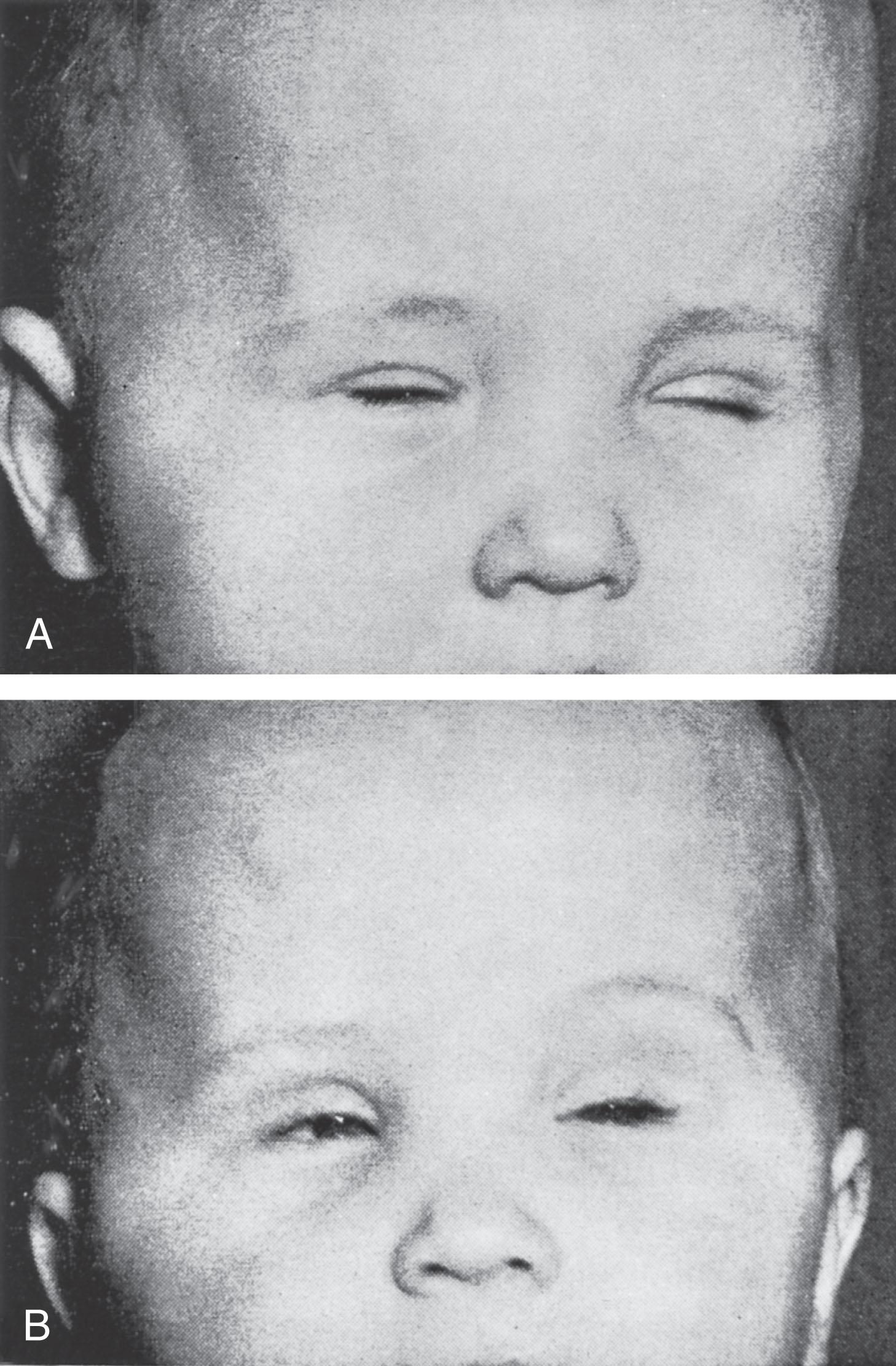
Impairment of motor and intellectual development is apparent in the first weeks of life (see Table 37.1 ). Intellectual disability is essentially invariable in infants with congenital myotonic dystrophy who survive the neonatal period. Intelligence quotient (IQ) scores in the 50 to 65 range have been generally observed. The disturbance of intellectual development is nonprogressive. The only available data on relatively long-term follow-up (i.e., ≈10 to 30 years) indicated that only 2 of 42 patients managed “normal education,” and only one was “gainfully employed.” Some improvement in motor function occurs in late infancy and childhood.
Transmission of myotonic dystrophy in patients with the neonatal form of the disease is essentially always through the mother (see Table 37.1 ). Only nine cases of congenital myotonic dystrophy transmitted through an affected father have been reported, but this apparent rarity is probably an underestimate because many paternal transmissions may not have been reported. At any rate, it is critical for the physician to be cognizant of the disorder as it appears in adults. Indeed, it is common for the mother with an affected infant to be unaware that she has the disease. Most helpful in recognition of the disease in the mother is the typical facies of myotonic dystrophy ( Fig. 37.6 ), characterized by atrophy of the masseter and temporalis muscles, ptosis, and a straight, stiff smile. Myotonia is prominent and readily elicited. Thus active myotonia is seen in the affected woman when she closes her eyes tightly and then is unable to open them fully for several seconds or more or when she clenches her fist tightly and then cannot extend her fingers immediately on command. Passive myotonia is demonstrable by percussion of such muscles as the deltoid, thenar eminence, and tongue and by observation of the prolonged dimpling caused by sustained muscle contraction ( , , , ). Other characteristics (e.g., cataracts, disturbance of intellect, and cardiac abnormality) may also be present. The essential point is that the physician should examine the mother when this diagnosis is considered in a newborn.
Serum creatine kinase (CK) level, cerebrospinal fluid (CSF) protein concentration, and nerve conduction studies are normal. Slender ribs are observed commonly on chest radiographs (see Fig. 37.2 ) and may suggest the diagnosis in an infant thought initially to have primary asphyxia. This finding can be observed with other congenital myopathic disorders, as well as with Werdnig-Hoffmann disease.
The diagnosis is supported particularly by demonstrating myotonic discharges on the electromyogram (EMG). These discharges are difficult to elicit in the neonatal period, although they have been observed as early as 5 days of age. Electrical myotonia is elicited by movement of the recording needle or by direct percussion of the recorded muscle; this procedure results in repetitive electrical potentials of up to 100/second that wax and wane in frequency and amplitude ( Fig. 37.7 ). These potentials produce the characteristic “dive bomber” sound. Later in infancy, particularly after the age of 2 to 3 years, myotonia is elicited more easily, and small-amplitude, short-duration, and polyphasic potentials, typical of myopathic disease, also can be demonstrated. Fibrillation potentials also may be observed.

Ventricular dilation has been observed in approximately 80% of newborns studied by ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI). In one study, macrocephaly was present in approximately 70% of the infants in the presence or absence of ventricular dilation. However, head circumference within the upper one-half of the normal range is more common than is overt macrocephaly. The ventricular dilation is nonprogressive and is not accompanied by rapid head growth or signs of increased intracranial pressure. The occasional occurrence of periventricular echodensities on ultrasound scans or hyperintensity on subsequent T2-weighted MRI scans may reflect periventricular leukomalacia occurring in association with neonatal respiratory disturbance. However, this white matter change also could reflect an abnormality of myelination. A small corpus callosum was documented in four of seven infants studied by MRI in another study. The possibility of an abnormality in neuronal development was raised by the demonstration by MR spectroscopy of decreased levels of N -acetyl aspartic acid, a neuronal marker (see Chapter 13 ), in five infants and children with congenital myotonic dystrophy.
Muscle pathology in congenital myotonic dystrophy is different from that observed in the adult with the disease and consists particularly of features of immaturity. In a detailed postmortem analysis of three infants, Sarnat and Silbert and others demonstrated particular involvement of limb muscles around arthrogrypotic joints, pharyngeal muscles, and the diaphragm. The features indicative of a disturbance of maturation included small round muscle fibers with large internal nuclei and sparse myofibrils, reminiscent of fetal myotubes. Moreover, differentiation of fibers into distinct histochemical types was incomplete. Similarly, electron microscopy showed dilated transverse tubules that were aligned longitudinally, as in fetal myotubes, as well as poorly formed Z-bands, simple mitochondria, peripheral halo of absent mitochondria, and many satellite cells, which are also features of immature muscle. Investigators have suggested that these changes principally represent an arrest in fetal muscle maturation. Others have viewed these changes as signs of abnormal development. Pathological findings in the pancreas (nesidioblastosis), kidney (persistent renal blastema), and other organs (cryptorchidism and patent ductus arteriosus) support the concept of a disturbance of maturation.
The neuropathological substrate for the intellectual failure is unclear. A study of three adult patients with subnormal IQ scores by Rosman and Rebeiz revealed pachygyria in two and disordered cortical architectonics, with neuronal heterotopias in all three. These findings were not observed in a later study of four infants from the same institution. However, another report from a different institution also described cerebral cortical neuronal heterotopias in four infants and neuronal heterotopias in two studied postmortem. Thus, the neuropathological basis for the consistent impairment of intellect currently is not established conclusively. It seems reasonable to postulate that an aberration of maturation, as in muscle and other organs, is present. A study of the organizational aspects of brain development (see Chapter 7 ) would be of interest.
The disorder is inherited as an autosomal dominant trait, in virtually every case through the mother . Additionally, the risk of congenital myotonic dystrophy may bear a relationship with the disease in the mother. Earlier work indicated that the earlier the onset and the more severe the maternal disease, the greater the risk for the severe congenital form of myotonic dystrophy in the child. This phenomenon is consistent with anticipation (i.e., the occurrence of progressively earlier onset and more severe disease in successive generations). However, subsequent studies have shown no correlation with the maternal disease state because most women were asymptomatic and diagnosed with myotonic dystrophy only after the birth of the affected infants. Specific intrauterine factors were not detected.
Molecular genetic studies have provided major insight into the reasons for anticipation and related aspects of congenital myotonic dystrophy ( Box 37.2 ). Thus myotonic dystrophy has been shown to be associated with an increase in the number of specific (CTG) trinucleotide repeats (more than 50) in an unstable DNA region of the 3′ untranslated region of the myotonic dystrophy gene, DMPK , which is located at chromosome 19q13.3. In infants with congenital myotonic dystrophy, the pathogenesis has been linked to a large CTG repeat expansion (>1000 repeats), but, as discussed later, this is not true in all cases. Moreover, albeit not always, the number of repeats increases with maternal transmission of the gene, and a correlation of the number of repeats (i.e., the extent of the so-called expansion of the triplet repeat region on chromosome 19) with severity and early onset of the disease in the infant has been suggested. In general, most affected infants have between 1000 and 6500 CTG repeats. Current data suggest that paternal transmission of the unstable trinucleotide repeat is associated with reduced amplification or even contraction of the region of repeats, in contrast to the situation with maternal transmission. This feature of paternal transmission and recently described epigenetic factors may explain the exclusively maternal transmission of congenital myotonic dystrophy. For example, CpG hypermethylation in the myotonic dystrophy gene region may alter chromatin structure and expression of genes, such as SIX5 , which is involved in spermatogenesis; this phenomenon may explain the maternal bias for transmission of congenital myotonic dystrophy and possibly other unique features of the disease. Unfortunately, determination of the number of repeats from DNA samples of a woman and her fetus cannot be used to estimate reliably the likelihood of transmitting severe congenital myotonic dystrophy, because in some affected infants, repeat lengths much shorter than 1000 repeats, or even shorter than the number of repeats in the blood of the transmitting mother, have been described. Genotype-phenotype correlations are not perfect, also in part because of somatic mosaicism (i.e., the extent of repeat expansion varies from tissue to tissue, even in postmitotic tissue), which has been documented in congenital myotonic dystrophy, although less pronounced than in the adult form of the disease.
a See text for references
The disease is associated with an abnormally high number of CTG trinucleotide repeats, usually more than a thousand, in the 3′-untranslated region of the myotonic dystrophy protein kinase gene (MDPK) on chromosome 19q13.3.
The increased number of repeats correlates approximately with earlier onset and more severe disease, but genotype-phenotype correlations are not always accurate.
The mutation is “dynamic” (i.e., does not remain of constant size within a pedigree or across generations).
Maternal transmission is usually associated with expansion (i.e., increased number) of the repeat region (1000–6500 repeats) and with more severe disease in the infant (i.e., “anticipation”).
The extent of repeat expansion varies among tissues (i.e., “somatic mosaicism”); thus determination of the number of repeats in DNA in blood or chorionic villus cannot predict severity of disease in muscle or other tissues with absolute accuracy.
Sequestration of multiple RNA and DNA binding proteins by the region of multiple repeats leads to dysregulation of splicing of premRNAs, such as the insulin receptor and skeletal muscle chloride channel, resulting in insulin resistance and myotonia.
The gene affected in myotonic dystrophy (MDPK) encodes a serine-threonine protein kinase, a member of the protein kinase family expressed in tissues affected by myotonic dystrophy. Considering the important role of protein kinases in multiple biochemical and cellular pathways, the involvement of the DMPK polypeptide in myotonic dystrophy is not surprising and, in fact, was predicted by Roses and Appel in 1974. In 1992, it was discovered that the DMPK gene contains an unstable triplet CTG repeat. Normal leukocyte DNA contains 7 to 37 copies of the CTG trinucleotide. In “permutation carriers,” the size of the unstable CTG array is 37 to 50 repeats; however, it varies between 50 and 6500 repeats in symptomatic individuals. Infants with congenital myotonic dystrophy are likely to have more than 1000 repeats in their leukocyte DNA, although, as described earlier, a number of these patients may have CTG expansions less than 1000 repeats. The features of congenital myotonic dystrophy appear to be related to a toxic RNA gain of function effect. Thus the trinucleotide repeats appear to bind and sequester multiple RNA- and DNA-binding proteins, such as the muscleblind-like (MBNL) protein family, and thereby to lead to multiple deleterious effects (see Box 37.2 ), including dysregulation of alternative splicing of pre-mRNAs. Sequestration of MBNL1 leads to increased synthesis of the less responsive neonatal isoform of the insulin receptor and overproduction of the neonatal isoform of the skeletal muscle chloride channel; this imbalance results in insulin resistance and significant reduction in chloride conductance, causing myotonia.
The diagnosis is suspected if the mother has clinical or EMG features of DM1 or if she has a confirmed genetic diagnosis, and it is made in the newborn by identifying the abnormally expanded CTG repeat in the DMPK gene.
