Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
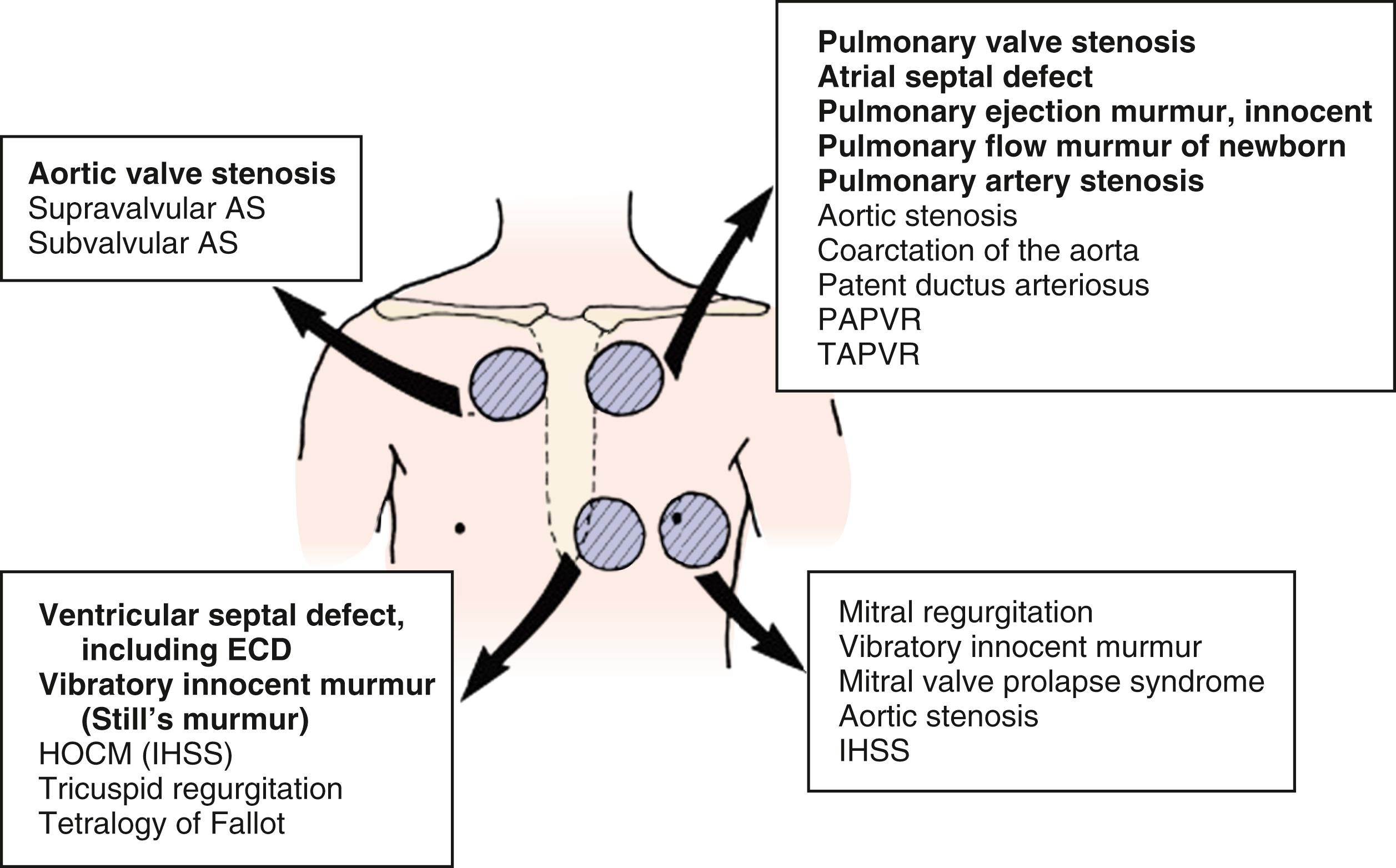
A heart murmur is a sign, the audible turbulence of blood flow through the heart or the major vessels; symptoms associated with heart murmurs vary by the underlying cause, as well as the nature and severity of any cardiac lesion producing the murmur. While the majority of heart murmurs are normal or innocent, they must be distinguished from the pathologic murmurs of congenital or acquired cardiac disease. Whereas <1% of the population has significant structural congenital cardiac disease, as much as 85% of the population may have a heart murmur during childhood; causes vary by the age of the patient at presentation ( Table 9.1 ). The causes of congenital heart disease are varied and include genetic disorders, metabolic disorders, teratogens, and syndrome complexes ( Table 9.2 ). The causes of acquired heart diseases in children include rheumatic fever, endocarditis, and cardiac injury caused by systemic illnesses. Whereas the echocardiogram defines the significance of pathologic heart abnormalities, the only way to definitively diagnose an innocent murmur is with a stethoscope.
| Neonate ∗ | Infant | Older Child |
|---|---|---|
| Transient patency of the ductus arteriosus Peripheral pulmonic stenosis Cyanotic congenital heart disease Congenital valvular obstruction Arteriovenous malformation (CNS, hepatic, pulmonary) Anemia Asphyxia-related myocardial ischemia (transient TI or MI) Pulmonary hypertension |
Congenital heart disease (L→R shunt or R→L shunt) † Ejection murmurs (normal) Anemia Arteriovenous malformation Infective endocarditis Kawasaki disease Hunter syndrome Hurler syndrome Fabry syndrome |
Congenital valvular obstruction Ejection murmurs (normal) Repaired congenital heart disease Anemia Mitral valve prolapse Venous hum Infective endocarditis Rheumatic fever Marfan syndrome Prosthetic valves Obstructive (hypertrophic) cardiomyopathy (subaortic stenosis) Carotid or abdominal bruit Tumor (atrial myxoma) Thyrotoxicosis Systemic lupus erythematosus Pericardial friction rub |
∗ Common causes of congenital heart disease in low birthweight infants include PDA, VSD, tetralogy of Fallot, coarctation of the aorta–interrupted aortic arch, hypoplastic left heart syndrome, heterotaxy, and dextrotransposition of the great arteries, in that order. Common causes of congenital heart disease in term infants include VSD, dextrotransposition of the great arteries, tetralogy of Fallot, coarctation of the aorta, pulmonary stenosis, hypoplastic left heart syndrome, and PDA; other causes represent a smaller percentage.
† The relative percentages of congenital heart lesions are VSD (25–30%); ASD (6–8%); PDA (6–8%); coarctation of aorta (5–7%); tetralogy of Fallot (5–7%); pulmonary valve stenosis (5–7%); aortic valve stenosis (5–7%); dextrotransposition of great arteries (3–5%); and hypoplastic left ventricle, truncus arteriosus, total anomalous venous return, tricuspid atresia, single ventricle, and double-outlet right ventricle representing 1–3% each. Other and more complex lesions (e.g., forms of heterotaxy) together represent 5–10% of all lesions.
