Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Patients with syndromic synostosis require protocolized treatment coordination from a multidisciplinary craniofacial team.
Treatment of cephalocranial disproportion requires early and sequential surgical intervention.
All protocols include monitoring for obstructive airway disease and hydrocephalus.
Sequential surgical treatment can be tailored to the presenting craniofacial dysmorphology.
Premature fusion of the cranial sutures can occur in isolation or may occur along with consistent associated anomalies as part of a genetic syndrome. Patients with syndromic synostosis require protocolized treatment coordination from a multidisciplinary craniofacial team to meet their needs, and in complex presentations can require urgent surgical management in the immediate neonatal period. Although there are several syndromes associated with craniosynostosis, this chapter will focus on the most common of these rare conditions, namely those named by Crouzon, Pfeiffer, Apert, Muenke, and Saethre-Chotzen ( Fig. 25.3.1 ). In the past, protocols for the surgical management of multisuture syndromic synostosis were shared with single suture non-syndromic synostosis resulting in suboptimal outcomes and a high rate of relapse with the need to repeat surgery. More recently, surgical protocols have been tailored to the unique needs and natural history of patients with syndromic synostosis, resulting in improved outcomes and a decreased surgical burden. These considerations include the importance of critical timing of staged surgeries and the coordination with treatment of associated conditions such as Chiari malformation, hydrocephalus, and obstructive upper airway disease. In addition to adapting to the unique nature of syndromic synostosis, protocols are also now recognizing that the morphology of each syndrome is distinct, and that treatment needs to be tailored to each presentation and not simply generalized to all syndromes ( Fig. 25.3.2 ). Finally, as we learn more about the influence of genes on craniofacial dysmorphology, the future of tailored surgical treatment, for example, will likely be based more on a specific mutation such as W290C rather than on a traditional eponymous diagnosis described by Rudolf Arthur Pfeiffer.
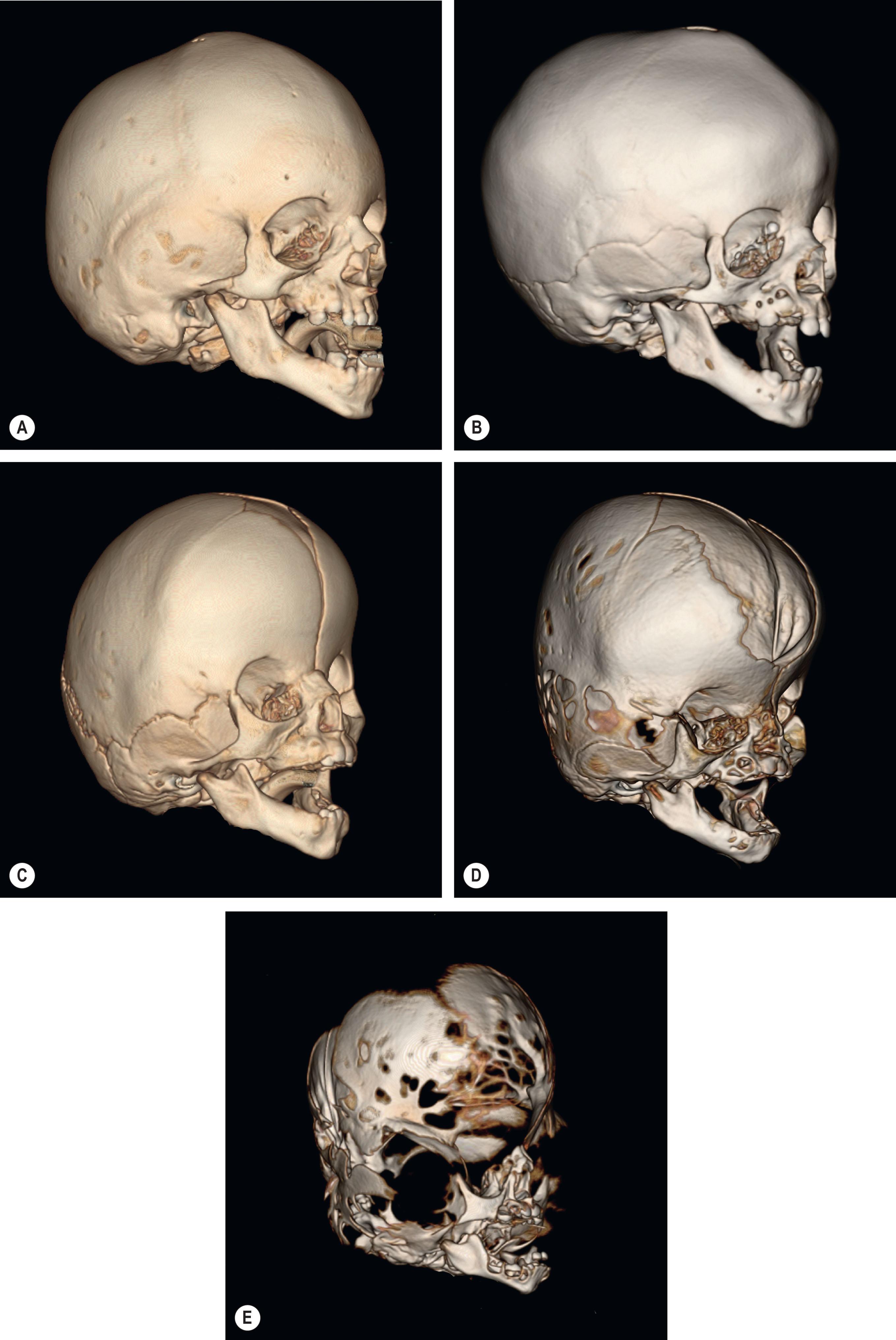
The purpose of this chapter is to describe an algorithmic approach to management of a patient with syndromic synostosis from infancy to maturity, with a goal of tailoring interventions to the unique needs of each condition.
The craniofacial growth sequence is initiated with rapid calvarial growth during the first few years of life, orbital and midface growth in early childhood, followed by mandibular growth in late adolescence. The brain triples in volume to approach 60% of its adult size by 1 year and 90% at 2 years. The rate of cranial growth then slows, with the brain reaching adult size by 10 years of age. The growing brain displaces the bone plates that are separated by patent sutures, with bone deposition occuring perpendicular to the suture line. In the presence of premature fusion of multiple sutures, the typical cephalocranial growth pattern is disrupted by regions of restricted growth countered by areas of compensation. Although dysmorphology is the most visible effect of this altered dynamic, the primary concern is that cephalocranial disproportion can result if the resulting intracranial volume is insufficient to accommodate the needs of the growing brain.
