Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The need to extract and utilize sufficient amounts of oxygen from the environment to sustain life has had an incredible impact on the evolution of the respiratory systems. In particular, breathing amphibians and mammals with their high metabolic rates have been forced to evolve a sophisticated organ to meet their oxygen requirements and get rid of carbon dioxide, the cellular waste of aerobic metabolism. We explore this process of lung development and examine a variety of important molecular regulators mediating lung formation.
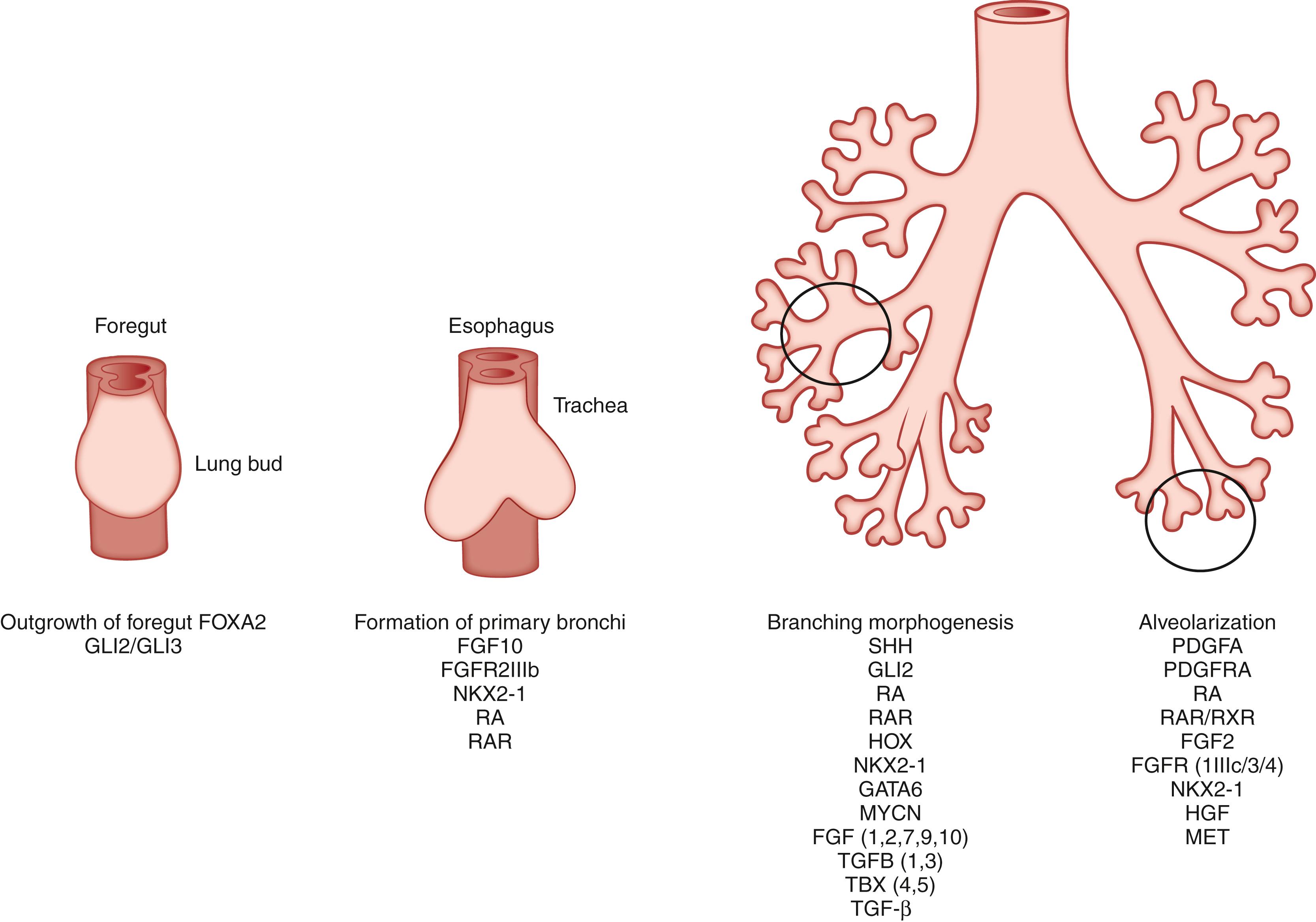
Lung development can be subdivided into six distinct stages ( Table 58.1 ). , The embryonic and fetal stages of lung formation comprise the embryonic, pseudoglandular, canalicular, and saccular stages. The embryonic stage encompasses organogenesis of the lung and formation of the major airways and pleura. During the pseudoglandular stage, the bronchial tree and respiratory parenchyma begin to form and the primitive airway epithelium starts to differentiate. Neuroendocrine, ciliated, and goblet cells appear, whereas mesenchymal cells begin to form cartilage and smooth muscle cells. In the subsequent canalicular period, the airway branching pattern is completed and the prospective gas exchange region starts to develop. During this period, respiratory bronchioles appear, interstitial tissue volume decreases, vascularization within the peripheral mesenchyme increases, distal cuboidal epithelium differentiates into type I and type II cells, and surfactant begins to appear. In the saccular period, growth of the pulmonary parenchyma, thinning of the connective tissue between the air spaces, and maturation of the surfactant system occur in preparation for life ex utero. Although capable of functional gas exchange, the lung is structurally immature. The distal airspaces at this time consist of smooth-walled transitory ducts and saccules with thick primitive septa containing a double capillary network.