| Syndrome | Features |
|---|---|
| Chromosomal Disorders | |
| Trisomy 21 (Down syndrome) | Endocardial cushion defect, VSD, ASD |
| Trisomy 21p (cat-eye syndrome) | Miscellaneous, total anomalous pulmonary venous return |
| Trisomy 18 | VSD, ASD, PDA, TOF, coarctation of aorta, bicuspid aortic or pulmonary valve |
| Trisomy 13 | VSD, ASD, PDA, coarctation of aorta, bicuspid aortic or pulmonary valve |
| Trisomy 9 | Miscellaneous, VSD |
| XXXXY | PDA, ASD |
| Penta X | PDA, VSD |
| Triploidy | VSD, ASD, PDA |
| XO (Turner syndrome) | Bicuspid aortic valve, coarctation of aorta |
| Fragile X | Mitral valve prolapse, aortic root dilatation |
| Duplication 3q2 | Miscellaneous |
| Deletion 4p (Wolf-Hirschhorn syndrome) | VSD, PDA, aortic stenosis |
| Deletion 9p | Miscellaneous |
| Deletion 5p (cri du chat syndrome) | VSD, PDA, ASD, TOF |
| Deletion 10q | VSD, TOF, conotruncal lesions ∗ |
| Deletion 13q | VSD |
| Deletion 18q | VSD |
| Deletion 1p36 | ASD, VSD, PDA, TOF, cardiomyopathy |
| Deletion/duplication 1q21.1 | ASD, VSD, PS |
| Deletion 7q11.23 (Williams syndrome) | Supravalvular AS, branch PS |
| Deletion 11q 24-25 (Jacobsen syndrome) | VSD, left-sided lesions |
| Syndrome Complexes | |
| CHARGE association ( c oloboma, h eart, a tresia choanae, growth r etardation, g enital, and e ar anomalies) | VSD, ASD, PDA, TOF, endocardial cushion defect |
| DiGeorge syndrome, CATCH 22 ( c ardiac defects, a bnormal facies, t hymic aplasia, c left palate, h ypocalcemia, and deletion 22q11) | Aortic arch anomalies, conotruncal anomalies |
| Alagille syndrome (arteriohepatic dysplasia) | Peripheral pulmonic stenosis, PS, TOF, abdominal coarctation |
| VATER association ( v ertebral, a nal, t racheo e sophageal, r adial, and r enal anomalies) † | VSD, TOF, ASD, PDA |
| OAVS ( o culo- a uriculo v ertebral s pectrum), including Goldenhar syndrome | TOF, VSD |
| CHILD ( c ongenital h emidysplasia with i chthyosiform erythroderma, l imb d efects) | Miscellaneous |
| Mulibrey nanism (muscle, liver, brain, eye) | Pericardial thickening, constrictive pericarditis |
| Asplenia syndrome | Complex cyanotic heart lesions with decreased pulmonary blood flow, transposition of great arteries, anomalous pulmonary venous return, dextrocardia, single ventricle, single atrioventricular valve |
| Polysplenia syndrome | Acyanotic lesions with increased pulmonary blood flow, azygos continuation of inferior vena cava, partial anomalous pulmonary venous return, dextrocardia, single ventricle, common atrioventricular valve |
| PHACE syndrome ( p osterior brain fossa anomalies, facial h emangiomas, a rterial anomalies, c ardiac anomalies and aortic coarctation, e ye anomalies) | VSD, PDA, coarctation of aorta, arterial aneurysms |
| Teratogenic Agents | |
| Congenital rubella | PDA, peripheral pulmonic stenosis |
| Fetal hydantoin/phenytoin syndrome | VSD, ASD, coarctation of aorta, PDA |
| Fetal alcohol syndrome | ASD, VSD, TOF |
| Fetal valproate effects | Coarctation of aorta, hypoplastic left side of heart, aortic stenosis, pulmonary atresia, VSD |
| Maternal phenylketonuria | VSD, ASD, PDA, coarctation of aorta |
| Retinoic acid embryopathy | Conotruncal anomalies |
| Others | |
| Apert syndrome | VSD |
| Autosomal dominant polycystic kidney disease | Mitral valve prolapse |
| Carpenter syndrome | PDA |
| Char syndrome | PDA |
| Conradi syndrome | VSD, PDA |
| Cornelia de Lange syndrome | VSD, TOF |
| Crouzon disease | PDA, coarctation of aorta |
| Cutis laxa | Pulmonary hypertension, pulmonic stenosis |
| Ellis–van Creveld syndrome | Single atrium, VSD |
| Holt-Oram syndrome | ASD, VSD, first-degree heart block |
| Infant of diabetic mother | Hypertrophic cardiomyopathy, VSD, conotruncal anomalies |
| Kartagener syndrome | Dextrocardia |
| Laurence-Moon | TOF, VSD |
| Marfan | Aortic root dissection, mitral valve prolapse |
| Meckel-Gruber syndrome | ASD, VSD |
| Noonan syndrome (with or without multiple lentigines) | Pulmonic stenosis, ASD, cardiomyopathy |
| Pallister-Hall syndrome | Endocardial cushion defect |
| Pierre Robin sequence | ASD, VSD, PDA, coarctation of aorta |
| Primary ciliary dyskinesia | Heterotaxy disorders |
| Rubinstein-Taybi syndrome | PDA, PS, coarctation of aorta, VSD |
| Scimitar syndrome | Hypoplasia of right lung, anomalous pulmonary venous return to inferior vena cava |
| Smith-Lemli-Opitz syndrome | VSD, PDA |
| TAR syndrome (thrombocytopenia and absent radius) | ASD, TOF, VSD |
| Treacher Collins syndrome | VSD, ASD, PDA |
∗ Conotruncal includes TOF, pulmonary atresia, truncus arteriosus, and transposition of great arteries.
† Also known as VACTERL ( v ertebral, a nal, c ardiac, t racheo-esophageal fistula, r enal, l imb).
Knowing the location of the heart chambers and valves within the thorax helps in the interpretation of the heart sounds ( Fig. 9.1 ). The left atrium is located posteriorly, close to the spine. The right atrium and right ventricle are located anteriorly, immediately beneath the sternum. The outflow tract of the right ventricle, which contains the pulmonary valve, rises to the left of the sternum. The parts of the left side of the heart that are close to the chest wall include the left ventricular apex and the ascending aorta as it passes up to the right of the sternum. In other areas, lung tissue lies between the heart and chest wall, which may diminish or distort the intensity of heart sounds.
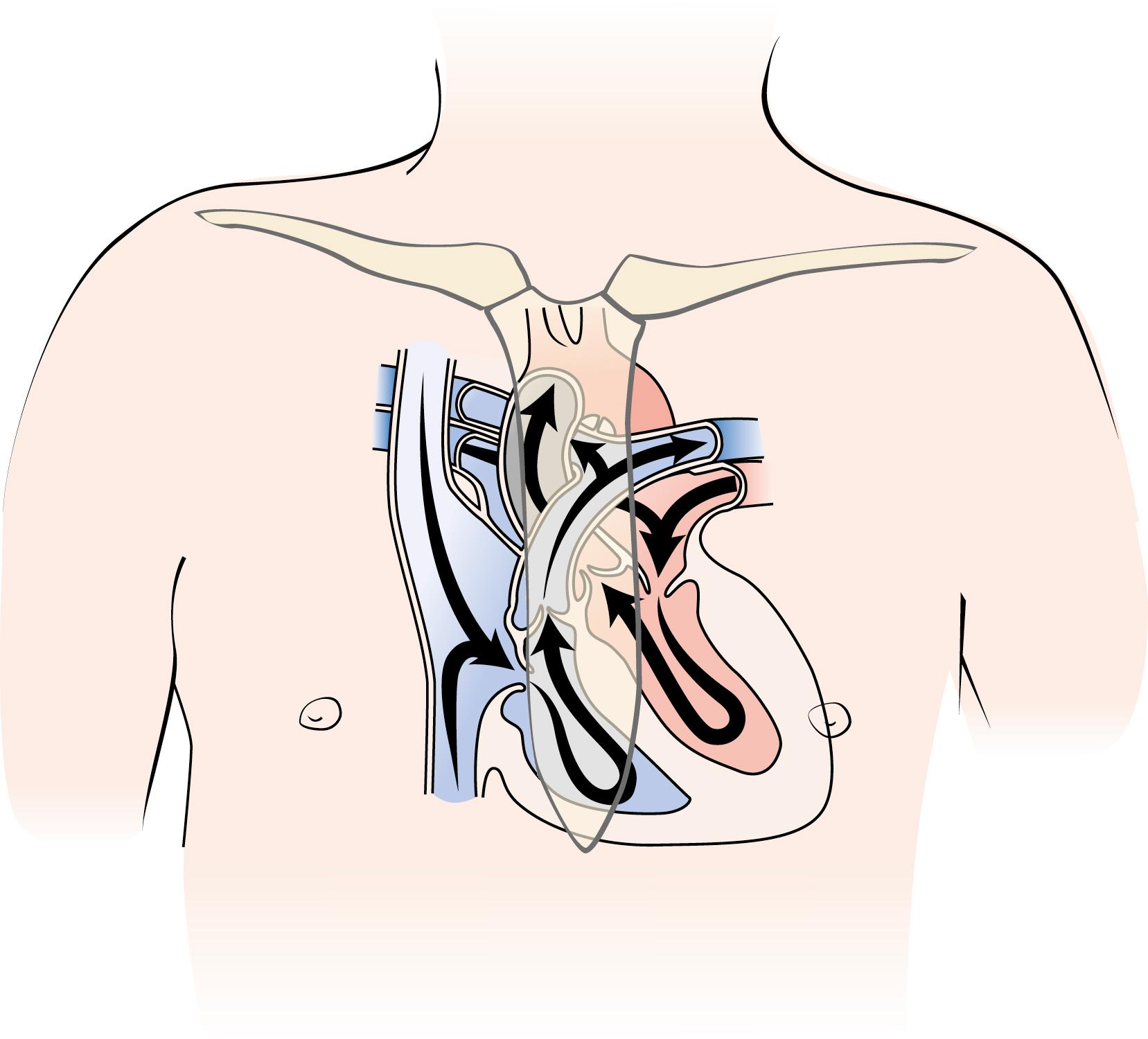
Normal heart sounds originate from the vibration of heart valves when they close and from heart chambers when they fill rapidly. The amount of pressure that forces valve closure influences the intensity of a heart sound. Other mechanical factors such as valve stiffness, thickness, and excursion have lesser effects on sound intensity.
