Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cancer is fundamentally a genetic disease caused by inherited germline variants coupled with the accumulation of somatic mutations in oncogenes and tumor suppressor genes. Some patients inherit gene mutations that predispose them to cancer, while others acquire all alterations somatically. In both inherited and sporadic cancers, neoplasms acquire numerous somatic alterations throughout their development; some are driver alterations that play crucial roles in tumorigenesis while others are passenger alterations with no known functional consequences. Knowledge of the genetic drivers of neoplasia has greatly advanced our understanding of basic tumor biology and has revolutionized the practice of oncological pathology, with mutation-specific diagnostic and treatment strategies in multiple tumor types.
Pancreatobiliary neoplasms are among the best genetically characterized tumors, and studies of their molecular features have shown that molecular changes match morphologically defined entities: genetics mirrors morphology. With this newly reached understanding of the molecular biology of pancreatobiliary neoplasms, the field has moved into an era of genomic medicine. In the coming decades, knowledge of the molecular underpinnings of a neoplasm will be crucial for a given patient’s clinical care, and pathologists will play an even greater role in patient care. Therefore a sound understanding of the molecular genetics of pancreatobiliary neoplasms is required to care effectively for patients.
Ductal (tubular) adenocarcinomas are malignant invasive gland-forming epithelial neoplasms.
Approximately 10% of pancreatic ductal adenocarcinomas have a familial basis, and several germline variants have been identified that lead to an increased risk of pancreatic cancer ( Table 35.1 ). , These germline variants are important to recognize for two reasons. First, they can be used to quantify cancer risk. Second, some cancers that arise in patients with a germline variant are remarkably sensitive to specific therapies. , Although a strong family history of cancer suggests an increased likelihood of a deleterious germline variant, many individuals with a germline variant do not have a family history of cancer. , The National Comprehensive Cancer Network (NCCN) has therefore updated its guidelines to recommend that all patients with pancreatic cancer should be offered germline testing. Although germline testing is powerful, it should also be noted that the genetic basis for the majority of familial pancreatic cancer remains unknown.
| Gene | Chromosome | Syndrome | Neoplasm |
|---|---|---|---|
| APC | 5 | Familial adenomatous polyposis (FAP) | SPN, PB |
| ATM | 11 | Ataxia-telangiectasia | PDA |
| BRCA1 | 17 | Familial breast cancer | PDA |
| BRCA2 | 13 | Familial breast cancer | PDA, acinar, cholangio |
| FANCC , FANCG | Multiple | Fanconi’s anemia pathway | PDA (?) |
| Highly imprinted area: effects IGF2 and CDKN1C expression | 11 | Beckwith-Wiedemann syndrome (BWS) | PB |
| hMSH2 , hMLH1 , hPMS1 , hPMS2 , hMSH6/GTB | Multiple | Lynch syndrome/hereditary nonpolyposis colorectal cancer (HNPCC) | PDA (medullary variant), PanNETs, cholangio |
| MEN1 | 11 | Multiple endocrine neoplasia type 1 (MEN1) | PanNET |
| NF1 | 17 | Neurofibromatosis type 1 (NF1) | PanNET |
| P16/CDKN2A | 9 | Familial atypical multiple mole melanoma syndrome (FAMMM) | PDA |
| PALB2 (FANCN) | 16 | Familial breast cancer | PDA |
| PRKAR1A | 17 | Carney complex | Acinar |
| PRSS1 , SPINK1 | 7, 5 | Hereditary pancreatitis | PDA |
| STK11/LKB1 | 19 | Peutz-Jeghers syndrome (PJS) | PDA, IPMN |
| TSC1 , TSC2 | 9, 16 | Tuberous sclerosis complex (TSC) | PanNET |
| VHL | 3 | von Hippel-Lindau syndrome (VHL) | SCA, PanNET |
Germline mutations in genes in the Fanconi anemia pathway, which encode proteins involved in repair of DNA cross-linking damage, have been strongly associated with familial pancreatic cancer. , Germline mutations in BRCA2 , a crucial component of the Fanconi anemia pathway, result in increased risk of breast, ovarian, pancreatic, and other cancers. , In addition to germline BRCA2 mutations, germline alterations in other genes in the Fanconi pathway also play important roles in familial pancreatic cancer. The gene PALB2 (also known as FANCN ) encodes a protein that interacts with the BRCA2 protein, and germline mutations in PALB2 account for a subset (approximately 3%) of patients with familial pancreatic cancer. , , Germline mutations in other Fanconi pathway genes, including BRCA1 , also predispose to familial pancreatic cancer. ,
In addition to syndromes associated with germline mutations in Fanconi pathway genes, increased risk of pancreatic cancer is also a key feature in several other inherited syndromes (see Table 35.1 ). Germline mutation in p16/CDKN2A cause familial atypical mole melanoma syndrome (FAMMM); patients with this syndrome have an increased risk of melanoma (with multiple nevi and atypical nevi) and pancreatic cancer. Individuals with germline mutations in p16/CDKN2A without a history of melanoma also have an increased risk of developing pancreatic cancer, and smoking appears to significantly increase risk in gene carriers. Germline mutations in STK11/LKB1 are associated with Peutz-Jeghers syndrome (PJS), a syndrome associated with gastrointestinal hamartomas and pigmented macules on the lips and buccal mucosa, as well as cancer predisposition, including an increased risk of pancreatic cancer. , Pancreatic ductal adenocarcinomas in these patients show somatic loss of the wild-type STK11/LKB1 allele, indicating the importance of biallelic inactivation of the gene for carcinoma development in these patients. Patients with hereditary pancreatitis are also at a markedly increased risk of pancreatic cancer. , In contrast to germline mutations in tumor suppressor genes, the increased risk of cancer in these patients appears to be the result of repeated bouts of inflammation and repair, and importantly, the increased cancer risk is limited to the pancreas.
