Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Muscle diseases, which are also called myopathies, are disorders of skeletal muscle structure or function. Myopathies can be primary and occur in isolation, or they can be part of a multisystem disorder.
Many muscle diseases ( Table 389-1 ) are inherited as autosomal dominant, autosomal recessive, X-linked, or maternal (mitochondrial) conditions. Environmental factors that may precipitate myopathies include recent infection, foreign travel, exposure to medications such as statins, and alcohol abuse ( Chapter 364 ). Exercise commonly precipitates symptoms in patients with metabolic myopathies, whereas exposure to cold and high-carbohydrate or potassium-rich food can precipitate weakness in muscle channelopathies.
| HEREDITARY |
| Muscular dystrophies Congenital myopathies Myotonia and channelopathies Metabolic myopathies Mitochondrial myopathies |
| ACQUIRED |
| Inflammatory myopathies Endocrine myopathies Myopathies associated with systemic illness Drug-induced/toxic myopathies |
The prevalence of muscle disease is estimated to be about 1 per 1000 people, including acute and transient disorders (e.g., myositis owing to infectious or toxic causes) and chronic inflammatory or genetic disorders that cause substantial morbidity over decades or a lifetime. Myopathies can cause premature death owing to neuromuscular weakness and secondary respiratory infections or to involvement of other organs in multisystem diseases. Myocardial involvement, which is particularly common in some muscle diseases, can cause heart failure or life-threatening arrhythmias.
Muscle disease can result from a perturbation in the anatomy or any of the physiologic processes required for muscle contraction or the genes that control them. Skeletal muscle is part of a motor unit, which is defined as the anterior horn cell body, its axon, the neuromuscular junction, and the skeletal muscle fibers innervated by the one axon. The motor unit is coordinated in a manner that allows efficient muscle contraction and function. The number of muscle fibers innervated by each motor unit varies from a few (e.g., in muscles controlling very precise movements, such as extraocular muscles) to more than 1000 (e.g., large and powerful but less precise muscles, such as the quadriceps).
Skeletal muscle is composed of myriad muscle fibers. Muscle fibers, which are multinucleated cells formed by fusion of myoblasts during development, are surrounded by a plasma membrane, the sarcolemma, which is surrounded by a basal lamina and endomysial connective tissue. Groups of muscle fibers compose the fascicles, which are surrounded by perimysium, and the groups of fascicles in turn are surrounded by epimysium. Nerve branches, blood vessels, muscle spindles, and fat cells lie within the connective tissue of the muscle.
Each muscle fiber is composed of myofibrils, which themselves are composed of repeat units a few microns long called sarcomeres. The sarcomere consists of a highly organized protein network that gives the muscle fiber its characteristic striated appearance. Each sarcomere is flanked by two Z discs. Z discs are composed of multiple proteins, including α-actinin. Emanating from the Z disc are thin filaments, composed of actin, troponin, and tropomyosin. Thick filaments consist of myosin. Other structures comprise subcellular organelles, including the mitochondria, which are the principal energy source, the endoplasmic reticulum, and the transverse tubules that communicate with the extracellular space.
Muscle function ( E-Fig. 389-1 ) is dependent on chemical energy from adenosine triphosphate (ATP). In the first 30 minutes of sustained activity, ATP is produced by the breakdown of glycogen (glycolysis), and after 30 minutes ATP is produced by fatty acid β-oxidation and oxidative phosphorylation within the mitochondria. The process that leads to muscle contraction begins with the generation of the muscle fiber action potential ( Chapter 41 ), which initiates muscle contraction after it is propagated into the interior of muscle fiber through the transverse tubular system. The release of calcium from the endoplasmic reticulum triggers a coordinated series of events that lead to the coupling of excitation to contraction. Calcium binds to troponin, which interacts with tropomyosin and results in actin-myosin binding. The repeated formation and cleavage of actin-tropomyosin cross-bridges, in an ATP-dependent process, results in sliding of thick and thin filaments and shortening of the sarcomere.
