Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The introduction of monoclonal antibody drugs has had major impact in medicine, leading to extraordinary progress in the treatment of a wide variety of diseases, particularly malignancies and chronic inflammatory and autoimmune diseases.
This chapter provides a general overview of monoclonal antibody drugs approved for clinical use, including the basic biology of antibodies, production, pharmacologic characteristics, nomenclature, and various clinical indications. Because of the widespread use of tumor necrosis factor (TNF) antagonists in clinical medicine, we focus on the clinical use of this class of monoclonal antibody drugs to explain the mechanisms that elicit immunogenicity and to describe methodologies for drug monitoring and immunogenicity testing in the clinical laboratory. Finally, interference of monoclonal antibodies with clinical laboratory testing is reviewed since this is an emerging issue increasingly faced by laboratories as the number of patients treated with these drugs is on the rise.
Monoclonal antibody therapeutics (MAT) were initially introduced in transplantation medicine , following the approval of the first monoclonal antibody drug, muromonab-CD3, by the United States Food and Drug Administration (FDA) in 1985 as an antirejection agent in renal transplant patients. Subsequently in the 1990s several other monoclonal antibodies were approved for clinical use such as rituximab in 1997, followed by trastuzumab and infliximab a year later. All three of these MAT achieved remarkable success, ranking among the top best-selling drugs ever since. The implementation of MAT led to unprecedented progress in the treatment of a wide variety of diseases, including malignancies and chronic inflammatory and autoimmune diseases. MAT are used in oncology, rheumatology, gastroenterology, neurology, and many other areas of medicine. In recent years there have been dramatic advancements in cancer immunotherapy with the introduction of antibody drugs targeting checkpoint inhibitors cytotoxic T lymphocyte-associated 4 (CTLA-4) and programmed cell death-1 (PD-1).
As of the end of 2019, there were 100 unique monoclonal antibody, antibody fragment, or Fc fusion-based drugs, and 20 biosimilars approved by the FDA for the treatment of various diseases. These numbers are expected to increase dramatically in coming years since many more are currently in development.
Antibodies, also known as immunoglobulins (Ig), are naturally occurring large glycoprotein molecules that can be found membrane-bound on the surface of B cells, or in secreted form produced by B cell-derived plasma cells. In addition to recognizing and binding specific antigens, they can induce effector functions such as complement binding and activation of the complement cascade, or induce antibody-dependent cellular cytotoxicity (ADCC). Both of these mechanisms are aimed at eliminating the antigen source by inducing cell killing. Diversity and specificity are essential features of antibodies: the immune system is genetically capable of producing antibodies to virtually any antigen that it encounters, and the antibodies produced are highly specific to that antigen. Antibody-mediated responses constitute crucial mechanisms of the adaptive immune system to provide protection against viruses, bacteria, parasites, and other pathogens.
Ig are large Y-shaped molecules. Each Ig consists of two identical heavy chains (H, 55 kD) and two light chains (L, 22 kD) ( Fig. 98.1 A). Light chains can be of either lambda (λ) or kappa (κ) isotype, which are functionally identical (for additional information, refer to Chapter 31 ). Ig heavy chains belong to five different isotypes, which are functionally and structurally different and define the five major Ig classes in humans: IgM, IgD, IgA, IgE, and IgG. IgG is the major type of Ig in normal human serum. It functions predominantly in the secondary phase of the immune response, since it takes at least 2 to 3 weeks for it to appear in the serum following antigenic encounter. IgG can be further subdivided into IgG1, IgG2, IgG3, and IgG4 subclasses in humans, which vary in abundance, the type of antigen they bind to, and in their ability to induce effector functions. Antibody subclass isotype determines the type of effector function induced; IgG3 and IgG1 are the most potent activators of the complement pathway by interacting with C1q and induce complement-dependent cytotoxicity (CDC). IgG3 and IgG1 also bind to Fc receptors on effector cells activating cellular responses such as ADCC. IgG1 is most abundant in serum, followed by IgG2, IgG3, and IgG4. Except for IgG3, which has a serum half-life of about 7 days, the rest of the IgG subclasses have half-lives of ~21 days. When effector functions such as ADCC are desired, IgG1 is the isotype of choice for MAT development (such as in oncology). When blockade rather than engaging immune effector functions is required, IgG2 and IgG4 have been used. Overall, IgG1 is the most frequently used isotype in MAT.
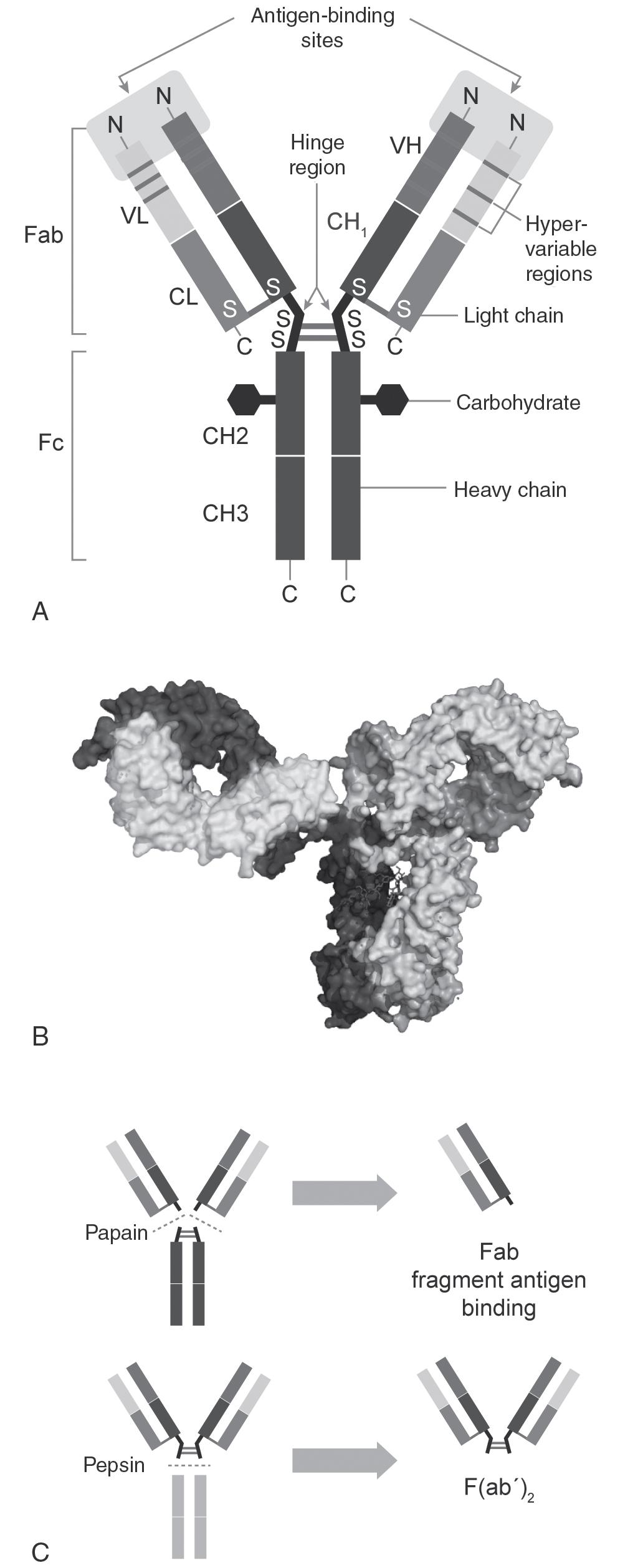
Structurally the IgG heavy and light chains are held together by interchain disulfide bonds in the hinge region (see Fig. 98.1 A). Although the structures are diverse, they contain well-defined domains such as the Ig variable (V) and constant (C) domains. These domains are composed of ~100 amino acid residues that share a common two-layered structure composed of two antiparallel β-sheets. The L chain folds into two domains, VL and CL, while the larger H chain consists of four domains, VH, CH1, CH2, and CH3. The antigen binding sites are located at the amino terminal variable region of both the H and the L chain, also called hypervariable regions or complementarity determining regions (CDR), which form a unique antigen binding site known as idiotype. Each variable domain contains three CDR regions, CDR1, CDR2, and CDR3, which show very high degree of sequence variability, and determine antibody specificity. Antibody diversity is generated by complex genetic mechanisms such as somatic hypermutation and affinity maturation. Despite sequence diversity, all Ig domains have highly similar three-dimensional structures. The available highest-resolution crystal structure of a typical full-length human IgG1 determined by x-ray crystallography is shown on Fig. 98.1 B. The structure indicates a highly asymmetric conformation ranging from Y shape to T shape, due to high molecular flexibility in the hinge region, which makes structure determination of complete IgG molecules difficult. However, mild enzymatic cleavage with proteolytic enzymes papain or pepsin results in fragments that are easier to crystallize ( Fig. 98.1 C). Cleavage of an intact antibody molecule with papain results in two monovalent Fab fragments and one dimeric crystallizable fragment (Fc). Cleavage with pepsin results in a bivalent, single F(ab’) fragment. In addition to intact Ig, these well-defined Ig fragments have medical and therapeutic applications themselves. The Ig domain structure is conserved in nature; its variants are commonly found as part of other proteins, including Fc receptors, adhesion molecules, costimulatory receptors and ligands, and many others.