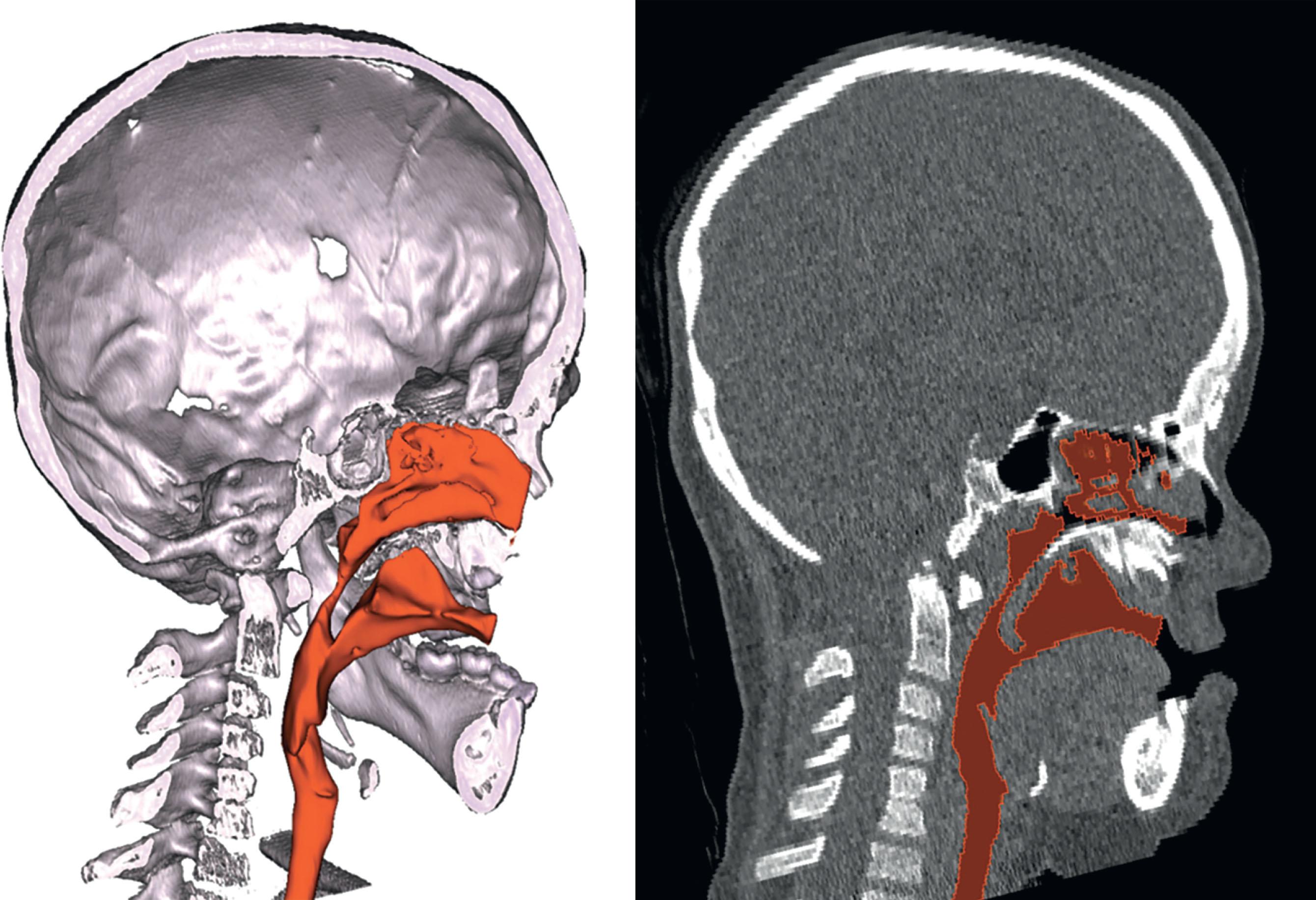
Cephalocranial disproportion is one potential cause of increased intracranial pressure (iICP), with the risk compounded exponentially with the number of sutures involved. Other contributors to iICP in syndromic synostosis include obstructive sleep apnea, abnormal venous drainage and cerebral venous hypertension, and ventriculomegaly. Obstructive sleep apnea in children with craniosynostosis is usually a multilevel problem, with the most common areas of obstruction at the nasopharynx and tongue base ( Fig. 25.3.3 ). Obstructive sleep apnea is believed to stir a cycle of hypoxia and hypercapnia during sleep, which causes cerebral vasodilatation, leading to an increase in cerebral blood flow and thus intracranial hypertension. Preoperative imaging is critical to evaluate for cerebral venous anomalies such as sinus pericrania ( Fig. 25.3.4 ). It is unclear whether abnormal venous drainage is compensatory or intrinsic to each syndrome, however, new research has shown that many cerebral venous abnormalities associated with syndromic craniosynostosis are not associated with papilloedema. This suggests that there may be an embryologic origin of the anomalous venous drainage, rather than a reflexive response to increased blood component of intracranial volume.
A delay in detection and treatment of iICP in syndromic synostosis can result in optic atrophy, blindness, and developmental delay. Early protocolized prevention with monitoring or early detection with intervention are therefore essential. Subjective signs of iICP include regular worsening morning headaches, recurrent vomiting, and a change in developmental milestone progression. However, these are nonspecific and can be absent despite the presence of iICP. The current gold standard of iICP detection is placement of a direct intraparenchymal pressure (IPP) monitor, but this invasive inpatient procedure includes the risks of bleeding, infection, mechanical failure, and cerebrospinal fluid leak and is not 100% sensitive. Although a properly placed IPP monitor can provide a direct measure of ICP while it is in place, the duration and frequency of monitoring required to give an accurate representation of day-to-day pressure remains controversial. Compounding this is the interpretation of the measured pressure level given the absence of a unanimously accepted threshold of normal and elevated IPC in patients with syndromic synostosis given their other associated cerebrovascular abnormalities.
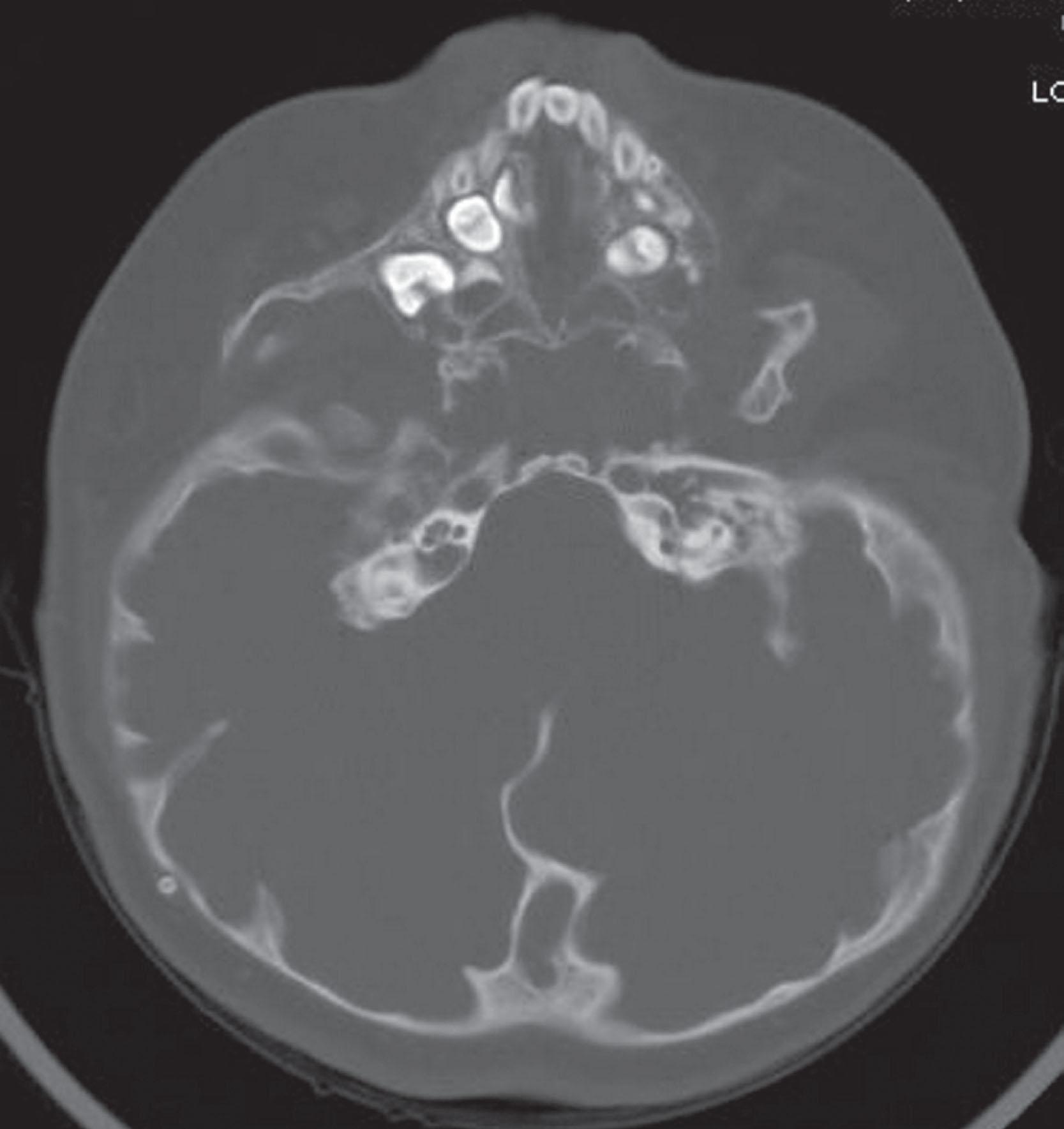
Other less invasive objective measures of iICP include imaging for cranial thickness and ventricular size, detection of papilloedema of the optic nerve, and calculation of visual evoked potentials. Imaging modalities such as computed tomography (CT) scans and plain radiographs can detect “thumb printing” or “copper beating” of the calvaria resulting from bone modulation by pressure of the gyri on the inner table ( Fig. 25.3.5 ). Although this is a finding in patients with syndromic synostosis and iICP, it can also be observed in normal children and normal pressure situations and is therefore nonspecific. Papilloedema and intracranial hypertension have also been associated with thinning of the cerebral cortex independently of hydrocephalus. Papilloedema is swelling of the optic nerve as a result of iICP and can be measured on dilated fundoscopy, transorbital ultrasound, and optical coherence tomography (OCT). A landmark study examined 122 children with both dilated fundoscopy and direct ICP monitoring found 100% of children older than 8 years with iICP had detectible papilloedema, compared to only 22% in patients less than 8 years old. Regular scheduled dilated fundoscopy therefore remains a cost-effective part of any craniofacial team monitoring program, but other measures are required for detecting iICP in younger children. Transorbital ultrasound can be used to measure optic nerve sheath diameter and is a recognized measure of acute changes in ICP in post-traumatic intensive care patients. However, one study on patients with craniosynostosis demonstrated fundoscopy was more sensitive than this modality in detecting documented iICP, noting that ultrasonography was highly specific at 97%, but had a low sensitivity of 11%. Spectral-domain OCT is a promising modality that can provide quantitative measures of the retinal structure surrounding the optic nerve which can be associated with papilloedema and iICP. When compared to direct, invasive monitoring of ICP, Swanson found that OCT had a sensitivity of 89% and a specificity of 62%. Analysis of visual evoked potentials (VEPs) is a noninvasive measure of the latency time of the averaged encephalographic response to a visual stimulus. VEPs can be abnormal in patients with syndromic synostosis without iICP, and therefore obtaining a measurement early in patient monitoring is important to establish a baseline. An increase in prolongation of the latency period over a patient’s established baseline is an indicator of axonal injury and has been shown to correlate with iICP.