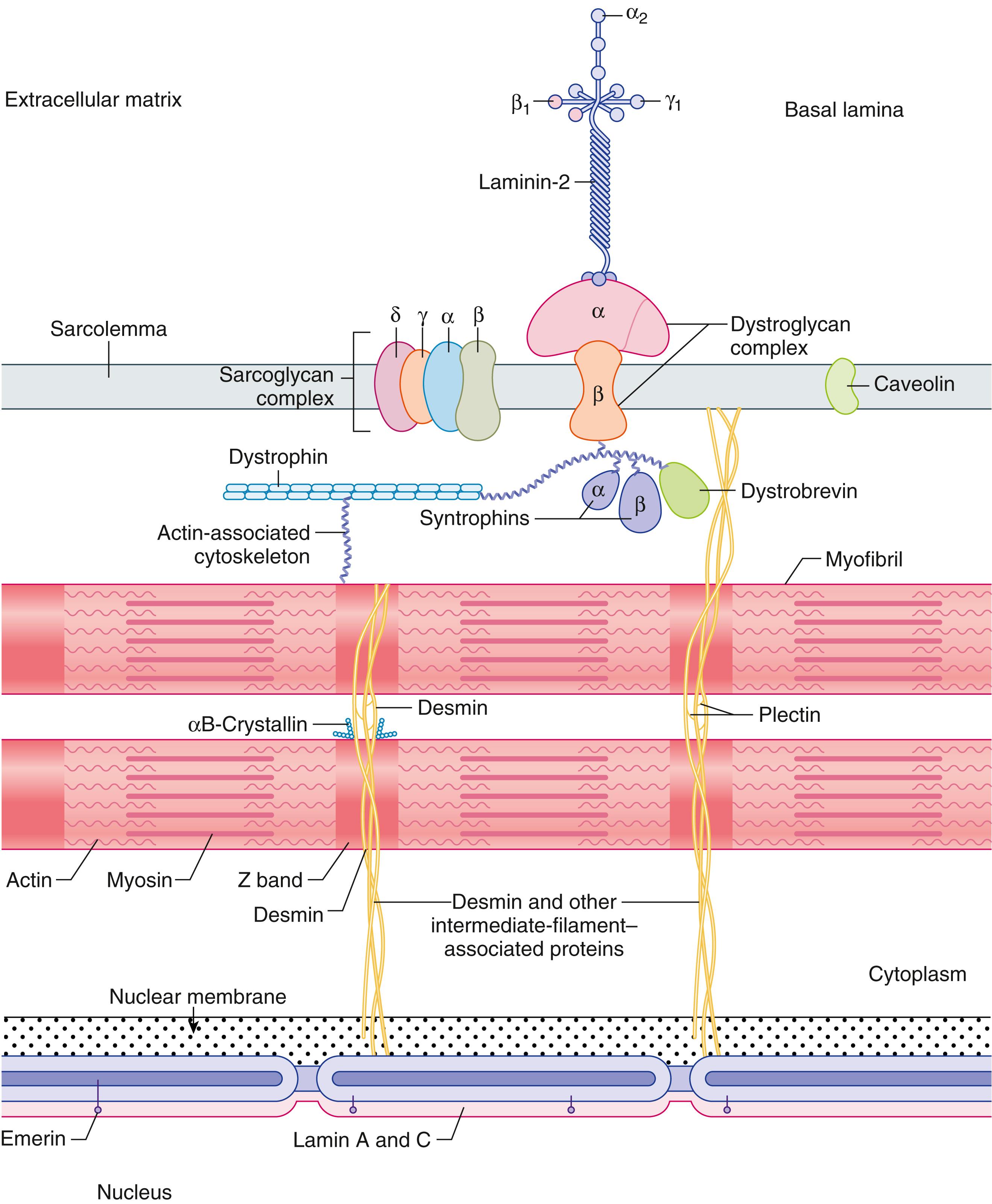
The structural integrity of the muscle fiber surface membrane is maintained by a network of proteins within the muscle. Dystrophin is a key component of the subsarcolemmal cytoskeleton. In combination with several glycoproteins called sarcoglycans (α, β, δ, γ), dystroglycans (α, β), and syntrophins (α,β1, β2), which form the dystrophin-sarcoglycan complex, it anchors the contractile elements of the muscle fiber to the sarcolemma and to the extracellular basal lamina. The basal lamina contains several important proteins, such as collagen, fibronectin, and laminin, which includes merosin and related proteins. The intermediate filament proteins, including desmin, connect the Z disc and other organelles to the subsarcolemmal cytoskeleton.
Muscle diseases often present with localized or diffuse muscle weakness, reduced exercise tolerance, resting or exercise-induced muscle pain, muscle enlargement or atrophy, cramps, delayed relaxation, or, rarely, myoglobinuria. These symptoms and signs can be masked by other neurologic or systemic features in patients with multisystem diseases.
The assessment of patients with neuromuscular diseases begins with a careful history, general physical examination, and detailed neurologic examination. The age of onset, the rate of progression, and whether the process is episodic, static, or progressive can provide important clues. Congenital and childhood-onset myopathies can be associated with reduced fetal movements, breech delivery, weak cry or suck, and the delayed acquisition of motor milestones. Weakness is the most common presenting symptom, but other symptoms of muscle disease include muscle pain, reduced exercise tolerance, change in the muscle bulk (hypertrophy or atrophy), abnormal spontaneous muscle activity, delayed relaxation, fatigue, or myoglobinuria. Weakness may be relatively static as in some congenital myopathies, progressive as in muscular dystrophies, intermittent as in periodic paralysis, fluctuating as in neuromuscular junction disorders ( Chapter 390 ), or exercise related as in metabolic myopathies. The most common distribution of weakness is proximal or limb-girdle weakness, which results in difficulties in getting out of low chairs, a bathtub, or a car seat; climbing up and down stairs; arising from a squat; or getting off the floor. Proximal arm weakness manifests as difficulty reaching to shelves, washing or brushing hair, or raising arms to put on a shirt. Distal leg weakness can lead to difficulty walking on uneven surfaces, tripping over curbs, difficulty standing on the toes, or slapping feet owing to footdrop. Distal upper limb weakness results in difficulty opening jars, typing at a keyboard, writing, or buttoning clothes. Bilateral facial weakness can result in difficulty whistling, blowing up balloons, or drinking through a straw. Predominant involvement of ocular muscles can produce ptosis and diplopia. Weakness of the bulbar muscles manifests as difficulties with speech and swallowing, neck weakness that can lead to a dropped head, and respiratory muscle weakness that can lead to symptoms suggestive of nocturnal hypoventilation or respiratory failure. The early recognition of progressive respiratory failure is essential because it is treatable with noninvasive positive-pressure ventilation.