| Stage | Gestational Age | Main Events | Epithelial Differentiation | |
|---|---|---|---|---|
| Human | Mouse | |||
| Embryonic | 3.5–8 wk | 9.5–14.5 days | Formation of lung bud, trachea, left and right primary bronchi, and major airways | Undifferentiated columnar epithelium |
| Pseudoglandular | 5–17 wk | 14.5–16.5 days | Establishment of the bronchial tree; all preacinar bronchi are formed | Proximal: columnar epithelium; ciliated, nonciliated, basal, neuroendocrine cells Distal: cuboidal epithelium; precursor type II cells |
| Canalicular | 16–26 wk | 16.5–17.5 days | Formation of the prospective pulmonary acinus by narrowing of terminal buds and increase of capillary bed | Proximal: columnar epithelium; ciliated, nonciliated, basal, neuroendocrine cells Distal: differentiation of cuboid type II cells to squamous type I cells |
| Saccular | 24–38 wk | 17.5 days to 5 dpn | Formation of alveoli precursors: saccules, alveolar ducts, and alveolar air sacs | Proximal: ciliated, nonciliated club, basal, neuroendocrine cells Distal: type I cells flatten and type II cells mature |
| Alveolar | 36 wk to 2 ypn | 5–30 dpn | Formation of alveoli by septation of alveolar air sacs, thinning of interalveolar septa | Proximal: columnar epithelium; ciliated, nonciliated, basal, neuroendocrine cells Distal: type I cells and type II cells mature |
| Microvascular maturation | Birth to 3 ypn | 14–21 dpn | Fusion of the capillary bed to a single layered network | |
The final two stages of lung development include alveolarization and microvascular maturation. Depending on the species, alveolarization starts before or after birth. Secondary septa are formed and fusion of the dual-layer capillaries into a single-layer network occurs. The microvascular maturation period ensues concurrently with alveolarization with the remodeling and maturation of the alveolar septa and transformation of the capillary network into a single layer. One of the important hallmarks of lung development is the signaling crosstalk between the epithelial and mesenchymal tissue layers. The combination, concentration, and spatiotemporal localization of a multitude of molecular signaling factors working in harmony determines the fate of branching, proliferation, and cellular differentiation of the developing lung. This chapter summarizes studies on lung development from the past two decades and explore the current dogma on the molecular mechanisms that determine lung pattern formation.
Lung development starts as an endodermal outgrowth of the ventral foregut around the fourth week of human development. This foregut mass rapidly elongates into a single tube dividing into a dorsal esophagus and a ventral trachea. The buds of the right and left lungs appear as two independent outpouchings around the tracheal bud. In a similar process, the mouse respiratory system develops from a pair of endodermal buds in the ventral half of the primitive foregut, just anterior to the developing stomach at 9.5 days of gestation. The two buds elongate in a posteroventral direction, whereas starting from the primary branch point, the gut tube begins to pinch into two, creating the dorsal esophagus and ventral trachea.
In humans, the left lung bud gives rise to two main stem bronchi and the right lung bud gives rise to three main stem bronchi. In the mouse, the right lung bud forms four stem bronchi, whereas the left lung consists of one stem bronchus. The secondary bronchi will then branch and rebranch in a process termed branching morphogenesis. Endoderm-derived epithelial cells line the airways, whereas the surrounding mesenchyme provides the elastic tissue, smooth muscles, cartilage, vascular system, and other connective tissues. The visceral pleura forms from splanchnic mesoderm, whereas the parietal pleura forms from the somatic mesoderm layer. The first pulmonary vessels form as a plexus surrounding the lung buds by vasculogenesis. The formation of the bronchial tree is finished at 16 days of gestation in the mouse and at 16 weeks of gestation in the human. At this stage of development, the tracheobronchial tree from the trachea to the terminal bronchioles resembles a system of branching tubules that terminate in exocrine gland–like structures.