Cardiac murmurs are the direct result of blood flow turbulence. The amount of turbulence and consequently the intensity of a cardiac murmur are directly proportional to both the pressure difference or gradient across a narrowing or defect and the blood flow or volume moving across the site. In contrast to intensity, the frequency or pitch of a cardiac murmur is proportional to pressure difference or gradient alone.
As sound radiates from its source, sound intensity diminishes with the square of the distance. Consequently, heart sounds should be loudest near the point of origin. However, factors other than distance may influence this relationship. Sound passage through the body is affected by the transmission characteristics of the tissues through which the sound is being transmitted. Fat has a more pronounced dampening effect on higher frequencies than does more dense tissue such as bone. If the difference in tissue density is significant—for example, between the heart and lungs—more sound energy is lost. Only the loudest sounds may be heard when lung tissue is positioned between the heart and chest wall.
The timing of events in the cardiac cycle allows for a more thorough understanding of heart sounds and murmurs. The relationship between the normal heart cycle and that of the heart sounds is noted in Figure 9.2 .
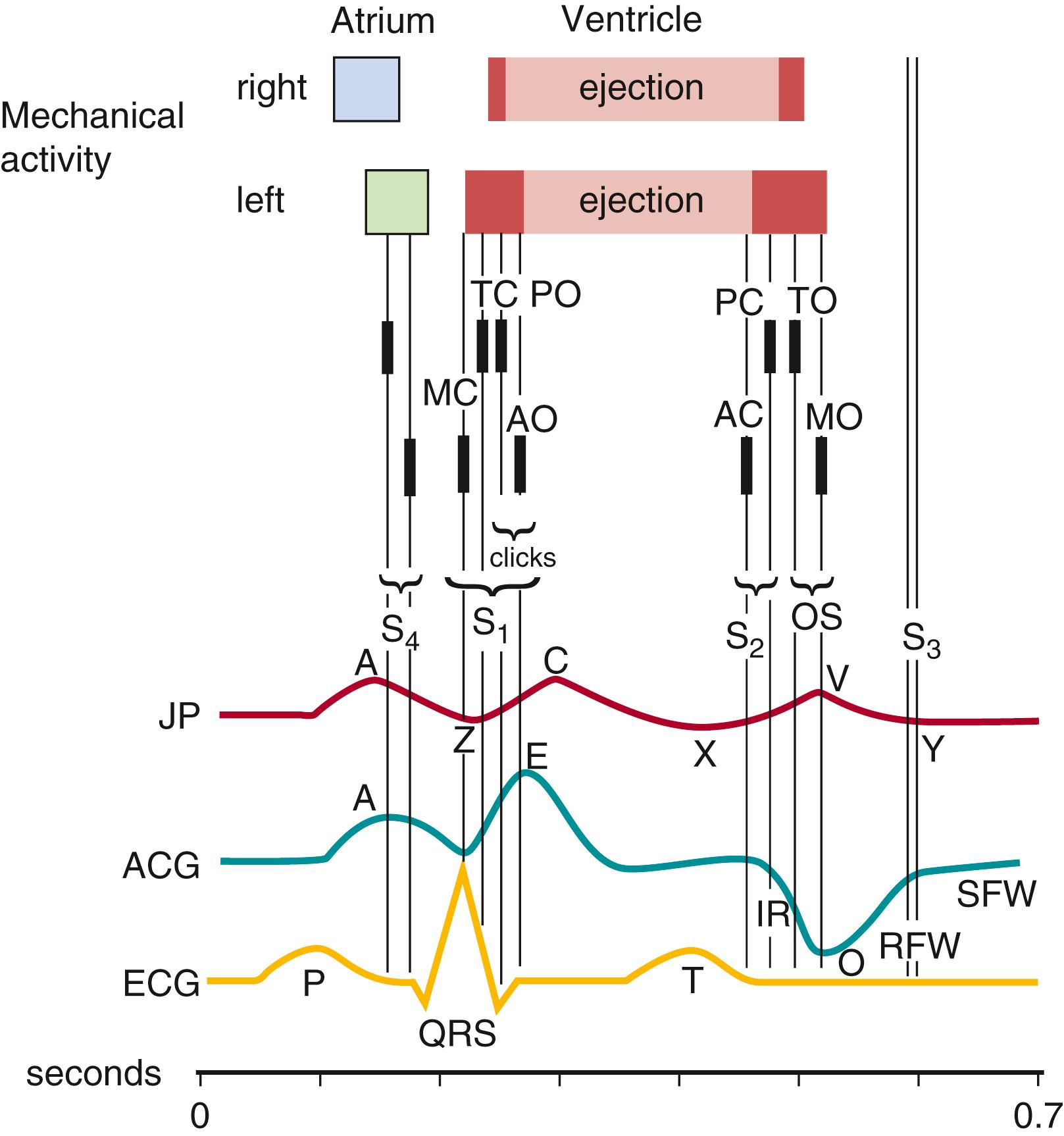
The cardiac cycle begins with atrial systole , the sequential activation and contraction of the two thin-walled upper chambers. Atrial systole is followed by the delayed contraction of the more powerful lower chambers, termed ventricular systole . Ventricular systole has three phases:
Isovolumic contraction: the short period of early contraction when the pressure builds within the ventricle but has yet to rise sufficiently to permit ejection
Ventricular ejection: when the ventricles eject blood to the body (via the aorta) and to the lungs (via the pulmonary artery)
Isovolumic relaxation: the period of ventricular relaxation when ejection ceases and pressure falls within the ventricles
During ventricular contraction, the atria relax ( atrial diastole ) and receive venous return from both the body and the lungs. Then, in ventricular diastole , the lower chambers relax, allowing initial passive filling of the thick-walled ventricles and emptying of the atria. Later, during the terminal period of ventricular relaxation, the atria contract. This atrial systole augments ventricular filling just before the onset of the next ventricular contraction.
The sequence of contractions generates pressure and blood flow through the heart. The relationship of blood volume, pressure, and flow determines opening and closing of heart valves and generates characteristic heart sounds and murmurs.
The majority of significant structural congenital heart disease is recognizable in the first few weeks of life. The age at recognition or presentation often correlates with the nature of the cardiac anomaly and the urgency with which assessment and treatment are necessary.
In the fetus, oxygen is acquired from the placenta and returns via the umbilical vein and through the ductus venosus to enter the inferior vena cava and right atrium ( Fig. 9.3 ). Preferentially, flow is directed across the foramen ovale to enter the left atrium and, subsequently, the left ventricle. Deoxygenated blood returning from the superior vena cava and upper body segment is preferentially directed by the flap of the eustachian valve to enter the right ventricle and then, via the ductus arteriosus, to enter the descending aorta to return via the umbilical arteries to the placenta. The pressures within both ventricles are essentially equal, inasmuch as both chambers pump to the systemic circulation. However, in utero, the right ventricle does the majority of the work, pumping 66% of the combined cardiac output. At transition (see Fig. 9.3 ), with the first breath, pulmonary arterial resistance begins to fall as the lungs begin the process of respiration. Pulmonary venous return to the left atrium closes the flap of the foramen ovale. Through mechanical and chemical mechanisms, the ductus arteriosus begins to close. In the normal full-term infant, this is accomplished by 10–15 hours after birth. Intermittent right-to-left atrial level shunting through the foramen ovale may occur, particularly if pulmonary vascular resistance fails to drop.