Fatigue and exercise intolerance can be presenting symptoms of muscle diseases, but they can also be multifactorial and nonspecific. In isolation, these symptoms usually do not indicate a primary muscle disease.
Muscle pain is another nonspecific symptom that can arise from many systemic and psychiatric conditions. Sometimes patients describe aching, stiffness, numbness, or burning as pain. Muscle diseases rarely cause diffuse, generalized, or persistent muscle pain. Muscle pain without muscle weakness is often a feature of fibromyalgia ( Chapter 253 ) or chronic fatigue syndrome. Diffuse myalgia can occur in inflammatory muscle disease such as polymyositis or dermatomyositis, vasculitis, or viral or parasitic myositis. Muscle pain precipitated by exercise usually suggests a metabolic myopathy.
Muscle cramps are involuntary painful contractions that may occur in healthy individuals. Dehydration, renal failure ( Chapter 116 ), and electrolyte imbalances ( Chapters 102 , 103 , 105 , and 227 ) can also produce muscle cramps. Muscle stiffness can occur in inflammatory, metabolic, and ion-channel diseases as well as in conditions such as multiple sclerosis ( Chapter 380 ), polymyalgia rheumatica ( Chapter 250 ), and connective tissue diseases ( Chapter 236 ).
Fasciculations are caused by spontaneous firing of muscle fibers that are innervated by a single motor unit. Fasciculations may occur in normal persons, in whom they are usually exacerbated by stress and increased caffeine intake. Fasciculations in association with muscle weakness suggest anterior horn cell disease. Myotonia, often described as muscle stiffness, is characterized by prolonged contraction and delayed relaxation of muscle.
Myotonia can affect limb, facial, or bulbar muscles, and it can lead to persistent limb muscle contraction, eyelid closure, or dysphagia. Myotonic dystrophy is the most common muscle disease associated with myotonia, but patients usually complain more of weakness than the myotonia. Conversely, the myotonia associated with sodium and chloride channelopathies can be disabling. Patients who describe locking of their hands but do not have objective myotonia rarely have a physical explanation for their symptoms.
Tetany is the most severe form of sustained muscle contraction. Tetany occurs in patients with hypocalcemia and hypomagnesemia ( Chapter 105 ), and it is aggravated by metabolic or respiratory alkalosis ( Chapter 104 ).
Severe acute muscle damage, termed rhabdomyolysis ( Chapter 99 ), results in myoglobinuria that presents as dark brown or red urine. Such discoloration must be distinguished from other causes of pigmenturia ( Chapter 100 ) such as hemolysis or porphyria.
The detailed family history should include questions about muscle disease, including specific questions about the use of canes, braces, or wheelchairs. It also should assess whether family members have had a cardiomyopathy, unexpected sudden death, diabetes, or cataracts.
A full physical examination must look for signs that may suggest any of the systemic diseases that are associated with myopathies. The skin examination can give clues to systemic illness, such as the heliotrope rash of dermatomyositis ( Chapter 248 ).