The outgrowth of the ventral foregut, formation of the trachea, and outgrowth of the main pulmonary bronchi occur during the embryonic period of lung development. The crucial event at this stage is the initiation of lung formation at the right place along the anteroposterior axis of the foregut. Genetic studies have implicated several transcription factors (TCFs), morphogens, peptide growth factors, and their cognate receptors in specifying the morphogenetic progenitor field of the lung along the foregut axis ( Fig. 58.1 ). One important TCF in this process is forkhead box (Fox) A2 (FOXA2). , Foxa2 is expressed in the ventral foregut endoderm before and immediately at the start of lung bud formation. Targeted ablation of Foxa2 in mice led to embryonic death between embryonic day 6.5 and embryonic day 9.5 before the onset of lung formation , ; however, chimeras rescued for the embryonic-extraembryonic constriction showed that Foxa2 was essential for foregut and lung formation.
Fibroblast growth factor (FGF) 10 is a member of the large family of FGFs essential to many processes during embryonic development. , In the murine lung, Fgf10 messenger RNA (mRNA) is dynamically expressed in the distal mesenchyme adjacent to the primitive lung buds. The importance of FGF10 for lung development was shown in Fgf1 0-deficient mice that die at birth because of respiratory failure. , The Fgf10 -deficient mice had complete lung agenesis; that is, lung development had stopped after the formation of the trachea. , FGF10 overexpression did not affect proper epithelial branching, which remained highly preserved. Instead, mesenchymal FGF10 maintained distal Sox-9 expressing epithelial progenitors in an undifferentiated state through the activation of β-catenin signaling. FGFs bind to and signal via FGF tyrosine kinase receptors (FGFRs). , The FGF10 receptor (FGFR2IIIb), an FGFR2 splice variant, is expressed in lung bud epithelium. FGFR2IIIb is also capable of binding FGF1 and FGF7, which have also been implicated in lung development. , Because targeted mutation of FGFR2 resulted in an early lethal phenotype caused by placental insufficiency, , Fgfr2 −/− chimeras were created to overcome this early lethality and allow lung development to be analyzed. Similarly to what occurred in the Fgf10 -deficient mice, only the trachea formed, without further pulmonary branching. Transgenic mice overexpressing a dominant negative FGFR2IIIb splice variant in distal lung epithelium show a severe pulmonary defect, forming only a trachea and two main bronchi, without any lateral branches. Moreover, Cre recombinase-mediated excision to generate mice lacking the IIIb form of FGFR2 while retaining expression of the IIIc splice form resulted in mice that had no lungs and thus died at birth. Taken together, these data indicate that FGF10 signaling via FGFR2IIIb plays a crucial role in the initiation of lung bud formation (see Fig. 58.1 ). The “no lung” phenotype as a result of inhibited FGF10 signaling shows a striking similarity to a phenotype resulting from the loss of function of either branchless (bnl) or breathless (btl) in Drosophila . The bnl gene encodes an FGF homologue that functions as a ligand for breathless, a Drosophila homologue of FGFR. Loss of function of either bnl or btl prevented tracheal branching in the fly. ,
Sonic hedgehog (SHH) is a secreted signaling molecule and a mammalian homologue of Drosophila hedgehog (HH) that is known to be involved in many fundamental processes during Drosophila embryonic development. Shh is expressed as early as ventral foregut endodermal development. It is induced by retinoic acid (RA) produced in the mesoderm and then signals back to activate the GLI2/3 TCFs, stimulating WNT2/2b and bone morphogenetic proteins (BMPs), which induce NKX2-1 expression. SHH functions via paracrine signaling. It is produced by the epithelial cells and is handled by the mesenchymal-located Patched (PTC) receptor, suggesting that the SHH pathway is an important regulator of epithelial-mesenchymal signaling during lung development. Its importance for lung development was shown when the Shh gene was genetically ablated, which resulted in a simplistic-looking lung consisting of only one lobe on each side of the trachea (pulmonary left isomerism). , In contrast to the FGF10-null mutant, the initiation of lung formation does occur in Shh -null mutants; however, there is an incorrect number of lobes and a subsequent failure of branching morphogenesis. , This was confirmed with generation of a lung-specific Shh -null mouse. In this mouse, when Shh expression was removed before embryonic day 13.5, tracheal, bronchial, and peripheral lung defects were seen similar to those of the Shh -null mouse. The mesenchyme appears to be the primary target of SHH deficiency, showing decreased cell proliferation and increased cell death. The effect of SHH on pulmonary mesenchyme was also demonstrated when Shh was overexpressed in distal lung epithelium with use of the surfactant protein C (Sftpc) promoter. Overexpression of SHH resulted in smaller lungs at birth that lacked functional alveoli, most likely due to hypercellularity of the mesenchyme. Together, these results reveal the important function of SHH in epithelial-mesenchymal signaling during early lung formation.