All of these signs and measures of cephalocranial disproportion and increased intracranial pressure are prone to error due to their limited observation duration and the variation in ICP that can occur with activity, stress, positioning, and the time of the day. In the absence of a clear noninvasive gold standard, the current practice of monitoring for progressive cephalocranial disproportion and iICP is to use a combination of modalities. Escalation to more invasive or expensive measures is based on the craniofacial team’s suspicion for iICP from clinical acumen gained from years of experience. In one study of older patients with craniosynostosis and concerning signs and symptoms of iICP, cranial vault expansion resolved nearly all of the symptoms associated with iICP. Headaches were the most common symptom found in these children, and 86% of these patients had resolution or improvement of their headaches following cranial vault expansion. Similarly, in patients who had preoperative visual changes, 82% of patients had improvements following cranial vault expansion. All patients who experienced preoperative nausea and vomiting had improvements of these symptoms.
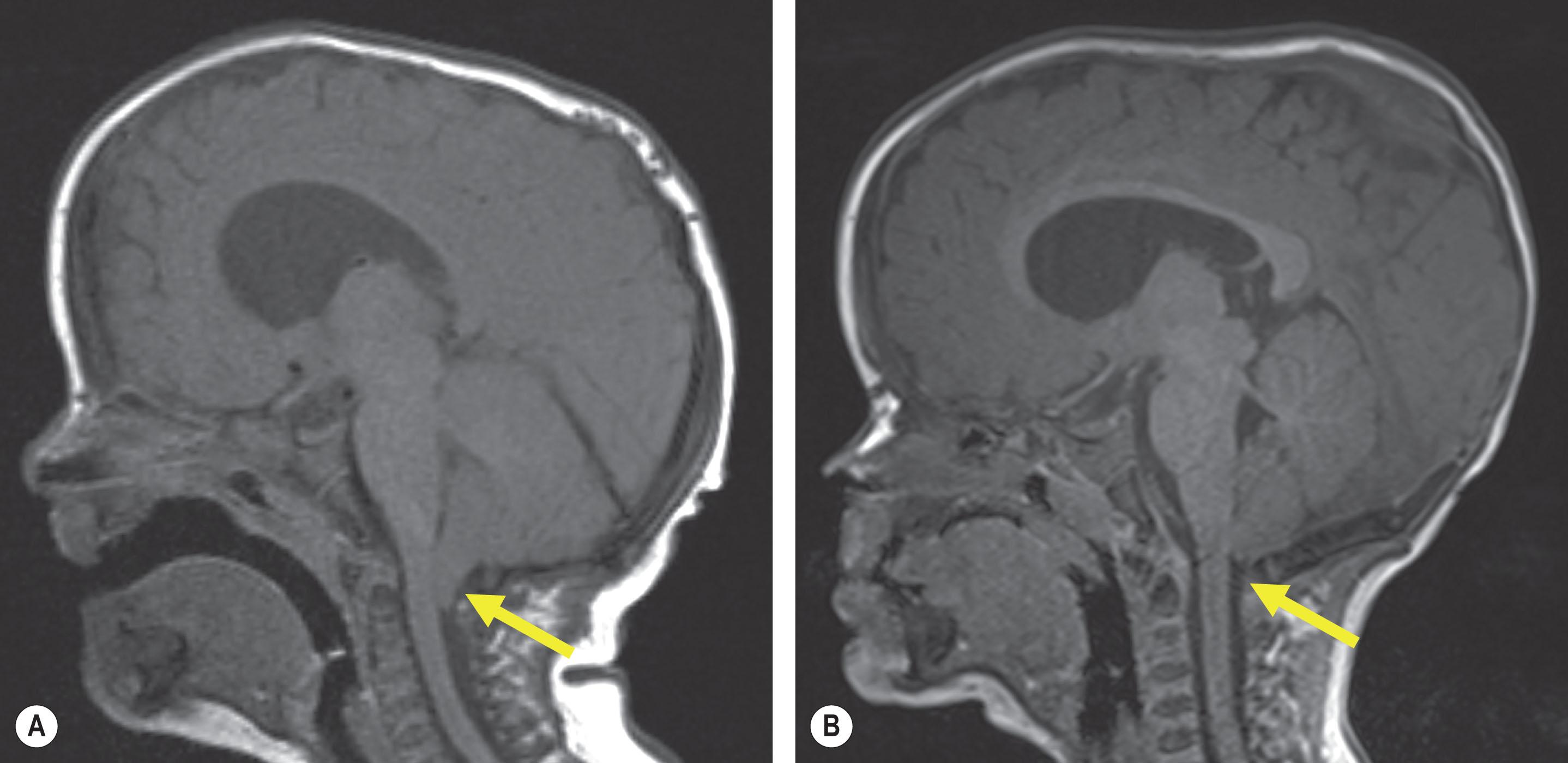
The Chiari I malformation is a downward displacement of the cerebellar tonsils through the foramen magnum and is present in up to 70% of patients with Crouzon syndrome, 82% with Pfeiffer syndrome, and 100% with the cloverleaf or Kleeblattshädel pattern of multiple suture synostosis. The malformation can result in noncommunicating hydrocephalus as a result of obstruction of cerebrospinal fluid outflow from the posterior fossa compression and can further progress to fluid accumulating within the spinal cord known as syringomyelia. Chiari malformation associated with syndromic synostosis has been considered an acquired condition developing in the first years of life secondary to hindbrain growth in an abnormally small posterior fossa from premature fusion of the lambdoid or cranial base sutures. However, a more recent study has suggested that posterior fossa volume is not predictive of Chiari malformations. Although a Chiari malformation can be suspected on a CT scan, the definitive imaging is a magnetic resonance (MR) scan that includes the upper spinal cord. Clinical symptoms if they do occur include headaches, dizziness, nausea, impaired coordination, muscle weakness, and in severe cases, paralysis.
Many patients with Chiari malformations remain asymptomatic and can be monitored with regular follow-up and repeat imaging for signs of deterioration. The recognized treatment of a symptomatic malformation is a bony decompression with a dura release and patch through a direct posterior neck approach by a neurosurgical team. The controversy over whether posterior fossa compression is the cause of the Chiari malformation adds to the debate whether prophylactic treatment of a non-symptomatic malformation through posterior vault expansion is warranted. Patients with Chiari malformations and craniosynostosis had similar posterior fossa volumes to patients with craniosynostosis alone. However, there have been a number of reports of resolution of a documented Chiari malformation with or without syringomyelia from an isolated posterior cranial vault expansion ( Fig. 25.3.6 ). Some centers advocate simultaneous removal of the posterior foramen magnum ring without dura release at time of posterior remodeling in high-risk cases. Our current approach is to perform this simultaneous bone decompression at the same time as a posterior vault distraction osteogenesis (PVDO) craniectomy in syndromic cases or cases with “Mercedes” (bilateral lambdoid with sagittal) synostosis with a documented Chiari malformation or CT evidence of regional bone invagination compressing the posterior fossa ( Fig. 25.3.7 ).