Hereditary nonpolyposis colorectal cancer (HNPCC, or Lynch syndrome) is caused by germline mutations in hMSH2 , hMLH1 , hPMS1 , hPMS2 , or hMSH6/GTB , leading to defects in DNA mismatch repair (and thus microsatellite instability), as well as markedly increased risk of carcinomas of the colon and other sites. There is a slight, but real, increased risk of pancreatic cancer in patients with HNPCC. , Some, but not all, pancreatic cancers with microsatellite instability have a characteristic “medullary” morphology. Germline mutations in the ATM gene, which encodes a protein with roles in DNA damage response as well as cell cycle regulation, also rarely occur in familial pancreatic cancer kindreds. , , Although bi-allelic germline mutations in ATM cause ataxia-telangiectasia, a syndrome characterized by cerebellar ataxia, sensitivity to ionizing radiation, and increased frequency of multiple malignancies, germline heterozygous ATM mutations have been reported in approximately 2% of patients with familial pancreatic cancer. , ,
As noted earlier, these germline variants are important to recognize for two reasons. First, they have implications for other family members who may have a significantly increased risk of developing cancer. Decisions can be made on whether or not to screen for pancreatic and extrapancreatic neoplasms, and some patients with an extremely high risk of cancer and minimal pancreas function, such as cigarette smokers with familial pancreatitis, even chose prophylactic surgery. Second, cancers that arise in patients with some of these germline variants, particularly those that code for proteins in the Fanconi anemia and DNA mismatch repair pathways, are exquisitely sensitive to specific forms of treatment. , , Cancers with microsatellite instability respond particularly well to immunotherapy, whereas cancers with mutations in a gene coding for a protein in the Fanconi anemia are often responsive to poly (ADP-ribose) polymerase (PARP) inhibitors. ,
The exomes and even genomes of several large well-characterized series of ductal adenocarcinomas have been sequenced and somatic alterations clearly play a crucial role in tumorigenesis ( Table 35.2 ). The somatic alterations driving pancreatic tumorigenesis are remarkably uniform, with four main driver genes ( KRAS , TP53 , SMAD4 , and p16 / CDKN2A ). Superimposed on these common genetic drivers are less prevalent alterations in a longer list of oncogenes and tumor suppressor genes (see Table 35.2 ).
| Neoplasm | Gene | Chromosome | Alteration Prevalence | Mechanisms of Alteration |
|---|---|---|---|---|
| PDA | KRAS | 12 | 95% | Missense mutation |
| P16/CDKN2A | 9 | 95% | Missense mutation with LOH, homozygous deletion, promoter methylation | |
| TP53 | 17 | 75% | Missense mutation with LOH | |
| SMAD4/DPC4 | 18 | 55% | Missense mutation with LOH, homozygous deletion | |
| IOPN | PRKACA | 19 | 45% | Gene fusions |
| PRKACB | 1 | 55% | Gene fusions | |
| IPMN | KRAS | 12 | 80% | Missense mutation |
| RNF43 | 17 | 75% | Missense mutation or nonsense mutation with LOH | |
| GNAS | 20 | 60% | Missense mutation | |
| P16/CDKN2A | 9 | Increase with dysplasia | Missense mutation with LOH, homozygous deletion, promoter methylation | |
| TP53 | 17 | only in HGD/carcinoma | Missense mutation with LOH | |
| SMAD4/DPC4 | 18 | only in HGD/carcinoma | Missense mutation with LOH, homozygous deletion | |
| PIK3CA | 3 | 10% | Missense mutation | |
| MCN | KRAS | 12 | 80% | Missense mutation |
| RNF43 | 17 | 40% | Missense mutation or nonsense mutation with LOH | |
| P16/CDKN2A | 9 | Increase with dysplasia | Missense mutation with LOH, homozygous deletion, promoter methylation | |
| TP53 | 17 | only in HGD/carcinoma | Missense mutation with LOH | |
| SMAD4/DPC4 | 18 | only in HGD/carcinoma | Missense mutation with LOH, homozygous deletion | |
| SCA | VHL | 3 | 50% | Missense mutation with LOH |
| PanNET | MEN1 | 11 | 45% | Missense mutation with LOH |
| DAXX/ATRX | 6/X | 45% | Missense or nonsense mutation with LOH | |
| mTOR pathway | Multiple | 15% | Multiple | |
| VHL | 3 | 25% | Promoter methylation | |
| SPN | CTNNB1 | 3 | 95% | Missense mutation |
| ACC | cMYC | 8 | 50% | Amplified |
| BRAF | 7 | 25% | Rearrangements | |
| APC | 5 | 15% | Inactivating/truncating mutation with LOH | |
| CTNNB1 | 3 | 5% | Missense mutation | |
| PB | Imprinted locus | 11 | 85% | Loss of heterozygosity |
| CTNNB1 | 3 | 55% | Missense mutation | |
| APC | 5 | 10% | Inactivating/truncating mutation with LOH | |
| Cholangio | P16/CDKN2A | 9 | 85% | Missense mutation with LOH, homozygous deletion, promoter methylation |
| TP53 | 17 | 50% | Missense mutation with LOH | |
| SMAD4/DPC4 | 18 | 50% | Missense mutation with LOH, homozygous deletion | |
| FGFR2 | 10 | 45% | Fusions, often with PPHLN1 | |
| IDH1/IDH2 | 2/15 | 20% | Missense mutation | |