Management of the affected infant is, in considerable part, an ethical decision. For optimal development, adequate nutrition and ventilation must be ensured, and respiratory support and tube feedings may be needed for days to weeks. Survivors usually are able to suck and swallow adequately for oral nutrition by 8 to 12 weeks of age. When ventilatory support is required for more than 3 to 4 weeks, the mortality rate exceeds 25%. However, the use of nasal continuous positive airway pressure has facilitated weaning in affected infants after such prolonged mechanical ventilation. In infants with particularly poor gastric motility, metoclopramide, which decreases the smooth muscle threshold for the action of acetylcholine, may be especially useful.
Subsequent problems relate to both the muscle and central nervous system (CNS). Supportive measures for the myopathy are required. Most of the joint deformities often can be managed with a nonsurgical approach, although more aggressive management of the foot and ankle deformities may prevent gait disturbances.
A number of potential molecular therapeutic interventions described include editing of the CTG expanded repeat, inactivation of the toxic mutant transcript, and modulation of MBNL and downstream signaling pathways. It is hoped that treatments for myotonic dystrophy will emerge in the future through ongoing investigation of these therapeutic strategies.
The term congenital muscular dystrophy (CMD) refers to a group of disorders that share clinical and myopathological features. In older patients, the term muscular dystrophy has been used for a group of progressive, inherited disorders of muscle that share a striking myopathology. Although some of the reported cases of CMD do not exhibit definite progression, and a familial nature cannot always be established, the disorder indeed does conform generally to an inherited involvement of muscle, progressing prenatally (and ultimately also postnatally) and sharing a myopathology similar to that of later-onset dystrophies.
Classification of CMDs has become particularly difficult, because in the past 25 years or so, largely through molecular genetic studies, increasing numbers of clinical phenotypes and responsible genes have been recognized. To date, pathogenic variants have been detected in 18 different genes. We have elected to use a fundamentally clinical categorization, and we focus on those disorders that may manifest in the neonatal period ( Table 37.2 ). We find it most useful to distinguish those CMD disorders that involve primarily muscle (i.e., without overt CNS abnormalities; e.g., manifested by major neurological deficits) from those CMD disorders that are accompanied by overt CNS abnormalities (with such associated deficits as intellectual disability). However, CMDs have been classified based on a combination of clinical, biochemical, pathological, and genetic features (see Table 37.2 ). Table 37.2 lists the main genes mutated in various CMDs and the respective phenotypes. We focus on those disorders that are most likely to be associated with a clear neonatal presentation—primary, merosin-deficient CMD—and the three major CMDs with overt CNS abnormalities: Walker-Warburg syndrome (WWS), muscle-eye-brain disease (MEB), and Fukuyama CMD. We describe only briefly those disorders that are particularly unusual or that do not generally result in marked neonatal features (i.e., Ullrich syndrome, congenital laminopathy, and rigid spine syndrome).
| BIOCHEMICAL DEFECT | LOCUS | GENE | DISEASE PHENOTYPE(S) |
|---|---|---|---|
| Extracellular matrix proteins | |||
| 6q22–23 | LAMA2 | Primary merosin deficiency (MDC1A) | |
|
|
Ullrich CMD | |
| Basal membrane proteins | |||
| 12q13 | ITGA7 | Integrin α7-related CMD | |
| 3p23–21 | ITGA9 | Integrin α9-related CMD | |
| Primary dystroglycanopathy | 3p21 | DAG1 | LGMD with early onset and intellectual disability, normal brain MRI |
| Secondary dystroglycanopathies | |||
| 9q34.1 | POMT1 | WWS, MEB, CMD with cerebellar involvement, CMD with intellectual disability and microcephaly | |
| 1q32–34 | POMGnT1 | WWS, MEB, CMD with cerebellar involvement | |
| 14q24.3 | POMT2 | WWS, MEB, CMD with cerebellar involvement, CMD with intellectual disability and microcephaly, CMD without intellectual disability | |
| 19q13.3 | FKRP | WWS, MEB, CMD with cerebellar involvement, CMD with intellectual disability and microcephaly, CMD with no intellectual disability and normal brain MRI | |
| 9q31 | FCMD | Fukuyama CMD, WWS, CMD without intellectual disability | |
| 22q12.3–13.1 | LARGE | WWS, MEB, white matter changes, CMD with intellectual disability | |
| 1q12–q21 | DPM2/DPM3 | CMD with intellectual disability and severe epilepsy | |
| 7p21.2 | ISPD | WWS, LGMD, polycystic kidneys | |
| 3p22.1 | GTDC2 | WWS | |
| 11q13.2 | B3GALNT2 | MEB, WWS, CMD with intellectual disability, epilepsy | |
| 3p21.23 | GMPPB | CMD with intellectual disability and severe epilepsy, MEB, LGMD, isolated CK elevation | |
| 8p11.21 | SGK196/POMK | MEB, WWS, LGMD | |
| Endoplasmic reticulum protein | |||
| 1p35–36 | SEPN1 | CMD with spinal rigidity (RSMD1) | |
| Nuclear envelope proteins | |||
| 6q25 | SYNE1 (nesprin 1) | CMD with adducted thumbs | |
| 1q21.2 | LMNA | Congenital laminopathy | |
| Sarcolemmal and mitochondrial membrane protein | |||
| 22q13 | CHKB | Mitochondrial CMD (CMDmt) | |
| 21q22.3 |
|
Ullrich CMD | |
| 2q37.3 | COL6A3 | Ullrich CMD |
CMD caused by a primary deficiency of merosin (i.e., laminin alpha-2 chain; see later), MDC1A, accounts for approximately 20% to 30% of cases of CMD (see Table 37.2 ). In 1979 an initial series of five infants with CMD was reported with no clinical signs of CNS disease (except for one with seizures) but with marked hypodensity of cerebral white matter on CT. Over the next decade, subsequent reports of a similar relationship between CMD and abnormal cerebral white matter, primarily on CT scans, were published. With the discovery that this disorder is associated with a deficiency of a critical basal lamina component (i.e., the laminin alpha-2 chain), recognition of the relative frequency of the disorder increased dramatically. The clinical presentation with weakness and hypotonia in merosin-deficient CMD is more severe than in other merosin-positive CMDs. Indeed, motor function usually does not evolve past sitting or standing with orthotic support; ambulation is hardly ever achieved. Contractures are common and involve the hips, knees, elbows, and ankles, although severe neonatal arthrogryposis is rare. Nevertheless, in our experience, merosin-deficient CMD and congenital myotonic dystrophy are the two most common muscle disorders leading to multiple congenital contractures. Weakness tends to affect the upper limbs more severely than lower limbs. Facial weakness is often prominent. A typical clinical sign is limitation of eye movements, particularly of the upgaze, but this sign is often not noted until the second decade of life. Disturbance of chewing and swallowing, with a tendency for aspiration, is very common; a feeding tube may be needed. In a recent database search, 41% of patients with laminin alpha-2 related CMD had cardiac abnormalities, primarily left ventricular systolic dysfunction and arrhythmias. As many as 20% to 30% die of cardiopulmonary complications in the first year or so of life. A less severe phenotype, associated with partial deficiency of merosin , has been reported. However, these patients usually have normal milestones in the first years of life and usually do not require enteral feeding and/or ventilator support.
Despite the uniform occurrence of diffusely abnormal cerebral white matter by brain imaging in merosin-deficient CMD (see later discussion), clinical neurological abnormalities are unusual. Approximately 20% to 30% of patients develop seizure disorders, usually later in childhood, and approximately 5% to 10% of patients exhibit cognitive deficits.
Muscle biopsy shows dystrophic changes, and immunofluorescence with antimerosin antibodies demonstrates decreased or absent staining; in severe infantile cases, there is total absence or only traces of the laminin alpha-2 chain protein. Sometimes, an extensive inflammatory infiltrate may be seen.
The diagnosis of merosin-deficient CMD is confirmed by detecting a recessive pathogenic variant(s) (homozygous or compound heterozygous state) in the laminin alpha-2 chain (LAMA2) gene.
Partial merosin deficiency may occur in the setting of other CMDs, such as dystroglycanopathies. CMD with secondary deficiency of merosin is rare in the neonatal period and likely represents a heterogenous disorder. Formerly, the best characterized of this group was CMD related to FKRP mutations (see later). However, the more recent recognition that this disorder may be associated with overt CNS abnormalities makes it more appropriate to discuss later.
Merosin-positive CMD encompasses multiple clinical phenotypes, but the three most likely to be encountered in the neonatal period are Ullrich CMD, congenital laminopathy, and rigid spine syndrome (see Table 37.2 ). The term classic merosin-positive CMD has become obsolete as molecular genetic studies characterize distinct disorders.
Ullrich CMD is a type of merosin-positive CMD with a characteristic clinical phenotype. Neonatal presentation with hypotonia, weakness, proximal joint contractures, torticollis, hip dislocation, spine kyphosis, and marked distal hyperlaxity is characteristic. In older infants and children, other typical features include prominent calcanei, keratosis pilaris, and hypertrophic scars (keloids). Subsequent motor development is severely delayed, and walking independently occurs in the minority. The defect involves the gene COL6A , which encodes collagen VI, an extracellular matrix protein. Collagen VI is composed of three alpha chains encoded by three genes: COL6A1, COL6A2 , and COL6A3 . Ullrich CMD is caused by either autosomal recessive or dominant mutations in any one of these three genes. Most neonatal onset cases are autosomal recessive.
Congenital laminopathy is another CMD (L-CMD) related to recessive or dominant de novo mutations in the lamin A/C gene (LMNA) (see Table 37.2 ). LMNA mutations cause the dominant form of Emery-Dreifuss muscular dystrophy. Early onset in infancy has been described with severe axial weakness, wasting of cervical-axial muscles, limited spontaneous movement, “dropped head” syndrome, as well as cardiac arrhythmias and feeding and respiratory difficulties. Muscle biopsy usually shows a dystrophic pattern and sometimes inflammatory infiltrates.
Rigid spine syndrome is a third merosin-positive CMD that usually presents clinically after the neonatal period with hypotonia and axial weakness. Neonatal onset has been reported. Subsequently, the characteristic spinal extensor contractures become apparent, with resulting spinal rigidity, scoliosis, and restrictive respiratory disease. The defect involves the gene SEPN1 for an endoplasmic reticulum protein, selenoprotein N, the function of which is unknown.
CMD may be accompanied by prominent involvement of the CNS, manifested by severe neurological deficits (e.g., intellectual disability; see Table 37.2 ). Although primary merosin deficiency is associated with a morphological disturbance of cerebral white matter (see later), no clinical neurological abnormalities occur in most cases. Because these disorders are related to abnormalities of glycosylation of alpha-dystroglycan, they are often grouped as dystroglycanopathies . The term dystroglycanopathies includes a group of muscle diseases, with hypoglycosylation of alpha-dystroglycan on muscle biopsy, often associated with central nervous system structural abnormalities and less frequently with eye pathology. Some of the entities in this category are associated with disorders of neuronal migration, cerebral white matter abnormalities, hindbrain anomalies, and marked clinical neurological abnormalities and are termed Fukuyama CMD (FCMD), WWS, and MEB. There are also mild dystroglycanopathies with late onset and no structural or functional brain or eye involvement, which will not be discussed here. The Online Mendelian Inheritance in Man (OMIM) database subdivides the group of muscular dystrophies with deficit of dystroglycan glycosylation or muscular dystrophy–dystroglycanopathy (MDDG) into three broad phenotypic groups, A, B, and C. MDDGA is the most severe phenotype and includes patients with WWS, MEB, and Fukuyama CMD who display eye, muscle, and brain anomalies with associated intellectual disability. Patients with MDDGB have primarily an intermediate muscle phenotype with various degrees of associated intellectual disability but without eye or brain structural defects. Patients with MDDGC present with limb-girdle muscular dystrophy usually of late onset. Primarily the type A severe phenotypes will be discussed here.
Fukuyama CMD (see Table 37.2 and Table 37.3 ) has been described in several hundred Japanese children and in a much smaller number of children in North America, Europe, and Australia. The disorder exhibits the same basic clinical features referable to muscle involvement as the CMDs without CNS involvement: (1) onset in the neonatal period, (2) marked diffuse weakness and hypotonia, (3) facial weakness, and (4) joint contractures that develop particularly in infancy after the neonatal period. The clinical course of the motor function is characterized generally by slow acquisition of skills, usually the ability to crawl or to stand with support, until approximately the age of 8 years, when motor functions begin to deteriorate, ultimately to a lower level. The progression relates both to worsening of contractures and to apparent increasing weakness. Most patients become bedridden by the age of 10 years, and life expectancy is usually approximately 20 years.
The hallmark of Fukuyama CMD is the evidence of major CNS disease. Intellectual disability is essentially constant, and the usual IQ level is between 30 and 50. Moreover, febrile and afebrile seizures occur in approximately 60% of the cases. CT and, in particular, MRI scanning and neuropathological studies (see subsequent discussions of diagnosis and pathology) establish the causes of these neural phenomena; that is, major neuronal migrational and other abnormalities.
Walker-Warburg syndrome is the more severe of the two CMDs with prominent ocular as well as CNS abnormalities (see Table 37.2 , Table 37.3 ) (see Chapter 6 ). Indeed, the life expectancy in this disorder is less than 3 years. In the newborn, the muscle disorder is often overshadowed by the severe CNS (cobblestone lissencephaly, pontocerebellar hypoplasia, severe white matter abnormality) and ocular (microphthalmia, hypoplastic optic nerves, colobomata, retinal detachment) abnormalities. Severe disturbances of feeding and tube or gastrostomy feeding are nearly invariable.
| TYPE OF CMD | |||
|---|---|---|---|
| CLINICAL FEATURE | FUKUYAMA | WALKER-WARBURG | MUSCLE-EYE-BRAIN |
| Neurological deficits | + | + | + |
| Microcephaly | + | – | – |
| Macrocephaly | – | + | + |
| Hypotonia | + | + | + |
| Ocular abnormalities (severe) | – | + | + |
| White matter abnormality on neuroimaging (persistent or progressive) | + | + | – |
| Lissencephaly pachygyria | + | + | + |
| Cortical mantle thin | ± | + | – |
| Corpus callosum absent | ± | + | – |
| Hydrocephalus | – | + | ± |
| Encephalocele | – | + a | – |
| Cerebellar malformation | – | + | ± |
a Characteristic when present but described in less than 50% of cases.