There are several functional issues faced by patients with syndromic synostosis, and the timing of surgeries should be designed to reduce morbidity from intracranial pressure, airway compromise, exorbitism, and psychosocial development. Cranial vault expansion has been shown to acutely lower intracranial pressure, but patients with syndromic craniosynostosis have a high recurrence rate of intracranial hypertension. Timing of surgery to alleviate this pressure is critical, both acutely and longitudinally. Earlier cranial vault expansion can avoid increased intracranial pressure, while the calvaria is malleable and bony defects more readily heal. Later intervention allows more rigid fixation, which increases the predictability of surgery and the stability of the postoperative result. Utria et al . proposed that the timing of cranial vault remodeling was optimized around 6–9 months of age, with earlier operations having five times greater odds of requiring a major reoperation.
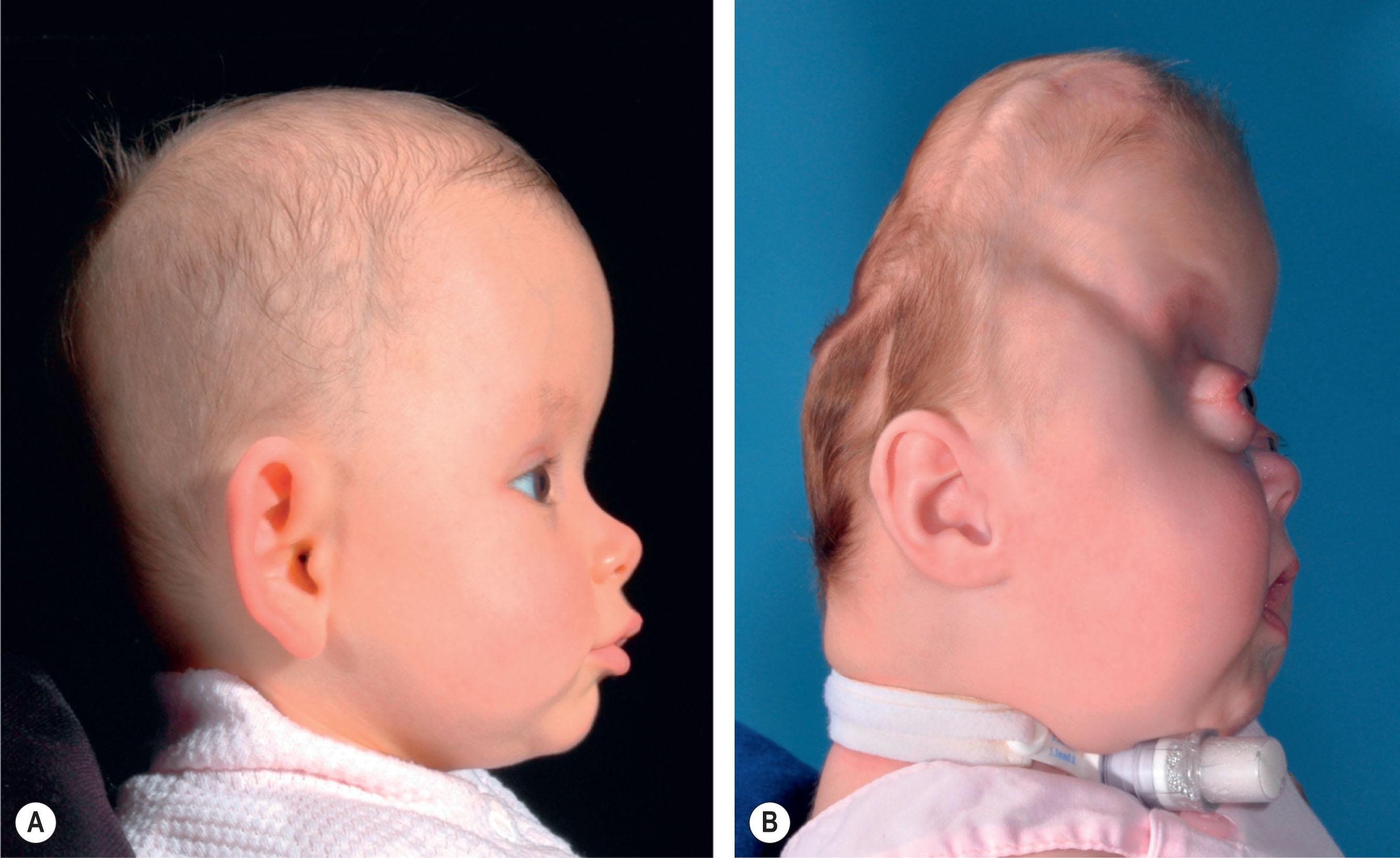
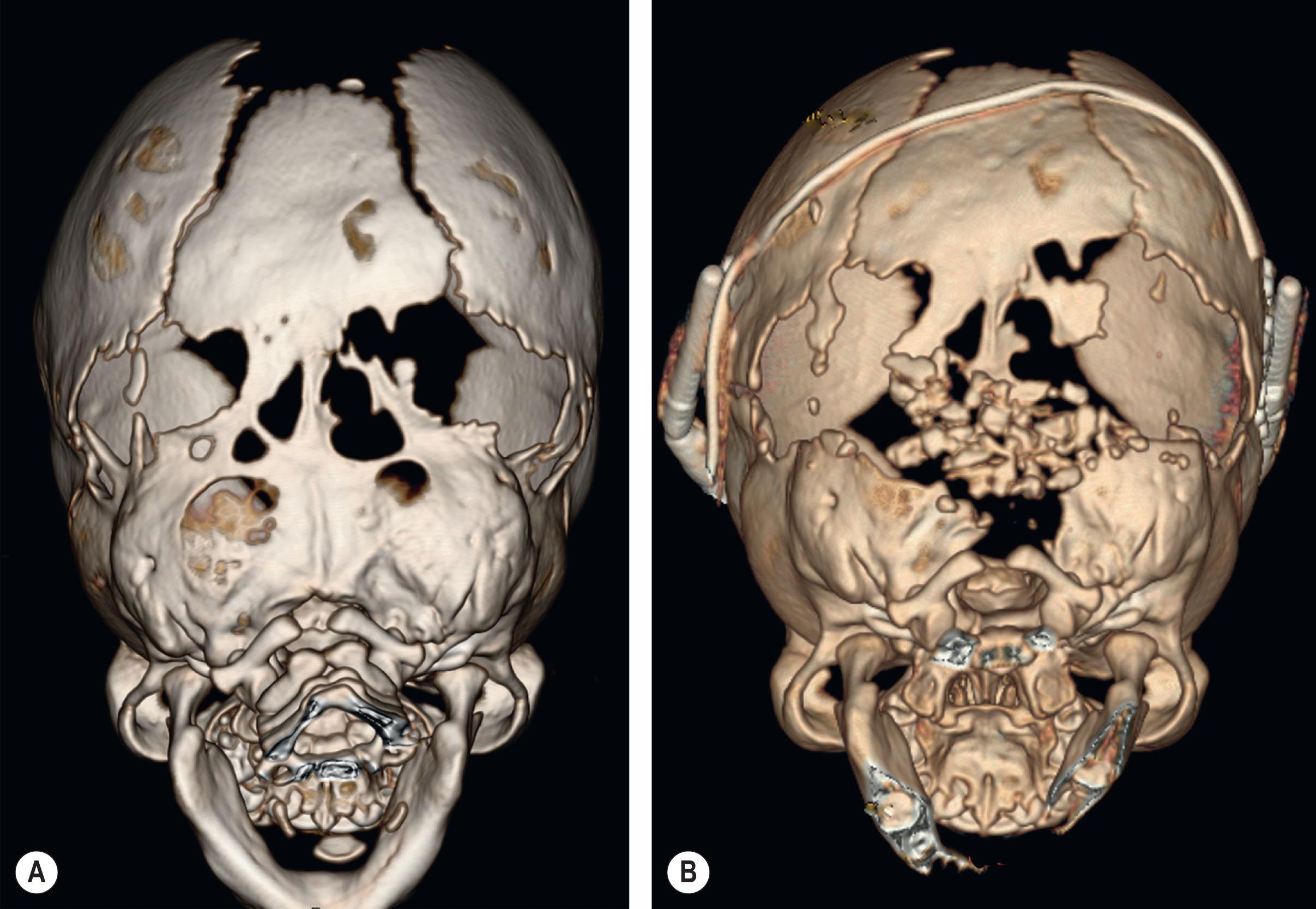
In severe cases of syndromic craniosynostosis, cephalocranial disproportion and iICP can be evident at birth, and staged interventions to relieve pressure on the intracranial contents must start in the first days or weeks of life. Left untreated, early cephalocranial disproportion in severe cases of syndromic synostosis will result in progressive loss of calvarial bone through compensatory over-expansion of patent minor sutures, such as the squamosal and frontozygomatic, as well as pressure erosion and thinning of the neonatal bone from the growing brain ( Fig. 25.3.8 ).