The concept of using serum therapy for treatment of disease dates back to the discovery of diphtheria antitoxin by Emil Adolf von Behring (1890), who was awarded a shared Nobel prize in 1901 for the development of serum therapies against diphtheria and tetanus. Serum polyclonal antibody preparations and pooled IgG from healthy individuals have been used for prevention of several infectious diseases (e.g., hepatitis A), or for replacement therapy in patients with Ig deficiencies. Intravenous Ig, approved in 1980, and subcutaneous Ig, approved in 2006, are widely used as replacement therapy, or for the treatment of various autoimmune diseases or as part of desensitization protocols or treatment of rejection in transplantation.
Polyclonal antibodies used for therapeutic interventions include Rh (D) Ig, an enriched fraction of antibodies directed to the D blood group antigen for the prevention of rhesus D alloimmunization in pregnancy. Anti-thymocyte globulin (ATG) is another example of a widely used polyclonal antibody therapeutic prepared by immunizing mammals (commonly rabbit or horse) with human thymic lymphocytes. ATG administered to patients binds to lymphocytes and depletes them, leading to a profound suppression of the cellular immune response. ATG is used for the treatment of aplastic anemia and organ rejection.
Development of the hybridoma technology in 1975 allowed for production of monoclonal antibodies in vitro, using a hybridoma cell obtained by fusing an immortal myeloma cell (plasma cell-derived tumor cell) and a splenic normal B-cell derived plasma cell. This technology allowed for in vitro production of large amounts of MAT against a wide range of targets, including soluble and cell surface proteins. Early MAT products contained large amounts of nonhuman (i.e., mouse) proteins due to the use of mouse spleen derived plasma cells, which was responsible for the development of adverse effects due to the generation of human anti-mouse antibodies. Clinical experience with the first MAT, murine anti-CD3 muromonab, indicated poor pharmacokinetics and high immunogenicity, making treatment ineffective, which eventually resulted in withdrawal of the drug.
Using recombinant DNA technologies and better understanding of antibody structure-function correlation allowed for the development of chimeric (>65% human on average), humanized (>80% human), and fully human (>95% human) antibodies. Chimeric antibodies contain murine sequences in the variable region since they are initially developed as mouse antibodies. The rest of the molecule is replaced with human sequences, which reduces, but does not eliminate immunogenicity, and improves effector functions due to the presence of the human Fc part. The first chimeric antibody, abciximab, a human-mouse Fab fragment, was approved in the US in 1994, followed by rituximab in 1997, the first chimeric full-length antibody. The next phase of technology development allowed for the exchange of rodent sequences almost entirely to human sequences throughout the molecule, by grafting of the rodent CDR onto human IgG. The first humanized antibody, daclizumab, an antibody to IL-2 receptor α subunit, was approved in the United States in 1997 for the treatment of transplant rejection (withdrawn in 2009). Generation of fully human antibodies became possible with the development of phage-display technology, and transgenic mouse platforms. Phage display platform has become a commodity since the technology patents expired recently. It allows for designing and manipulating the repertoire of antibody genes used as antibody sources, followed by in vitro selection. The first antibody to reach the US market developed by this technology was the tumor necrosis factor (TNF) antagonist adalimumab, approved in 2002. In contrast to phage display platform, transgenic animal technology allows for in vivo selection process and requires less optimization and shorter timelines to reach clinical development. Antibody-producing plasma cells isolated from the spleen of immunized, genetically modified mice that express fully human monoclonal antibodies but are unable to produce mouse antibodies, are used to create hybridomas, which are used for subsequent large-scale production. MAT produced by transgenic technologies include panitumumab, approved in 2006 for oncology, and golimumab, a TNF antagonist approved in 2009.
As of the end of 2019, there were 100 unique monoclonal antibody-based drugs approved in the United States ( Table 98.1 ). Eighty-six of these are monoclonal antibodies or antibody fragments, 12 are Fc fusion proteins or peptides, and 2 are engineered chimeric antigen receptor T-cell (CAR T) therapies. Of the 86 monoclonal antibody drugs 4 are murine, 10 are chimeric, 40 humanized (human-murine, human-rat, human-camelid), and 32 are fully human. Most of these drugs are full-length antibodies, the most common isotype being IgG1, which occurs in 61 (71%) of approved antibodies; 8 are IgG2 (9%), 2 are IgG2/IgG4 hybrid (2%), and 13 are IgG4 (15%). Additional structures include camelid, and single chain Fv fragments. 11 out of the 12 approved Fc fusion drugs contain IgG1, and one is fused to IgG4 Fc. Engineering the Fc part is becoming more common in order to modulate effector functions, such as using glycoengineering to enhance effector function. As an example, afucosylation of IgG1 Fc in benralizumab facilitates binding to FcγRIII on natural killer (NK) cells, enhancing ADCC-mediated apoptosis of eosinophils and basophils, which is beneficial for the treatment of severe eosinophilic asthma. The Fc part can also be modified to silence effector functions such as in abatacept or eculizumab. Another approach for improving pharmacokinetics is to incorporate mutations to increase neonatal Fc receptor (FcRn) binding, such as the YTE mutation in some antibodies in development. Fusing IgG Fc with other proteins or peptides improves their stability and half-life. This approach is used in 12 currently approved MAT including TNF receptor, enzymes (phosphatase), peptides (glucagon-like), and clotting factors (VIII, IX). In addition to the full-length antibodies, five of the MAT are Fab antibody fragments. Some Fabs are fused to stabilizer molecules to improve pharmacokinetics in the absence of the Fc part, such as certolizumab pegol, which is PEGylated. Another innovative approach takes advantage of bispecific monoclonal antibodies designed as single-chain variable fragments of two different antibodies combined, such as in blinatumomab (approved in 2014 for acute leukemia), which is a bi-specific T-cell engager, connecting T cells via CD3 with tumor cells expressing CD19, facilitating immunologic synapse formation and tumor cell killing. Most of the approved MAT are nonconjugated intact IgG. However, ten of the MAT used in oncology are conjugated with drug molecules such as antimitotic agents, enzymes, or radioisotopes, which provide additional therapeutic benefits aimed at killing the tumor cell.