Cubitus interruptus (CI) has been identified as a downstream target in HH signaling in Drosophila . Mammalian Gli genes are the putative homologues of Drosophila ci and have also been implicated in mammalian SHH signaling. Three Gli genes have been described in mice: Gli1 , Gli2, and Gli3 , all of which are expressed in the early pulmonary mesenchyme. These TCFs are posttranslationally converted into their activator or repressor forms to regulate the expression of SHH target genes. Gli3 -null mice show a mild lung defect with a slight reduction in size. However, Gli2 -null mice have respiratory failure at birth, with defective airway branching, left pulmonary isomerism, and severe hypoplasia. Concurrent deletion of both Gli2 and Gli3 revealed a more dramatic phenotype. These Gli2 / Gli3 −/− double-mutant mice have no lung, trachea, or esophagus and die early in gestation. Other foregut derivatives such as the pancreas, thymus, and stomach do develop in Gli2 / Gli3 −/− mutant mice, although they are hypoplastic. These findings indicate that GLI2 and GLI3 have distinct, overlapping, and vital functions during initiation of lung bud formation. The complete absence of trachea and lung formation was ameliorated by the presence of one Gli3 gene, as the Gli2 −/− / Gli3 +/− mutant had a lung consisting of one hypoplastic lobe. Because the ablation of both Gli2 and Gli3 resulted in a far worse lung phenotype than Shh deficiency alone, SHH is likely not the only regulator of Gli genes during lung development. The complete absence of a lung in Gli2 / Gli3 −/− mutant mice is similar to the “no lung” phenotype seen in Fgf10 -deficient mice. The difference is that the trachea and esophagus are present in Fgf10 -deficient mice but absent in Gli2 / Gli3 -deficient mice, implicating different signaling pathways. Other single or compound mutants for the three Gli genes show a variable degree of lung hypoplasia with an aberrant number of lung lobes and decreased branching morphogenesis. , However, another compound mutant recently analyzed revealed an interesting relationship between SHH and GLI3 during lung development. The Shh / Gli3 −/− mouse shows a phenotype that is less severe than the Shh -null lung alone, with enhanced vasculogenesis and growth potential. For a complete review of the SHH-GLI pathway interactions and effect of mutations on lung development, see Rutter and Post.
RA plays a crucial role during gestation and is involved in the developmental processes of almost every organ. , Both a deficiency and an excess of RA cause congenital defects during human development in a variety of organs. , RA exerts its effects via retinoic acid receptors (RARs) and retinoid X receptors (RXRs), tyrosine kinase receptors, which function as transcriptional regulators of RA target genes. The RAR family is composed of three genes producing several isoforms: RARA 1,2 , RARB 1–4 , and RARG 1,2 , all activated by both all- trans -RA and 9- cis -RA, whereas the three isoforms from the RXR family, RXRA, RXRB, and RXRG, are activated only by 9- cis -RA. RARs require heterodimerization with RXRs for DNA binding to RA response elements (RAREs). Mice deficient for only one of the isoforms show a milder phenotype than expected on the basis of their expression patterns, indicating a high degree of redundancy among the RARs. In contrast, compound mutant mice had similar congenital defects as seen with fetal vitamin A deficiency. , Rara2/Rarb2 −/− double-mutant mice die soon after birth with agenesis of the left lung and hypoplasia of the right lung. Lung hypoplasia was also reported in Rara1/Rarb − / − and Rxra/Rara − / − double mutants. , RA has profound influences on lung development during branching morphogenesis and alveolarization. The localized expression of FGF10 in the mesenchyme requires RA signaling and may regulate the Hox genes. Hox genes form a large family of homeobox-containing TCFs that are implicated in the specification of cells that form morphologic structures along an anteroposterior axis. Hox genes are arranged in four chromosomal clusters, and the 3′ to 5′ position of each gene within a cluster corresponds to their expression pattern along the anteroposterior axis of the developing body. Genes of the 3′ regions of Hox clusters A and B are expressed in the developing lung, with the Hoxb cluster predominantly expressed in the early pulmonary mesenchyme. , Within the mesenchyme, Hoxb genes express in a proximal-distal expression gradient, suggesting a role for Hoxb genes in specifying proximal from distal pulmonary mesenchyme. Several mutant models have been created that illustrate the importance of Hox genes during lung development. Single-mutant mice for Hox genes are generally normal, most likely because of redundancy. However, compound Hoxa1 / Hoxb1 −/− mutants have severe lung hypoplasia with phenotypes ranging from five hypoplastic lung lobes to a lung with only two lobes. In the Hox5 paralog group, Hoxb5 − / − mice have lung phenotypes that are less severe than those in Hoxa5 mutants. Hoxa5 −/− mice die in the perinatal period and have laryngotracheal malformations, a reduced tracheal lumen, and lung hypoplasia. Hoxa5 also plays specific roles in lung microvascular development and diaphragm innervation.