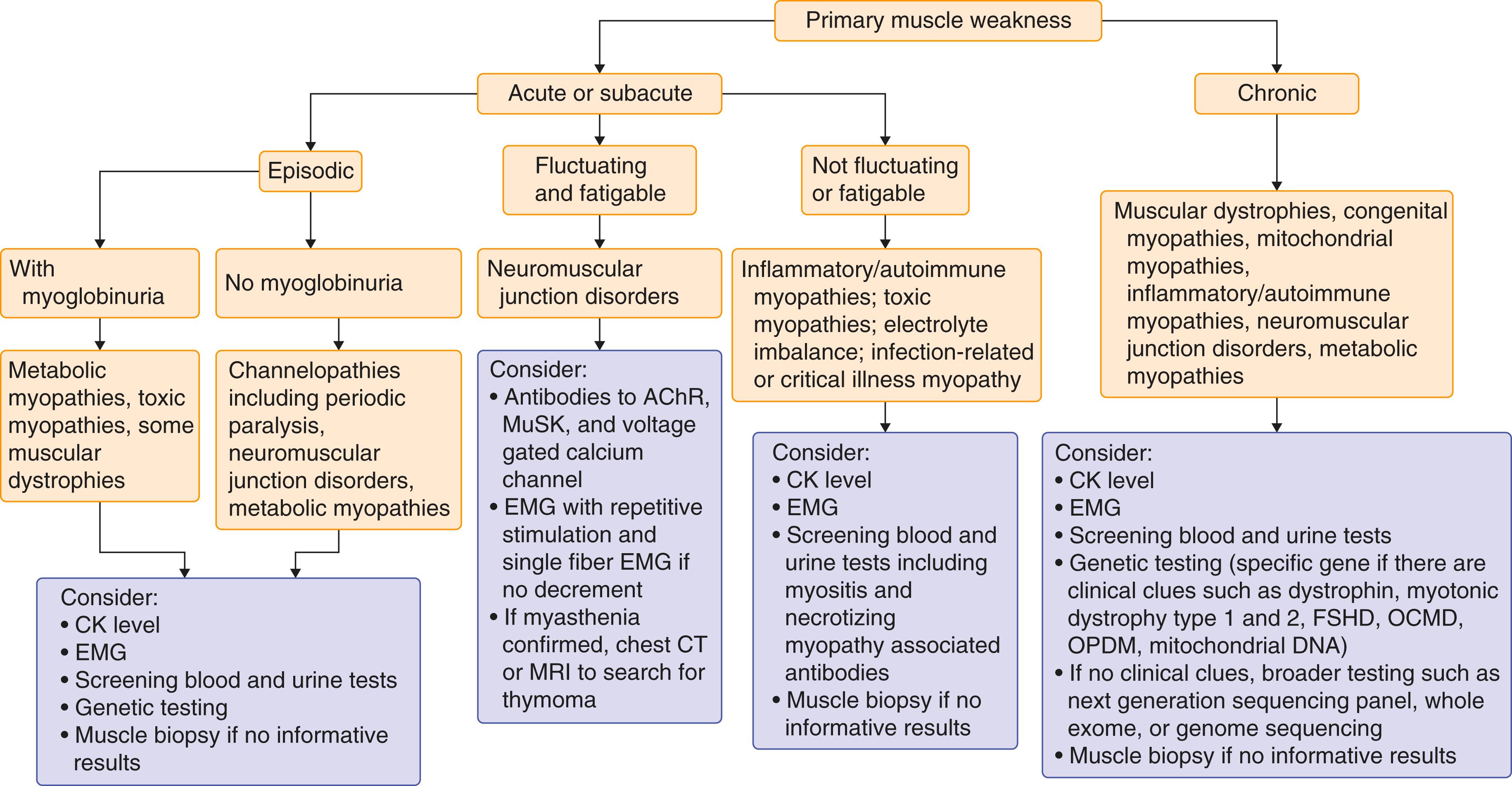
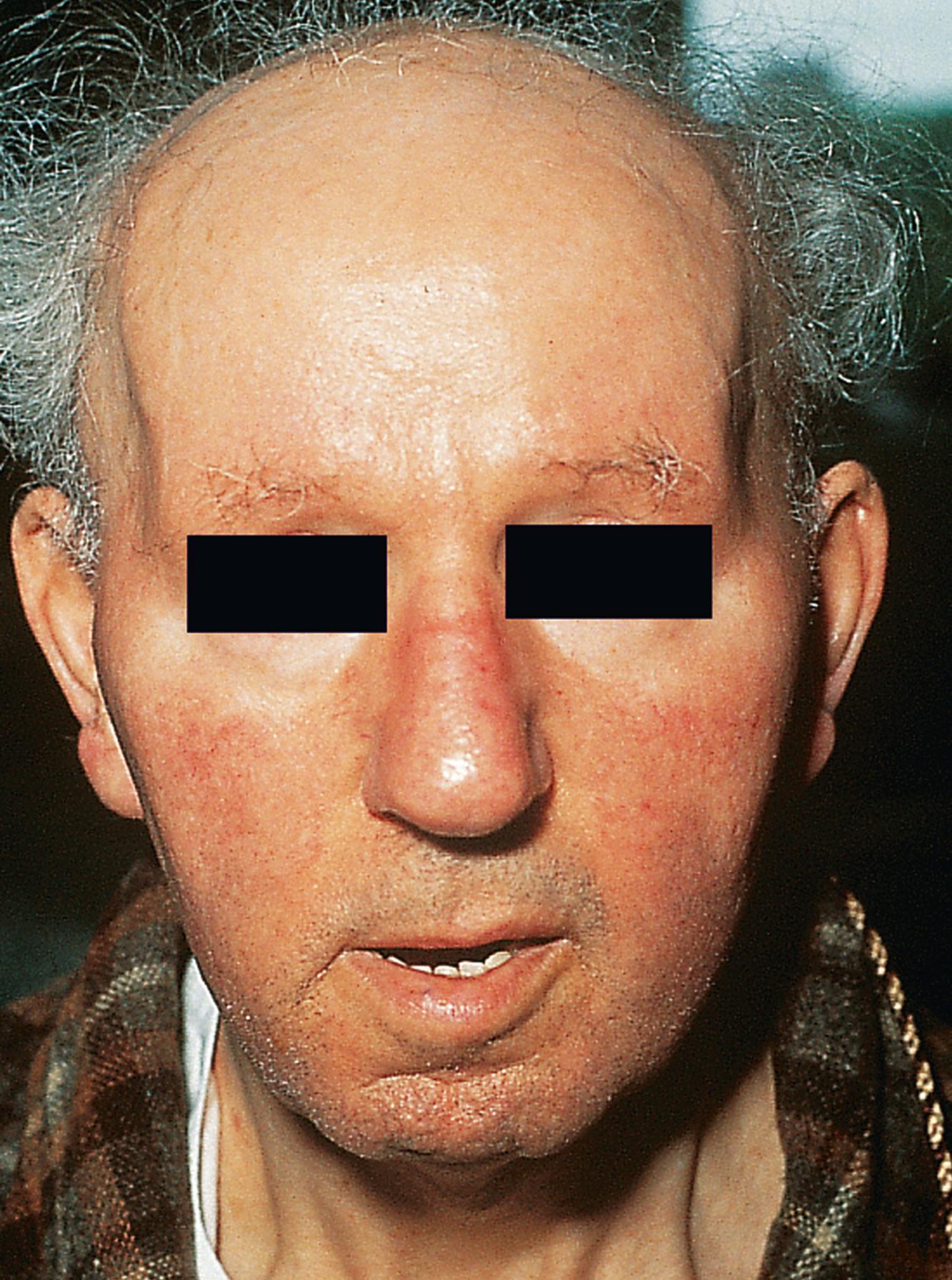
A comprehensive neurologic examination should be performed in each patient to exclude possible central or peripheral nervous system disorders ( Table 389-2 ). Once the muscle disease is confirmed, further testing and diagnosis can be guided by the history and physical examination ( Fig. 389-1 ). The examination begins as soon as the patient enters the examination room. Proximal leg muscle weakness may be evident if patients push themselves up on their thighs or have a waddling gait. Patients should be examined for possible facial muscle weakness or wasting, ptosis, or characteristic dysmorphic features, such as with myotonic dystrophy ( Fig. 389-2 ), that can lead to an immediate clinical diagnosis.
| FINDING | MYOPATHY | ANTERIOR HORN CELL DISEASE | PERIPHERAL NEUROPATHY | NEUROMUSCULAR JUNCTION DISEASE |
|---|---|---|---|---|
| Distribution | Usually proximal and symmetrical but can be distal or asymmetrical at onset | Proximal, asymmetrical, and bulbar | Distal, symmetrical | Extraocular, bulbar, proximal limb, but sometimes distal |
| Atrophy | Slight early, marked late | Marked early | Moderate | Occasionally |
| Fasciculations | Absent | Frequent | Sometimes present | Absent |
| Reflexes | Lost late | Variable, can be hyperreflexic | Lost early | Normal or hyporeflexic |
| Pain | Variable | Absent | Variable, distal when present | Absent |
| Cramps | Rare | Frequent | Occasional | Absent |
| Sensory loss | Absent | Absent | Usually present | Absent |
| Serum creatine kinase | Usually elevated | Occasionally mildly elevated | Normal | Occasionally mildly elevated |
Patients should be asked to rise from a squatting position and walk on their toes to assess possible calf weakness and on their heels to assess ankle dorsiflexion weakness. Patients should be asked to stand to assess posture and any evidence of rigidity or scoliosis. Joints should be moved passively to assess for contractures.