| Product (Proper) Name | Proprietary Name | Date of Licensure (month/day/year) | Molecular Target | Protein Format | Source | Major Indication |
|---|---|---|---|---|---|---|
| Oncology | ||||||
| Capromab pendetide | ProstaScint | 10/28/1996 | Prostate specific membrane antigen (PSMA), or glutamate carboxypep-tidase 2 | Murine IgG1, conjugated to Indium-111-pendetide | Mouse hybridoma | Imaging of prostate cancer |
| Rituximab | Rituxan | 11/26/1997 | CD20 | Chimeric human-murine IgG1κ | CHO cells | NHL, CLL, RA |
| Trastuzumab | Herceptin | 09/25/1998 | EGFR (HER2) | Humanized IgG1κ | CHO cells | HER2-positive breast cancer |
| Alemtuzumab | Campath, Lemtrada | 05/07/2001 | CD52 | Humanized rat IgG1κ | CHO cells | B-cell CLL |
| Ibritumomab tiuxetan | Zevalin | 02/19/2002 | CD20 | Murine IgG1 conjugated to Indium or yttrium | CHO cells | NH lymphoma |
| Cetuximab | Erbitux | 02/12/2004 | EGFR | Chimeric murine-human mAb Fab | SP2/0 murine myeloma | Metastatic colorectal carcinoma (EGFR+) |
| Bevacizumab | Avastin | 02/26/2004 | VEGF | Humanized IgG1 | CHO | Metastatic colorectal cancer, Her2 negative metastatic breast cancer |
| Panitumumab | Vectibix | 09/27/2006 | EGFR | Human IgG2 | Transgenic technology/Hybridoma cells | Metastatic colorectal carcinoma |
| Ofatumumab | Arzerra | 10/26/2009 | CD20 | Human IgG1κ | Mouse hybridoma | CLL |
| Ipilimumab | Yervoy | 03/25/2011 | CTLA-4 | Human IgG1κ | CHO cells | Metastatic melanoma |
| Brentuximab vedotin | Adcetris | 08/19/2011 | CD30 | Chimeric human-murine IgG1 coupled with monomethyl auristatin E (MMAE), a microtubule disrupting agent, through a protease-cleavable linker | CHO cells for the mAb | HL, anaplastic LCL |
| Pertuzumab | Perjeta | 06/08/2012 | EGFR (HER2) | Humanized IgG1 | CHO cells | HER2-positive breast cancer |
| Ziv-aflibercept | Zaltrap | 08/03/2012 | VEGF | VEGFR fused to human IgG1 Fc | CHO cells | Metastatic colorectal cancer |
| Ado-trastuzumab emtansine | Kadcyla | 02/22/2013 | EGFR (HER2) | Humanized IgG1k linked to anti-microtubule agent DM1 | CHO cells for the mAb | HER2-positive breast cancer |
| Obinutuzumab | Gazyva | 11/01/2013 | CD20 | Humanized IgG1 | CHO cells | CLL |
| Ramucirumab | Cyramza | 04/21/2014 | VEGFR2 | Human IgG1 | Hybridoma cells | Gastric adenocarcinoma |
| Pembrolizumab | Keytruda | 09/04/2014 | PD-1 (CD279) | Humanized IgG4κ | Hybridoma | Melanoma, NSCLC, Head and Neck cancer |
| Blinatumomab | Blincyto | 12/03/2014 | CD19, CD3d | Murine antibody, constructed BiTE (bi-specific T-cell engager) | CHO cells | B-cell ALL |
| Nivolumab | Opdivo | 12/22/2014 | PD-1 (CD279) | Human IgG4 | Transgenic mouse technology | Metastatic melanoma |
| Dinutuximab | Unituxin | 03/10/2015 | GD2, disialogan-glioside | Chimeric IgG1κ | Murine hybridoma | Neuroblastoma |
| Daratumumab | Darzalex | 11/16/2015 | CD38 | Human IgG1κ | CHO cells | Multiple myeloma |
| Necitumumab | Portrazza | 11/24/2015 | EGFR | Human IgG1κ | NSO Myeloma cells | NSCLC |
| Elotuzumab | Empliciti | 11/30/2015 | SLAMF7 | Humanized IgG1κ | NSO Myeloma cells | Multiple myeloma |
| Atezolizumab | Tecentriq | 05/18/2016 | PD-L1 (CD274) | Humanized IgG1, Fc engineered (nonglycosylated) | Mouse hybridoma | Urothelial carcinoma |
| Olaratumab | Lartruvo | 10/19/2016 | PDGFRa | Human IgG1 | NSO Myeloma cells | Soft tissue sarcoma (STS) |
| Avelumab | Bavencio | 03/23/2017 | PD-L1 (CD274) | Human IgG1λ | CHO cells | Metastatic Merkel cell carcinoma |
| Durvalumab | Imfinzi | 05/01/2017 | PD-L1 (CD274) | Human IgG1κ | CHO cells | Urothelial carcinoma |
| Rituximab and Hyaluronidase human | Rituxan Hycela | 06/22/2017 | CD20 | Chimeric human-murine IgG1κ | CHO cells | Follicular lymphoma, diffuse large B-cell lymphoma, CLL |
| Inotuzumab Ozogamicin | Besponsa | 08/17/2017 | CD22 | Humanized IgG4κ attached to N-acetyl-γ-calicheamicin dimethylhydrazide | CHO cells | ALL |
| Tisagenle-cleucel | Kymriah | 08/30/2017 | CD19 | Chimeric antigen receptor (CAR) | Expressed by patient’s own cells by in vitro molecular engineering | ALL |
| Gemtuzumab ozogamicin | Mylotarg | 09/01/2017 | CD33 | Humanized IgG4κ attached to calicheamicin | NSO Myeloma cells | CD33 positive AML |
| Axicabtagene ciloleucel | Yescarta | 10/18/2017 | CD19 | Chimeric antigen receptor (CAR) | Expressed by patient’s own cells by in vitro molecular engineering | Large B-cell lymphoma |
| Mogamulizumab-kpkc | Poteligeo | 08/08/2018 | CCR4 | Humanized IgG1κ | CHO cells | T-cell malignancies: mycosis fungoides or Sézary syndrome |
| Moxetumomab pasudotox-tdfk | Lumoxiti | 09/13/2018 | CD22 | Murine IgV fused to Pseudomonas exotoxin PE38 | Escherichia coli | Hairy cell leukemia |
| Cemiplimab-rwlc | Libtayo | 09/28/2018 | PD-1 (CD279) | Human IgG4 | CHO cells | Cutaneous squamous cell carcinoma (CSCC) |
| Trastuzumab and Hyaluronidase-oysk | Herceptin Hylecta | 02/28/2019 | EGFR (HER2) | Humanized IgG1κ | CHO cells | HER2-positive breast cancer |
| Polatuzumab vedotin-piiq | Polivy | 06/10/2019 | CD79b | Humanized IgG1 fused to anti-mitotic agent MMAE through protease-cleavable linker | CHO cells | Diffuse large B-cell lymphoma |
| Enfortumab vedotin-ejfv | Padcev | 12/18/2019 | Nectin 4 | Human IgG1κ conjugated to ani-mitotic agent monomethyl auristatin E (MMAE) through protease-cleavable linker | CHO cells | Advanced or metastatic urothelial cancer |
| Fam-trastuzumab deruxtecan-nxki | Enhertu | 12/20/2019 | Her2 | Humanized IgG1 linked to topoisomerase inhibitor DXd through protease cleavable linker | CHO cells | Metastatic breast cancer |
| Rheumatology | ||||||
| Infliximab | Remicade | 08/24/1998 | TNF | Chimeric human-murine IgG1κ | Mouse hybridoma | RA, PsA, AS, Crohn disease, PsO |
| Etanercept | Enbrel | 11/02/1998 | TNF | p75 TNFR2—huIgG1 Fc fusion | CHO cells | RA, PsO, AS, JIA, plaque psoriasis |
| Adalimumab | Humira | 12/31/2002 | TNF | Human IgG1κ | CHO cells | RA, PsA, AS, JIA, Crohn disease, plaque psoriasis, hidradenitis suppurativa, uveitis |
| Abatacept | Orencia | 12/23/2005 | CTLA-4 ligands CD80 (B7-1), CD86 (B7-2) | CTLA-4 fused to IgG1 Fc | CHO cells | RA, PsA, JIA |
| Certolizumab pegol | Cimzia | 04/22/2008 | TNF | Humanized IgG4 Fab | E. coli | RA, PsA, AS, Crohn disease |
| Golimumab | Simponi | 04/24/2009 | TNF | Human IgG1κ | Mouse SP2/0 hybridoma | RA, PsA, AS, Spondyloarthritis, UC |