Early timely intervention to temporarily re-establish cephalocranial proportion will restore the balance between the brain, with its osteogenic dura, and the calvarium resulting in calvarial bone formation. Unfortunately, in the face of rapid brain growth in the first 2 years of life, the associated cerebrovascular, ventricular, and cranial suture abnormalities in syndromic synostosis make any early treatment a temporary fix, and disproportion and pressure will appear again, resulting in recurrent bone erosion and progressive decompensation. Timely detection of this recurrence of cephalocranial disproportion and staged treatment will reset the balance, restoring appropriate bone formation and cranial volume until the next occurrence of disproportion. We have coined this concept of ebb and flow of detecting cephalocranial disproportion and restoring balance through carefully time-staged surgical intervention in the first 2 years of life “surfing the bone pressure wave”. This wave can be represented by a graph of the amount of cranial bone surface area over time, and illustrates how the calvarial bone decreases in response to cephalocranial disproportion and iICP, but then increases in response to surgical interventions tailored to the patient’s age and situation ( Fig. 25.3.9 ). For example, if severe cephalocranial disproportion presents soon after birth in a patient with syndromic synostosis, surgical options are limited due to the fragility of the patient’s medical condition and cranium. A cranial strip release of the thickened bone band that forms from the sphenoid to the anterior fontanelle can stop progression of bone erosion and allow the brain to grow and deposit new bone. In a few months, the “wave” may rise again as the deposited bone fails to compensate from the rapidly growing brain that is compounded by early ventriculomegaly from hydrocephalus. The patient’s bone may still be too thin to allow a sustainable cranial expansion surgery, but a timely placed ventriculoperitoneal (VP) shunt in a patient with early signs of hydrocephalus can alleviate this pressure and temporarily restore the balance to allow bone formation to continue. In the second year of life, the imbalance may manifest again despite treatment of the hydrocephalus and frontal surgery is contraindicated due to the evidence of high relapse when performed at this age. However, sufficient bone has now formed for a craniectomy and placement of devices for posterior vault expansion from distraction osteogenesis. These early interventions have “surfed” bone pressure wave ebbs and flows into the second year of life, where a frontofacial procedure will have a more stable result in treating any residual disproportion and associated frontal abnormalities.
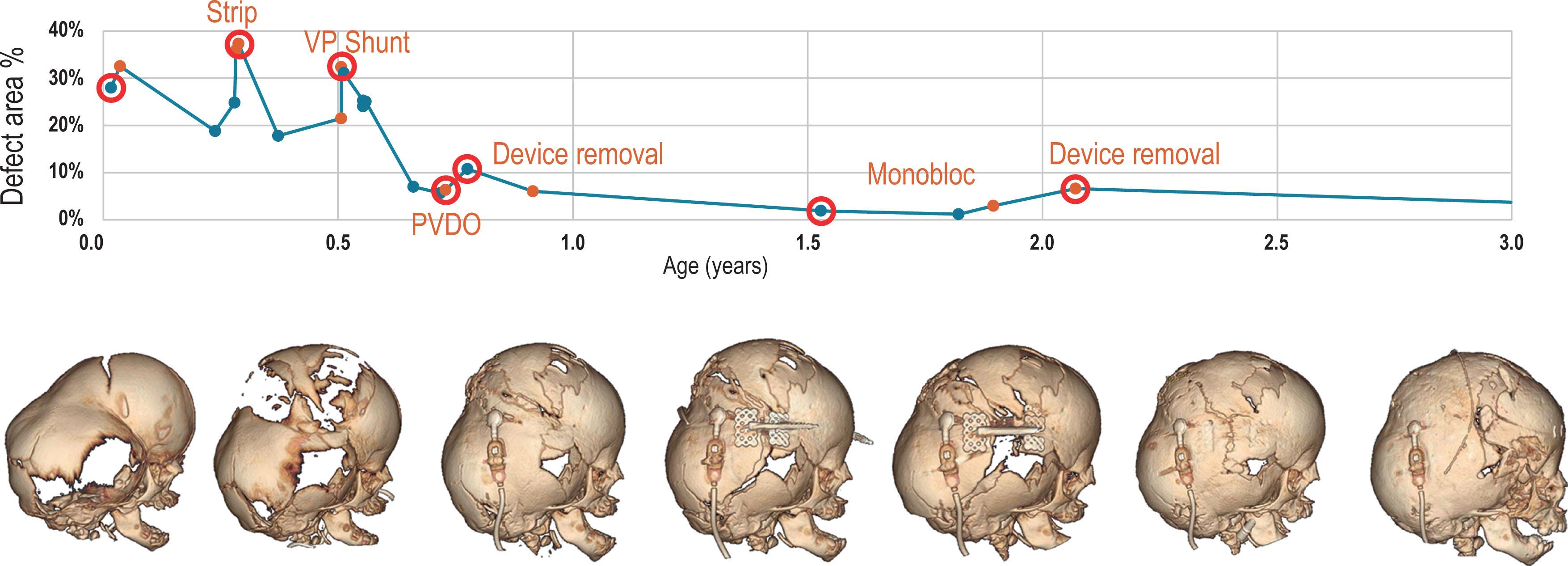
The rapid brain growth that achieves 90% of adult size in the first 2 years of life marks a critical period of surgical decision-making and tailored treatment. Adopting this “surfing the bone pressure wave concept” has resulted in patients historically thought to have extremely poor prognosis, such as the rare W290C variant of Pfeiffer syndrome, to achieve milestones that approach and, in some cases, exceed their age-matched non-synostotic peers. The next sections of this chapter focuses on specific surgeries that allow “surfing of the bone pressure wave”, two different approaches to detect or prevent cephalocranial disproportion, and a description of tailored sequences of craniofacial surgeries based on patient presentations.