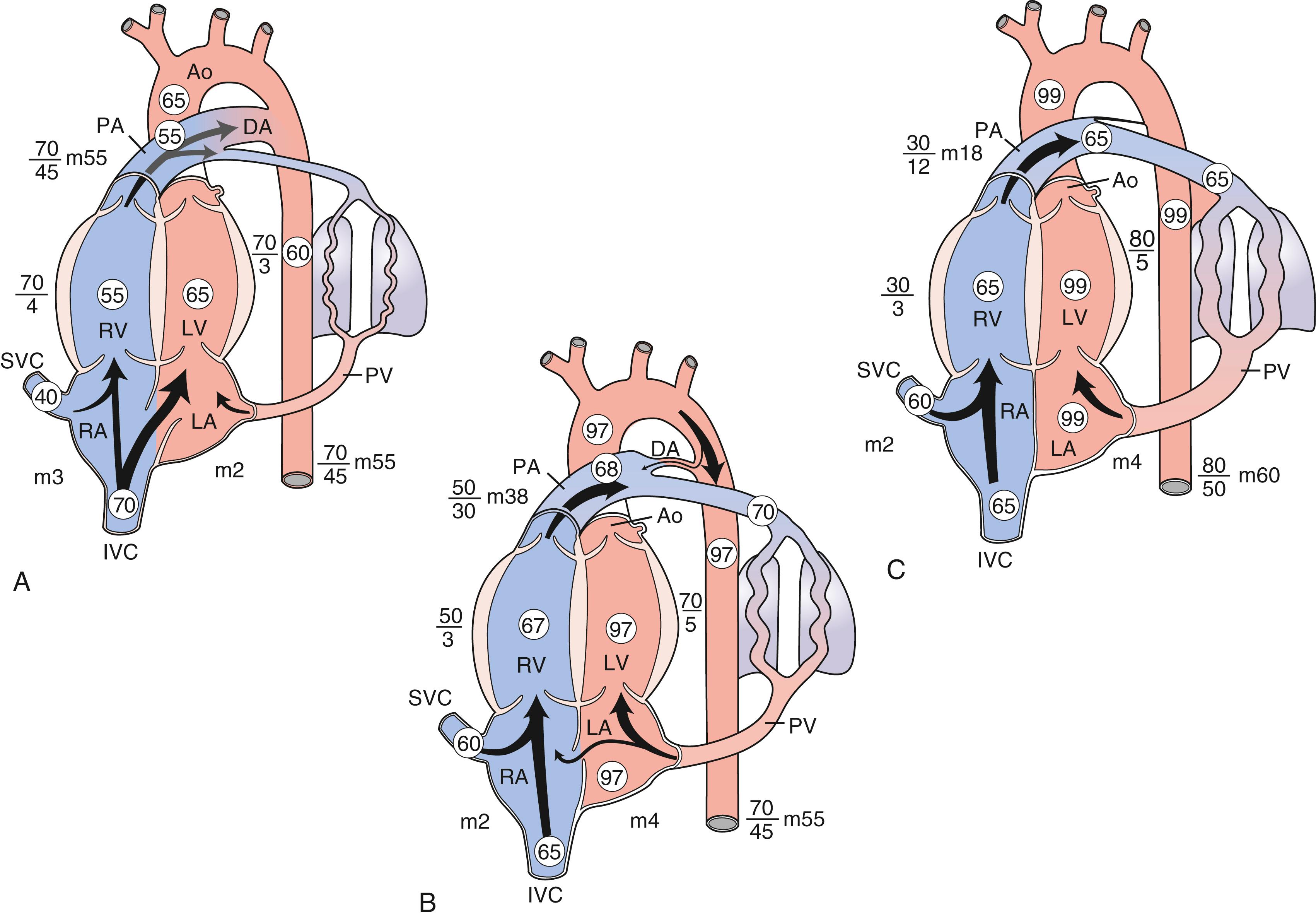
The time when congenital heart disease becomes symptomatic is influenced by the specific lesion. Some structural cardiac abnormalities require patency of the ductus arteriosus for maintenance of either pulmonary blood flow (e.g., pulmonary atresia) or systemic blood flow (e.g., hypoplastic left heart syndrome). These and other ductus-dependent abnormalities, such as transposition of the great arteries, coarctation of the aorta, or significant outflow obstruction (e.g., critical aortic valve stenosis), manifest in the first few hours or days after birth when the ductus arteriosus begins to close. In the absence of an associated anomaly, hemodynamically significant ventricular septal defects (VSDs) do not manifest before 2–4 weeks after birth. Atrial septal defects (ASDs) are seldom symptomatic in infancy.
In the neonate, after birth and successful transition, resistance to flow in the pulmonary circuit is much lower than in the systemic circuit. As such, the pressures in the right-sided chambers are lower than those in the left-sided chambers. The higher values within the ventricles reflect the pressure during the period of ventricular systole in a normal heart (see Fig. 9.3 ). Pressure in the great vessels during systole is identical to that in the corresponding ventricles, though this relationship changes if there is outflow obstruction. In ventricular diastole, the semilunar valves (aortic and pulmonary) close. Resistance to blood flow in the vascular bed determines the diastolic pressures in the great arteries. The thin-walled atria generate much lower pressures than do the ventricles, both during the phase of passive atrial filling ( v wave ) and during atrial contraction ( a wave ). Only the mean (m) or average atrial pressure is shown in Figure 9.3 . During ventricular relaxation, the diastolic pressures are lower than those in the atria, enabling filling.
Historical assessment of the pediatric patient referred for evaluation of a cardiac murmur should include questions about the family history, pregnancy (including fetal ultrasonography), and perinatal course, in addition to questions about symptoms of cardiovascular disease. An assessment of exercise or play or feeding capacity should be sought, as should an assessment of growth and development. The presence of congenital abnormalities of other major organ systems is associated with structural cardiac problems in as many as 25% of patients.
Structural heart disease is frequently seen in association with recognizable syndromes (see Table 9.2 ). Children with clearly definable chromosomal disorders known to be strongly associated with structural cardiac abnormalities, such as trisomy 21 or Turner syndrome, should be referred for further diagnostic evaluation. Family history of sudden unexplained death, rheumatic fever, sudden infant death syndrome, or a structural cardiac abnormality in a first-degree relative may be relevant. Hypertrophic cardiomyopathy in a first-degree relative is associated with a high incidence of inheritance, and this condition is sufficiently subtle that echocardiographic screening is mandatory. Unexplained fever, lethargy, a history of intravenous drug use, or additional symptoms arising after recent dental work should arouse suspicion of possible endocarditis.
Infants born to mothers with diabetes mellitus (type 1 or gestational) have as high as a 30% chance of transient hypertrophic cardiomyopathy and are also at risk for congenital structural abnormalities. Additional maternal risk factors for congenital structural heart disease include acute or chronic maternal illness, vertically transmitted infections, illicit drug use, or the use of certain teratogenic medications.
The general health of a child with a suspected cardiac malformation is important to assess. Particularly relevant are the rate of growth and history of past illnesses. Although symptoms of failure to thrive are nonspecific, patterns of growth reflect the severity of the disease and effectiveness of treatment (see Chapter 12 ). In an infant, feeding difficulties are often the first evidence of congestive heart failure. Feeding problems are common manifestations of cardiac disease and may be evidenced as disinterest, excessive fatigue, long feeding duration, diaphoresis, tachypnea, dyspnea, or a change in the pattern of respiration. It is important to obtain an objective measure of caloric intake by quantitating the number and/or volume of feedings. Some index of exertional tolerance should be sought in all children as an index of cardiovascular fitness and a sign of functional capability. This index should be age relevant and, in an infant, might include assessment of the vigor and duration of feeding and the time period of interactive play. In a toddler, the index might include the ability to keep up with peers, climb stairs, or walk for extended periods. In an older child, a comparison with peer sporting interactions, level of function in physical education, and an index of aerobic ability should be sought.