| Tocilizumab | Actemra | 01/08/2010 10/21/2013 |
IL-6R (CD126) | Humanized IgG1κ | Hybridoma | RA, JIA, Giant cell arteritis, Cytokine release syndrome |
| Golimumab | Simponi Aria | 07/18/2013 | TNF | Human IgG1κ | Transgenic mouse technology | RA, PsA, AS |
| Sarilumab | Kevzara | 05/22/2017 | IL-6R (CD126) | Human IgG1 | CHO cells | RA |
| Secukinumab | Cosentyx | 01/21/2015 | IL-17A | Human IgG1κ | CHO cells | Psoriasis, AS |
| Ustekinumab | Stelara | 09/25/2009 09/23/2016 |
p40 subunit of IL-12 and Il-23 | Human IgG1κ | SP2/0 murine myeloma | Plaque psoriasis, PsA, Crohn disease |
| Canakinumab | Ilaris | 06/17/2009 | IL-1β | Human IgG1κ | Mouse Sp2/0-Ag14 cell line | Cryopyrin-associated periodic syndromes: Familial cold autoinflammatory syndrome and Muckle-Wells syndrome; JIA |
| Dermatology | ||||||
| Guselkumab | Tremfya | 07/13/2017 | IL-23 p19 | Human IgG1λ | Mammalian cell line | Plaque psoriasis |
| Tildrakizumab-asmn | Ilumya | 03/20/2018 | IL-23 p19 | Humanized IgG1κ | CHO cells | Plaque psoriasis |
| Risankizumab-rzaa | Skyrizi | 04/23/2019 | IL-23 p19 | Humanized IgG1 | Mammalian cell line | Plaque psoriasis |
| Ustekinumab | Stelara | 09/25/2009 9/23/2016 |
p40 subunit of IL-12 and Il-23 | Human IgG1κ | SP2/0 murine myeloma | Plaque psoriasis, PsA, Crohn disease |
| Secukinumab | Cosentyx | 01/21/2015 | IL-17A | Human IgG1κ | CHO cells | Psoriasis, AS |
| Ixekizumab | Taltz | 03/22/2016 | IL-17A | Humanized IgG4κ | Mouse hybridoma | Plaque psoriasis |
| Brodalumab | Siliq | 02/15/2017 | IL-17RA | Human IgG2 | CHO cells | Plaque psoriasis |
| Dupilumab | Dupixent | 03/28/2017 | IL-4Ra, IL-4 and IL-13 receptor | Human IgG4 | CHO cells | Eczema, atopic dermatitis |
| Blood Disease | ||||||
| Emicizumab-kxwh | Hemlibra | 11/16/2017 | Factor Ixa and Factor X | Humanized IgG4, bispecific | CHO cells | Hemophilia |
| Caplacizumab-yhdp | Cablivi | 02/06/2019 | von Willebrand factor | Humanized bivalent antibody fragment (Nanobody, heavy chain fragment from Camelidae) | E. coli | Acquired thrombotic thrombocytopenic purpura (aTTP) |
| Efmoroctocog alfa | Eloctate | 06/06/2014 | Factor VIII substitute | Factor VII fused to human IgG1 Fc | HEK cell line | Hemophilia A |
| Luspatercept-aamt | Reblozyl | 11/08/2019 | TGF-β superfamily ligands | Activin receptor type IIB linked to human IgG1 Fc domain | CHO cells | Erythroid maturation agent |
| Crizanlizumab-tmca | Adakveo | 11/15/2019 | P-selectin | Humanized IgG2κ | CHO cells | Sickle cell disease |
| Romiplostim | Nplate | 08/22/2008 | Thrombopoietin receptor c-Mpl | Peptibody, fused to IgG1 Fc | E. coli | chronic immune thrombocytopenic purpura |
| Eftrenonacog alfa | Alprolix | 03/28/2014 | Factor IX substitute | Factor IX fused to human IgG1 Fc | HEK293H cell line | Hemophilia B |
| Idarucizumab | Praxbind | 10/16/2015 | Dabigatran, thrombin inhibitor drug | Humanized IgG1 Fab | CHO cells | Anticoagulant reversal |
| Gastroenterology | ||||||
| Infliximab | Remicade | 08/24/1998 | TNF | Chimeric human-murine IgG1κ | Mouse hybridoma | RA, PsA, AS, Crohn disease, plaque psoriasis |
| Adalimumab | Humira | 12/31/2002 | TNF | Human IgG1κ | CHO cells | RA, PsA, AS, JIA, Crohn disease, plaque psoriasis, hidradenitis suppurativa, uveitis |
| Certolizumab pegol | Cimzia | 04/22/2008 | TNF | Humanized IgG4 Fab | E. coli | RA, PsA, AS, Crohn disease |
| Golimumab | Simponi | 04/24/2009 | TNF | Human IgG1κ | Mouse SP2/0 hybridoma | RA, PsA, AS, Spondyloarthritis, UC |
| Ustekinumab | Stelara | 09/25/2009 09/23/2016 |
p40 subunit of IL-12 and Il-23 | Human IgG1κ | SP2/0 murine myeloma | Plaque psoriasis, PsA, Crohn disease |
| Natalizumab | Tysabri | 11/23/2004 | α4-integrin | Humanized IgG4κ | Murine myeloma | MS, Crohn disease |
| Vedolizumab | Entyvio | 05/20/2014 | a4b7 integrin | Humanized IgG1 | CHO cells | UC, Crohn disease |
| Autoinflammatory and Other Immunologic Disease | ||||||
| Lanadelumab-flyo | Takhzyro | 08/23/2018 | Plasma kallikrein | Human IgG1κ | CHO cells | Hereditary angioedema (HAE) |
| Emapalumab-lzsg | Gamifant | 11/20/2018 | Interferon-γ | Human IgG1 | CHO cells | Hemophagocytic lymphohistiocytosis (HLH) |
| Ravulizumab-cwvz | Ultomiris | 12/21/2018 | Complement C5 | Humanized IgG2/4κ | CHO cells | Paroxysmal nocturnal hemoglobinuria (PNH) |
| Eculizumab | Soliris | 03/16/2007 | C5 | Humanized IgG2/4κ | Murine hybridoma | PNH |
| Canakinumab | Ilaris | 06/17/2009 | IL-1β | Human IgG1κ | Mouse Sp2/0-Ag14 cell line | Cryopyrin-associated periodic syndromes: Familial cold autoinflammatory syndrome and Muckle-Wells syndrome; JIA |
| Siltuximab | Sylvant | 04/23/2014 | IL-6 | Chimeric IgG1 | CHO cells | multicentric Castleman’s disease |
| Rilonacept | Arcalyst | 02/27/2008 | IL-1 | IL-1R and IL-1R accessory protein linked to hu IgG1 Fc | CHO cells | Cryopyrin-associated periodic syndromes: Familial cold autoinflammatory syndrome and Muckle-Wells syndrome |
| Neurologic Disease | ||||||
| Ocrelizumab | Ocrevus | 03/28/2017 | CD20 | Humanized IgG1 | CHO cells | Progressive MS |
| Natalizumab | Tysabri | 11/23/2004 | α4-integrin | Humanized IgG4κ | Murine myeloma | MS, Crohn disease |
| Erenumab-aooe | Aimovig | 05/17/2018 | Calcitonin gene-related peptide receptor (CGRP) | Human IgG2λ | CHO cells | Migraine |
| Fremanezumab-vfrm | Ajovy | 09/14/2018 | Calcitonin gene-related peptide (CGRP) ligand | Humanized IgG2Δa/κ | CHO cells | Migraine |
| Galcanezumab-gnlm | Emgality | 09/27/2018 | Calcitonin gene-related peptide (CGRP) ligand | Humanized IgG4κ | CHO cells | Migraine |
| Allergy and Asthma | ||||||
| Reslizumab | Cinqair | 03/23/2016 | IL-5 | Humanized IgG4κ | NSO Myeloma cells | Severe eosinophilic asthma |
| Omalizumab | Xolair | 06/20/2003 | IgE | Humanized IgG1κ | CHO cells | Asthma caused by allergies |
| Benralizumab | Fasenra | 11/14/2017 | IL-5Ra | Humanized IgG1κ, Fc engineered (afucosylated) | CHO cells | Severe eosinophilic asthma |
| Mepolizumab | Nucala | 11/04/2015 | IL-5 | Humanized IgG1κ | CHO cells | Severe eosinophilic asthma; Eosinophilic granulomatosis with polyangiitis (EGPA), appr 6/6/19 |
| Dupilumab | Dupixent | 03/28/2017 | IL-4Ra, IL-4 and IL-13 receptor | Human IgG4 | CHO cells | Eczema, atopic dermatitis |