Another homeodomain TCF expressed at the onset of lung morphogenesis is NKX2-1 (also known as TTF1 ). Expression of Nkx2-1 mRNA is localized to epithelial cells of the developing pulmonary tubules. , , NKX2-1 continues to be expressed in adult bronchiolar and alveolar epithelial type II cells, where it plays an important role in the regulation of secretoglobin protein, SCGB1A1 (also known as club cell secretory protein ), and surfactant protein synthesis. Targeted disruption of NKX2-1 results in severe hypoplasia of the lung lacking separation of the trachea from the esophagus, arrest of branching morphogenesis, and epithelial cell differentiation at the pseudoglandular stage. ,
The formation of a tracheoesophageal septum divides the ventral trachea from the dorsal esophagus. Failure of the trachea and esophagus to separate results in a congenital defect in humans called tracheoesophageal fistula. Deficiency of the growth and TCFs implicated in lung agenesis or hypoplasia also results in tracheoesophageal fistula with different gradations of severity ( Table 58.2 ). Gli2 / Gli3 −/− mutant mice have no trachea, nor do they form an esophagus, and Gli2 −/− / Gli3 +/− mutants have a single tracheoesophageal tube connected to the stomach. In Shh −/− mutant mice, the trachea and esophagus fail to separate, resulting in tracheoesophageal fistula. , Mice haploinsufficient for the TCF Foxf1 exhibit foregut abnormalities including narrowing and, sometimes, atresia of the esophagus, as well as frequent fusion of the trachea and esophagus. Foxf1 expression was absent in foregut derivates (trachea, esophagus, oral cavity, lungs) of Shh −/− mutants, indicating that SHH signaling is required for activation of FOXF1 in these tissues. Surprisingly, separation of the trachea and esophagus occurs normally in Fgf10 -deficient mice, , whereas Nkx2-1 -deficient mice have a complete tracheoesophageal defect. Rara/Rarb2 −/− , Rara1/Rarb −/− , and Rxra / Rara −/− mutant mice all exhibit a tracheoesophageal septal defect and other tracheal malformations such as disorganized cartilaginous rings and shortening of the trachea. The end result of RA signaling in relation to lung development appears to be mediated through FGF10. This became evident in studies using the pan-RAR antagonist BMS493 in foregut explant cultures, which resulted in failure of initial lung bud outgrowth in the prospective lung field due to inhibition of Fgf10 expression in the foregut mesenchyme.
| Condition | Trachea | Esophagus | Remarks |
|---|---|---|---|
| Fgf10 −/− | + | + | Trachea-esophagus separation; trachea ends blind |
| Shh −/− | + | + | Trachea-esophagus septal defect |
| Gli2 −/− | + | + | Trachea-esophagus separation with stenosis |
| Gli2 −/− /Gli3 −/+ | + | − | Single (tracheal) tube connecting to the stomach, esophageal atresia |
| Gli2 −/− /Gli3 −/− | − | − | No esophagus, trachea, or lung |
| Rara −/− /Rarb2 −/− Rara1 −/− /Rarb −/− |
+ | + | Trachea-esophagus septal defect, tracheal cartilage malformations |
| Nkx2-1 −/− | + | + | Trachea-esophagus septal defect |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here