Tachypnea may occur as a consequence of increased pulmonary blood flow. With increasing pulmonary congestion, particularly obstruction to pulmonary venous drainage, dyspnea is manifested as an anxious look with grunting, flaring of the alae nasi, head bobbing, and intercostal, suprasternal, and subcostal retractions. Respiratory rates and the pattern of breathing should be assessed for a full minute in the quiet infant because rates may vary considerably with activity and feeding ( Table 9.3 ), or even over shorter time periods in a quiet infant. Cardiac asthma or exercise-inducible reactive airway disease may occur as a consequence of passive or active pulmonary congestion (see Chapter 4 ). Compression of airways by plethoric vessels may contribute to the stasis of secretions and atelectasis, which predisposes to respiratory tract infections.
| AGE | |||||
|---|---|---|---|---|---|
| Birth–6 Wk | 6 Wk–2 Yr | 2–6 Yr | 6–10 Yr | Older Than 10 Yr | |
| Respiratory rate | 45–60/min | 40/min | 30/min | 25/min | 20/min |
| Heart rate | 125 ± 30/min | 115 ± 25/min | 100 ± 20/min | 90 ± 15/min | 85 ± 15/min |
Cyanosis in association with a cardiac murmur suggests a structural lesion with restriction to pulmonary blood flow ( Table 9.4 ). Cyanosis, or a blue discoloration of the skin and mucous membranes, is a consequence of reduced hemoglobin being present in >5 g/dL of hemoglobin and is evident in one third of infants with potentially lethal congenital heart disease. Central cyanosis is distinguished from acrocyanosis (peripheral cyanosis) by involvement of the warm mucous membranes, including the tongue and buccal mucosa. Acrocyanosis or peripheral cyanosis is generally confined to the perioral and perinasal regions, extremities, or nail beds and occurs in the child who is cold, vasoconstricted, or at rest. A distinctive feature is that central cyanosis generally worsens with activity and increasing cardiac output, whereas acrocyanosis generally improves or resolves with increased activity.
| Group | Heart Size | Pulmonary Blood Flow | Low Cardiac Output | Respiratory Distress | Examples |
|---|---|---|---|---|---|
| I | Small | Reduced | No | None | Hypoplastic RV with pulmonary atresia |
| Hypoplastic RV with tricuspid atresia | |||||
| Tetralogy of Fallot (severe) | |||||
| II | Small or slight cardiomegaly | Increased | No | Moderate | Transposition of great arteries with intact ventricular septum |
| III | Large | Increased | Yes | Yes | Complicated coarctation of aorta with VSD, hypoplastic LV |
| IV | Small | Pulmonary venous congestion | Yes | Yes | Obstructed total anomalous pulmonary veins |
Height and weight should be measured and plotted on a growth chart. An assessment of the child’s overall growth, appearance, and state of distress serves as a guide to the urgency of further investigation and management. The sick infant often appears anxious, fretful, diaphoretic, pale, or breathless and is seldom consolable. Cyanosis, pallor, digital clubbing, an abnormal pattern of respiration, and possible dysmorphic features may suggest specific structural cardiac anomalies.
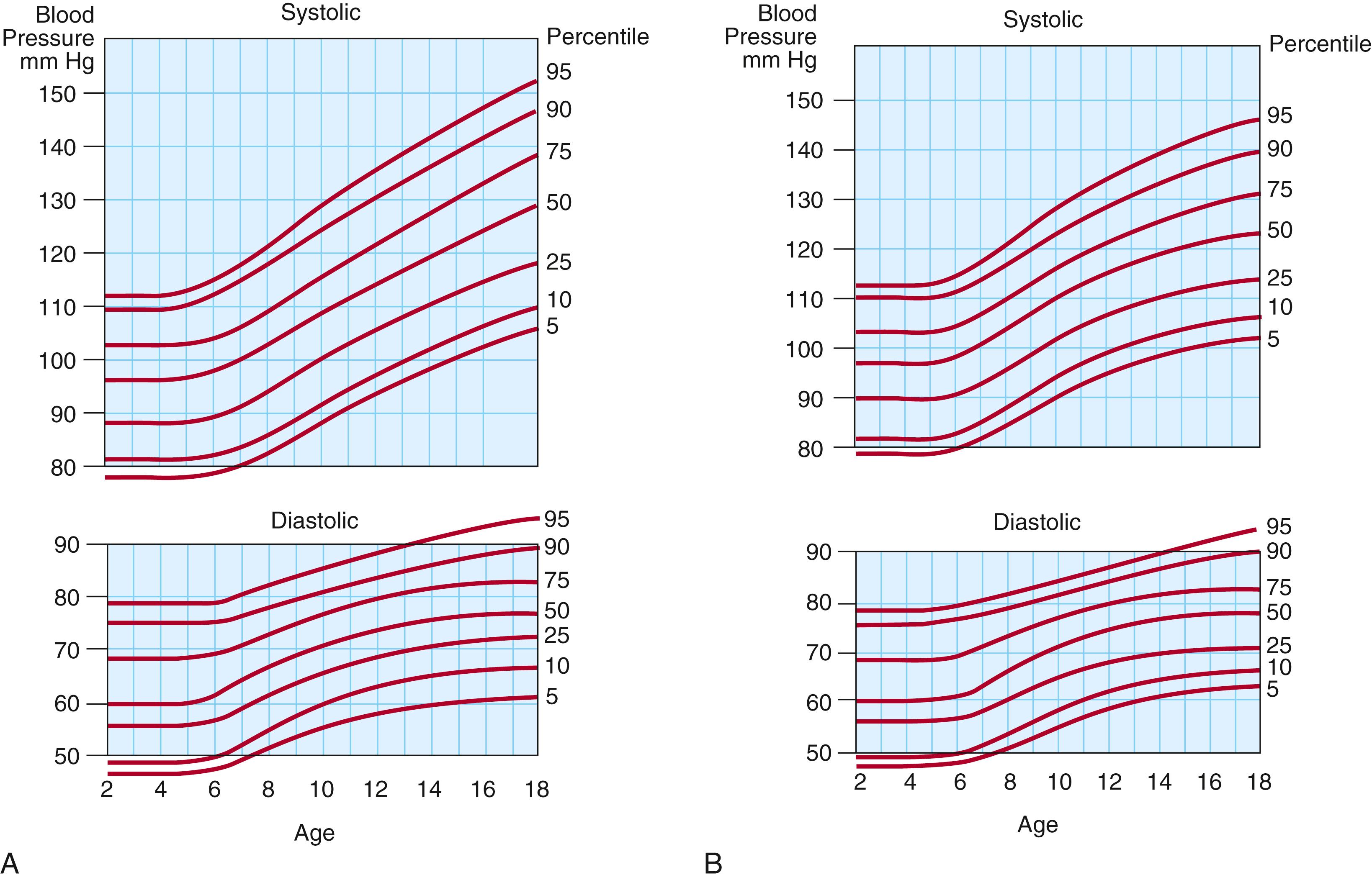
Normal resting heart rates and respiratory rate values for age are presented in Table 9.3 . Blood pressure should be measured using an appropriately sized cuff. Every child should have a comparison of upper and lower blood pressures on at least one occasion. The lower limb systolic blood pressure is normally 10 mm Hg higher than the upper limb pressure in older children. On occasion, the subclavian arteries may arise aberrantly beyond the site of ductal ligament insertion. Therefore, both upper limb pressures should be measured and compared with the lower limb pressure. Normal values for blood pressure in children are presented in Figure 9.4 .
Respiratory distress may suggest cardiac disease. In addition to noting the rate, depth, and effort of respiration, the inspection should include observation for evidence of air trapping, increased chest diameter, or the presence of Harrison sulci (horizontal grooves in the anterior chest at the diaphragm insertion site along the sixth and seventh costal cartilages) as an indication of chronic upper airway obstruction. Midfacial hypoplasia with chronic mouth breathing may also suggest upper airway obstructive disease with predisposition to hypercapnia and pulmonary hypertension. Although crackles in the lungs in infants and even young children usually indicate infection, pulmonary edema should also be a consideration.