All muscle groups should be inspected for evidence of involuntary movements, atrophy, or hypertrophy. Muscles should be palpated for tenderness or unusual texture. Myotonia can be assessed by the inability to relax the muscle belly after percussion with a reflex hammer or the inability to relax the fingers from a firm grip.
Strength should be graded ( Table 389-3 ) in each muscle group. Observing children and infants when they play with toys and how they stand up and walk usually reveals more than formal manual muscle strength testing. The pattern of muscle involvement can provide clues for the diagnosis of a specific myopathy.
| GRADE | DEGREE OF STRENGTH |
|---|---|
| 5 4 3 0 |
Normal power Active movement against gravity and resistance (often subdivided into 4−, 4, and 4+) Active movement against gravity, but not against resistance Active movement, with gravity eliminated Observable muscle contraction, but not capable of initiating movement No contraction |
Neurophysiologic testing, measurement of serum creatine kinase (CK), muscle biopsy, and genetic testing help guide the diagnosis of muscle diseases (see Fig. 389-1 and Table 389-4 ).
|
|
|
A complete blood count and serum levels of alanine aminotransferase, aspartate aminotransferase, and creatinine can assess possible systemic involvement. An elevated erythrocyte sedimentation rate or C-reactive protein level is found in some inflammatory myopathies and is typical of connective tissue disorders ( Chapters 236 and 237 ). Additional tests to evaluate patients suspected of having inflammatory myopathy or connective tissue disease can include antinuclear antibodies, extractable nuclear antigens, rheumatoid factor, and antineutrophilic cytoplasmic antibodies, as well as anti-Jo-1, anti-Mi2, anti-MDA5, anti-centromere, anti-SCL-70, anti-PM/Scl-100, anti-Ku, anti-PL-7, anti-PL-12, anti-MDA5, anti-NXP-2, anti-TIF-1gamma, anti-U1 RNP, anti-U2 RNP, anti-U3 RNP, anti-SAE1, anti-SAE2, anti-EJ, anti-OJ, anti-SSA, anti-SSB, anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase, or anti–signal recognition particle antibodies ( Chapter 237 ).