Muscle-eye-brain disease is a similar CMD, with ocular and CNS abnormalities, although it is milder than WWS (see Table 37.2 and Table 37.3 ). Thus an initial report from Finland described 18 affected patients with the clinical features referable to CMD, intellectual disability of variable severity in 15 of the 18 reported patients, “occasional” seizures, and, distinctively, a wide variety of ocular abnormalities. These ocular defects included high myopia, congenital or infantile glaucoma, hypoplasia of the retina and optic nerve, coloboma of the optic nerve, and cataracts. Many subsequent reports further delineated this type of CMD.
Although patients with MEB disease that is overt at birth may exhibit severe abnormalities, in general the features of this disorder are milder than those observed with WWS, and both of these cerebro-ocular types of CMD exhibit differences from Fukuyama CMD (see Table 37.3 ).
There is also a fourth group of patients with CMD (OMIM classification Type B) with posterior fossa/cerebellar abnormalities on imaging or a normal MRI; these patients may be intellectually normal or have an associated intellectual disability. In this group, cerebellar abnormalities may be the only structural brain defects and include cerebellar cysts, hypoplasia, or dysplasia. Although supratentorial defects may also occur, cerebellar cysts in the absence of supratentorial white matter or cortical involvement have been described mostly in patients with mutations in the fukutin-related protein (FKRP) gene. Although FKRP-related CMD usually manifests beyond the neonatal period and is not considered in detail, neonatal onset is well documented. Weakness and hypotonia are apparent in the first weeks or months of life, although a phenotype as severe as primary deficiency of merosin has been observed. Subsequent motor development is markedly impaired, and respiratory muscle weakness leading to ventilatory failure occurs in the first or second decade of life. In neonatal-onset cases, severe cognitive deficits are common. Muscle biopsy shows a partial deficiency of laminin alpha-2 protein and thus this disorder previously was classified as CMD with secondary deficiency of merosin. However, the principal defect involves fukutin-related protein, presumed to be a glycosyl transferase, with alpha-dystroglycan the likely substrate. More than half of 25 reported cases with FKRP mutations have had prominent CNS abnormalities and thus should be included with the CMD group with overt CNS abnormalities. The neurological abnormalities have included intellectual disability, and the CNS structural deficits have included subependymal heterotopias, focal pachygyria, white matter abnormalities, cerebellar cysts, marked cerebellar dysplasia, and pontine hypoplasia. These CNS deficits are consistent, at least in part, with neuronal migrational disturbance and overlap with those observed in Fukuyama CMD, WWS, and MEB disease. This similarity is noteworthy because, as discussed earlier, the latter three types of CMD with overt CNS abnormalities all also involve disturbances of glycosylation of alpha-dystroglycan.
When all types of CMD (see Table 37.2 ) are considered, the serum CK level is elevated in most cases, particularly early in the course of the disease. However, the presence and degree of elevation vary with the type of disorder. In the largest series of cases ( n = 50) of the now obsolete entity known as merosin-positive classic (pure) CMD , 54% of infants had elevated CK levels, and the average elevation was approximately three times the upper limit of normal. In another series of 24 cases, CK elevation was 2.5-fold above the normal upper limit. The important clinical point is that a normal CK level does not exclude the diagnosis. By contrast, in merosin-negative CMD , the CK levels are consistently and markedly elevated to approximately 10-fold to 20-fold higher than the upper limit of normal. In Fukuyama CMD, CK levels generally range from 10-fold to 50-fold higher than the normal upper limit. Levels are similarly very high in MEB disease and in WWS. In all cases, CK levels tend to decline with the patient’s age. The EMG reveals the changes of myopathy (see Table 34.4 ), including small-amplitude, brief-duration, and polyphasic motor unit potentials.
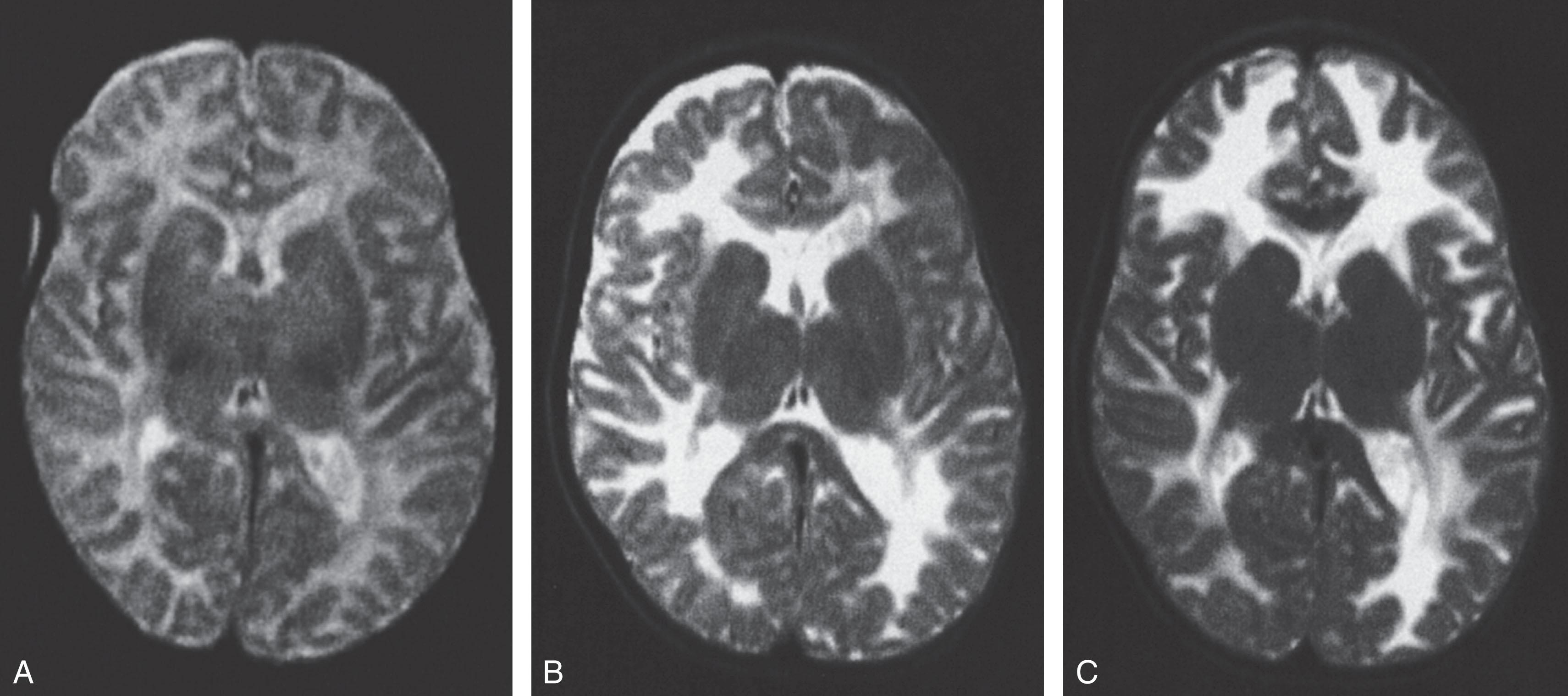
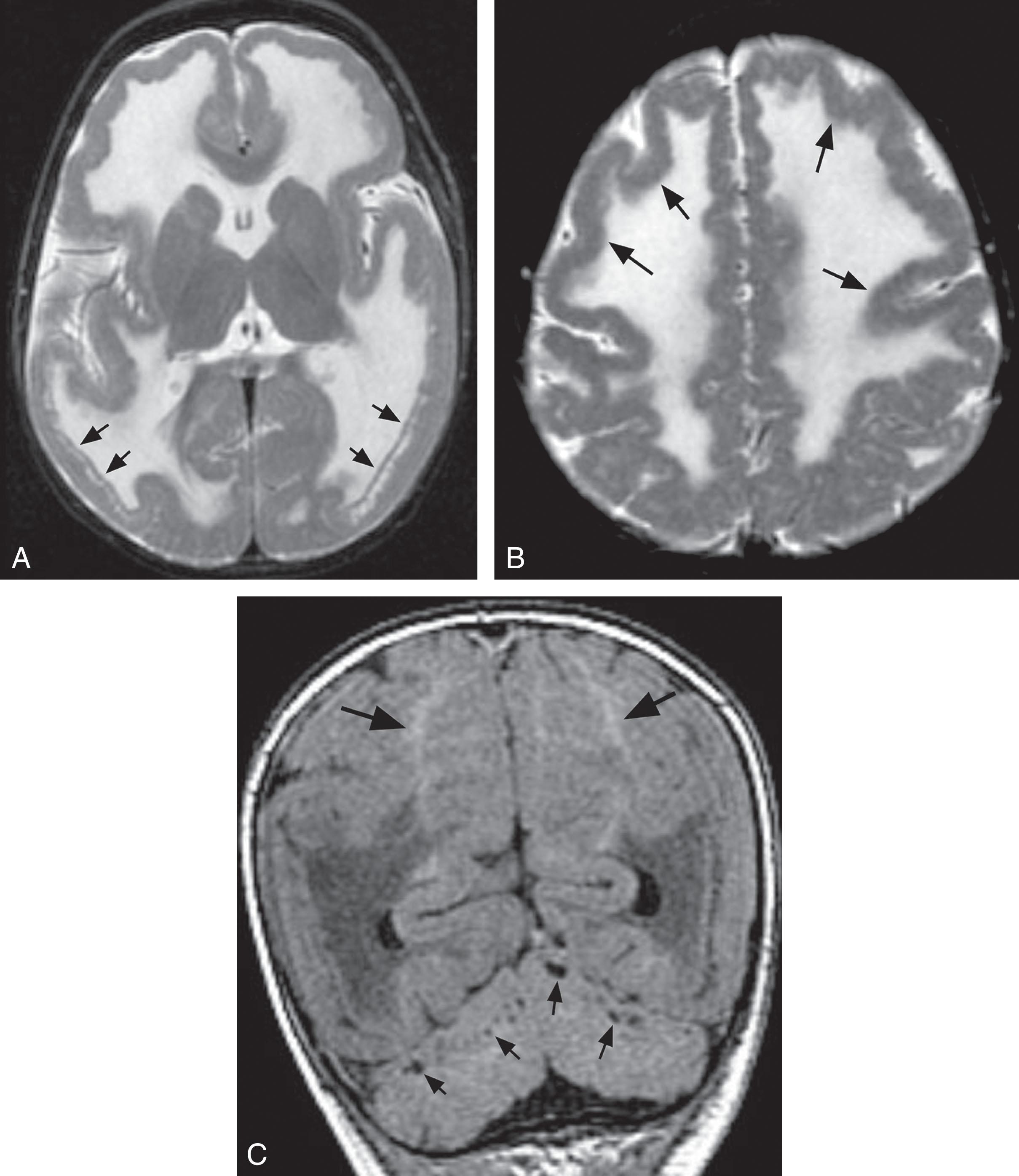
CT and, preferably, MRI scans of the head are useful in identification of the cerebral white matter abnormality characteristic of primary merosin-deficient CMD ( Fig. 37.8 ). The appearance on CT is decreased attenuation of cerebral white matter, and on MRI, increased signal on T2-weighted images. The cerebral white matter is affected diffusely, and the cerebellar white matter is unaffected. The cerebral white matter abnormality generally is not apparent in the first weeks of life and becomes apparent by approximately 6 months of life (see Fig. 37.8 ). However, utilization of T2-weighted images with a fast spin-echo sequence has demonstrated the white matter abnormality at 5 days of age. The abnormal white matter signal is most prominent in structures myelinated after birth (e.g., cerebral white matter), rather than in those structures myelinated before birth (e.g., brainstem and cerebellar white matter). Thus the abnormality may reflect a delay or arrest in myelination, and the finding in the affected white matter of abnormally increased apparent diffusion coefficients, characteristic of immature white matter, is perhaps consistent with this notion. Although the cerebral white matter abnormality is the unifying feature of primary merosin-negative cases, several reports documented the occurrence of cortical gyral dysplasia, especially in the occipital region, in isolated cases (~<10%). Hypoplasia of the cerebellum was observed by MRI to varying degrees in 6 of 14 well-studied cases, and associated cerebellar cysts have also been described.
In Fukuyama CMD , the most striking abnormality is the gyral disturbance ( Fig. 37.9 ). The two major findings detectable by MRI are as follows: (1) a thick cortex with shallow sulci in the frontal regions, consistent with polymicrogyria, and (2) a thick cortex with a smooth surface in the temporo-occipital regions, consistent with lissencephaly pachygyria. These findings correlate well with the neuropathology (see later discussion). Additional consistent features are white matter changes similar to those described earlier for merosin-deficient CMD. However, distinction of these two entities by MRI is straightforward because of the marked gyral abnormalities in Fukuyama CMD. Moreover, the white matter abnormality in the latter diminishes with age. The corpus callosum is slightly hypoplastic in most cases of Fukuyama CMD.