| Infectious Disease | ||||||
| Palivizumab | Synagis | 06/19/1998 | RSV F protein, A antigenic site | Humanized IgG1κ | Mouse hybridoma | RSV infection |
| Raxibacumab | Raxibacumab | 12/14/2012 | Bacillus anthracis PA toxin | Human IgG1λ | Murine hybridoma | Anthrax |
| Ibalizumab-uiyk | Trogarzo | 03/06/2018 | CD4 | Humanized IgG4 | NSO Myeloma cells | HIV infection |
| Obiltoxaximab | Anthim | 03/18/2016 | B. anthracis PA toxin | Chimeric IgG1κ, affinity enhanced | Mouse hybridoma | Anthrax—Biodefense |
| Bezlotoxumab | Zinplava | 10/21/2016 | Clostridium difficile toxin B | Human IgG1 | CHO cells | C. difficile infection |
| Bone Disease | ||||||
| Burosumab-twza | Crysvita | 04/17/2018 | Fibroblast Growth Factor (FGF23) | Human IgG1κ | CHO cells | X-linked hypophosphatemia (XLH), a form of rickets |
| Romosozumab-aqqg | Evenity | 04/09/2019 | Sclerostin | Humanized IgG2 | CHO cells | Osteoporosis in postmenopausal women |
| Denosumab | Prolia, Xgeva | 06/01/2010 | RANKL, NF-κB ligand | Human IgG2 | CHO cells | Osteoporosis, bone metastasis from solid tumors |
| Asfotase alfa | Strensiq | 10/23/2015 | TNSALP enzyme replacement | Catalytic domain of human tissue nonspecific alkaline phosphatase (TNSALP), fused to human IgG1 Fc, and a deca-aspartate peptide as bone targeting domain | CHO cells | Hypophosphatasia |
| Cardiovascular Disease | ||||||
| Abciximab | ReoPro | 12/22/1994 | GP IIb/IIIa platelet receptor | Chimeric human-murine mAb Fab | Mouse hybridoma | CVD |
| Alirocumab | Praluent | 07/24/2015 | PCSK9, paraprotein convertase subtilisin/kexin type 9 | Human IgG1 | CHO cells | Familial hypercholesterolemia, CVD |
| Evolocumab | Repatha | 08/27/2015 | PCSK9, paraprotein convertase subtilisin/kexin type 10 | Human IgG2 | CHO cells | Familial hypercholesterolemia, CVD |
| Ophthalmology | ||||||
| Brolucizumab-dbll | Beovu | 10/07/2019 | VEGF | Humanized single-chain Fv (scFv) antibody fragment | E. coli | Neovascular (Wet) age-related macular degeneration (AMD) |
| Ranibizumab | Lucentis | 06/30/2006 | VEGF-A | Humanized IgG1κ mAb fragment | E. coli | Macular edema, age-related macular degeneration |
| Aflibercept | Eylea | 11/18/2011 | VEGF | VEGFR fused to human IgG1 Fc | CHO cells | Macular edema |
| Transplant | ||||||
| Basiliximab | Simulect | 05/12/1998 | CD25, IL-2Rα | Chimeric human-murine IgG1κ | Mouse hybridoma | Transplant rejection |
| Belatacept | Nulojix | 06/15/2011 | CTLA-4 ligands CD80 (B7-1), CD86 (B7-2) | CTLA-4 (2 amino acid mutant) fused to IgG1 Fc | CHO cells | Transplant rejection |
| Systemic Autoimmune | ||||||
| Belimumab | Benlysta | 03/09/2011 iv 07/20/2017 sc |
B lymphocyte stimulator protein (BLyS)/TNFSF13B/BAFF | Human IgG1λ | NSO Myeloma cells | SLE |
| Mepolizumab | Nucala | 11/04/2015 | IL-5 | Humanized IgG1κ | CHO cells | Severe eosinophilic asthma; Eosinophilic granulomatosis with polyangiitis (EGPA), appr 6/6/19 |
| Metabolic Disease | ||||||
| Dulaglutide | Trulicity | 09/18/2014 | GLP-1R | Glucagon-like peptide-1 agonist (GLP-1) fused to human IgG4 Fc | HEK293H cell line | Type II diabetes |
As a major breakthrough for cancer immunotherapy, two CAR T-cell therapy agents were approved in 2017: tisagenlecleucel for advanced leukemia and axicabtagene ciloleucel for lymphoma. Chimeric antigen receptor (CAR) is engineered as a single-chain variable fragment molecule fused to signaling domains, which is then expressed on host T cells, grafting monoclonal antibody specificity to the T cells. When injected back to the body, engineered host T cells expressing CAR receptors will recognize the target antigen on the cancer cells, such as CD19 on leukemic or lymphoma cells, and will induce tumor cell killing.
MAT are administered parenterally by intravenous, subcutaneous, or intramuscular routes. The intravenous route has the advantage of offering systemic delivery, the ability to deliver high volumes, and most importantly, assures complete bioavailability. However, limitations include inconvenience and adverse effects due to infusion reactions. Subcutaneous and intramuscular delivery offers lower bioavailability (24 to 95%) but allows self-administration and decreased rate of infusion-related adverse events. Although MAT distribute to most tissues to varying degrees, due to their large molecular weight (typically ~150 kDa) they are not able to cross the blood brain barrier and cannot enter the central nervous system.
MAT are eliminated from circulation through various mechanisms: antigen-specific target-mediated disposition and elimination through endothelial cells of the reticuloendothelial system. Target-mediated disposition involves antigen recognition and binding, typically to a membrane bound target, and subsequent clearance by endocytosis and lysosomal degradation. Since the number of antigenic targets is limited, this process is saturable and nonlinear. Elimination through the reticuloendothelial system, however, is non-saturable and linear, and it involves both free and bound drug. These two processes typically occur in parallel, for MAT targeting a membrane-bound antigen. In contrast to small molecule drugs, the liver and kidneys are not considered essential in the elimination of MAT under normal physiologic conditions, but clearance may be affected in the context of kidney injury leading to disruption of endothelial lining of the glomeruli (such as glomerulonephritis). Other routes affecting MAT clearance include loss of the drug through the gastrointestinal tract in protein losing enteropathy in patients with inflammatory bowel disease (IBD).
Since MAT are typically IgG molecules, their expected half-lives are quite long. This is explained by FcRn mediated uptake and endosomal recycling, a process that substantially extends the half-life of IgG ( Fig. 98.2 ). The binding affinity of FcRn for IgG is negligible at physiologic pH, but when IgG is internalized and enters the endosomes, in the acidic environment of endocytic vacuoles (pH 6.5) it binds to FcRn with high affinity, which rescues IgG from degradation, recycling it through transcytosis. , The poor pharmacokinetics of mouse antibody-based drugs observed in earlier trials can be explained by their low affinity to human FcRn, which makes the recycling process less efficient.