| PIK3CA | 3 | Variable | Missense mutation | |
| Chromatin remodeling genes (ARID1A, BAP1 , PBRM1 , etc.) | Multiple | Variable | Missense mutation with LOH | |
| KRAS | 12 | Variable | Missense mutation | |
| GBC | ||||
| P16/CDKN2A | 9 | 75% | Missense mutation with LOH, homozygous deletion, promoter methylation | |
| TP53 | 17 | 60% | Missense mutation with LOH | |
| BRAF | 7 | 30% | Missense mutation | |
| KEAP1 | 19 | 30% | Inactivating/truncating mutation with LOH | |
| SMAD4/DPC4 | 18 | 20% | Missense mutation with LOH, homozygous deletion | |
| PIK3CA | 3 | 10% | Missense mutation | |
| CTNNB1 | 3 | 10% | Missense mutation | |
| KRAS | 12 | Variable | Missense mutation | |
KRAS is the most commonly altered oncogene in ductal adenocarcinoma, with somatic KRAS mutations in more than 90% of cancers, clearly indicating that this gene is a driver of tumorigenesis in the pancreas. KRAS codes for a small GTPase that mediates downstream signaling from growth factor receptors, and somatic mutations in KRAS cluster in a few specific hotspots (most commonly in codon 12), confirming the role of KRAS as an oncogene. Mutations have been reported in other oncogenes in the same cell-signaling pathway (including BRAF ) in rare KRAS wild-type carcinomas. , , , Recently, germline mutations in the RABL3 gene, which encodes for a protein that regulates RAS prenylation, have been associated with hereditary pancreatic cancer.
Several frequently inactivated tumor suppressor genes have also been identified in ductal adenocarcinoma of the pancreas, including p16/CDKN2A , TP53 , and SMAD4 ; these genes also represent drivers in pancreatic tumorigenesis. , P16/CDKN2A is the most frequently altered tumor suppressor gene in ductal adenocarcinoma, with loss of p16 protein function in more than 90% of carcinomas. , Multiple genetic and epigenetic mechanisms underlie this loss of p16 protein expression, including intragenic mutation coupled with loss of the second allele, homozygous deletion of both copies of the gene, and promoter methylation. Loss of p16 results in cell cycle dysregulation, as this protein normally blocks cell cycle progression by preventing inactivation of Rb, another important cell cycle regulator. Immunolabeling for the p16 protein has been reported, but the labeling is often hard to interpret and therefore not useful in clinical practice.
TP53 , which encodes a protein with a pivotal role in the cellular stress response, is another key tumor suppressor gene in ductal adenocarcinoma of the pancreas, with somatic mutations reported in approximately 75% of cases. , These somatic mutations almost always occur through intragenic mutation followed by loss of the wild-type allele. Immunolabeling for the p53 protein can be useful, as overexpression or complete loss of labeling correlates with the presence of TP53 gene mutations.
Somatic inactivation of SMAD4 also occurs frequently in invasive ductal adenocarcinomas, with homozygous deletion or intragenic mutation followed by loss of the wild-type allele occurring in approximately 55% of carcinomas. , , The Smad4 protein mediates cellular signaling downstream of the transforming growth factor beta (TGF-β) receptor, and less frequent somatic mutations occur in other components of the same signaling pathway, including TGFBR2 and ALK5 . , , , Immunolabeling for the Smad4 protein can be useful, as complete loss of labeling correlates with the presence of SMAD4 gene mutations. Of all of the genes targeted in pancreatic cancer, loss of SMAD4 has been the most closely tied to poor prognosis.
In addition to these known driver genes, somatic mutations are present at low prevalence in numerous other genes in pancreatic ductal adenocarcinoma. The role of these genes as drivers or passengers is more difficult to establish when only a few mutations are identified; however, some are likely to play a functional role and can be important in the individual patients in whose cancers they are targeted. For example, ARID1A and other chromatin-regulating genes, such as MLL2 and MLL3 , are mutated in a minority of pancreatic cancers, but these mutations may have prognostic significance and some may be therapeutically targetable. , ,
In addition to small somatic mutations, large chromosomal gains and losses as well as complex chromosome rearrangements also occur frequently in ductal adenocarcinoma. Some alterations target known driver genes, while many others may be a manifestation of widespread chromosomal instability. Chromothripsis, the cataclysmic shattering and abnormal reassembling of selected chromosomes, has also been described in pancreatic cancer. Notta et al. have suggested that chromothripsis may lead to abrupt progression of disease; however, most chromothriptic events do not target known driver genes.