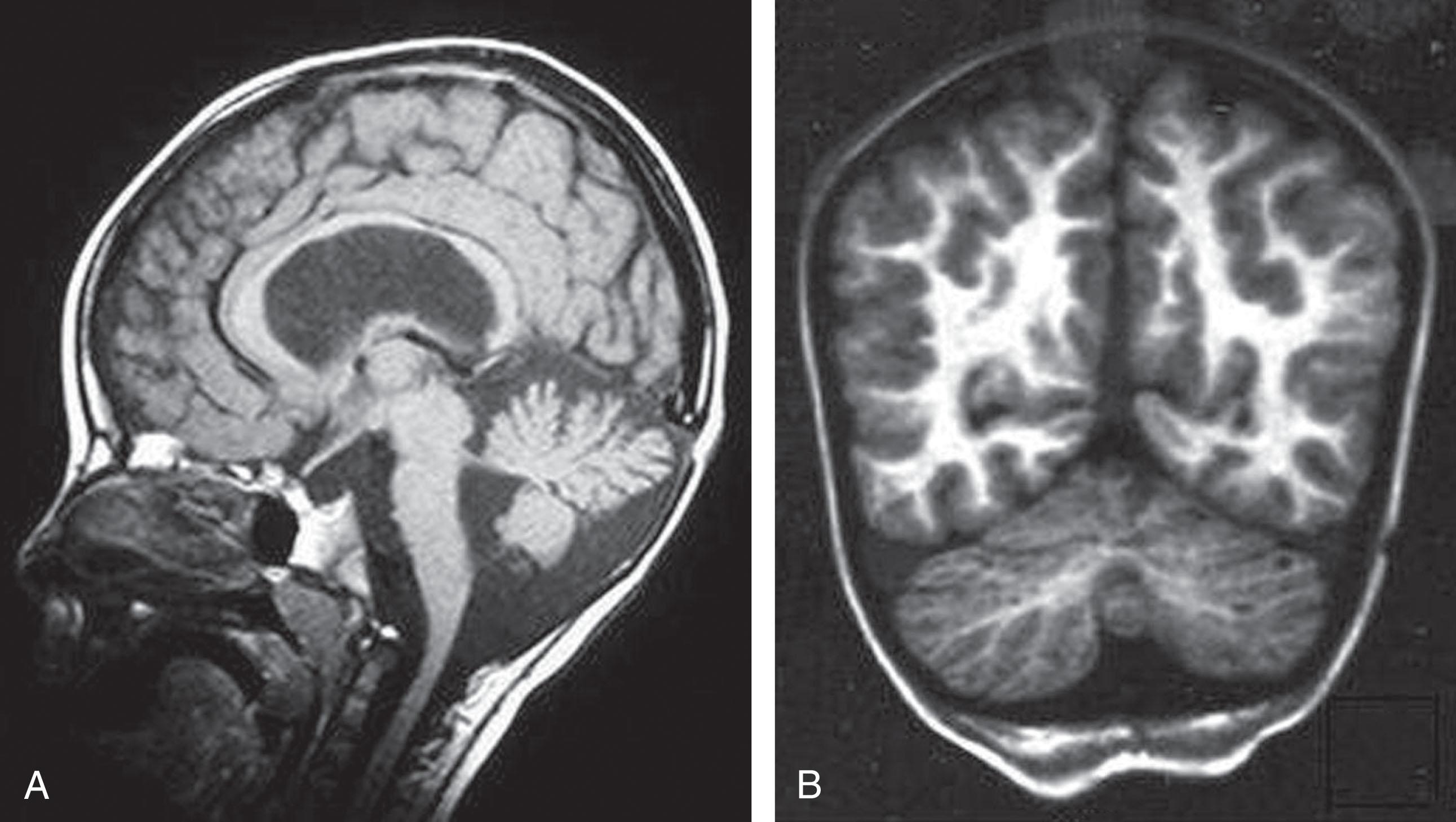
In WWS and MEB disease , the dominant finding is the gyral abnormality. In its full form, as in WWS, the appearance of type II lissencephaly (“cobblestone” lissencephaly) and pachygyria is striking and is associated with diffuse white matter abnormality of the type observed in merosin-deficient CMD. Again, the distinction by MRI from the latter disorder is straightforward because of the presence of the gyral disturbance, as well as marked dysgenesis of cerebellum and hypoplasia of the pons, especially in WWS.
Additional laboratory studies of interest include measurements of cerebral visual and somatosensory evoked responses and of peripheral nerve conduction velocities in merosin-deficient CMD ( Table 37.4 ). Thus for somatosensory evoked responses, latencies have been delayed in all patients studied, and for visual evoked responses, latencies have been delayed in approximately 90% of patients. Nerve conduction velocity studies showed moderately slow values in more than 80% of merosin-deficient cases studied thus far. One infant studied in the newborn period had normal values but exhibited slowed conduction when studied again at 6 months of age. No abnormalities in cerebral evoked responses or motor nerve conduction velocities have been observed in merosin-positive CMD.
| FEATURES | ||
|---|---|---|
| Clinical severity | Marked | |
| Ambulation | Rare | |
| Creatine kinase elevation | Marked | |
| Myopathology | Severe | |
| Nerve conduction velocity | Slow | |
| White matter abnormality | Marked | |
| Structural brain abnormalities | ≈< 10% | |
| Epilepsy | ≈20% | |
| Chromosomal locus | 6q2 | |
| Laminin alpha–2 (merosin) | Absent b | |
b Partial but not total deficiency of laminin alpha-2 chain is associated with less severe phenotype (see text).
The demonstration of laminin alpha-2 deficiency in skin biopsy and in cultured fibroblasts in primary merosin-deficient cases indicates the potential value of this approach for the diagnosis of merosin-deficient CMD. Additionally, the prenatal demonstration of merosin deficiency in trophoblastic tissue obtained from chorionic villus samples indicates the value of this approach (with simultaneous linkage analysis) in prenatal diagnosis.
Muscle biopsy in all types of CMD shows striking changes, consistent with a dystrophic process. Notable are marked variation in fiber size in a nongrouped distribution, internal nuclei, and marked replacement of muscle by fat and connective tissue ( Fig. 37.10 ). Necrosis of fibers and evidence of regeneration are common. Striking changes not apparent on initial biopsies may appear on subsequent biopsies. The fiber type pattern is retained; the muscle does not exhibit the prominent signs of maturational disturbance observed in myotonic dystrophy (see earlier discussion). Although comparisons are difficult, the myopathic changes reported in primary merosin-deficient CMD are more striking than those in merosin-positive CMD. Several reports have emphasized the presence of inflammatory cellular infiltrates, especially early in the disease and perhaps related to necrosis; this finding should not lead to the mistaken diagnosis of infantile polymyositis, a treatable and self-limited disorder (see later discussion). The diagnostic feature critical for merosin-deficient CMD is the lack of staining for this molecule on immunocytochemical study ( Fig. 37.11 ). Partial absence is observed in the infants with the less severe, partial type of merosin-deficient CMD or in secondary merosin deficiencies.
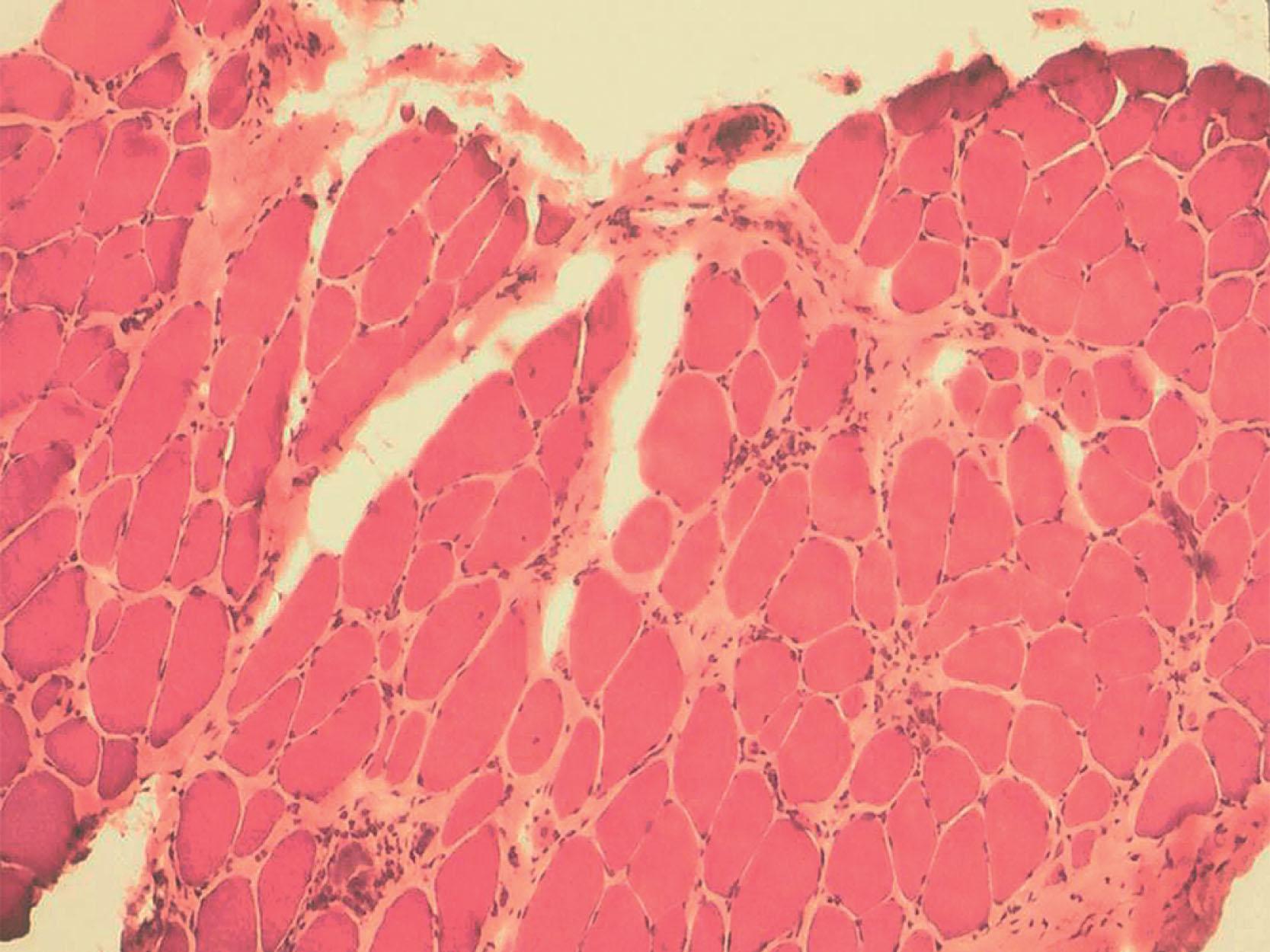
The neuropathology in infants with merosin-deficient CMD with cerebral white matter abnormalities is not clearly known. The little neuropathological information available is inconclusive. The finding that the white matter abnormality becomes most apparent later in infancy and is associated with disturbances of visual and somatosensory evoked potentials and the MRI findings suggesting a defect in postnatal myelin development (see earlier discussions) suggest that myelination is altered in some way. Laminin alpha-2 chain is expressed in the basement membrane of blood vessels that form the blood-brain barrier and also along developing axonal tracts where interaction with oligodendrocytes may occur. Disturbances at either or both loci could lead to disturbed myelin development and the abnormal signal with increased water diffusion on MRI or the “edema” by MR spectroscopy.
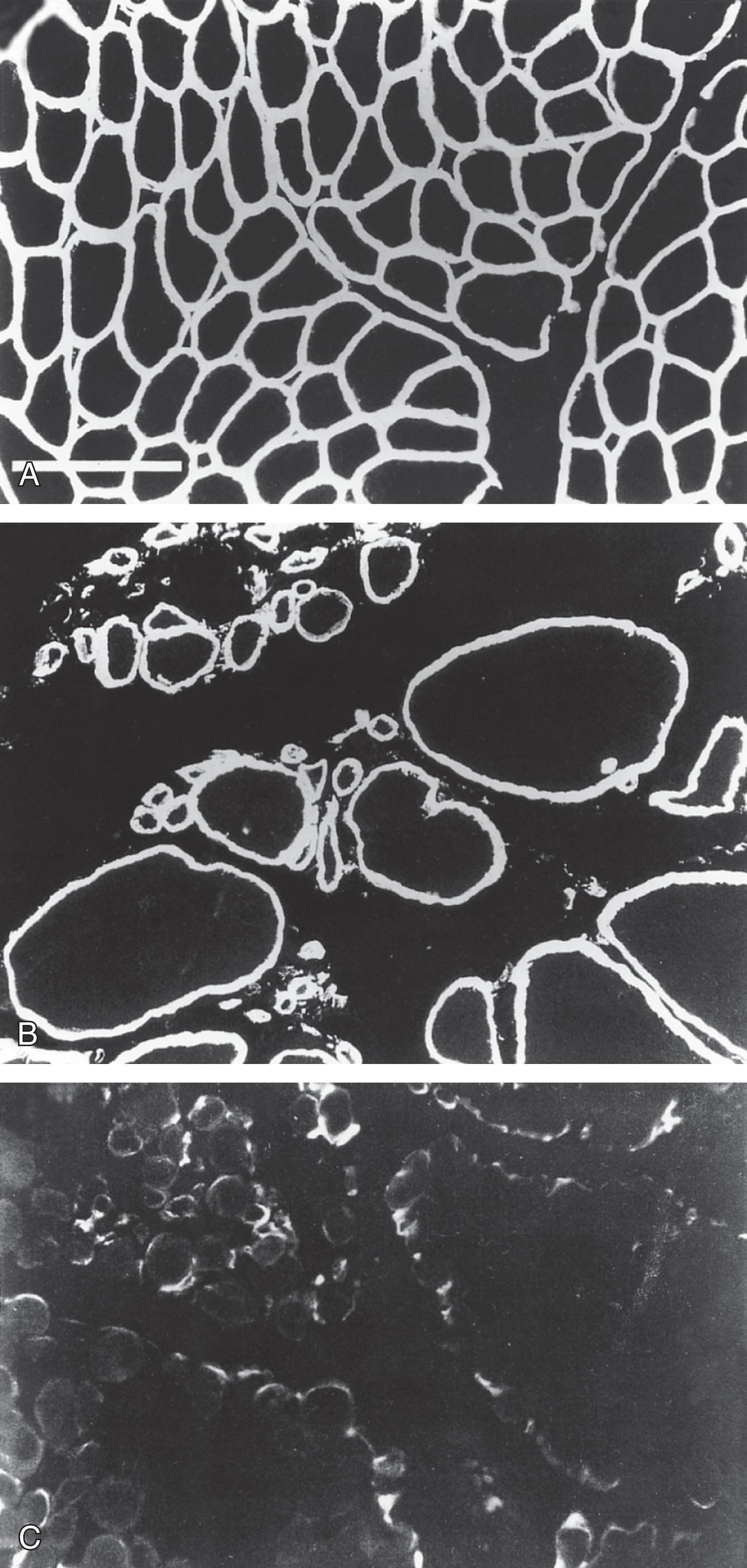
The neuropathology in infants with Fukuyama CMD is striking. The dominant finding is cerebral gyral abnormality, most consistently polymicrogyria ( Fig. 37.12A ), pachygyria, or agyria (type II lissencephaly). The disorder indicates a disturbance of neuronal migration, as described in Chapter 6 . Other neuropathological abnormalities include subpial neuronal-glial heterotopias, cerebellar polymicrogyria ( Fig. 37.12B ), aberrant myelinated fascicles over the surface of the cerebellum, hypoplasia and aberrant course of the pyramidal tracts, leptomeningeal thickening, and occasional hydrocephalus. No distinct changes have been described in cerebral white matter, except for a delay in myelination, and hence the basis for the white matter abnormality detected by CT or MRI is unclear (see earlier discussion).
The neuropathology in the two CMDs with prominent ocular and brain abnormalities has been described. The findings bear similarities to those described in Fukuyama CMD, including the following: type II lissencephaly; polymicrogyria, pachygyria, and heterotopias in the basal meninges; hypoplasia of the pyramidal tracts; and aqueductal stenosis. Distinguishing features in the more severe form of cerebro-ocular CMD, WWS, include more severe cortical migrational disturbances, hydrocephalus, cerebellar dysplasia-hypoplasia (including absence of vermis), and encephalocele (see Table 37.3 ). In general, the findings in MEB disease are milder than those in WWS.