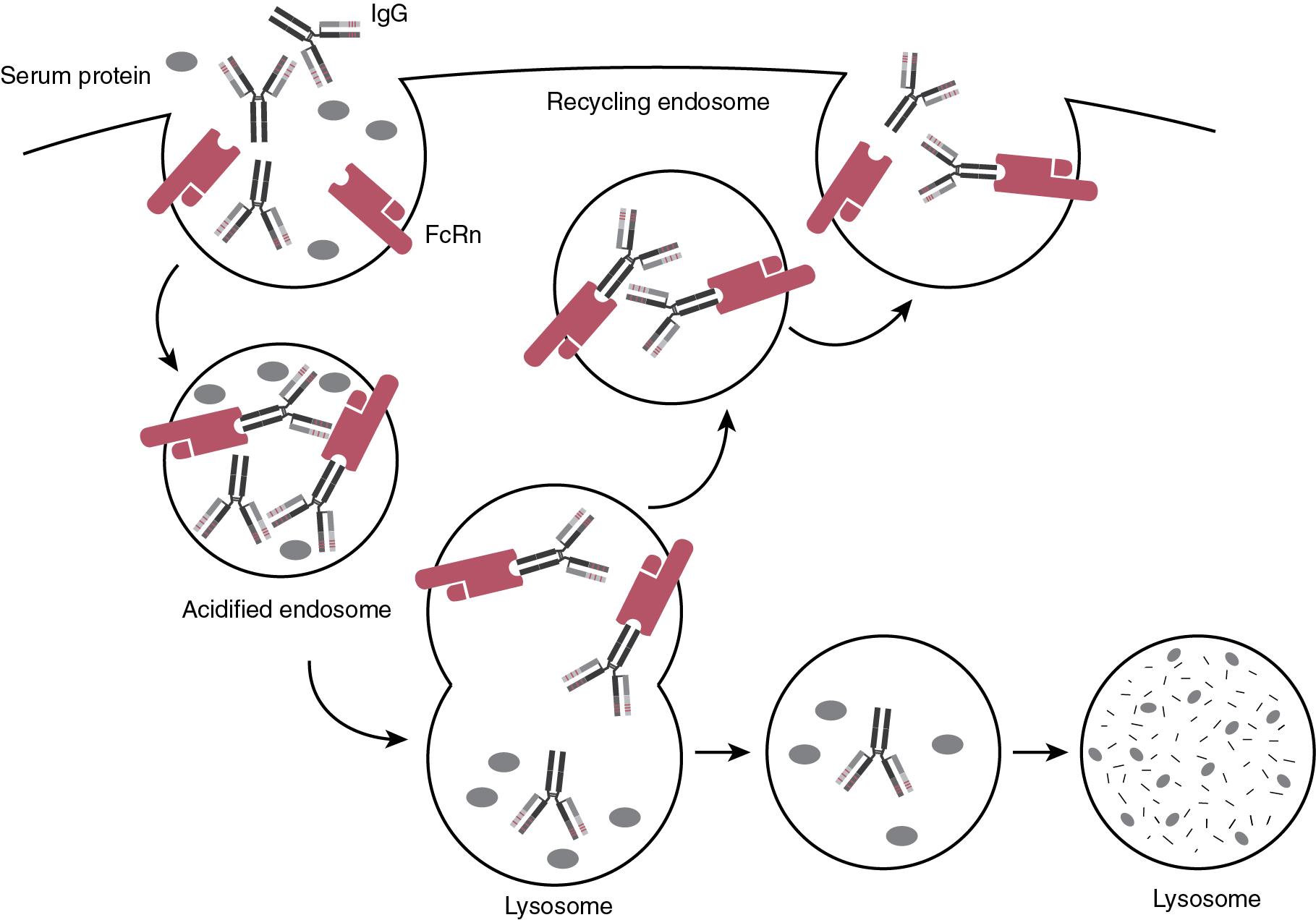
Of interest, high interindividual variability has been observed in pharmacokinetics of several MAT drugs. Based on multiple clinical trials, the most commonly identified covariates on MAT pharmacokinetics include body weight/surface area, gender, antidrug antibodies (ADA), creatinine clearance, age, disease severity, and inflammation markers such as C-reactive protein. Other factors influencing inter-individual variability include influence of disease elements such as proteinuria and protein losing enteropathy as mentioned above, or injury to blood-brain barrier, and influence of co-administered drugs. Better understanding of pharmacokinetics and patient-specific covariates paves the way toward personalized MAT therapy, with the goal of increasing efficacy in a cost-effective way, while decreasing toxicity.
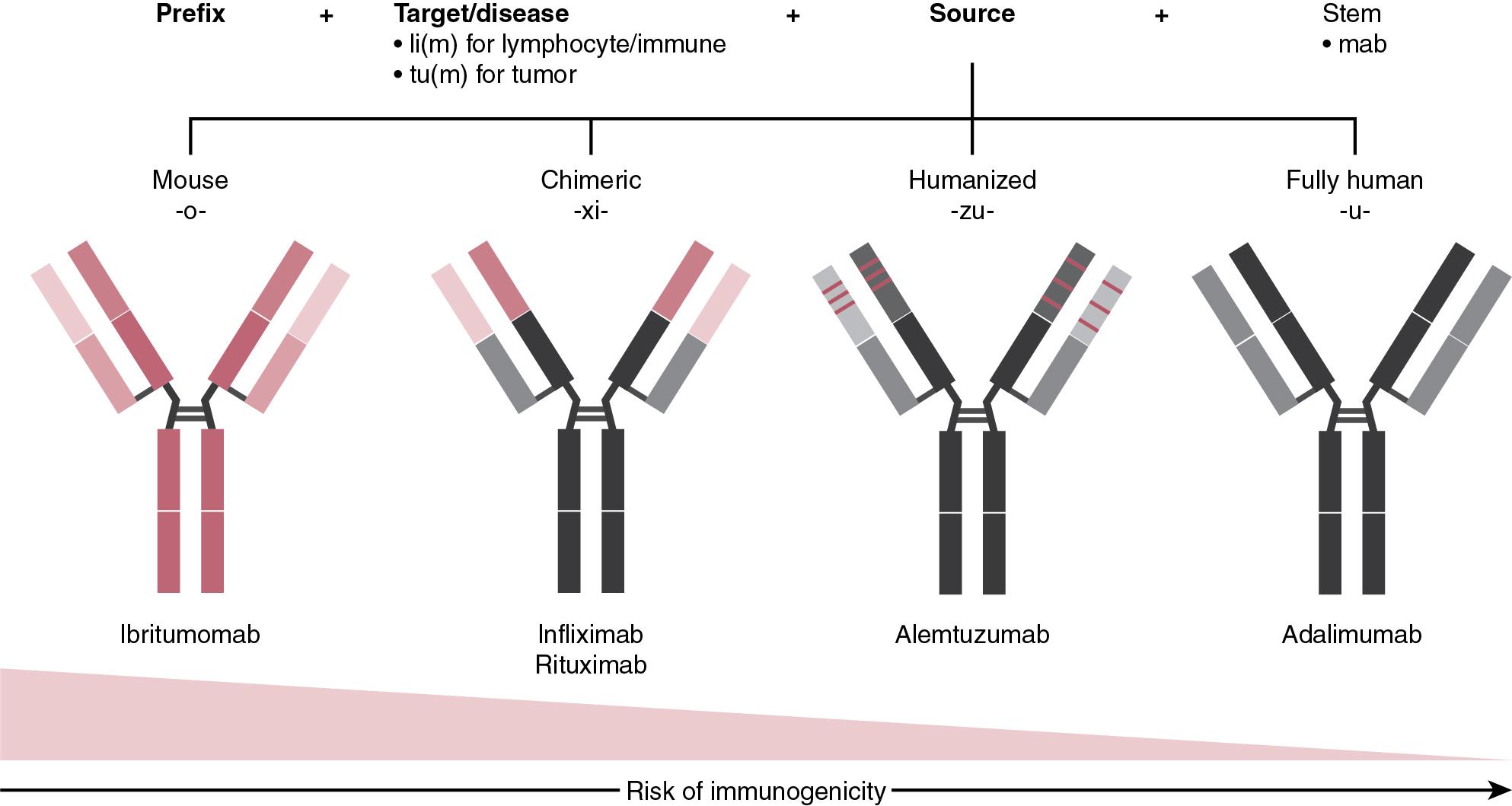
Nomenclature of MAT is currently standardized by international guidelines adopted by the American Medical Association’s United States Adopted Names Council. All monoclonal antibody product names end in the suffix –mab ( Fig. 98.3 ). The nomenclature includes a distinct starting prefix to create a unique name, which is followed by an infix representing the target or disease. Infixes indicating the target class, or disease the antibody is used to treat for include tu-/t- for tumor, li-/l- for immunomodulator, vi- for viral, ba- for bacterial, etc. Since the source of the antibody has important safety consideration in that it may induce immunogenicity in patients, a second infix included indicates the source of the antibody (the species on which the Ig sequence of the antibody is based): o for mouse, a for rat, u for human, i for primate. In addition, the degree of presence and origin of nonhuman sequences is further indicated by additional letters in the source infix such as –xi- indicating chimeric (~65% human), –zu- indicating humanized (~80% human), and -u- indicating fully human (>95% human) antibody. For engineered fusion proteins, suffix –cept indicates the presence of receptor molecules as part of the recombinant Fc fusion (such as etanercept for TNF receptor Ig fusion).
MAT were developed against a number of unique antigen targets. Some antigens show overlap between clinical areas, for example, TNF antagonists are used in rheumatology, as well as gastroenterology for the treatment of Crohn disease (CD). Some antigens are more frequently targeted than others (100 drugs for 61 targets), with TNF and CD20 being the most popular with 6 drugs approved for each, followed by HER2 and VEGF with 5 drugs, and CD19, EGFR, IL-17RA, IL-23 p19, PD-1, and PD-L1 with three antibodies for each. The majority of approved MAT are directed against targets in oncology (35%). Other areas include rheumatology (11%), dermatology (7%), blood disorders (7%), gastroenterology (6%), autoinflammatory and other immunologic disease (6%), allergy and asthma (4%), infectious disease (4%), neurologic disease including multiple sclerosis (4%), bone disorders (4%), cardiovascular disease (3%), ophthalmology (3%), transplant and systemic autoimmune disease (2% each), and type II diabetes (1%).