The expression of mRNAs and of proteins also changes as neoplastic cells progress to invasive carcinoma, and the patterns of gene expression have been used to define subtypes of ductal adenocarcinoma. , Although several studies establishing these subtypes were confounded by the inclusion of mRNAs expressed in contaminating nonneoplastic stroma, at least two subtypes appear to be reproducible. The “squamous” and “basal-like” subtypes correlate well with squamous differentiation by light microscopy and with decreased GATA6 expression. , The second subtype, the “classical” subtype, is associated with a better prognosis. , , , Rather than relying on a single marker, a panel of immunomarkers that includes GATA6, p40 (deltaN-p63), cytokeratin 5 (CK5), and claudin 18 may be most useful in separating these subtypes.
Ductal adenocarcinomas also express microRNAs, small noncoding RNAs that regulate gene expression. Differential expression of multiple microRNAs has been reported in ductal adenocarcinoma compared with nonneoplastic pancreatic tissue.
Although pancreatic intraepithelial neoplasia (PanIN) was recognized histologically for years, data on the genetic alterations in PanIN lesions firmly established them as noninvasive precursors to ductal adenocarcinoma. , Just as PanINs acquire increasing cytological and architectural abnormalities with increasing grade, they also acquire the same genetic alterations that occur in invasive carcinoma. Although some molecular changes occur early and are present in low-grade PanINs, other alterations are limited to severely dysplastic and invasive lesions. KRAS mutation and loss of p16 expression are early events that are present in even low-grade PanINs. When extremely sensitive techniques are employed, KRAS mutations are present in more than 90% of even low-grade PanINs, suggesting that KRAS mutations may represent a key initiating step in pancreatic neoplasia. In contrast, loss of Smad4 and TP53 mutation are late events, occurring only in high-grade PanIN and invasive carcinoma. Alterations other than those in known driver genes also occur in PanINs; telomere shortening is one of the most common early events in pancreatic tumorigenesis, with shortening in approximately 90% of low-grade PanINs. Early telomere shortening may make the cells susceptible to chromosomal fusion and anaphase bridges, which may produce some of the chromosomal abnormalities observed in invasive pancreatic cancer.
In addition to expanding our knowledge of precursors to ductal adenocarcinoma, detailed study of somatic mutations in primary tumors and metastases has deepened our understanding of the process of clonal evolution within tumors, enabling the estimation of evolutionary time in tumors. , These studies suggest a time window of approximately 15 years between tumor initiation and the acquisition of metastatic ability, providing a broad time window for early detection and subsequent clinical intervention. ,
Knowledge of the genetic underpinnings of familial pancreatic cancer has direct clinical implications for pathologists in several ways. First, the pathologist will be the first to recognize subtypes of pancreatic cancer (such as “medullary” carcinoma) that are associated with specific familial syndromes. When a pathologist diagnoses a medullary carcinoma, he or she should suggest clinical germline testing. The reality, however, is that there is not a specific histological phenotype for cancers caused by germline variants, and universal consideration of germline testing is therefore now recommended for all patients with pancreatic cancer. Second, as noted earlier, some familial syndromes have specific treatment implications, highlighting the importance that they be clinically recognized at the time of diagnosis. For example, carcinomas in patients with germline alterations in the Fanconi anemia pathway (BRCA2 , BRCA1 , PALB2) are exquisitely sensitive to drugs that target their specific DNA repair defect, such as PARP inhibitors. , Carcinomas in patients with HNPCC also possess specific recommendations for treatment: tumors with microsatellite instability are resistant to fluorouracil-based chemotherapy but extremely sensitive to immune checkpoint blockade. , , Finally, clinical recognition of familial pancreatic cancer syndromes will lead to increased screening of at-risk patients. The screening for select extrapancreatic neoplasms can be guided by the specific germline variant identified.