The pathogenesis and etiology of this group of disorders focus on the sarcolemma membrane and the related proteins that link the extracellular matrix to the actin filaments of the myocytic cytoskeleton. The key sarcolemma protein involved in linking to the extracellular matrix is alpha-dystroglycan; the latter interacts with the key extracellular matrix protein, laminin, which, in turn, interacts with collagen type VI ( Fig. 37.13 ). The intracellular connection is mediated by the interaction of alpha-dystroglycan with the sarcolemma proteins, sarcoglycans, which are linked to dystrophin and thereby to the actin cytoskeleton (see Fig. 37.13 ). In the CMDs, the key proteins affected are alpha-dystroglycan, laminin , and collagen type VI , defects of which interrupt the vital relationship between the extracellular matrix and the muscle cytoskeleton and result in the muscle degeneration of CMD.
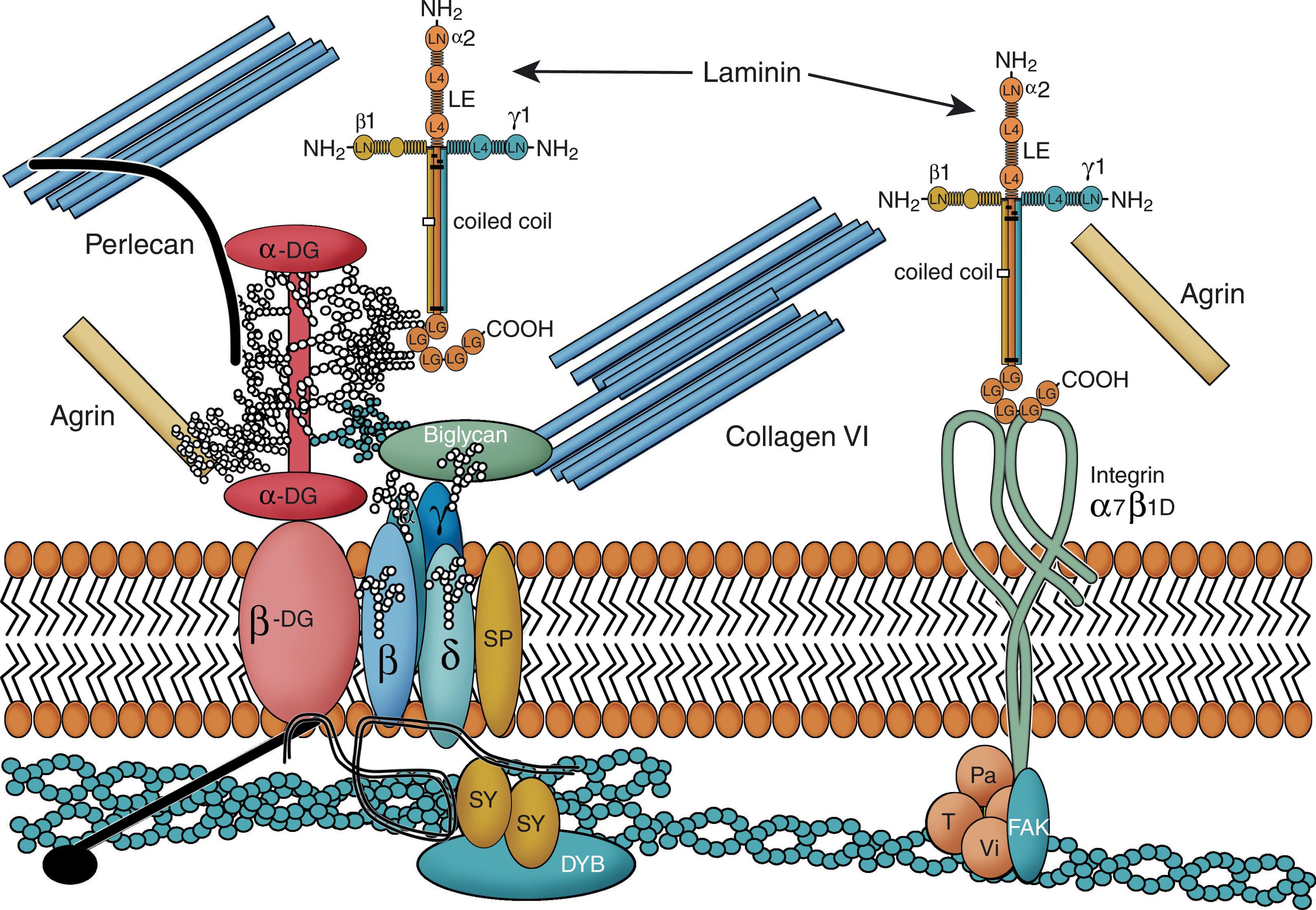
The molecular basis of primary merosin-deficient CMD is well established. Laminin-2 or merosin is the critical alpha-dystroglycan–binding basement membrane protein of the muscle extracellular matrix (see Fig. 37.13 ). The critical laminin alpha-2 chain of laminin 2 is encoded by the LAMA2 gene on chromosome 6q2, and this is the gene defective in merosin-deficient CMD. Laminin alpha-2 chain is also present in basement membrane of Schwann cells, and thus its absence may underlie the defect of peripheral nerve described earlier. Moreover, as noted earlier, laminin alpha-2 is present in blood vessels and along developing axons in human brain (see earlier). How these observations relate to the cerebral white matter abnormality remains to be clearly elucidated. Demonstration of the deficient protein in muscle and skin facilitates diagnosis in the infant, and demonstration of the defect in chorionic villus samples facilitates prenatal diagnosis.
The molecular basis of classic (pure) merosin-positive CMD is heterogeneous and reflects the nature of the underlying genetic defects.
As discussed earlier, the molecular basis of Ullrich CMD involves the three genes (COL6A1, COL6A2, COL6A3) for collagen VI (see earlier). This defect results in a prominent loss of the basal lamina of muscle fibers and interrupts the vital link between the extracellular space and the intracellular cytoskeleton, as described earlier.
The molecular basis of rigid spine syndrome involves a deficiency of the gene selenoprotein N (SEPN1) , an endoplasmic reticulum protein (see earlier). The function of this protein remains elusive; however, it may be involved in protection of cells against oxidative stress.
The molecular bases for the CMDs with overt CNS abnormalities (i.e., Fukuyama CMD, WWS, MEB disease , and others), the dystroglycanopathies, all involve defects in dystroglycan and multiple glycosyl transferase genes catalyzing the O -linked glycosylation of alpha-dystroglycan (see Table 37.2 ). POMT1 encodes the protein O -mannosyl transferase; POMGnT1 encodes the protein O -mannosyl- N -acetylglucosaminyl transferase. Multiple O -linked glycosylation sites are present in certain serine-threonine rich domains of alpha-dystroglycan. Fukuyama CMD is caused by a defect in a gene (FCMD) that encodes fukutin (FKTN), a putative glycosyltransferase, the exact function of which is yet unclear.
Mutations in the FKTN, FKRP , and ISPD genes can result in severe WWS or MEB phenotypes, in intermediate phenotypes such as those associated with cerebellar cysts or normal brain MRI, or even in milder limb-girdle muscular dystrophies. Although the mutated gene cannot be predicted from the clinical phenotype, there are certain features that may suggest a particular gene or number of genes.
In WWS, although POMT1 was the first genetic defect to be described, mutations in all known dystroglycanopathy genes have been described.
In MEB, mutations have been identified in POMGnT1 (the first linked to MEB), POMT1, POMT2, ISPD, and FKRP.
Cerebellar cysts have been described in association with mutations in FKRP, POMGnT1, and ISPD genes but rarely also may be seen with POMT1 and POMT2 mutations.
Disturbances in glycosylation of alpha-dystroglycan impair its interaction with laminin and with sarcolemmal proteins, especially beta-dystroglycan (see Fig. 37.14 ). Muscle degeneration is the result. Because alpha-dystroglycan also is present in the basement membrane of the glia limitans in the developing cerebral cortex, defective glycosylation results in the disturbances in neuronal migration that underlie the occurrence of cobblestone lissencephaly (see Chapter 6 ). As noted earlier, more than one-half of cases of CMD related to FKRP mutations and fukutin-related protein deficiency, which exhibit CNS abnormalities milder but mechanistically similar to those of the three main disorders discussed here (WWS, MEB, FCMD), are relevant in this context, because the fukutin-related protein also appears to be a glycosyltransferase for alpha-dystroglycan.
Because the course in most patients with any of the types of CMD is nonprogressive for many months to years, it is important to attempt to correct existing contractures by physical therapy (i.e., passive stretching or serial splints) and to prevent the development of contractures by active and passive exercises. The development of fixed deformities is often more deleterious to future motor abilities than is muscle weakness. Although no specific therapies are available, more details concerning supportive management are available in specialized sources.
FSHD is usually recognized as an autosomal dominantly inherited myopathy with prominent involvement of the face and upper extremities, onset in adolescence or early adulthood, and a slowly progressive or apparently static course. A rare type of FSHD with onset in early infancy and a more severe course has been delineated.
The infantile clinical syndrome , first described by Carroll and Brooke in 1979, is characterized by onset in early infancy of facial weakness. The weakness is distinctive, in that most patients are unable to move the upper lip and later have a peculiar horizontal smile. Difficulty in closing the eyes is prominent, and the infant may sleep with the eyes open. The whole face tends to be smooth and expressionless. Difficulty in sucking is common. However, motor milestones usually are not significantly delayed, and 8 of 11 patients in the largest series walked alone by 15 months of age. Later in infancy and early childhood, progressive weakness and wasting of axial and limb muscles supervene, particularly in the proximal upper extremities, but then also in the lower extremities. Of 5 patients who had reached 15 years of age or more, 4 were no longer walking, and the remaining patient was walking with difficulty. A similarly progressive disease course has been documented by other investigators. In a report of 7 infantile-onset cases, the age at onset was between infancy and 4.5 years and the mean duration between onset of first symptoms and wheelchair dependency was 9.9 years. In a recently reported series of patients enrolled in the Italian FSHD Registry, 54.5% showed clinical signs during the first 10 years; however, no patients had perinatal onset. The other clinical accompaniment, observed in the series of Carroll and Brooke, was sensorineural hearing loss in 6 of 11 children; this loss was severe in 2 children. Approximately 50% of subsequently reported cases have exhibited sensorineural hearing loss. Later in childhood, some patients have developed retinal telangiectasia, exudation, and detachment (Coats syndrome) in addition to sensorineural hearing loss. Intellectual disability was observed in 30% of cases in one series, and it was also documented with epilepsy in other cases.
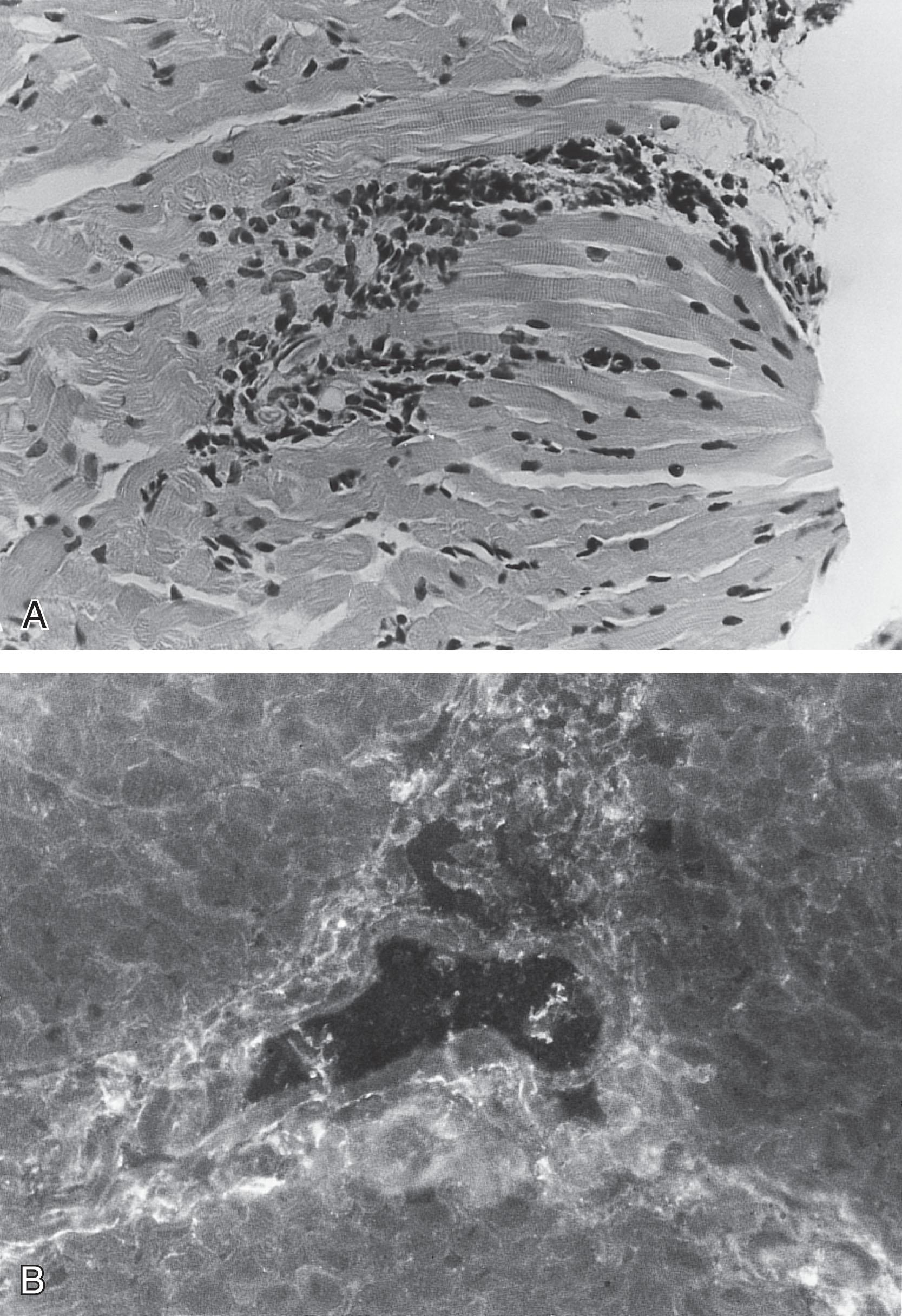
Muscle pathology was striking in six of the nine patients studied in detail by Carroll and Brooke, and it exhibited an inflammatory response suggestive of polymyositis ( Fig. 37.14 ). Inflammatory changes in FSHD have been noted by others. This finding may lead to treatment with steroids, which results in no objective benefit. Indeed, it is clear from the relative frequencies of infantile FSHD and of polymyositis (see later) that inflammatory changes in muscle in an infant should raise the question of FSHD (or other congenital myopathy) rather than polymyositis, especially if the face is particularly involved .