Antigenic targets for MAT are frequently cell membrane bound receptors such as GPIIb/IIIa, integrins, EGFR, CD20, CD52, CTLA-4, PD-1, PD-L1, or soluble targets including growth factors (VEGF), cytokines (TNF, IL-6, IL-17, IL-12/23), complement (C5), or IgE. While most MAT are designed to recognize human targets, a few of them that are used to treat or prevent infectious diseases bind to microbial protein targets (RSV-F protein, Bacillus anthracis PA toxin, Clostridium difficile toxin).
MAT targets for oncology can be classified into three different categories. The first category includes tumor-specific antigens, adhesion molecules serving as “postal addresses” for which killing mechanisms can be targeted. Tumor cell killing can be achieved by activation of immunologic mechanisms following antibody binding to tumor cells (e.g., ADCC or CDC), or by conjugating the antibody to another molecule mediating the killing such as toxins or radioactive isotopes. The second target category overlaps with the first, since it includes tumor cell receptors, which are targeted by MAT with the goal to block ligand binding and signal transduction, for example Her2. The third category includes immunotherapy agents such as checkpoint inhibitors designed to block co-inhibitory signals and thus directly stimulate tumor-specific T-cell responses, or CAR-T cells that express single chain chimeric antigen receptors specific to tumor antigen and are used to induce tumor cell killing.
MAT targeting cytokines include antibodies to pro-inflammatory cytokines IL-1, IL-6, IL-17, and TNF, which play crucial roles in the pathogenesis of numerous chronic inflammatory and autoimmune diseases. These MAT typically block the biological effect by either neutralizing the cytokine or blocking the cytokine receptor that mediates signaling. Due to their pleiotropic biological effects, which include playing a crucial role in host defense against pathogens, systemic pro-inflammatory cytokine blockade may interfere with host defense against infections, which is an unwanted adverse effect.
Immunoassays, cell-based assays, and mass spectrometry approaches are available to measure monoclonal antibody therapeutics (MAT) concentrations in the clinical lab.
It is important to interpret MAT concentration and antidrug antibodies results in the context of the assay used, and timing of blood samples.
Therapeutic drug monitoring of MAT is relevant in the setting of loss of response to therapy (reactive monitoring) and newer evidence suggests a role for proactive continuous monitoring during different time-points in the course of treatment to adjust therapy before loss of response occurs.
Interference of MAT with clinical laboratory tests is an emerging concern that can trigger unnecessary additional testing and may delay important therapeutic decisions.
MAT are mostly of the IgG κ isotype and some of them are given in high enough doses that they show up as monoclonal bands in protein electrophoresis and IgG κ bands upon immunofixation testing. Approaches to differentiate the MAT from an endogenous monoclonal protein have been developed.
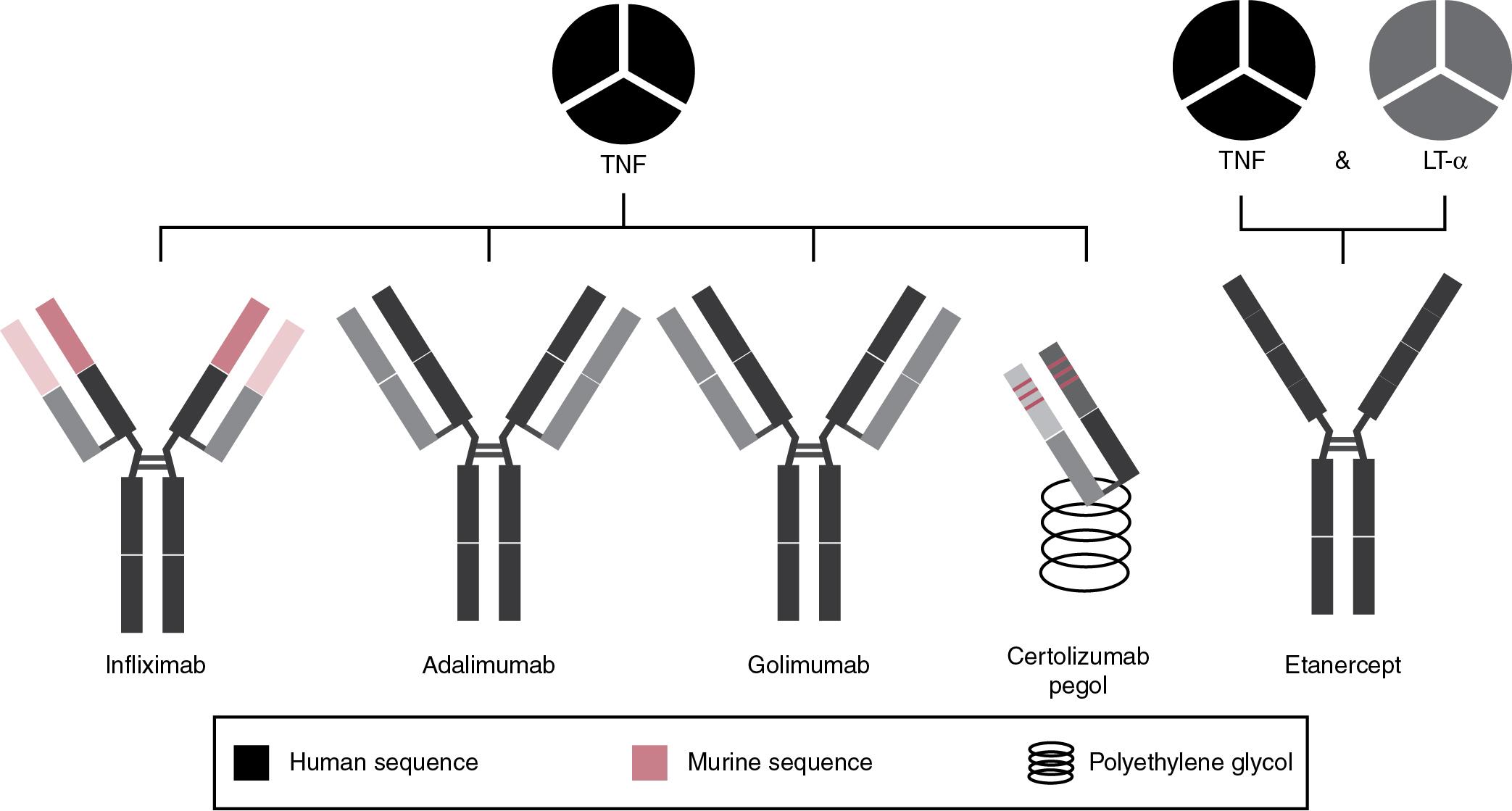
Due to the central role of TNF in inflammation and the pathogenesis of autoimmune and chronic inflammatory diseases, TNF antagonists have revolutionized the treatment of rheumatoid arthritis (RA), ankylosing spondylitis, psoriasis, and IBD including CD and ulcerative colitis. , The therapeutic benefits have been so dramatic that TNF antagonists are still among the best-selling, most prescribed pharmaceuticals. In recent decades several monoclonal antibody-based TNF antagonists have been developed ( Fig. 98.4 , Table 98.2 ). Infliximab is a chimeric mouse/human antibody in which the mouse variable region is preserved, but the rest of the molecule is replaced by human sequences. Adalimumab and golimumab are fully human IgG1 antibodies, produced by phage display or transgenic mouse technology, respectively. Despite containing predominantly human sequences, these molecules may show structural features that are different from the endogenous proteins, such as glycosylation patterns that are characteristic of the producing cells (Chinese Hamster Ovary cells [CHO] or mouse myeloma cells), not necessarily identical to the human glycosylation pattern, which may contribute to immunogenicity. Certolizumab pegol is a humanized antibody Fab fragment, designed by engrafting the mouse CDR into a human IgG4 κ Fab framework, and it has a polyethylene glycol (PEG) molecule attached that prolongs the half-life in the circulation. Etanercept is a fusion protein composed of the extracellular part of human TNF type 2 receptor (TNF-R2, p75) fused to dimeric human IgG1 Fc. Unlike the antibody drugs targeting TNF, etanercept is able to neutralize both TNF receptor ligands, TNF and Lymphotoxin-α.