Pulses should be assessed for rate, rhythm, volume, and character. The dynamic character of the pulse may provide information about the cardiac output. A clinical index of cardiac output includes the warmth of the digits and measured capillary refill time, obtained by blanching the nail beds or digits and estimating the time to full reperfusion, which is normally <2 seconds. Initially, the radial and brachial pulses should be assessed simultaneously in the upper limb. By palpating the pulse at two sites and altering the pressure applied by the palpating fingers, a more accurate assessment of the rate of rise, volume, and contour may be obtained. Assessment of the femoral pulse requires that the infant be quiet. Palpating parallel to the inguinal crease and allowing the leg to continue to flex is generally more effective than extending the leg. Blood pressures in the arm and leg should be assessed, and the radial and femoral pulses should be palpated simultaneously. Whenever possible, the radial pulse should be brought in close apposition to the femoral pulse to compare for any delay, allowing for a more accurate appreciation of any temporal delay and enabling more accurate detection of the presence of coarctation of the aorta . The presence of a palpable femoral pulse is by itself an inadequate screen for coarctation because a widely patent ductus arteriosus (PDA) or collateral vessels, particularly in the older patient, may provide delayed perfusion. Previous arterial instrumentation, injury, or congenital variability may account for reduction in palpable peripheral pulses.
In infants and young children, the liver size and character offer more reliable indicators of right atrial pressure and systemic congestion than does the jugular venous pressure. The position, size, and consistency of the liver should be assessed with quiet respiration. The character of the normal liver margin is generally likened to that of the cartilage of the external pinna, and the margin should be sharp and angulated. In the newborn, the liver may be normally palpable at 1.5–2.5 cm below the right costal margin in the midclavicular line. This distance decreases to approximately 1–2 cm by 1 year of age and remains just palpable until school-entrance age. In the presence of congestive heart failure, the liver enlarges and distends downward. The congested liver margin becomes rounded and firm and is often more difficult to feel. An enlarged liver may be tender, and aggressive palpation may cause discomfort and tensing of the abdominal musculature, making accurate assessment difficult. A transverse liver is suggestive of a heterotaxy syndrome with abnormal abdominal organ location (i.e., situs abnormalities) and complex congenital heart lesions. The spleen should always be sought; enlargement suggests endocarditis in the patient with a heart murmur. Splenic enlargement in association with congestive heart failure is unusual (see Chapter 17 ).
Inspection of the chest may suggest the presence of a precordial bulge of long-standing right ventricular volume overload. The examiner’s entire palm and hand should be warmed and then fully applied to the patient’s chest wall to maximize ability to detect thrills or heaves. Whereas the examiner’s fingertips are best utilized to localize an abnormality, the palmar surface of the metacarpals and first phalanges is more sensitive for the detection of low-frequency events. The fingertips should be used to localize the most lateral displacement of the apical impulse. In patients of all ages, the apical impulse should be confined to one intercostal interspace, in which case the impulse would be described as localized; however, if the apical impulse is equally dynamic in two or more interspaces, then it is best described as diffuse. In the neonate, a right ventricular impulse may be felt close to the sternum. Later in life, the same degree of parasternal activity is likely to suggest pulmonary hypertension, right-sided heart volume overload, or right ventricular outflow obstruction. The lateral displacement of the apex, normally located in the midclavicular line, should be compared to existing landmarks. A dynamic or thrusting character to an apical impulse may be detected in association with an elevated cardiac output or various forms of obstruction to left ventricular outflow. On occasion, an apical filling impulse, coinciding with an audible S 3 , may be normally palpable, particularly in the adolescent or athlete with a relative bradycardia and increased stroke volume.
A thrill is a palpable murmur and should be sought in the precordial and suprasternal areas. The palmar surface of the examiner’s hand is most sensitive in detection of a thrill; however, only the tips of the digits fit in the patient’s suprasternal notch. A palpable second heart sound (S 2 ), indicative of a significant level of pulmonary hypertension, may be detected as a sharp or distinctive impulse in the pulmonary outflow.
Thorough auscultation in the cooperative patient may take as long as 5–10 minutes and should include listening in the principal areas of the precordial auscultation (tricuspid, pulmonary, mitral, and aortic) with both the bell and diaphragm of the stethoscope, with the patient in the supine, sitting, and standing positions. These four areas serve as a guide to auscultation of the heart ( Fig. 9.5 ). These are the optimal sites for listening to sounds that arise within the chambers and great vessels:
The tricuspid area is represented by the fourth and fifth intercostal spaces along the left sternal edge but extends to the right of the sternum as well as downward to the subxiphisternal area.
The pulmonary area is the second intercostal space along the left sternal border. Murmurs that are best heard in this area may also extend to the left infraclavicular area and often lower, along the left sternal edge to the third intercostal space.
The mitral area involves the region of the cardiac apex and generally is at the fifth intercostal space in the midclavicular line. This area may also extend medially to the left sternal edge and laterally to the region of the axilla.
The aortic area , although centered at the second right intercostal space, may extend to the suprasternal area, to the neck, and inferiorly to the third left intercostal space.
The margins of these areas are ill defined, and auscultation should not be limited to these sites and may extend to the axillae, neck, back, or infraclavicular areas.
A step-by-step auscultation—first for heart sounds, subsequently for systolic murmurs, and then separately for diastolic murmurs—is essential. The ability to clearly characterize the S 2 is perhaps more crucial than for any other sound; the effects of respiration are important. The components of the S 2 in childhood are normally split with inspiration and become single on expiration. A loud pulmonary closure sound should suggest the possibility of pulmonary artery hypertension. The S 2 may be widely split and/or fixed in association with right ventricular volume overload or delayed right ventricular conduction. Normal inspiratory splitting of the S 2 should be sought and established in all patients. As timing may be difficult in the infant with a rapid respiratory rate, the presence of splitting at any time during the respiratory cycle may be accepted as normal.
The right ventricle is normally just beneath the sternum. This proximity generally makes sounds emanating from the right heart louder and less diffuse. In addition, right heart sounds and murmurs are more influenced by the effects of respiration.
Examination should first focus on identifying the normal heart sounds in sequence; subsequent attention should be directed at establishing the effects of inspiration and expiration on the heart sounds. Next, examination should address the presence or absence of additional heart sounds and murmurs. Finally, examination should ascertain any variability that occurs with a change of body position.
The first heart sound (S 1 ) (see Fig. 9.2 ) arises from closure of the atrioventricular (mitral and tricuspid) valves in early isovolumic ventricular contraction and, consequently, is best heard in the mitral and tricuspid valve areas. Mitral valve closure occurs slightly in advance of tricuspid valve closure, and, on rare occasion, near the lower left sternal edge two components (splitting) of the S 1 may be heard. There is usually a single first heart sound. The S 1 is most easily heard when the heart rate is slow because the interval between the S 1 and S 2 is shorter than the interval between the S 2 and subsequent S 1 . The intensity of the S 1 is influenced by the position of the atrioventricular valve at the onset of ventricular contraction.
Shortly after the onset of ventricular contraction, the semilunar valves (aortic and pulmonary) open and permit ventricular ejection. This opening does not usually generate any sound. The atrioventricular valves remain tightly closed during ventricular ejection. As ventricular ejection nears completion, the pressure begins to fall within the ventricles, and the semilunar valves snap closed. This prevents regurgitation from the aorta and pulmonary artery back into the heart. The closure of the semilunar valves generates the S 2 (see Fig. 9.2 ). The S 2 usually consists of a louder and earlier aortic valve closure sound (A 2 ), followed by a later and quieter pulmonary valve closure sound (P 2 ). Normal physiologic splitting or variability is generally only appreciated in the pulmonary area during or near the end of inspiration. During expiration, the aortic and pulmonary valves close almost synchronously and produce a single or narrowly split S 2 . Normal splitting of S 2 is caused by (1) increased right-sided heart filling during inspiration because of increased blood volume returning via the venae cavae and (2) diminished left-sided heart filling because blood is retained within the small blood vessels of the lungs when the thorax expands. During inspiration, when the right ventricle is filled more than the left, it takes slightly longer to empty. This causes the noticeable inspiratory delay in P 2 in relation to A 2 . Splitting of the S 2 during inspiration is a normal finding and should be sought in all patients.