The genetic abnormality in this dominantly inherited disease is located at chromosome 4q35. The frequency of de novo mutations is high. However, as many as one-half of apparently sporadic cases were found to have an affected parent on careful examination. Frequently, parents are carriers with somatic mosaicism of the mutation and thus may appear unaffected. The defect is particularly interesting and involves a reduction in the number of repeats of a 3.3-kb repeat sequence, D4Z4, at the 4q35 locus on one of their chromosomes 4. Normal individuals carry 11 or more D4Z4 repeats (11–100); patients with FSHD have 1 to 10 copies. There is an inverse and coarse relationship between the onset and severity of the disease and the size of the D4Z4 repeat array. Most patients with 1 to 3 D4Z4 units are usually severely affected with seizures, cognitive delay, and hearing loss and are typically sporadic cases. However, approximately only one-half of the contractions are pathogenic because the array needs to be on an FSHD-permissive chromosome 4 (4qA haplotype) that contains a polyadenylation signal for DUX4. This gene is embedded within each D4Z4 repeat and encodes a germline transcription factor. In somatic cells, the D4Z4 repeat is epigenetically repressed, which results in silencing of the DUX4 gene. When the repeat contracts in FSHD1 patients, the epigenetic silencing is released, causing partial D4Z4 chromatin relaxation evidenced by DNA hypomethylation and expression of the DUX4 gene and protein, which is toxic to mature skeletal muscle. In <5% of patients, normal-sized D4Z4 repeat arrays can also be depressed by heterozygous pathogenic variants in the structural maintenance of chromosomes flexible hinge domain containing 1 (SMCHD1) gene on chromosome 18, which encodes for a chromatin modifier; this defect explains the pathogenesis of FSHD2, in which the D4Z4 repeat is of normal size but hypomethylated on both chromosomes 4 and 10. SMCHD1 pathogenic variants in patients with FSHD1-sized D4Z4 repeat arrays of 9 to 10 units cause a more severe phenotype ( Fig. 37.15 ).
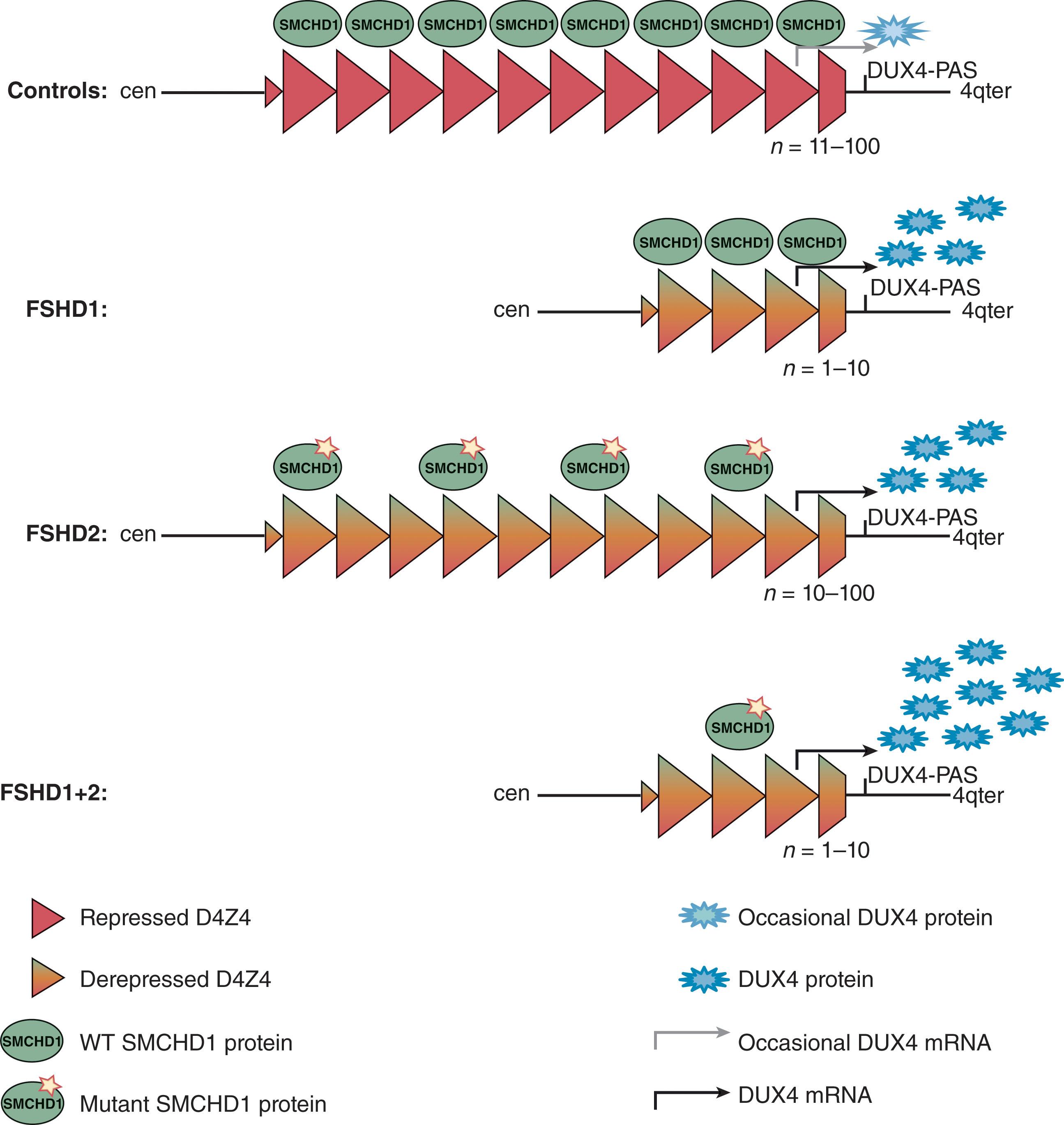
Pathogenic variants in the same SMCHD1 gene have also been linked to the arrhinia microphthalmia syndrome. Two more genes, LRIF1 and DNMT3B , have been associated with FSHD2 as well.
The serum CK level is moderately elevated in nearly all patients. The EMG reveals low-amplitude and short-duration motor unit potentials, indicative of myopathy. Nerve conduction studies are normal. However, these tests have become obsolete because in suspected cases the diagnosis can be confirmed by examination of leukocyte DNA for the D4Z4 repeat contraction and SMCHD1 mutations.
Management is similar to that of progressive myopathy in late childhood and adolescence. Of importance for the physician evaluating the newborn with facial weakness is the recognition that FSHD may be present; family members must be examined and perhaps have their leukocyte DNA evaluated. The parents should be counseled that although the disorder in the affected parent is mild, a distinct possibility exists of a more severe form in their children (i.e., the phenomenon of anticipation).
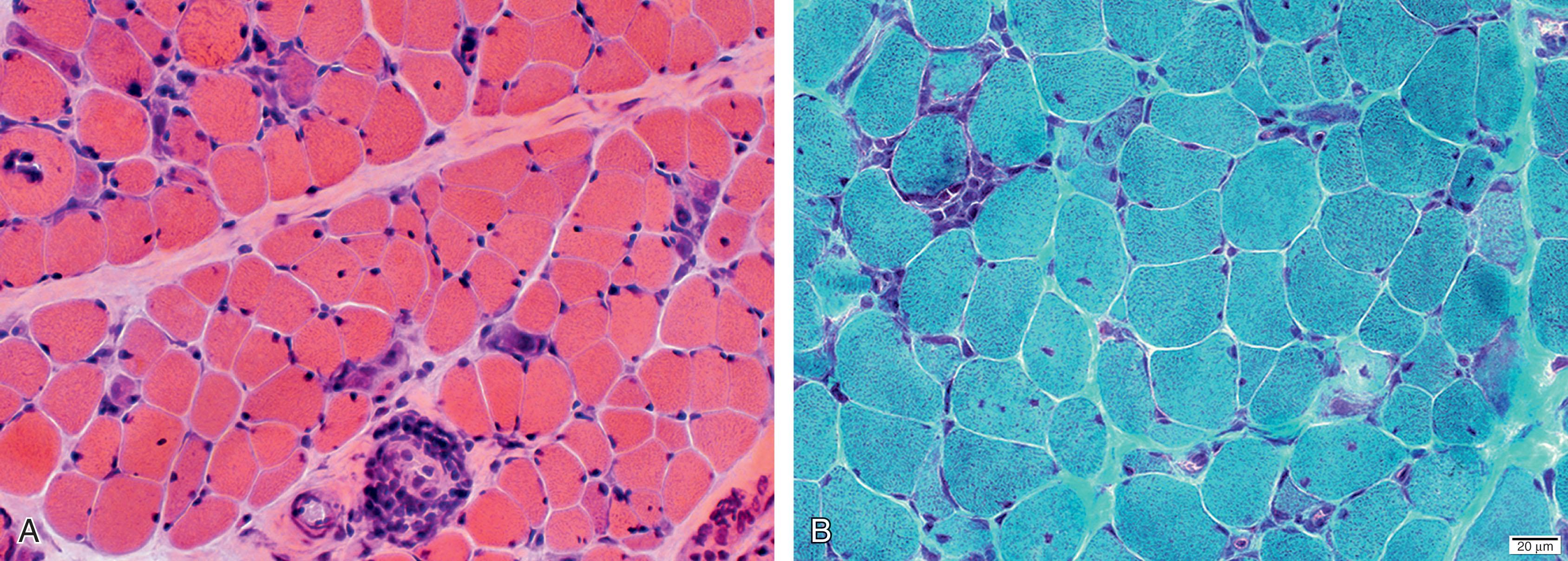
Previously reported in infants no younger than 3 months of age, polymyositis was described in two newborns in 1982. The newborns exhibited marked hypotonia and weakness, weak cry, and poor sucking and feeding ability. Neck flexors were affected markedly, as in older patients with polymyositis. Particularly striking were markedly elevated levels of creatine phosphokinase (CK; 2500 and 3600 mU/mL), and muscle biopsy findings that included lymphocytic infiltration as well as myopathic changes. Improvement in overall strength and decrease in CK levels followed treatment with steroids at 6 and 15 months of age. The observations suggested that polymyositis may occur in the newborn, that the diagnosis should be suspected with the findings of markedly elevated CK levels and inflammatory cells in the muscle biopsy specimens, and that treatment with steroids may be beneficial. Subsequent reports supported these general conclusions. The myopathic changes are consistent with an inflammatory myopathy with an immunological basis ( Fig. 37.16 ). The finding of perifascicular myopathy, generally considered a feature of such autoimmune myopathies as dermatomyositis, is particularly characteristic. It is important to rule out other neonatal myopathic disorders with inflammatory changes (e.g., FSHD, merosin-deficient CMD, or infantile-onset LMNA-associated myopathy), but other features of these latter disorders should make this distinction possible (see earlier discussions).
Minimal change myopathy , or nonspecific congenital myopathy , is the designation used for those infants with congenital hypotonia and weakness who exhibit minor (or even no) abnormalities of serum enzyme levels, EMG, or muscle biopsy. Myopathic illness is inferred from the clinical features (e.g., proximal more than distal weakness, preserved tendon reflexes, normal sensation, and absence of fasciculations), as well as from the occasional myopathic changes observed on the EMG (e.g., small amplitude, brief duration, and polyphasic motor unit potentials) and abnormal muscle biopsy (e.g., modest variation in fiber size). The clinical features are not as prominent as those observed in more common myopathies (e.g., congenital myotonic dystrophy) and in most cases of CMD. Many of these infants have good prognoses and improve over time. Considering the lack of pathological markers, the molecular basis for most of these myopathies may become easier to delineate with the use of extensive panel-based genetic testing. Therefore this category will become increasingly less common.
The histology diagnostic myopathic disorders are characterized by histological changes so striking and distinctive that a specific diagnosis usually can be made. An enormous literature has developed in this area since the 1970s, and several books and reviews deal with the details and complexities of the problem. Because most of the designations for these disorders are derived from histological and not clinical criteria, the clinical syndromes associated with each histological change vary. Moreover, considerable overlap exists among the disorders on both clinical and histological grounds. Nevertheless, certain specific congenital myopathies are well defined clinically, morphologically, and genetically, and several conclusions relevant to the neonatal patient can be drawn.