The muscle isoform (MM) of CK is frequently elevated in patients with muscle disease, although a normal level is seen in metabolic myopathies and in some chronic myopathies. A mild to moderate increase in the serum CK level can occur in patients with peripheral neuropathy, radiculopathy, and anterior horn cell diseases. The serum CK level is markedly increased in dystrophinopathies, dysferlinopathy, some of the sarcoglycanopathies, α-dystroglycanopathies, and during rhabdomyolysis ( Chapter 99 ). However, it can decline later in muscular dystrophies as the disease progresses. When the serum CK level exceeds the upper limit of normal by about 10-fold, levels of alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase also can be elevated, and some patients can be initially misdiagnosed as having hepatitis ( Chapters 133 and 134 ) before the serum CK level is measured. The blood lactate level can be increased in patients with a mitochondrial myopathy, but a normal value does not exclude this diagnosis. In patients with acute muscle pain and/or weakness, electrolytes and thyroid function tests should be checked.
In patients who are diagnosed with dermatomyositis ( Chapter 248 ), the evaluation should include a search for an underlying malignancy. In patients with suspected mitochondrial cytopathy, a serum and/or spinal fluid lactate level may be useful for diagnosis. In patients with suspected fatty acid β-oxidation defects, blood acylcarnitine profile may be useful for the diagnosis.
Electromyography (EMG) consists of nerve conduction studies, repetitive nerve stimulation, and needle examination of muscles ( Chapter 366 ). Nerve conduction studies ( Chapter 388 ) are normal in patients with myopathies. In myopathies, the needle examination typically shows complex, polyphasic, low-amplitude motor unit potentials. Myotonia is caused by recurrent depolarization of the muscle fiber surface membrane and has characteristic waxing and waning rhythmical discharges or fibrillation potentials during the needle examination on an EMG. Contractures are electrically silent on EMGs, but muscle cramps are associated with high-amplitude, high-frequency bursts of motor unit activity. The EMG can be normal in some focal myopathies (such as inflammatory myositis), in metabolic myopathies, and in some congenital myopathies.
The widespread availability of molecular genetic testing with commercial panels and whole-exome and -genome sequencing has revolutionized the approach to patients who are suspected of having hereditary muscle disease. Dystrophin genetic testing can identify the defects in dystrophin in 90 to 95% of the patients with Duchenne and Becker muscular dystrophy. The sensitivity of the whole-exome sequencing is higher if multiple affected and some unaffected family members and more than one family are analyzed at the same time. Novel genes causing muscular dystrophies are still being discovered.
Despite advances in genetics and molecular biology, muscle biopsy remains a key component in the diagnosis of most muscle diseases. The biopsy site should be carefully chosen from a clinically affected but not too severely involved muscle. Fresh-frozen sections of the specimens should be used for histochemical studies because even marked morphologic alterations may be undetected in paraffin-embedded tissue. Immunocytochemical localization of specific proteins is useful and diagnostic in some forms of muscular dystrophies. Specific enzyme histochemistry and biochemistry can be used for metabolic myopathies. Genetic analysis of the muscle specimen can be more informative than blood specimens for mitochondrial myopathies.
Muscle computed tomography (CT) and magnetic resonance imaging (MRI) are of limited utility for evaluating muscle diseases but can be very useful in excluding spinal cord abnormalities that may cause weakness. Some inherited myopathies are associated with patterns of atrophy and the replacement of muscle with fat. In patchy or focal inflammatory myopathy, MRI can guide muscle biopsy. Functional MRI is sometimes useful in patients with suspected mitochondrial disorders.
The four main categories of inherited muscle diseases are muscular dystrophies ( E-Table 389-1 ), congenital myopathies ( E-Table 389-2 ), muscle ion-channel disorders ( E-Table 389-3 ), and metabolic myopathies ( E-Table 389-4 ). Some gene defects cause specific phenotypes that are instantly recognizable at the bedside by an experienced clinician, but less specific phenotypes can be caused by defects in more than one gene. A systematic approach is critical for an efficient and successful investigation of these disorders.