The aortic and pulmonary pressure in diastole close the semilunar valves. Many forms of congenital or acquired heart disease have an impact on the pulmonary circulation and, consequently, often affect the S 2 . Thus, the higher the pulmonary artery diastolic pressure, the more intense and earlier the P 2 is. Pulmonary hypertension in children is suggested when the P 2 is palpable, loud, and narrowly split or cannot be separated from A 2 . If the P 2 is audible outside of the pulmonary area, particularly at the apex, then pulmonary hypertension is likely. A single or narrow split S 2 may also be noted in patients with severe pulmonic or aortic valve stenosis, tetralogy of Fallot, truncus arteriosus, pulmonary atresia, hypoplastic left heart syndrome, tricuspid valve atresia, or Eisenmenger syndrome with a VSD. In the presence of moderate to severe pulmonic stenosis, there is low pulmonary artery diastolic pressure. The pulmonary valve closure is therefore delayed and of decreased intensity and is occasionally inaudible.
The S 2 may be widely split and/or fixed in association with right ventricular volume overload or delayed right ventricular conduction.
The third heart sound (S 3 ) (see Fig. 9.2 ), which is of very low frequency, occurs about a third of the way into diastole, at the time of the most rapid filling of the ventricles. It is most likely caused by sudden tension of the ventricles, enough to produce sound vibrations within the myocardial wall. Vibrations in the atrioventricular valve itself, as well as in the chordae, may also contribute to the sound. The amplitude of S 3 increases with an increased ventricular filling rate. When heard at the apex, S 3 is considered left ventricular in origin, and when heard at the lower left sternal border, S 3 is likely to be right ventricular in origin. An apical S 3 of soft to moderate intensity is readily heard in most children and young adults. An S 3 in association with tachycardia is termed a gallop and may be caused by lesions associated with left or right ventricular diastolic overload or diminished ventricular compliance.
The fourth heart sound (S 4 ) (see Fig. 9.2 ) is also of low frequency and can be both left-sided and right-sided in origin. It occurs with atrial contraction against a high resistance and is therefore heard just before S 1 . It is more difficult to hear than S 3 , particularly in children, in whom the PR interval is usually shorter than that in the adult. The S 4 is thought to be caused by a forceful atrial contraction against a poorly compliant left ventricle (e.g., as in diastolic overload). The sound is readily heard in adults with significant chronic hypertension or left ventricular cardiomyopathy and, except for its timing, sounds much like an S 3 . In a young baby with total anomalous pulmonary venous return, low pulmonary vascular resistance, and significantly increased right ventricular and pulmonary blood flow, a loud right ventricular S 4 (as well as S 3 ) may be heard as part of a quadruple rhythm at the lower left sternal border. An intermittent S 4 may be heard in children with complete atrioventricular block. Whereas an S 3 may be heard in a normal adolescent and can be physiologic, the S 4 only occurs in a pathologic condition.
An audible ejection click (see Fig. 9.2 ) is abnormal and is related to either the hemodynamics associated with a dilated root of the aorta ( aortic ejection click ) or a dilated root of the pulmonary artery ( pulmonary ejection click ) or the effects of a thickened and immobile semilunar valve. The sound is sharp and of very high frequency. The pulmonary ejection click is best heard at the upper left sternal border, whereas the aortic ejection click is usually best heard at the apex. It may also be heard at the upper right sternal border, but if so, it is always louder at the apex or the lower left sternal border. The click arises either from sudden tension of the semilunar valve or from sudden distention with lateral pressure at the root of the aorta or pulmonary artery. The sound is present in aortic or pulmonary valve stenosis. In such cases, the rapid movement of the stenotic valve is suddenly checked. An aortic ejection click may be heard in the presence of a normal aortic valve (as in severe tetralogy of Fallot with a large aortic root); a pulmonary ejection click may be heard with a normal pulmonic valve (as in Eisenmenger syndrome with a large pulmonary root). The aortic ejection click, best heard at the apex, does not vary with respirations. However, the pulmonary ejection click, best heard at the upper left sternal border, is better heard on expiration than inspiration.
An ejection click or a sharp sound present at the upper left sternal border, louder with expiration or heard only on expiration, is characteristic of pulmonary valve stenosis . The ejection click follows the period of isovolumic contraction and occurs as a consequence of restricted semilunar pulmonary valve excursion at the onset of ventricular ejection. When the ejection sound occurs at the upper right sternal border or at the apex, a bicuspid or stenotic aortic valve disease is suggested. In contrast to ejection clicks, right-sided cardiac murmurs are accentuated with inspiration. Left-sided heart auscultatory abnormalities vary little with the respiratory cycle.
In the case of the aortic ejection click, the sound is usually well separated from S 1 . However, the pulmonary ejection click is usually closer to S 1 than is an aortic click. In some moderate to severe cases, the pulmonary ejection click occurs at the same time as S 1 . If one perceives a split S 1 , one is most likely hearing an ejection click as the causes of a true split S 1 are very rare.
The opening snap is present only in rheumatic mitral valve stenosis when the anteromedial leaflet is immobile; is heard early in diastole, usually above the apex; and is of medium frequency. Because the leaflets are fused, the downward movement of the opening valve is suddenly checked, resulting in the opening snap. This sound is often confused with an S 3 . The frequency is somewhat higher and the timing is earlier than those of an S 3 . The opening snap and the S 3 , although similar in timing, can never occur together in the same patient.
Non-ejection clicks are heard at the apex and occur one third to half of the way between S 1 and S 2 . Thus, they are commonly called mid-systolic clicks . The sounds are of medium to high frequency. The sound is caused by the sudden tensing of the posterior mitral valve leaflet as it prolapses into the left atrium; in rare cases, there may be multiple mid-systolic clicks. The clicks may be loud, but they may also be soft and easily missed.
Heart murmurs are the consequence of turbulent blood flow. Turbulence may arise as a result of
high flow through abnormal or normal valves
normal flow through narrow or stenotic valves or vessels
backward or regurgitant flow through incompetent leaky valves
flow through congenital or surgical communications
anemia with high flows and decreased blood viscosity
Not all cardiac murmurs indicate heart problems.
The clinician should be able to determine and describe the following seven characteristics of heart murmurs:
Timing: the relative position within the cardiac cycle relative to S 1 and S 2
Intensity or loudness: murmurs are graded as
grade I: heard only with intense concentration
grade II: faint but heard immediately
grade III: easily heard, of intermediate intensity
grade IV: easily heard and associated with a thrill (a palpable vibration on the chest wall)
grade V: very loud, with a thrill present, and audible with only the edge of the stethoscope on the chest wall
grade VI: audible with the stethoscope off the chest wall
Location: on the chest wall with regard to
area where the sound is loudest (point of maximal intensity)
area over which the sound is audible (extent of radiation)
Shape: to include the duration (the length of the murmur from beginning to end) and configuration (the dynamic changing nature of the murmur)
Pitch: the frequency range of the murmur, generally described as low, medium, or high pitched
Quality: aspect that relates to the presence of harmonics and overtones
Physiologic effects: of different positions, manipulations, or maneuvers
After the neonatal period, an innocent murmur may be detected at some time in the majority of children before school age. The clinical diagnosis of a normal ejection or innocent murmur should only occur in the setting of an otherwise normal history, physical examination, and appearance ( Table 9.5 and Fig. 9.6 ).