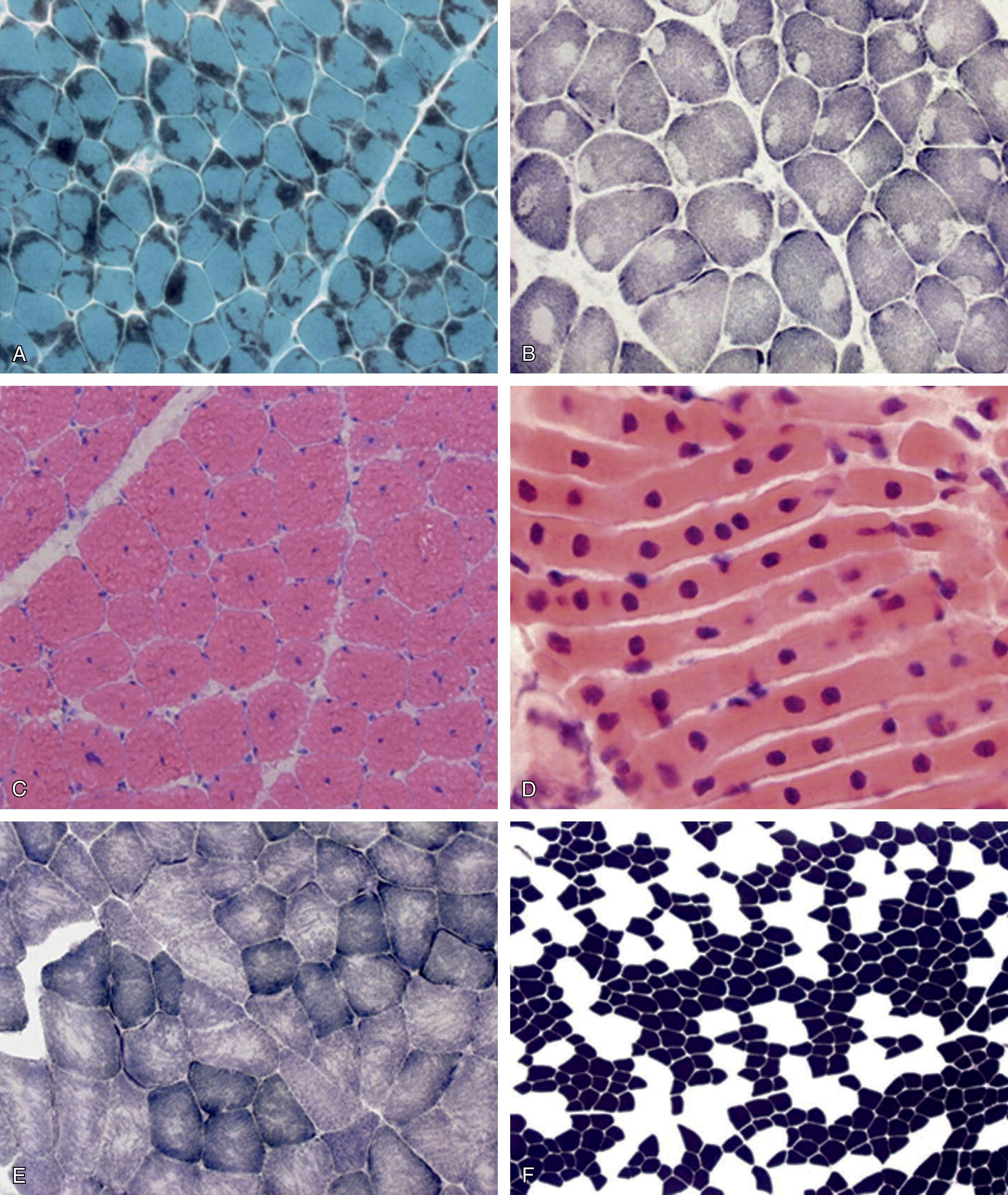
In the following discussion, we emphasize the most common features of each disorder, particularly those disorders with prominent clinical manifestations in the neonatal period. Of the seven categories listed in Box 37.1 , the first five refer to the disorders with morphologically distinctive changes by standard histological and histochemical techniques (i.e., specific congenital myopathies ) ( Fig. 37.17 ). Certain features are commonly observed among these disorders ( Box 37.3 ). Central core disease, nemaline myopathy, myotubular myopathy, and congenital fiber type disproportion are emphasized because, although uncommon, they are the most common in this category. Multiminicore myopathy is rare in the newborn and is described only briefly. The mitochondrial myopathies may be suspected by standard histological techniques, but electron microscopic studies are needed to define the distinctive mitochondrial abnormalities, if present. Biochemical studies may identify a specific abnormality of a mitochondrial enzyme. The metabolic myopathies may show no distinctive morphological change, and thus one could argue that these myopathies should be classified as histology not diagnostic . However, they are included here because the biopsy specimen may indicate glycogen or lipid deposition in muscle, which is usually the critical initial finding in the definition of the specific biochemical lesion.
Inheritance: autosomal dominant > recessive > X-linked recessive
Onset in neonatal period
Recognition often after the neonatal period
Hypotonia
Weakness: proximal > distal
Tendon reflexes decreased in proportion to weakness
Muscle enzymes: normal or mildly/slightly elevated
Electromyogram: myopathic or normal
Muscle biopsy: diagnostic
Genetic testing: may be diagnostic
In core myopathies, the defining pathological feature is the presence of large regions devoid of oxidative staining in the center of myofibers (in central core disease) or the presence of multiple smaller “minicores” (in multiminicore disease). Central cores or minicores may be seen in association with other pathological findings, such as nemaline rods, in the so-called core-rod myopathies often associated with pathogenic variants in various genes, including RYR1 ( Table 37.5 ).
| SUBTYPE | GENE | OMIM DESIGNATION | CHROMOSOME LOCATION | INHERITANCE PATTERN |
|---|---|---|---|---|
| Nemaline myopathy |
|
|
|
|
| Cap disease (NM variant) |
|
|
||
| Zebra body myopathy | ACTA1 | AD | ||
| Hyaline body myopathy (myosin storage myopathy) | MYH7 | 14q11.2 | AD | |
| Core-rod myopathy |
|
|
||
| Central core disease | RYR1 | CCD | 19q13.2 | AD, AR |
| Multiminicore disease |
|
1p36.11 |
|
|
| Centronuclear myopathy |
|
|
|
|
| Congenital fiber type disproportion |
|
|
||
| Autophagic vacuolar myopathies |
|
|
|
|
| Myofibrillar myopathies |
|
|
|
|
Central core disease , originally described by Shy and Magee in 1956, was the first of the specific congenital myopathies to be defined. Subsequent reports further delineated a fairly discrete clinical syndrome.
The clinical syndrome consists of hypotonia and weakness, apparent usually in the neonatal period. However, many infants are not recognized as exhibiting muscle disease until months later. Muscle weakness is usually more prominent in the lower limbs and in the proximal muscles ( Fig. 37.18A ), and tendon reflexes are usually preserved, although diminished or absent in proportion to the weakness. Congenital hip dislocation is also common. Mild facial weakness is not unusual. Ptosis, extraocular muscle weakness, dysphagia, and respiratory difficulties have not been features. However, severe neonatal weakness, arthrogryposis, and respiratory failure have been described in a subset of infants with central core disease.
With the exception of the last subgroup of infants with severe neonatal presentation, who do not walk independently and continue to require respiratory support, the course of the disease is usually nonprogressive, and infants attain motor milestones, albeit at a slow rate. Slow progression of weakness may occur, but even without this progression, contractures, pes cavus or planus, and kyphoscoliosis may result. In a series of 11 patients followed to the age of 4 to 20 years, 2 were unable to walk alone, and 2 had difficulty climbing stairs. An increased risk of malignant hyperthermia (e.g., during anesthesia) must be recognized to avoid unexpected death.
Laboratory investigations , aside from muscle biopsy and genetic testing, are not particularly helpful. The serum CK level is usually normal or slightly raised, and the EMG is usually normal or shows myopathic changes, although frequently minor.
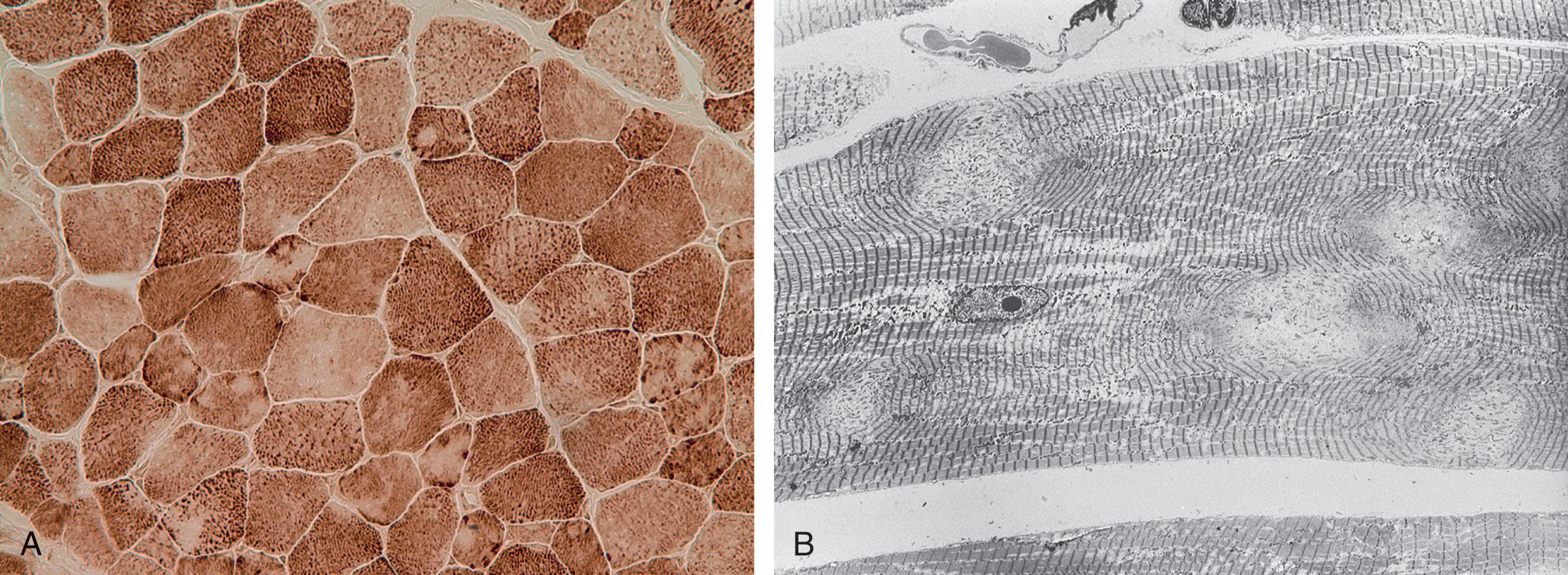
Muscle pathology is distinctive and diagnostic in the presence of the clinical findings. Many muscle fibers (20%–100%) exhibit cores that are central or somewhat eccentric in location ( Fig. 37.18B ). Single or multiple cores may be observed in a given fiber and are well demonstrated with the histochemical stains for oxidative enzymes (e.g., reduced nicotinamide adenine dinucleotide-tetrazolium reductase [NADH-TR] or succinic dehydrogenase [SDH]), which are absent in the core region. Indeed, electron microscopy shows an absence of mitochondria and sarcoplasmic reticulum in the core region. The cores contain densely packed and disorganized myofibrils. The cores have a particular predilection for type I fibers and, in some cases, type I fibers predominate or are the only fiber type observable.
The pathogenesis relates to a genetic defect that is usually inherited in an autosomal dominant manner, although less frequently autosomal recessive inheritance has been documented as well. The gene involved is RYR1 , which is located on chromosome 19q13.2 and encodes the skeletal muscle ryanodine receptor (see Table 37.5 ). The latter is a ligand-related release channel for internally stored calcium and thereby plays a crucial role in excitation-contraction coupling. More than 90% of central core disease patients have mutations in RYR1, which are most commonly dominant or de novo dominant; de novo dominant and autosomal recessive mutations are more common in the severe cases. In a recent study, facial weakness was more common in patients with biallelic variants, suggesting that such weakness might be a clinical marker for autosomal recessive forms of central core disease.
Management is the same as that for nonprogressive or slowly progressive myopathy. The prevention of contractures is important.
Multiminicore disease (MmD) should be discussed briefly in this context of core myopathies. The classic form of multiminicore disease typically has its clinical onset in the neonatal period or early infancy. Neonatal hypotonia and weakness, although generalized, tend to predominate in the axial muscles. Mild facial weakness is common. Respiratory difficulties generally appear later, as do spinal abnormalities, especially scoliosis. Some patients with MmD become ventilator dependent while still ambulant. Motor development is prominently delayed. The clinical course is static in most patients, although approximately 20% of patients exhibit mild progression. The pathology is distinctive and consists of the occurrence of multifocal core-like areas, seen best as areas of reduced activity on oxidative enzyme stains ( Fig. 37.19 ).
Pathogenesis is likely heterogeneous with primarily sporadic and autosomal recessive cases reported. In separate cohorts, defects in the SEPN1 gene, the gene mutated in rigid spine syndrome (see discussion of CMD earlier) and in some later-onset forms of congenital fiber type disproportion, and in the RYR1 gene, the gene mutated in central core disease, have been identified. Additionally, mutations in beta-myosin heavy chain gene (MYH7) , the satellite cell gene MEGF10 , and the giant sarcomeric protein titin (TTN) have been described in patients with MmD (see Table 37.5 ). The clinical overlap with CMD with rigid spine syndrome and the histological overlap with central core disease are consistent with these initial genetic findings.
Nemaline (rod body) myopathy , described initially by Shy and colleagues in 1963, was the second of the specific congenital myopathies to be defined. The term nemaline is derived from the Greek word nema , meaning “thread.” Numerous subsequent reports emphasized a considerable variation in clinical expression.
The clinical presentation in infants with manifestations from the neonatal period can be divided into three syndromes. First, as in typical central core disease, hypotonia and weakness are relatively mild and are sometimes even overlooked in the newborn period. This variety is termed typical congenital or classical form of nemaline myopathy . Second, marked neonatal hypotonia and weakness occur with no spontaneous movements or respiration at birth. Severe contractures or arthrogryposis and sometimes fractures complete this syndrome of severe congenital nemaline myopathy . Cardiac involvement in the form of dilated or hypertrophic cardiomyopathy may occur rarely, but in general cardiac contractility is normal. The pregnancy may be complicated by decreased fetal movements and polyhydramnios, and death in utero associated with fetal akinesia may occur. Third, marked neonatal hypotonia and weakness occur but with some movement and respiratory effort and no severe contractures. This serious disorder, although not so marked as the “severe” cases, is termed intermediate congenital nemaline myopathy . In one large series of nemaline myopathy ( n = 143), approximately 50% of patients presented in the neonatal period, and, of these, 35% had the severe congenital form, 45% had the intermediate congenital form, and 20% had the typical congenital form. Accompanying the severe and intermediate forms were marked facial diplegia and feeding disturbances. The milder, typical form is associated with varying degrees of facial diparesis and feeding difficulties, as well as distal involvement in addition to the proximal muscle weakness. Various skeletal anomalies, including high-arched palate, long dysmorphic face, prognathism malocclusion, pectus excavatum, and “pigeon chest,” may be seen. In surviving infants, pes cavus, kyphoscoliosis, and lumbar lordosis evolve. In 2019, Sewry et al. proposed a new classification that eliminates the “intermediate” and “other” categories and adds the genetic mutations in each class to assist in severity classification. However, there is significant overlap as certain genes are involved in both severe and mild forms of the disease.
The course of the typical, milder nemaline myopathy is considered generally to be nonprogressive or slowly progressive. However, in a study with a 5- to 25-year follow-up, 10 of 12 patients exhibited clinical deterioration, and only 8 of 12 were walking unsupported. Ten of 12 patients had developed scoliosis. Similar findings with 3- to 24-year follow-up were reported in a later study.
The course in those infants with the two more severe forms of the disease (i.e., the severe and intermediate forms) is generally unfavorable. Thus, in one series, 74% of patients with severe congenital cases died, generally before 1 year of age, and 28% of patients with intermediate congenital cases died. Among the infants with the severe form, a requirement for ventilation at birth or in the first month was followed by death in more than 90% of cases. Such infants fail to establish adequate ventilation after birth because of weakness of respiratory muscles. This weakness results in failure of full expansion of lungs, and, secondary to difficulty with secretions, aspiration often is added. Infants usually die in the neonatal period or in the first months of life. However, long-term survival in these patients, who are usually bedridden and are receiving mechanical ventilation, has been observed. Moreover, rare affected infants with the need for mechanical ventilation at birth, severe weakness, and markedly impaired feeding have improved over the ensuing 2 to 3 years to become independent of mechanical ventilation and to achieve the ability to stand or even walk.