| TNF Inhibitors | Etanercept | Infliximab | Adalimumab | Certolizumab Pegol | Golimumab |
|---|---|---|---|---|---|
| Molecule | hTNF receptor Fc fusion protein | Chimeric TNF antibody | Human TNF antibody | Humanized antibody Fab, PEGylated | Human TNF antibody |
| Protein Format | Dimeric hIgG1 Fc fused to hTNF-R2 (p75) | hIgG1κ, murine variable region | hIgG1κ, selected by phage display | hIgG4 Fab, murine CDR, conjugated to 40 kDa PEG | hIgG1κ, produced by transgenic mouse technology |
| Source | CHO cells | Sp2/0 hybridoma | CHO cells | Escherichia coli | SP2/0 hybridoma |
| Sequence origin | Human | ~75% human | Human | ~80% human | Human |
| Method of administration | sc 50 mg once weekly | iv 5 mg/kg at 0, 2, 6, and then every 8 weeks | 40 mg every 2 weeks | sc 400 mg at 0, 2, 4, and then every 4 weeks | sc 50 mg once a month; iv 2 mg/kg at 0 and 4, then every 8 weeks |
| Half-life (days) | 3–5 | 8–10 | 10–20 | 14 | 14 |
| Year of first FDA approval | 1998 | 1998 | 2002 | 2008 | 2009 |
| Indications | RA, JIA, PsA, AS, PsO | CD, UC, RA, AS, PsA, PsO | RA, JIA, PsA, AS, CD, PsO | CD, RA, PsA, AS, | RA, PsA, AS |
Patent expiration of biological drugs created the opportunity for manufacturers to develop biosimilar products, which are highly similar to and have no clinically meaningful differences from existing FDA-approved reference products. An abbreviated pathway for approval was created by Congress through the Biologics Price Competition and Innovation Act of 2009, assuring a shorter and less costly development program with the goal of providing more treatment options, lowering cost through competition, and increasing patient access to treatment. Similarity is determined by extensive structural and functional analysis, comparison of purity, chemical identity, and bioactivity; minor differences are however acceptable (e.g., stabilizer, buffer). For approval of a biosimilar, the manufacturer must demonstrate that there are no clinically meaningful differences compared to the reference product in pharmacokinetics, clinical efficacy, and safety. Assessment of clinical immunogenicity is important during biosimilar development. Due to the complexity of the manufacturing processes for MAT, involving recombinant DNA technology and production by living cells, it cannot be assumed that biosimilars are completely identical to the reference drug (in contrast to small molecule generic drugs, which have chemically identical structure to the reference drug).
Since 2015, 20 biosimilars to MAT have been approved in the US, 18 of which are monoclonal antibodies, along with 2 Fc fusions ( Table 98.3 ). The majority of them are TNF antagonists: four are biosimilars for infliximab, five for adalimumab, and two for etanercept. Biosimilars are expected to show similar immunogenicity profile compared to the reference drug, which is verified during clinical trials. However, due to the variety of assay platforms used across clinical laboratories, internal validation to verify assay performance for the biosimilar compared to the reference drug should be performed before implementing testing for biosimilar drug or ADA to biosimilars.
| Product Name | Proprietary Name | Date of Licensure (month/day/year) | Molecular Target | Protein Format | Source | Major Indication | Reference Drug | Reference Drug Proprietary Name | Reference Drug Date of Licensure (month/day/year) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Infliximab-dyyb | Inflectra | 04/05/2016 | TNF | Chimeric IgG1κ | Murine hybridoma | RA, PsA, AS, Crohn disease, UC, PsO | Infliximab | Remicade | 08/24/1998 |
| 2 | Etanercept-szzs | Erelzi | 08/30/2016 | TNF | Fc fusion, p75 TNFR2—huIgG1 Fc | CHO cells | RA, PsA, AS, JIA, PsO | Etanercept | Enbrel | 11/02/1998 |
| 3 | Adalimumab-atto | Amjevita | 09/23/2016 | TNF | Human IgG1κ | CHO cells | RA, PsA, AS, JIA, Crohn disease, UC, PsO | Adalimumab | Humira | 12/31/2002 |
| 4 | Infliximab-abda | Renflexis | 04/21/2017 | TNF | Chimeric IgG1κ | Murine hybridoma | RA, PsA, AS, Crohn disease, UC, PsO | Infliximab | Remicade | 08/24/1998 |
| 5 | Adalimumab-adbm | Cyltezo | 08/25/2017 | TNF | Human IgG1κ | CHO cells | RA, PsA, AS, JIA, Crohn disease, UC, PsO | Adalimumab | Humira | 12/31/2002 |
| 6 | Bevacizumab-awwb | Mvasi | 09/14/2017 | VEGF | Humanized IgG1 | CHO | Metastatic colorectal cancer, Her2 negative metastatic breast cancer | Bevacizumab | Avastin | 02/26/2004 |
| 7 | Trastuzumab-dkst | Ogivri | 12/01/2017 | EGFR (HER2) | Humanized IgG1κ | CHO cells | HER2-positive breast cancer | Trastuzumab | Herceptin | 09/25/1998 |
| 8 | Infliximab-qbtx | Ixifi | 12/13/2017 | TNF | Chimeric IgG1κ | Murine hybridoma | RA, PsA, AS, Crohn disease, UC, PsO | Infliximab | Remicade | 08/24/1998 |
| 9 | Adalimumab-adaz | Hyrimoz | 10/30/2018 | TNF | Human IgG1κ | CHO cells | RA, PsA, AS, JIA, Crohn disease, UC, plaque psoriasis | Adalimumab | Humira | 12/31/2002 |
| 10 | Rituximab-abbs | Truxima | 11/28/2018 | CD20 | Chimeric IgG1k | CHO cells | NHL | Rituximab | Rituxan | 11/26/1997 |
| 11 | Trastuzumab-pkrb | Herzuma | 12/14/2018 | EGFR (HER2) | Humanized IgG1κ | CHO cells | HER2-positive breast cancer | Trastuzumab | Herceptin | 09/25/1998 |
| 12 | Trastuzumab-dttb | Ontruzant | 01/18/2019 | EGFR (HER2) | Humanized IgG1κ | CHO cells | HER2-positive breast cancer, and gastric adenocarcinoma | Trastuzumab | Herceptin | 09/25/1998 |
| 13 | Trastuzumab-qyyp | Trazimera | 03/11/2019 | EGFR (HER2) | Humanized IgG1κ | CHO cells | HER2-positive breast cancer, and gastric adenocarcinoma | Trastuzumab | Herceptin | 09/25/1998 |
| 14 | Etanercept-ykro | Eticovo | 04/26/2019 | TNF | Fc fusion, p75 TNFR2—huIgG1 Fc | CHO cells | RA, PsA, AS, JIA, PsO | Etanercept | Enbrel | 11/02/1998 |
| 15 | Trastuzumab-anns | Kanjinti | 06/13/2019 | EGFR (HER2) | Humanized IgG1κ | CHO cells | HER2-positive breast cancer, and gastric adenocarcinoma | Trastuzumab | Herceptin | 09/25/1998 |
| 16 | Bevacizumab-bvzr | Zirabev | 06/28/2019 | VEGF | Humanized IgG1 | CHO | Metastatic colorectal cancer, NSCLC, recurrent glioblastoma, RCC, cervical cancer | Bevacizumab | Avastin | 02/26/2004 |
| 17 | Rituximab-pvvr | Ruxience | 07/23/2019 | CD20 | Chimeric IgG1κ | CHO cells | NHL, CLL, GPA, MPA | Rituximab | Rituxan | 11/26/1997 |
| 18 | Adalimumab-bwwd | Hadlima | 07/25/2019 | TNF | Human IgG1κ | Mammalian cells | RA, JIA, PsA, AS, Crohn disease, UC, PsO | Adalimumab | Humira | 12/31/2002 |
| 19 | Adalimumab-afzb | Abrilada | 11/18/2019 | TNF | Human IgG1κ | CHO cells | RA, JIA, PsA, AS, Crohn disease, UC, PsO | Adalimumab | Humira | 12/31/2002 |
| 20 | Infliximab-axxq | Avsola | 12/06/2019 | TNF | Chimeric IgG1κ | CHO cells | Crohn disease, UC, RA, AS, PsA, PsO | Infliximab | Remicade | 08/24/1998 |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here