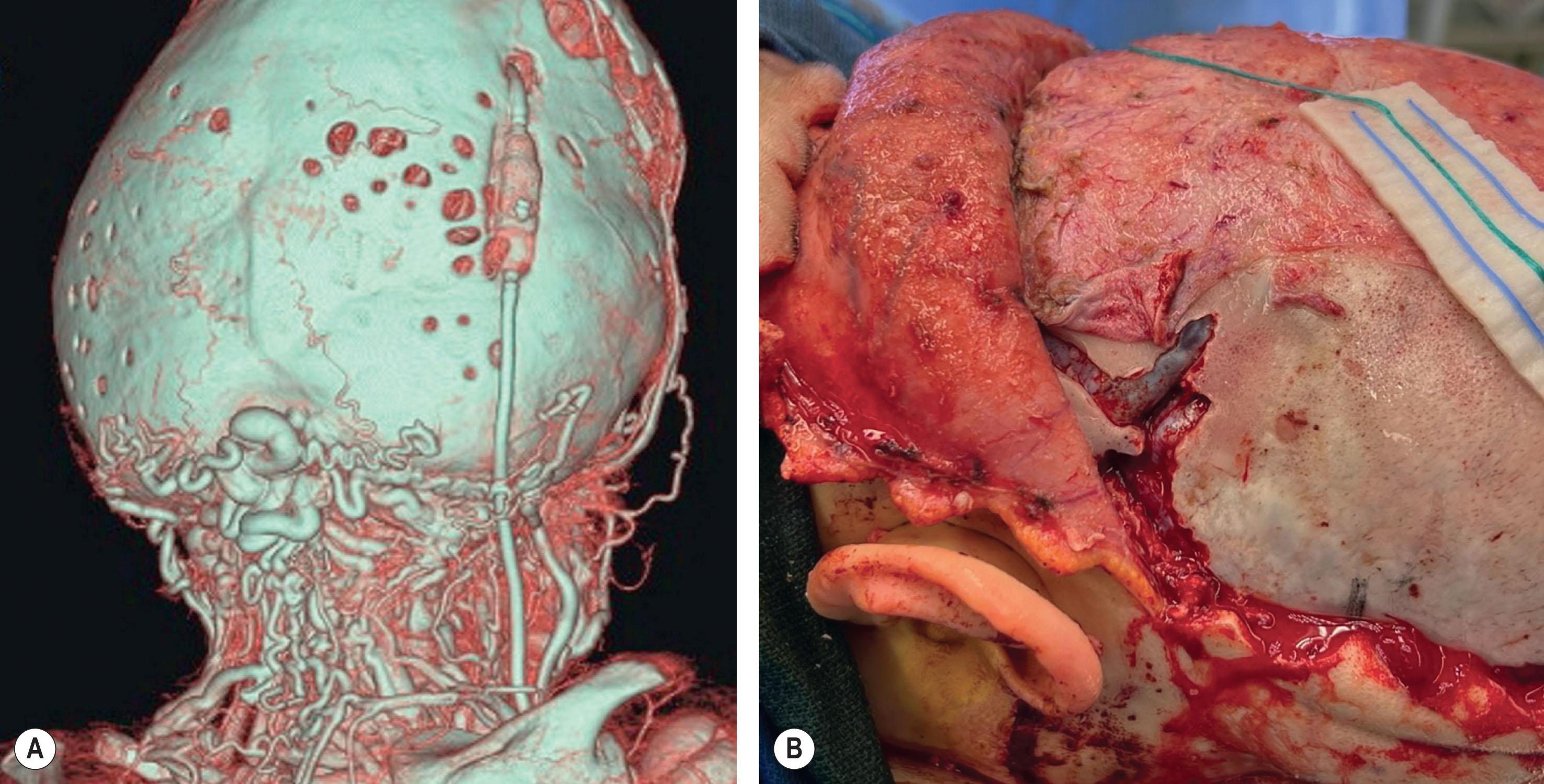
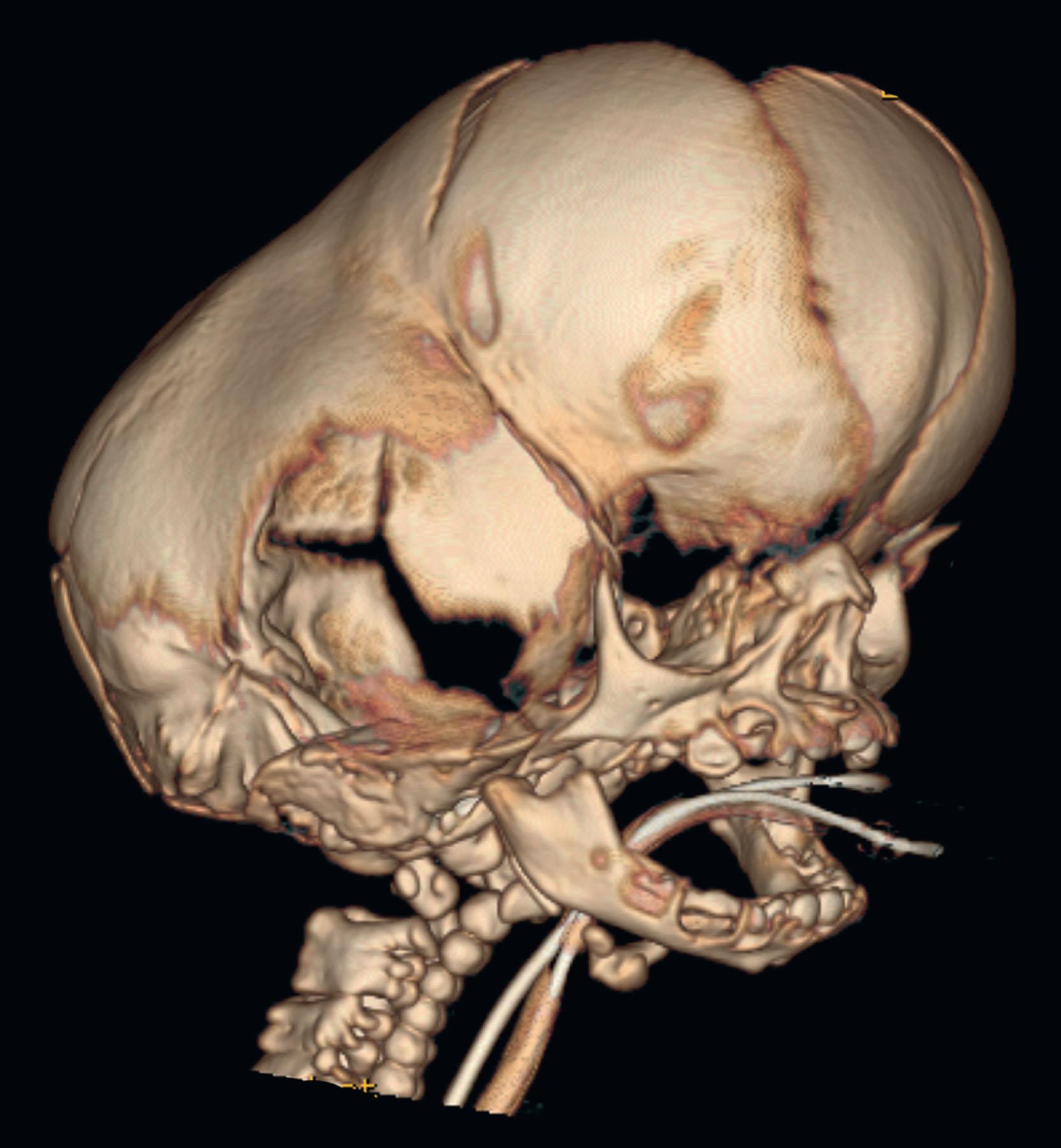
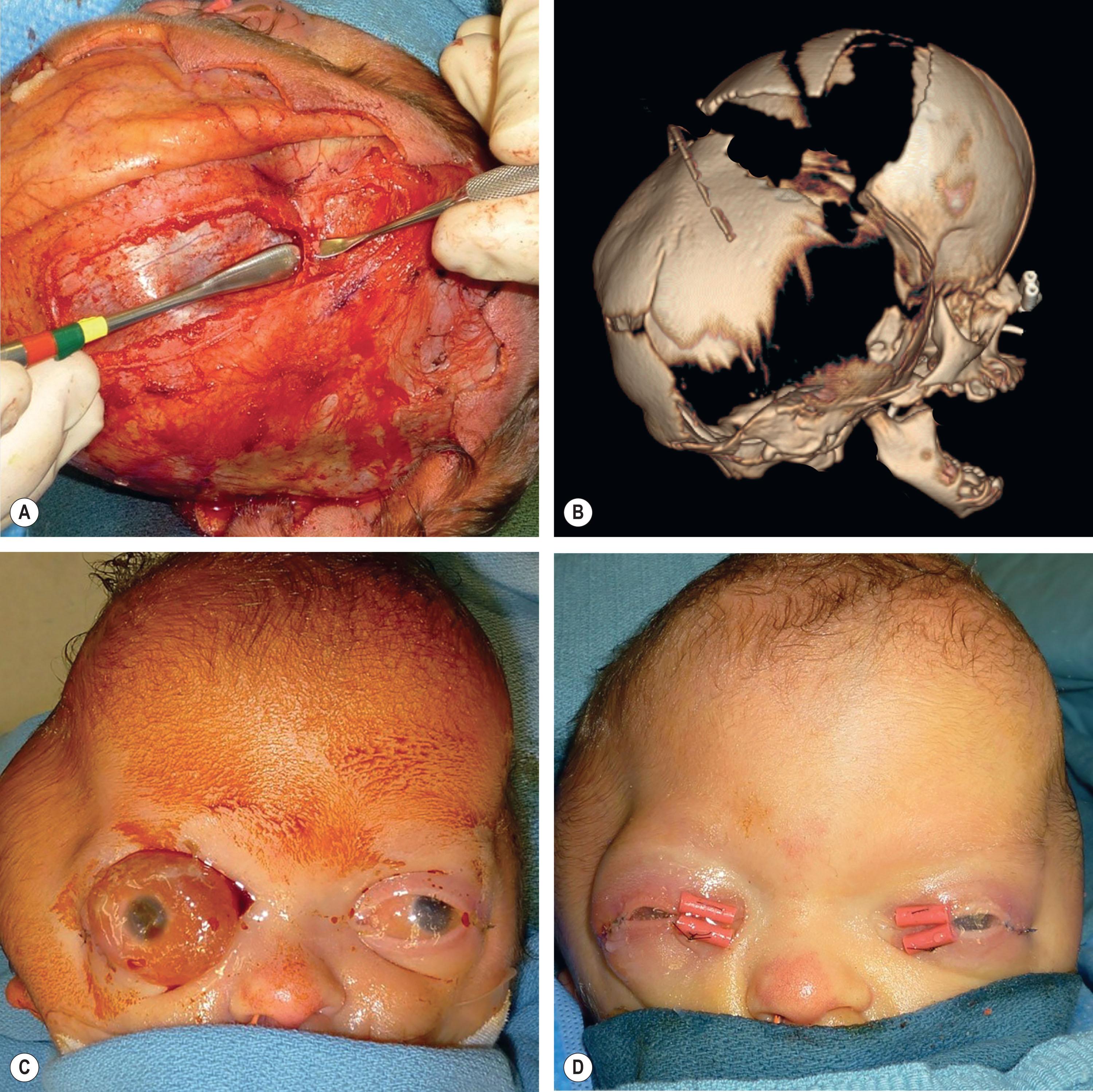
Neonatal strip craniectomy may be required in severe cases to stop early progressive cranial bone effacement due to iICP and cephalocranial disproportion following the “bone pressure wave” concept. Due to the higher risk of performing a craniectomy in the first month of life, this procedure should be reserved for the most severe phenotypes when other more conservative options are not available. Patients benefitting from neonatal strip craniectomy typically involve a combination of multiple suture synostosis, abnormal venous drainage, and are usually cases that eventually require shunting for hydrocephalus. Under these conditions, the expanding brain will compensate at the areas of lowest resistance, such as the squamosal and frontozygomatic suture as well as the anterior and posterior fontanelles but will be restricted by defined bony constriction bands from the pterion to the vertex. During neonatal strip craniectomy, these bands of thickened and constricting bone are identified and released to relieve temporarily the intracranial pressure and permit dural osteogenesis to occur ( Fig. 25.3.10 ). We perform neonatal strip craniectomy as an open procedure through the same coronal incision that will be used for future cranial surgeries. This allows direct visualization of the thinned and adherent dura but does result in increased blood loss and the need for transfusion.