| X-LINKED |
| Dystrophinopathies (Duchenne/Becker muscular dystrophy) Emery-Dreifuss (Emerin) FHL1-related (FHL1) |
| AUTOSOMAL DOMINANT |
| Facioscapulohumeral dystrophy (D4Z4 repeat deletions in 4q35 subtelomeric region in 95%; toxic gain of function in DUX4 in 5%) and with SMCHD1 mutation Myotonic dystrophy, type 1 (CTG repeats in DMPK ) Myotonic dystrophy, type 2 (CCTG repeats in ZNF9 ) Oculopharyngeal muscular dystrophy ( PABPN1 ) Myotilinopathy ∗ ( MYOT ) Laminopathy ( LMNA ) Caveolinopathy ( CAV3 ) DNAJB6opathy ∗ ( DNAJB6 ) Desminopathy ∗ ( DES ) Zaspopathy ∗ ( ZASP ) Bag3opathy ∗ ( BAG3 ) Filaminopathy ∗ ( FLNC ) α-B-crystallinopathy ∗ ( CRYAB ) Titinopathy ∗ ( TTN ) VCPopathy † [valosin-containing protein] ( VCP ) MYH7-myopathy † , ‡ [Laing myopathy] ( MYH7 ) MYH2-myopathy † ( MYH2 ) Nesprinopathy ( SYNE1, SYNE2 ) TIA1opathy [Welander distal myopathy] ( TIA1 ) KLHL9opathy [kelch-like homologue-9] ( KLHL9 ) LUMA related ( TMEM43 ) TNPO3opathy ( TNPO3 ) HNRNPDLopathy ( HNRNPDL ) CollagenVIopathy § ( COL6A1, COL6A2, COL6A3 ) Collagen 12-related § ( COL12A1 ) Calpainopathy ( CAPN3 ) HSPB8 related ( HSPB8 ) Oculopharyngodistal myopathy (trinucleotide repeats in LRP12 , GIPC1 , NOTCH2NLC ) |
| AUTOSOMAL RECESSIVE |
| Calpainopathy ( CAPN3 ) Dysferlinopathy ( DYSF ) Anoctaminopathy ( ANO5 ) α-sarcoglycanopathy ( SGCA ) β-sarcoglycanopathy ( SGCB ) δ-sarcoglycanopathy ( SGCD ) γ-sarcoglycanopathy ( SGCG ) Telethoninopathy § ( TCAP ) TRIM32opathy ( TRIM32 ) α-Dystroglycanopathies § ( FKRP, POMT1, POMT2, FKTN, POMGNT1, POMGNT2, LARGE1, DAG1, DPM1, DPM2, DPM3, GMPPB, ISPD, B3GNT1, ALG13, B3GALNT2, B4GAT1, TMEM5, POMK, POGLUT1, MPDU1, RXYLT1, GOSR2 ) Titinopathy ( TTN ) Laminopathy § ( LMNA ) Plectinopathy ( PLEC ) Merosin deficiency § (LAMA2) Selenoproteinopathy ‡ , § ( SELENON ) CollagenVIopathy § ( COL6A1, COL6A2, COL6A3 ) Collagen 12-related § ( COL12A1 ) With generalized lipodystrophy ( PTRF ) Integrinα7opathy § ( ITGA7 ) Integrinα9opathy § ( ITGA9 ) With mitochondrial structural abnormalities § ( CHKB ) GNE-opathy † [glucosamine (UDP- N -acetyl)-2-epimerase/ N -acetylmannosamine kinase] ( GNE ) Pompe disease ( GAA ) Matrin3opathy ( MATR3 ) LAP1Bopathy ( TOR1AIP1 ) Desminopathy ( DES ) TRAPPC11opathy § ( TRAPPC11 ) BVESopathy ( BVES ) CHKBopathy ¶ ( CHKB ) VCP related ( VCP ) POPDC3 related ( POPDC3 ) LIMS2 related ( LIMS2 ) PYROXD1 related ( PYROXD1 ) |
∗ Associated with pathologic features of myofibrillar myopathy.
† Associated with pathologic features of inclusion body myopathy.
‡ Can also cause congenital myopathy.
§ Can also cause congenital muscular dystrophy.
¶ Causes congenital muscular dystrophy with mitochondrial structural abnormalities (megaconial type).
| Central core, multi-minicore disease ( RYR1, SELENON, MYH7, ACTA1, LMNA, MEGF10, TTN, FXR1, ACTN2 ) |
| Centronuclear myopathy ( MTM1, DNM2, RYR1, BIN1, TTN, SPEG, CCDC78 ) |
| Nemaline rod myopathy ( NEB, ACTA1, TPM3, TPM2, TNNT1, CFL2, KLHL40, KLHL41, KBTBD13, LMOD3, MYPN, MYO18B, RYR3 ) |
| Congenital fiber-type disproportion ( ACTA1, SELENON, RYR1, MYH7, TPM3, MYH7, MYL2, ZAK ) |
| Myosin storage myopathy ( MYH7 ) |
| Other structural and nonspecific myopathies: cap myopathy, zebra body myopathy, sarcotubular myopathy, spheroid body myopathy, fingerprint body myopathy, trilaminar myopathy, cylindrical spiral myopathy, myopathy with muscle spindle excess, myopathy with tubular aggregates, Danon disease, myopathy with excessive autophagy, SCN4A related |
| DISORDER | PATTERN OF CLINICAL FEATURES |
|---|---|
| Thomsen disease ( CLCN1 ) | Myotonia |
| Becker disease ∗ ( CLCN1 ) | Myotonia and weakness |
| Paramyotonia congenita (SCN4A) | Paramyotonia |
| Hyperkalemic periodic paralysis ( SCN4A ) | Periodic paralysis, sometimes with myotonia and paramyotonia |
| Hypokalemic periodic paralysis ( SCN4A, ATP1A2, CACNA1S, KCNE3, KCNJ18 ) | Periodic paralysis |
| Andersen-Tawil syndrome ( KCNJ2 ) | Periodic paralysis, cardiac arrhythmias, skeletal abnormalities |
| Potassium-aggravated myotonias (myotonia fluctuans, myotonia permanens, and acetazolamide-sensitive myotonia) ( SCN4A ) | Myotonia |
| Schwartz-Jampel syndrome ∗ (Chondrodystrophic myotonia) ( HSPG2 ) | Pseudomyotonia, dysmorphism, blepharospasm |
| Rippling muscle disease ( CAV3 ) | Muscle mounding/stiffness |
| Brody disease ∗ ( ATP2A1 ) | Delayed relaxation, no myotonia on electromyogram |
| Malignant hyperthermia ( RYR1 , STAC3 , CACNA1S ) | Anesthetic-induced excessive calcium release by sarcoplasmic reticulum |
∗ Autosomal recessive; all other listed diseases are autosomal dominant.