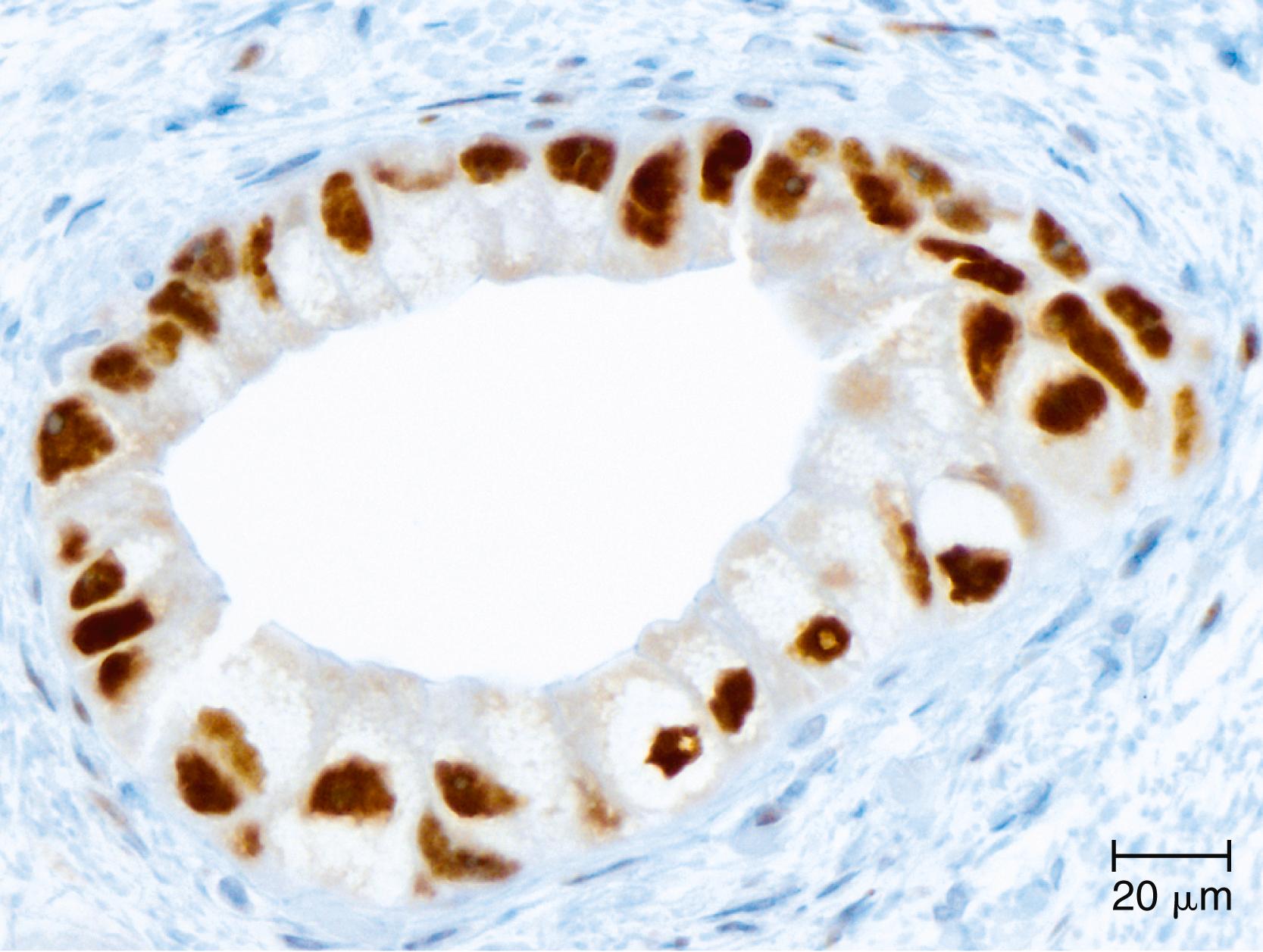
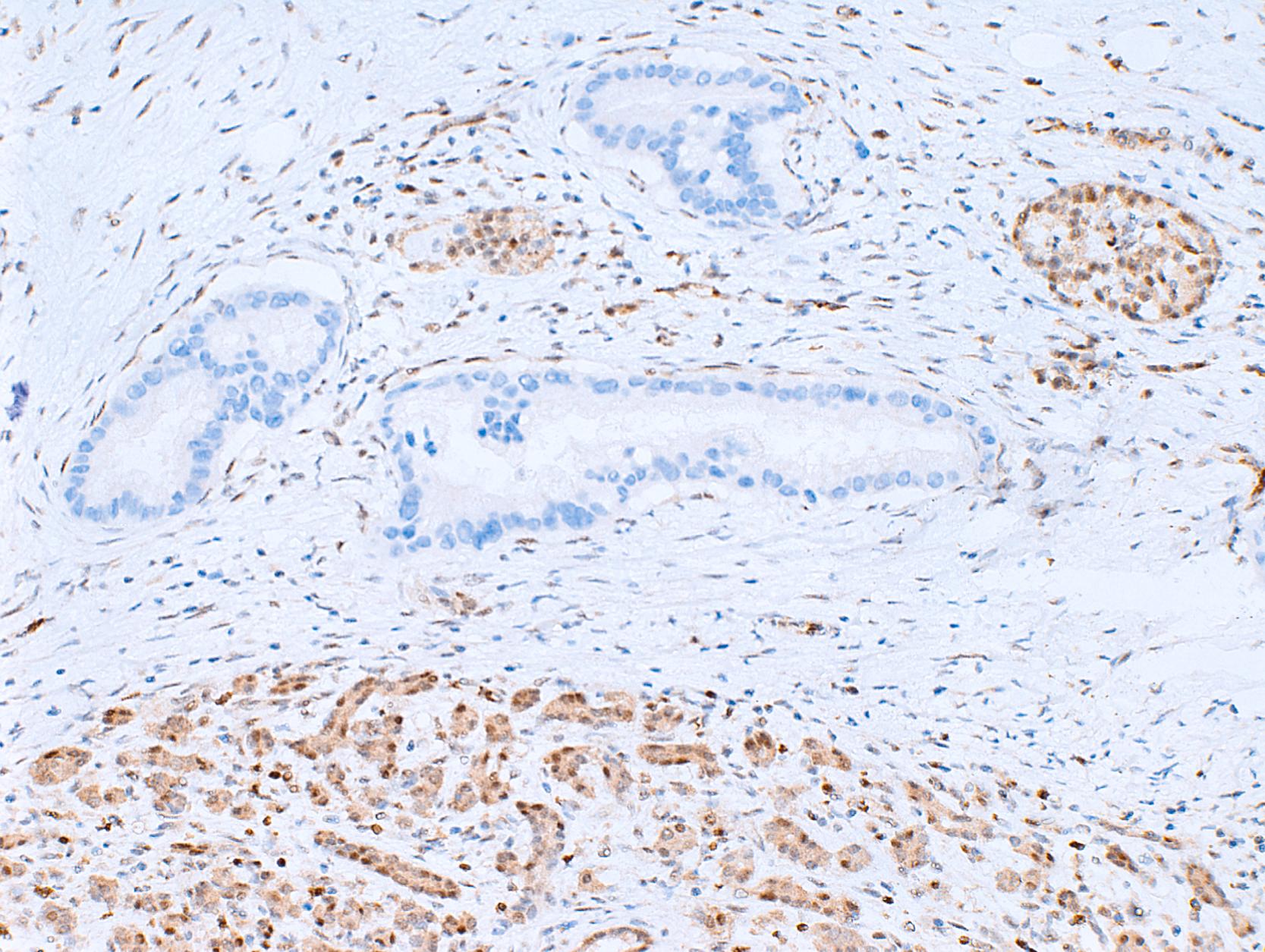
Somatic alterations in sporadic pancreatic cancer also impact pathology practice. Immunohistochemistry can be used to demonstrate protein expression alterations indicative of characteristic gene mutations. For example, TP53 mutations result in strong diffuse or complete loss of p53 nuclear labeling by immunohistochemistry, providing a histological surrogate for the genetic alteration and a potential technique for the identification of neoplastic pancreatic cells ( Fig. 35.1 ). In addition, loss of Smad4 protein expression by immunohistochemistry is correlated with genetic alterations in the SMAD4 gene ( Fig. 35.2 ). This technique can be used to distinguish ductal adenocarcinoma (with loss of Smad4) from atypical, but reactive, nonneoplastic pancreatic diseases (with retention of Smad4), and to suggest that a metastatic adenocarcinoma is of pancreatobiliary origin. , Although extremely promising, somatic mutations have, to date, not had a great impact on guiding therapy. The two largest trials of personalized therapy based on somatic mutations, IMPACT and COMPASS, both proved disappointing. ,
In addition, molecular studies interrogating genetic alterations also demonstrate clinical utility. For example, molecular analyses of KRAS , TP53 , and SMAD4 can be used to supplement morphological diagnosis in cytology specimens to increase sensitivity of fine-needle aspiration. In addition, with the continuous development of targeted therapies, molecular studies will likely serve a critical role in determining eligibility for therapy.
There are multiple uncommon morphological variants of ductal adenocarcinoma. While some share the molecular features of ductal adenocarcinoma, others harbor unique genetic alterations, and these entities have clinical implications.
Adenosquamous carcinoma has molecular features similar to those of ductal adenocarcinoma, with prevalent alterations in KRAS , p16/CDKN2A , SMAD4 , and TP53 , but it is an aggressive neoplasm with a poor prognosis. As noted earlier, GATA6 expression is decreased in these carcinomas compared with carcinomas of the “classical” subtype. , , It has been suggested that adenosquamous carcinomas respond best to platinum-based chemotherapies.
Colloid carcinoma (mucinous noncystic carcinoma) has a better prognosis than pure ductal (tubular) adenocarcinoma of the pancreas. These carcinomas almost always arise in association with an intestinal-type intraductal papillary mucinous neoplasm (IPMN), and, not surprisingly, the pattern of genetic alterations in colloid carcinomas parallels that of IPMNs. Colloid carcinomas, in addition to frequently harboring mutations in the genes targeted in ductal adenocarcinomas ( KRAS , TP53 etc.), also often harbor mutations in GNAS , an oncogene frequently altered in intraductal papillary mucinous neoplasms.