Endoscopic strip craniectomy has been suggested as a surgical option in the first year for patients with syndromic craniosynostosis as a primary procedure. This differs from the indications for neonatal strip craniectomy for severe cephalocranial disproportion, but is instead proposed as an alternative to open procedures such as posterior expansion and fronto-orbital advancement. Proponents of endoscopic strip craniectomies report that it can slow or halt the progression of turricephaly, however, more than half of syndromic patients still require subsequent open anterior expansion. Isolated strip craniectomy in syndromic cases with bilateral coronal craniosynostosis relies on brain growth during helmet molding to correct the frontal bone dysmorphology. A direct comparison on morphometrics will be required to determine its relative efficacy in establishing nasofrontal relationship compared to open fronto-orbital advancement. Open or endoscopic strip craniectomy may not be a single stage solution for patients with syndromic craniosynostosis, but is believed to mitigate the effects of craniosynostosis in early infancy to delay subsequent posterior and anterior open corrections to a more appropriate age.
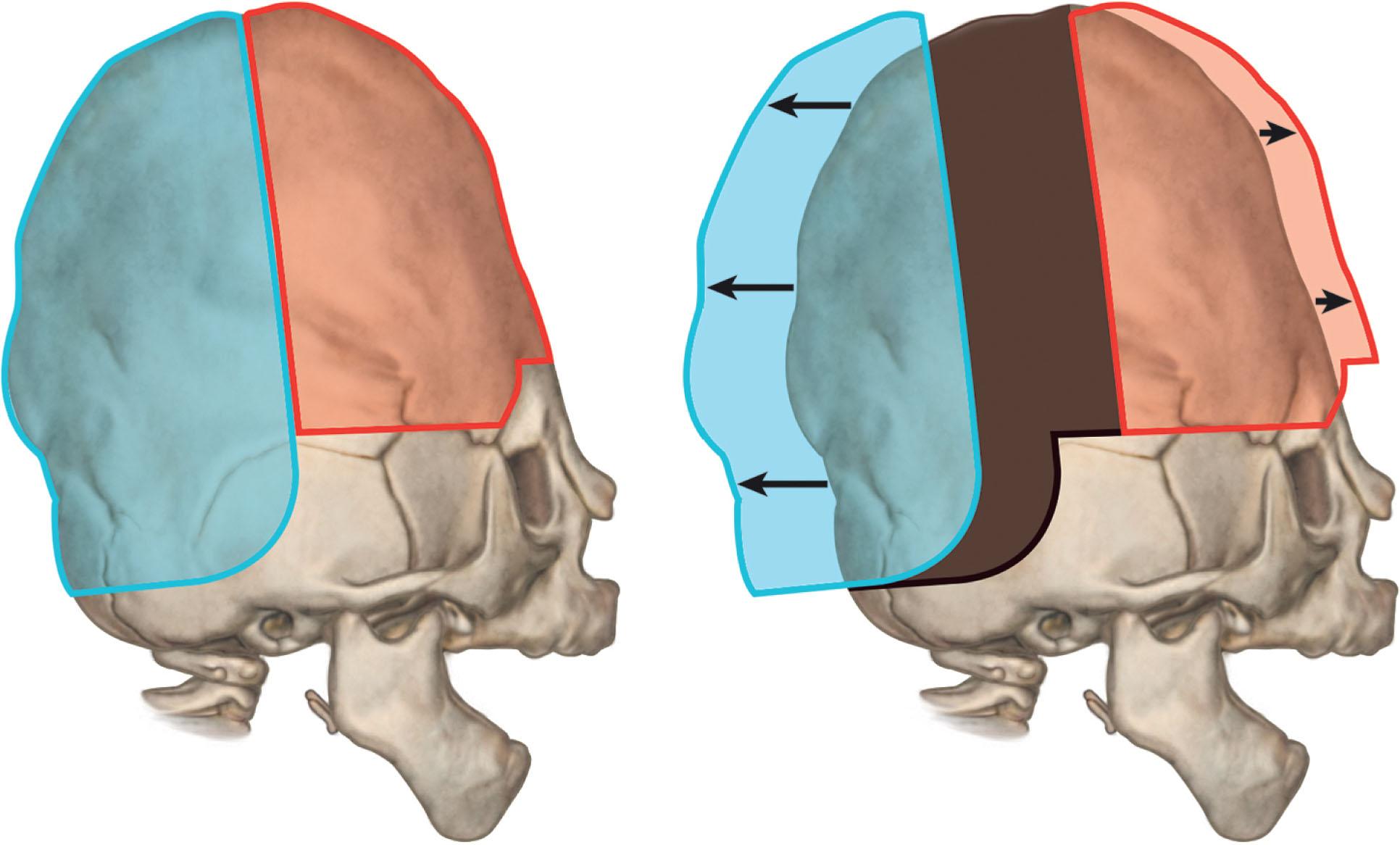
Historically, there has been debate on the efficacy of posterior versus anterior vault expansion as the first cranial procedure. The goals of early intervention are to enlarge the cranial volume to optimize brain growth and prevent any neurological complications of iICP. Proponents of anterior-first cranial expansion cite the importance of treating exorbitism with corneal protection provided by a fronto-orbital advancement (FOA). However, early frontal surgery has been recognized to have high relapse and reoperation rates. Proponents of posterior-first expansion, such as our team, note that it results in more than double the volume expansion compared to a fronto-orbital advancement ( Fig. 25.3.11 ) and results in less incidence of tonsillar herniation and papilloedema. Some techniques such as PVDO have also been associated with a decrease in the incidence of new Chiari malformations and improvement or resolution of existing Chiari malformations. Some centers are even examining posterior expansion as a first-line treatment in children with Chiari malformations. Early posterior expansion has been demonstrated to prevent the progression of turricephaly, improve frontal bossing, and further delay the need for a fronto-orbital advancement until an age when more stable results can be achieved. Overall, this staged approach of early posterior expansion followed by later anterior advancement may reduce the total number of transcranial surgeries that a child with severe syndromic craniosynostosis needs throughout their life. There are several techniques described for posterior cranial vault expansion, including open posterior cranial vault expansion, spring-assisted posterior vault expansion, and PVDO. It is important to note that not all syndromic patients require a posterior cranial expansion, and if a posterior-first approach is used, patients at risk of corneal ulceration from severe exorbitism will require a tarsorrhaphy until the second stage anterior expansion is performed. In an accepted but unpublished review, 23% of the Apert and Crouzon cases in the Seattle Children’s database and 35% at Great Ormond Street London underwent posterior vault expansion surgeries in the first 2 years of life.