| DISORDERS OF GLYCOGEN METABOLISM |
| Type II: α-1,4-Glucosidase (acid maltase) ( GAA ) Type III: Debrancher ( AGL ) Type IV: Branching ( GBE1 ) Type V: Myophosphorylase ( PYGM ) Type VII: Phosphofructokinase ( PFKM ) Type VIII: Phosphorylase b kinase ( PHBK ) Type IXd: Phosphorylase kinase, alpha 1 subunit ( PHKA1 ) Type IX: Phosphoglycerate kinase ( PGK ) Type X: Phosphoglycerate mutase ( PGAM-M ) Type XI: Lactate dehydrogenase ( LDHA ) Type XII: Aldolase A ( ALDOA ) Type XIII: β-Enolase ( ENO3 ) Type XIV: Phosphoglucomutase 1 ( PGM1 ) Type XV: Glycogenin 1 ( GYG1 ) Type 0: Glycogen synthetase 1 ( GYS1 ) |
| DISORDERS OF LIPID METABOLISM |
| Carnitine palmitoyltransferase II ( CPT2 ) Primary systemic carnitine deficiency ( SCL22A5 ) Carnitine/acyl-carnitine translocase deficiency ( SLC25A20 ) Secondary carnitine deficiency Very-long-chain acyl coenzyme A dehydrogenase deficiency ( ACADVL ) Long-chain acyl coenzyme A dehydrogenase deficiency ( ACADL ) Medium-chain acyl coenzyme A dehydrogenase deficiency ( ACADM ) Short-chain acyl coenzyme A dehydrogenase deficiency ( ACAD5 ) Long-chain hydroxyl/acyl coenzyme A dehydrogenase deficiency ( LCHAD ) Multiple acyl coenzyme A dehydrogenase deficiency ( ETFA, ETFB, ETFDH ) Mitochondrial complex I deficiency ( ACAD9 ) Triglyceride storage disease with ichthyosis ( ABHD5 ) Neutral lipid storage with myopathy ( PNPLA2 ) Acute recurrent myoglobinuria ( LPIN1 ) Flavin adenine dinucleotide synthetase deficiency ( FLAD1 ) Medications (valproic acid) |
| MITOCHONDRIAL MYOPATHIES |
| Chronic progressive external ophthalmoplegia Kearns-Sayre syndrome Mitochondrial encephalopathy, lactic acidosis, and strokelike episodes (MELAS) Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) Myoclonic epilepsy with ragged red fibers (MERRF) Infantile myopathy and lactic acidosis Cytochrome c oxidase deficiency |
The term muscular dystrophy refers to the primary degeneration of the muscle fiber, usually associated with an increase in fatty and fibrous connective tissue. The common clinical presentation is progressive muscle weakness.
Duchenne and Becker muscular dystrophies are caused by mutations in the dystrophin gene, which is located on the X chromosome. Female carriers can develop variable phenotypes, including a severe Duchenne-like presentation, mild adult-onset limb-girdle weakness, asymptomatic CK elevation, and cardiomyopathy.
Duchenne muscular dystrophy is the most common inherited muscle disease, with an incidence of about 1 in 5000 male births. About one third of patients carry a de novo mutation without a family history. In most patients, a frameshift mutation in the dystrophin gene results in a complete absence of the dystrophin protein. This absence of dystrophin disrupts the mechanical link between the sarcomere and the sarcolemma, thereby causing a calcium leak that leads to necrosis of muscle fibers.
Duchenne dystrophy typically presents in young boys who are between 2 and 5 years of age with delayed motor milestones, difficulty running, increasing falls, and enlarged calves. The disorder is relentlessly progressive and can cause a cardiomyopathy ( Chapter 47 ) that leads to heart failure and fatal arrhythmias. Intellectual disability, learning disorders, autism, and attention-deficit/hyperactivity disorder can be associated features. By 12 years of age, most affected individuals can no longer ambulate. By the age of 20 years, most patients develop joint contractures and kyphoscoliosis that lead to further respiratory compromise.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here