| Type (Timing) | Description of Murmur | Age Group |
|---|---|---|
| Classic vibratory murmur (Still murmur) (systolic) | Maximal at MLSB or between LLSB and apex Grade 2–3/6 in intensity Low-frequency vibratory, “twanging string,” groaning, squeaking, or musical |
3–6 yr Occasionally in infancy |
| Pulmonary ejection murmur (systolic) | Maximal at ULSB Early to mid-systolic Grade 1–3/6 in intensity Blowing in quality |
8–14 yr |
| Pulmonary flow murmur of newborn (systolic) | Maximal at ULSB Transmits well to the left and right chest, axilla, and back Grade 1–2/6 in intensity |
Premature and full-term newborns Usually disappears by 3–6 mo of age |
| Venous hum (continuous) | Maximal at right (or left) supraclavicular and infraclavicular areas Grade 1–3/6 in intensity Inaudible in the supine position Intensity changes with rotation of the head and compression of the jugular vein |
3–6 yr |
| Carotid bruit (systolic) | Right supraclavicular area and over the carotids Grade 2–3/6 in intensity Occasional thrill over a carotid |
Any age |
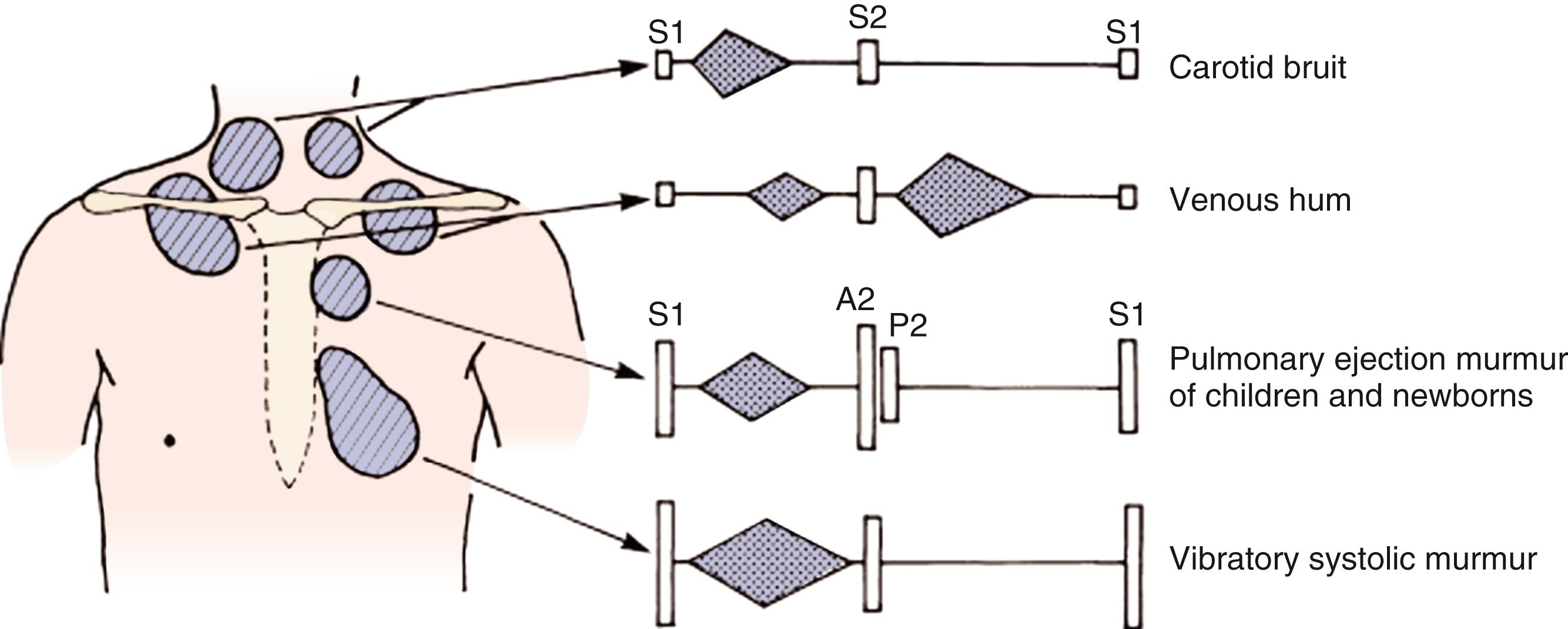
Thorough auscultation in the cooperative patient should include listening in the principal areas (tricuspid, pulmonary, mitral, and aortic) of the precordium with both the bell and diaphragm of the stethoscope and with the patient in the supine, sitting, and standing positions.
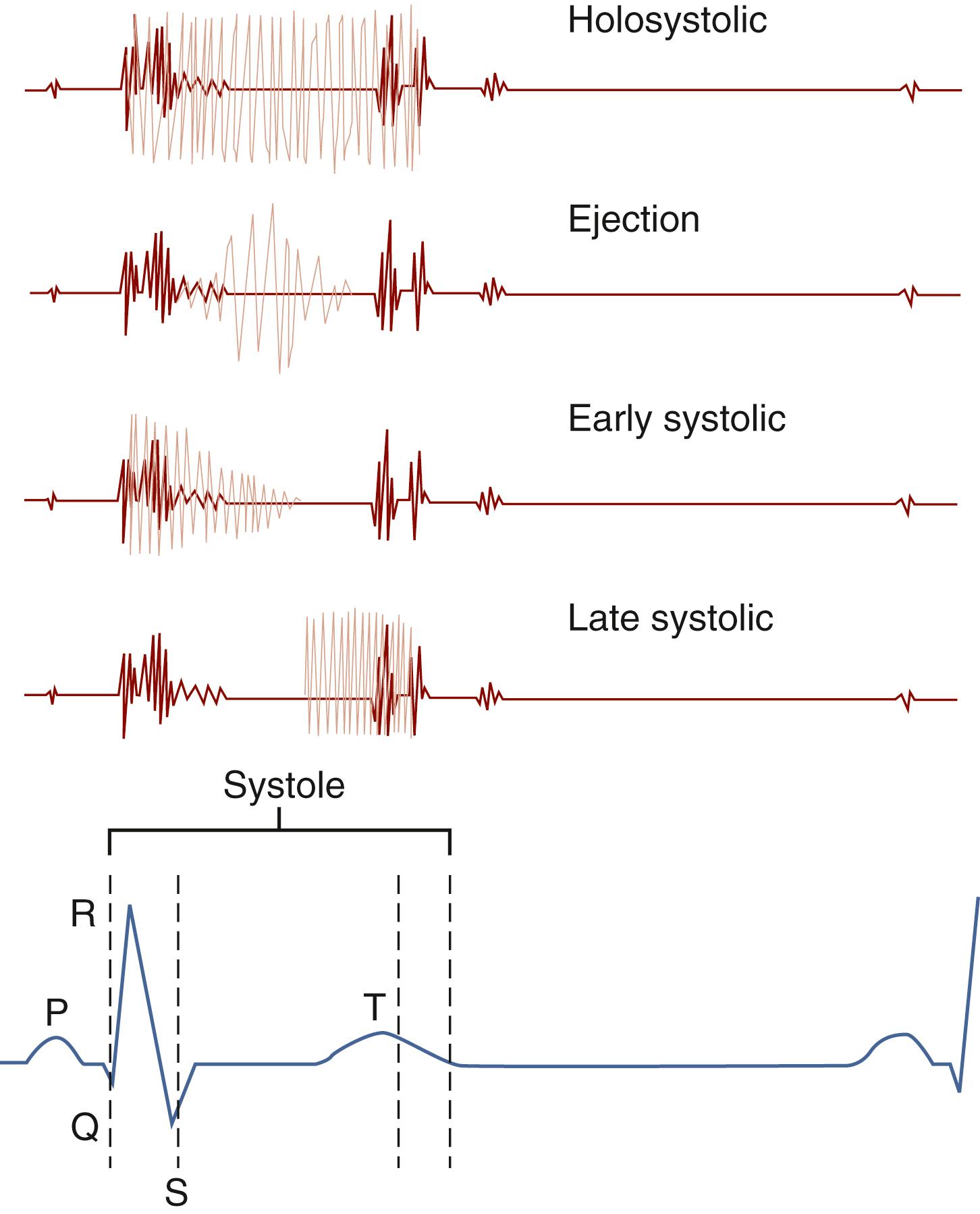
Systolic murmurs begin with or follow the S 1 and end before the S 2 ( Fig. 9.7 ).
Holosystolic murmurs , beginning abruptly with S 1 and continuing at the same intensity to S 2 , are graphically shown as a rectangle. This murmur begins during the period of isovolumic contraction and thus occurs when there is a regurgitant atrioventricular valve (tricuspid or mitral) or in association with a VSD.
Ejection murmurs are crescendo-decrescendo or diamond-shaped murmurs that may arise from narrowing of the semilunar valves or outflow tracts. The rising-and-falling nature of the murmur reflects the periods of lower flow at the beginning and near the end of ventricular systole.
Innocent murmurs are almost exclusively ejection systolic in nature (see Table 9.5 ). They are generally soft, are never associated with a palpable thrill, and are subject to considerable variation with positioning changes.
Early systolic murmurs start abruptly with S 1 but taper and disappear before the S 2 and are exclusively associated with small muscular VSDs.
Mid-systolic to late systolic murmurs begin midway through systole and are often heard in association with the mid-systolic clicks and insufficiency of mitral valve prolapse.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here