Open posterior cranial vault expansion (PCVE) involves an open approach craniectomy for removal of the posterior cranium down to the skull base. Although there are variations to the osteotomy pattern, the overall goal is to use techniques such as opening and closing wedge osteotomies and switch cranioplasty (substituting flattened areas with curved bone plates) to create symmetry, and then to remodel the posterior cranium into a more expanded shape using resorbable plate fixation. The benefit of PCVE is that the entire procedure is completed in a single stage and can be modified as needed to address regional asymmetries. Disadvantages of this approach are that the procedure often involves removal of bone from the sagittal and transverse sinuses along with the inherent risk of bleeding, and that the stability of the new construct will be limited to the strength of the bone plates. In young patients this instability is compounded by the risk of deformational flattening when the child rests supine in the postoperative period.
Posterior vault distraction osteogenesis (PVDO) was first described using devices designed for mandible distraction osteogenesis to apply a gradual opening force across a circumferential cranial osteotomy. Although the same distraction protocol is followed as has been used for other facial bones, with a brief latency phase, followed by activation at 1 mm/day, and a prolonged consolidation phase, it is theorized that the bone that forms in the expanding cranial gap is not due to the undifferentiated bony regenerate tissue typically associated with distraction. Instead, the bone is likely regenerated when the pre-osteoblasts at the osteotomy interact with the FGF-2-rich environment created by the juvenile dura. Unlike open PVCE, in PVDO the osteotomy is limited to what is needed to create mobility of the posterior cranial transport segment so that the bone plate can remain attached to the underlying dura without the need to dissect over the torcula. Care must be taken to avoid injury to the transverse venous sinuses which can be invaginated between endocranial bone ridges ( Fig. 25.3.4B ). Similar to other forms of distraction osteogenesis, gradual controlled expansion is thought to maintain vascularity of the transported bone and prevent a sudden increase in dead space between the bone and brain. While one of the challenges of open PCVE is to achieve soft tissue closure over the expanded cranial volume, in PVDO, tension-free skin closure can be achieved since the expansion only occurs after surgery when the devices are activated. The devices also act as internal stabilizers of the opening osteotomies when the child is in the supine position. Unlike PVCE which can be modified to any cranial shape, PVDO provides a unidirectional expansion which cannot correct for multiple vectors of deformity.
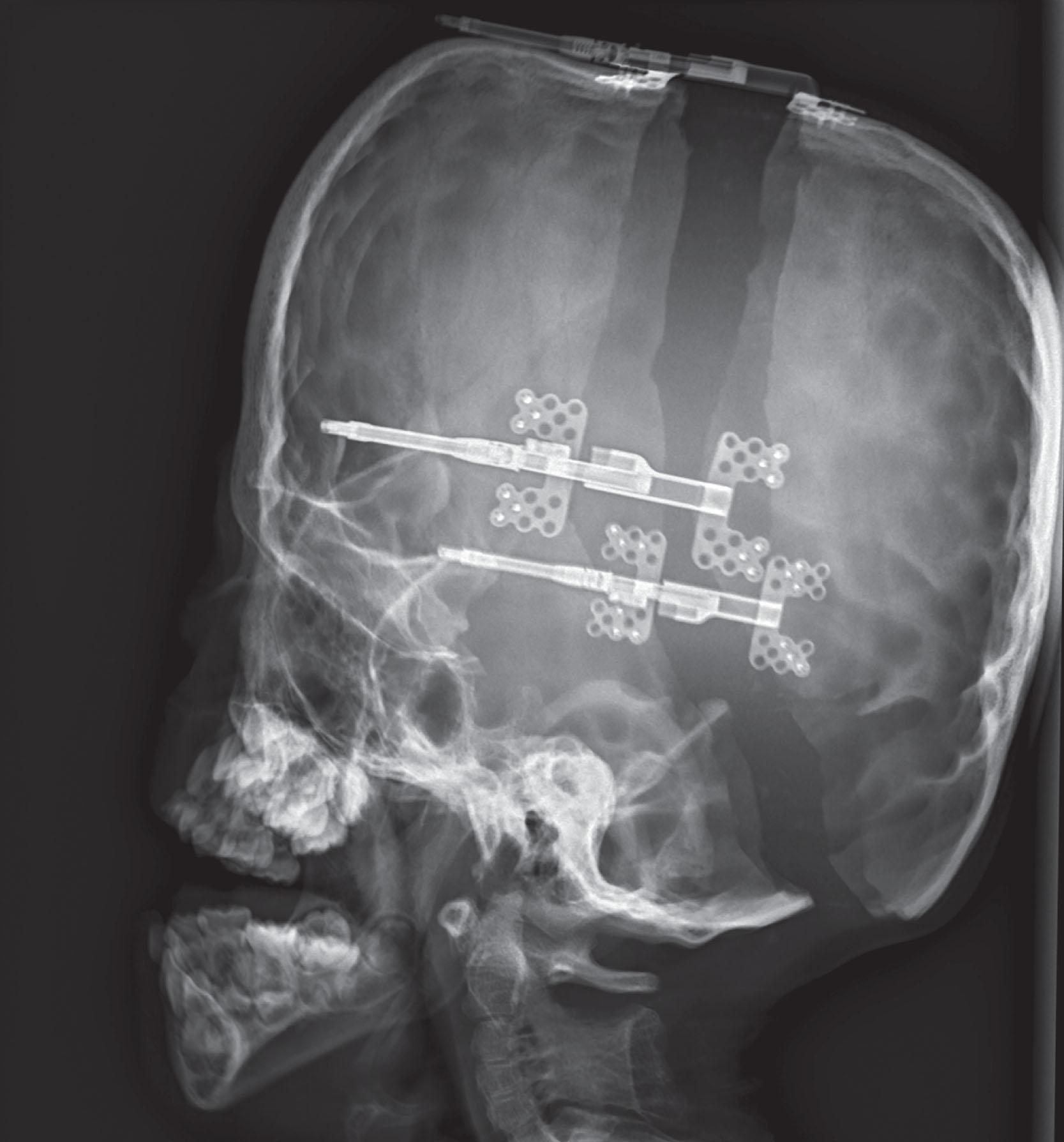
The PVDO technique involves a circumferential osteotomy from the vertex, inferior to the squamosal suture, posterior above the petrous ridge, then inferior to the torcula as low as possible and continuing in a symmetric pattern on the contralateral side. No epidural dissection is performed except for that needed for the osteotomy. Paired internal distraction devices are then placed parallel to the desired direction of expansion, with detachable percutaneous activation arms extending either anteriorly or posteriorly ( Fig. 25.3.12 ). The most stable bone for mounting the devices is typically just superior to the squamosal suture. A third device can be placed parasagittal if there would otherwise be asymmetric expansion due to a patent lambdoid suture or instability of the bone flap. A systematic review of PVDO protocols reported that latency periods ranged between 1 to 7 days, with most undergoing a latency period of 5 to 7 days. Distraction rates ranged from 0.5 mm to 2 mm per day, with the majority distracting at 1 mm per day. The consolidation period ranged from 28 to 156 days, with most patients undergoing 2–3 months of consolidation.
Spring-assisted posterior vault expansion (SAPVE) is achieved by applying a continuous force across an osteotomy or patent suture using a spring. It was first described as a treatment for sagittal synostosis, but Lauritzen and others have advocated for its use in any symmetric dysmorphology of craniosynostosis, including syndromic craniosynostosis through the use of multiple osteotomies and springs. SAPVE is typically used in the first year of life when the bones are more pliable and receptive to the spring forces, but it has been used successfully in older ages. Compared to PVDO, the force applied to the bone during SAPVE is not consistent with time, and is a result of the tension built within the spring against the changing resistance of the osteotomy gap. Early advocates of SAPVE created their springs manually, resulting in an unpredictable variation between springs and between surgeons. More recently, groups have researched ways to standardize the springs to exert a more consistent and predictable force and commercial springs are now available.
SAPVE can be performed either through a coronal scalp incision or via limited incisions with endoscopic visualization. The curvilinear craniectomy creates a posteriorly based bone flap that is then dissected from the dura a few centimeters until the “give” as tested by manual pressure is appropriate. To increase mobility, the osteotomies can be extended further towards the foramen magnum or more dural dissection is performed. Paired springs are then placed into grooves in the bone approximately 2 centimeters either side of the midline. Spring strength is chosen by the operating surgeon; if two springs are felt to be insufficient, further springs are placed. The pericranial flap and skin are then closed while compressing the springs. The springs are self-activating and are estimated to complete maximal opening over a period of ten days. Removal of the springs is performed as a separate procedure under general anesthesia once sufficient bone formation is felt to have occurred.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here