Laboratory investigations have included either normal or slightly elevated values for the serum CK level. The EMG may be normal but often exhibits short-duration and small-amplitude potentials of myopathy; signs of denervation have also been observed, particularly late in the disease, but nerve conduction studies are normal. Ultrasonography usually shows increased echogenicity in affected muscles. MRI reveals fatty infiltration of muscle tissue and can be valuable in the selection of a muscle to biopsy.
The pathogenesis of nemaline myopathy relates to defects in one of 11 genes, 7 of which encode proteins of skeletal muscle thin filaments (see Table 37.5 ). Approximately 60% to 75% of cases of severe congenital nemaline myopathy are related to defects of ACTA1 , the gene for alpha-actin. Most of the remaining cases are related to nebulin, KLHL40, KLHL41, and leiomodin-3 defects and are principally autosomal recessive. Mutations in the genes for alpha-tropomyosin, beta-tropomyosin, and troponin are rare causes of severe disease. A severe form of congenital nemaline myopathy with a paradoxical combination of weakness and chest wall stiffness leading to early severe hypoventilation has been described in patients with pathogenic variants in the troponin T type 1 (TNNT1) gene. KLHL40 and KLHL41, which belong to the kelch protein family, are thought to be involved in ubiquitination and protein degradation, and leiomodin-3 is considered essential for the organization of sarcomeric thin filaments ( Fig. 37.20 ).
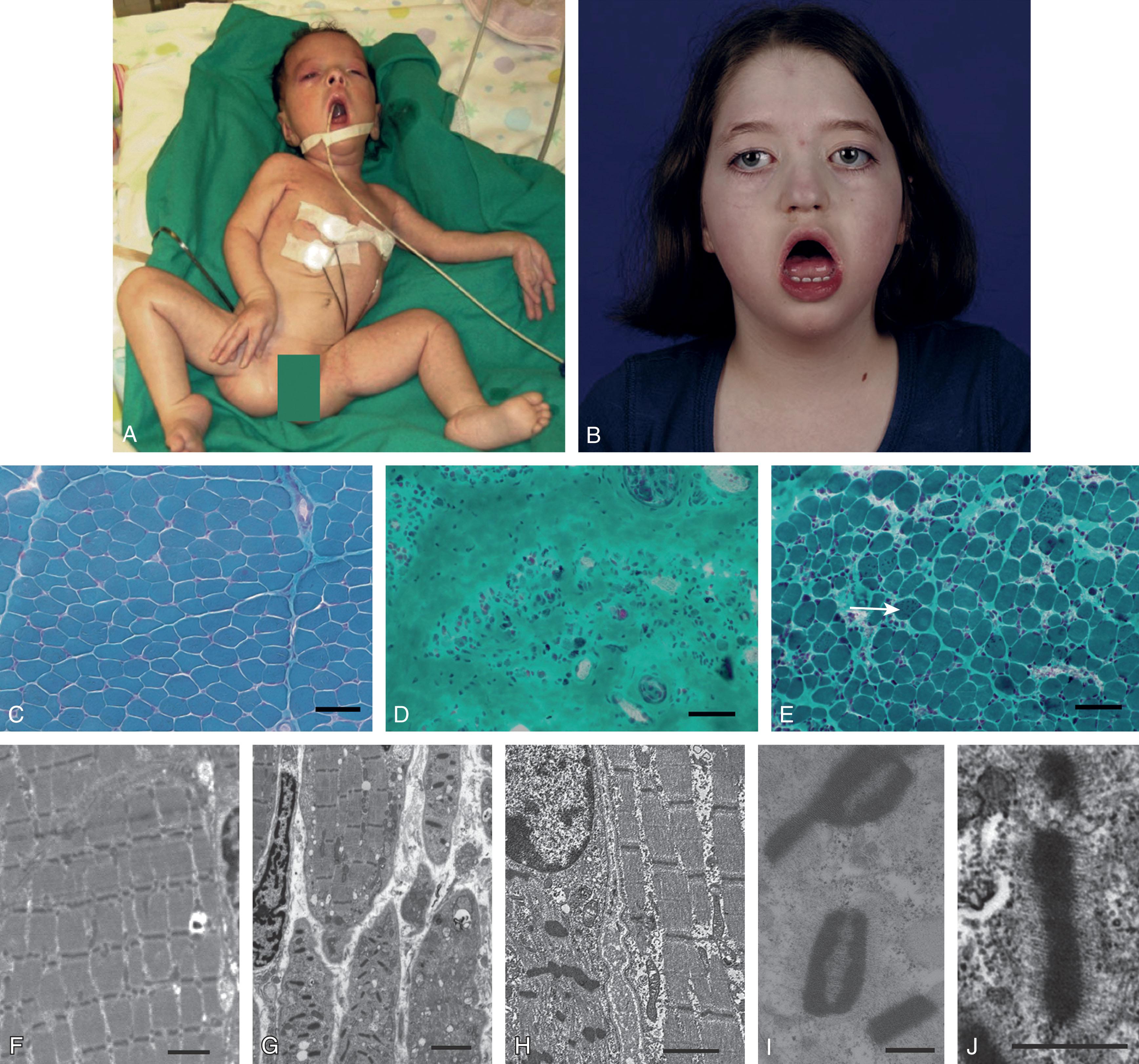
Muscle pathology is distinctive and diagnostic in the presence of the clinical features. Although the rod bodies are readily overlooked on routine hematoxylin and eosin staining, Gomori trichrome, toluidine blue, and other selected stains demonstrate them well ( Fig. 37.21 ). Predominance of type I fibers, which also are small, is consistent.
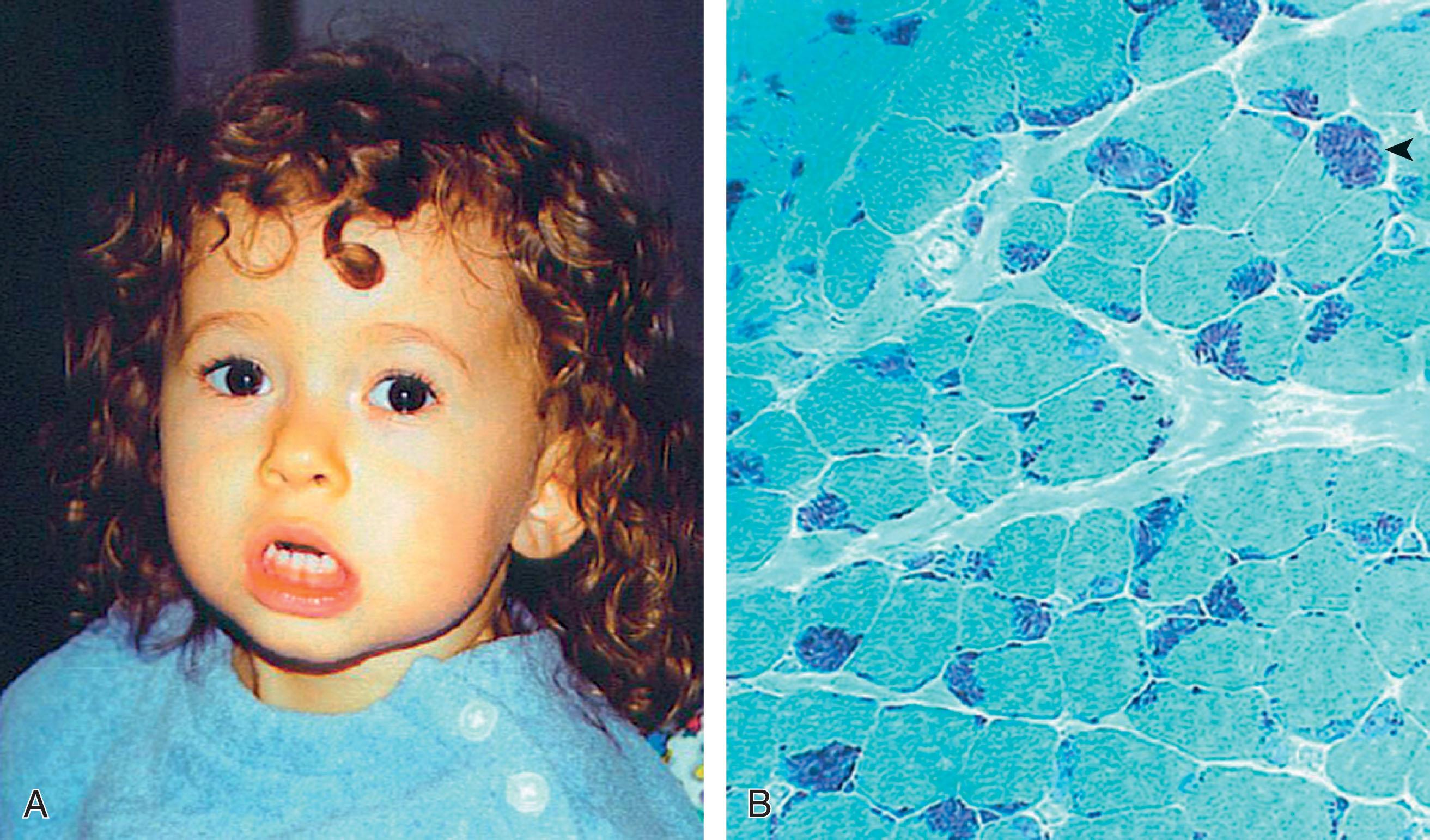
The rod body is derived from lateral expansion of the Z-disk, the band found normally at either end of the sarcomere. Thus the rod bodies have been shown by electron microscopy to be in structural continuity with the Z-disk ( Fig. 37.22 ). The rod’s major protein component is alpha-actin, the main protein of the Z-disk, and the bodies are penetrated by actin filaments, which normally penetrate the Z-disk. The rods are surrounded by desmin, the structural component of muscle-specific intermediate filaments. A combination of cores and rods has been reported in certain patients (core-rod myopathy). Intranuclear rods have been detected in some severe fatal neonatal cases ( Fig. 37.23 ). In general, the severity of the myopathological features correlate only imperfectly with the clinical course. However, in nebulin-related nemaline myopathy a correlation between the severity of the disease and genotype has been reported.

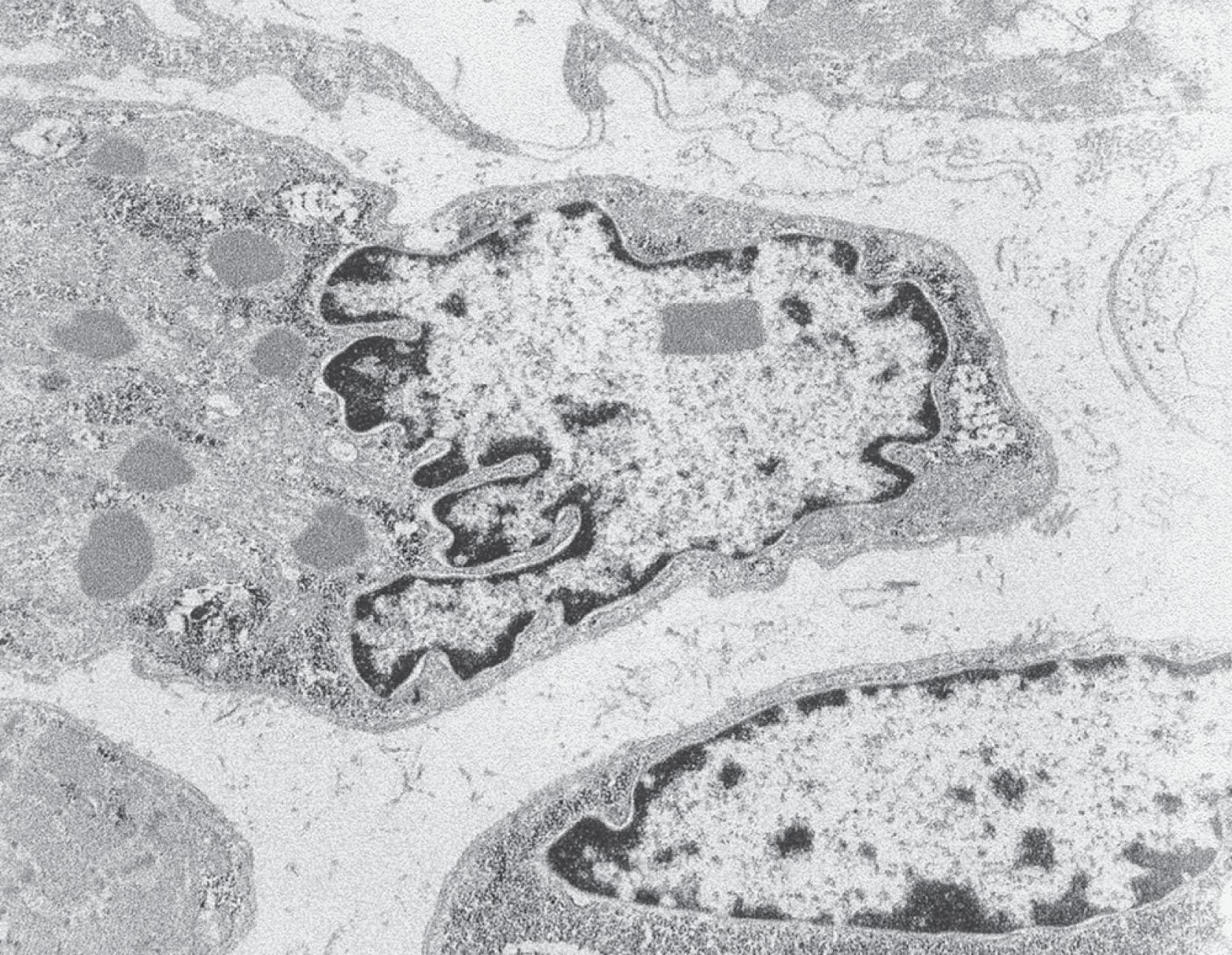
Management of the typical, nonprogressive, or slowly progressive form of nemaline myopathy depends principally on physical therapy and related techniques. However, respiratory failure may occur during infancy, rapidly and unexpectedly, with a fatal outcome, even in apparently stable or improving patients. Thus careful follow-up is necessary. Infants with the severe neonatal form require prompt recognition and vigorous ventilatory and nutritional support if they are to survive. However, on the basis of the data available, appreciable improvement, despite diligent supportive care, is unlikely.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here