Medullary carcinoma , another variant with a better prognosis than ductal adenocarcinoma, has a high prevalence of microsatellite instability and lacks somatic mutations in KRAS , though oncogenic BRAF mutations have been reported. , As noted earlier, these cancers with microsatellite instability may be exquisitely sensitive to immunotherapy. ,
Undifferentiated carcinoma is an aggressive neoplasm with a poor prognosis; in addition to prevalent KRAS mutations, these carcinomas also exhibit frequent loss of E-cadherin protein expression, providing a possible explanation for the carcinoma’s poorly cohesive morphology ( Fig. 35.3 ). , Undifferentiated rhabdoid carcinomas have been described, and the monomorphic variants of this carcinoma have SMARCB1 loss. ,
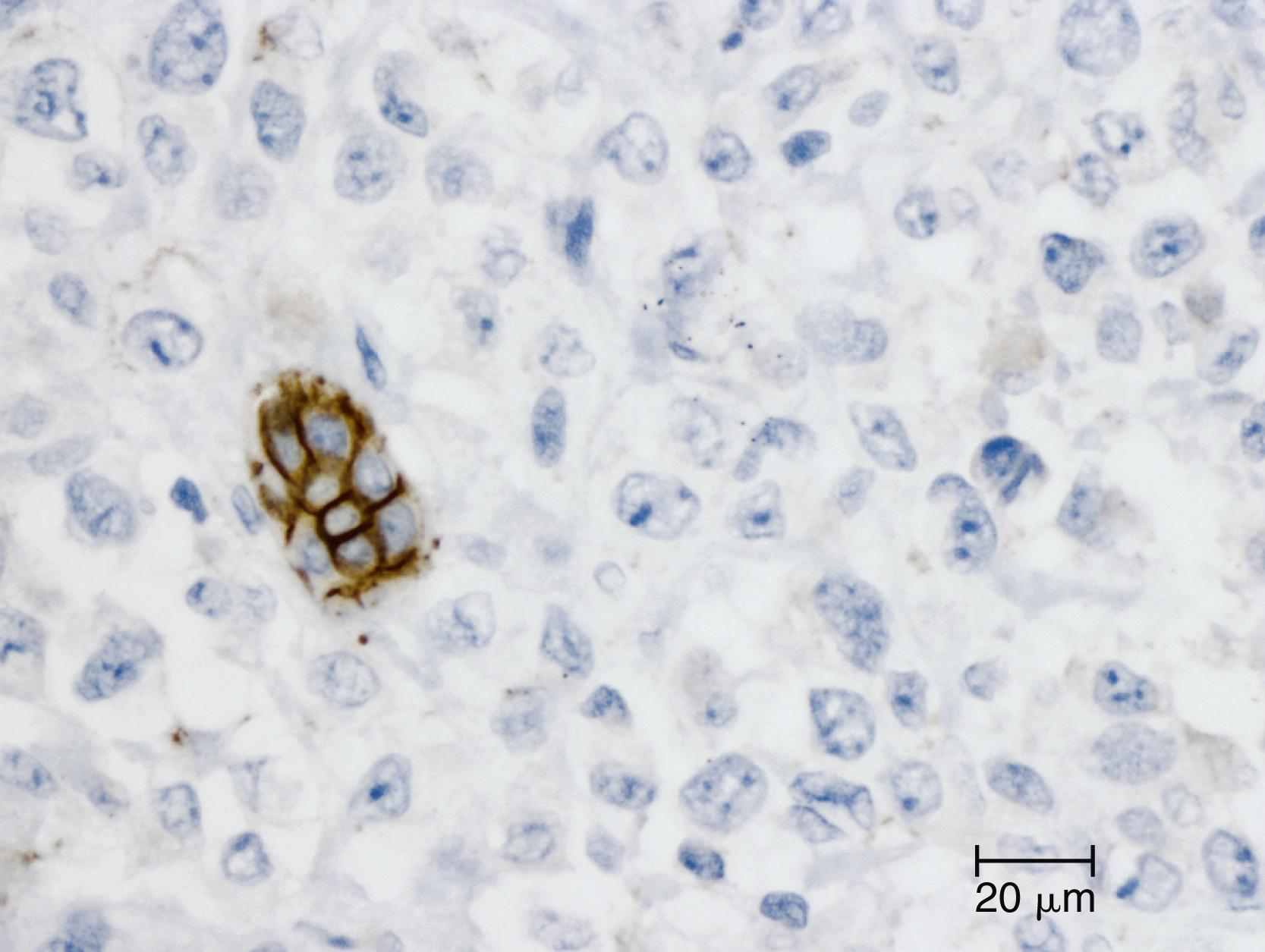
Undifferentiated carcinoma with osteoclast-like giant cells , another aggressive carcinoma with poor prognosis, contains two distinct cell populations: while the neoplastic mononuclear cells contain frequent KRAS mutations and p53 overexpression, the osteoclast-like giant cells are nonneoplastic and contain only mutant KRAS from phagocytized neoplastic mononuclear cell DNA. , Indeed, recent whole exome sequencing has highlighted that undifferentiated carcinomas with osteoclast-like giant cells are genetically very similar to invasive ductal adenocarcinomas.
Thus careful integration of molecular findings with histopathology has helped explain some of the morphological variants of ductal adenocarcinoma and is forming the basis for a new classification of pancreatic neoplasia, one that integrates molecular genetics together with tumor histopathology. In contrast to those in other organs, molecular findings have tended to support, and not supplant, existing morphological classifications.
Intraductal papillary mucinous neoplasms (IPMNs) are large (≥1.0 cm), noninvasive mucin-producing precancerous lesions that arise in the larger pancreatic ducts. Over time, some IPMNs progress to invasive carcinoma.
IPMNs occur rarely in inherited cancer predisposition syndromes (see Table 35.1 ). , For example, Skaro et al. identified germline mutations in 7% of 315 patients with an IPMN. These included germline mutations in ATM , BRCA2 , MSH6 , BUB1B , PALB2 , and other Fanconi anemia pathway genes. A 3-day-old infant with an IPMN was reported to have germline mutations in the SKIL and TUBB5 genes, and TUBB5 codes for a protein in the Robo-Slit pathway. These germline mutations in patients with IPMNs are important for the same reasons given earlier for germline mutations in patients with invasive carcinoma. In addition, patients with a surgically resected IPMN with a germline mutation are more likely to have concurrent invasive pancreatic carcinoma than are IPMN patients without a germline mutation.
Many of the genes mutated in IPMNs are the same genes commonly mutated in pancreatic ductal adenocarcinoma (see Table 35.2 ). , These include somatic mutations in KRAS , p16 / CDKN2A , TP53 , and SMAD4 . , , , KRAS mutations occur early, in lesions with low-grade dysplasia, p16 / CDKN2A an intermediate event, and TP53 and SMAD4 inactivation are late events. , , In addition to alterations in genes frequently altered in ductal adenocarcinoma, IPMNs also contain mutations in genes unique among pancreatic neoplasms (see Table 35.2 ). Somatic mutations in GNAS have been reported in approximately 60% of IPMNs. , , , Intriguingly, the GNAS mutations in IPMNs all occur in a previously described oncogenic hotspot (codon 201), providing strong evidence for their functional importance in IPMNs. Mutations in GNAS are most prevalent in intestinal-type IPMNs but occur in most other histological subtypes, with the possible exception of oncocytic IPMNs. , , In IPMNs with associated invasive carcinoma, GNAS mutations can be identified in both the noninvasive and invasive components, providing further genetic evidence that IPMNs give rise to invasive carcinoma. , ,
The gene RNF43 is also frequently altered in IPMNs: up to 75% of IPMNs contain somatic mutations in RNF43 , which encodes an E3 ubiquitin ligase. , , The majority of these alterations are nonsense substitution mutations, leading to the insertion of stop codons and thus loss of function of the encoded protein. This mutation pattern, along with frequent loss of heterozygosity at the RNF43 locus on chromosome 17q, provides strong evidence that RNF43 is a tumor suppressor gene in IPMNs.
In addition, approximately 10% of intestinal-type IPMNs contain somatic mutations in PIK3CA , the catalytic component of a crucial cell signaling pathway known to be an oncogene in several other tumor types, and some somatic mutations in IPMNs occur at previously described oncogenic hotspots in PIK3CA . , , Other less commonly targeted genes include STK11/LKB1 (the locus of PJS), EGFR , and ERBB2 .
The neoplastic cells of intraductal oncocytic papillary neoplasms (IOPNs) have a distinctive morphology with abundant eosinophilic cytoplasm, and they have been an enigma, as they lack the genetic alterations commonly found in other IPMNs. Singhi et al. recently solved this mystery when they discovered that almost all IOPNs harbor recurrent PRKACA or PRKACB gene rearrangements. These included ATP1B1 - PRKACB , DNAJB1 - PRKACA , and ATP1B1 - PRKACA fusion genes, the same fusion genes reported in fibrolamellar carcinomas of the liver. These fusion genes were not detected in other neoplasms of the pancreas but were detectable in cyst fluid aspirated from IOPNs.
Genetic mechanisms other than somatic mutation also play a role in the development of IPMNs. Promoter hypermethylation occurs in several genes in IPMNs, including p16/CDKN2A hypermethylation, and the expression of a number of microRNAs is altered in IPMNs. ,
These genetic data provide strong evidence for the role of IPMNs as a precursor to invasive adenocarcinoma, a role strongly supported by clinical data as well. Knowledge of the genetic drivers of IPMNs can be utilized in the development of diagnostic assays. For example, mutations shed from neoplastic cells can be detected in aspirated cyst fluid, indicating that molecular analyses of cyst fluid may be useful as a preoperative diagnostic tool in pancreatic cysts. , In specific, more than 95% of IPMNs contain a somatic mutation in either KRAS or GNAS , suggesting that a molecular assay involving these two genes would be a highly sensitive assay for the identification of IPMNs. , , Similarly, as described later, VHL gene mutations in cyst fluid are highly suggestive of a serous cystadenoma. It is likely that a combination of markers, one that includes clinical information as well as genetic analyses, will be the most useful in preoperative cyst classification.
Genetic analyses have also provided novel insights into the heterogeneity of IPMNs. Co-occurring IPMNs and invasive cancers sometimes have distinct somatic mutations, establishing that while IPMNs can progress to invasive cancer, not all invasive cancers arise from the IPMN that they happen to be adjacent to. Complicating things even more, IPMNs appear to be genetically very heterogeneous and some even have a polyclonal origin. This heterogeneity needs to be considered when interpreting tests of cyst fluid.
Mucinous cystic neoplasms (MCNs) are mucin-producing epithelial neoplasms that do not communicate with the duct system. They are characterized by a distinctive ovarian-type stroma.
There is no known genetic predisposition or association with particular genetic syndromes for mucinous cystic neoplasms.
The exomes of MCNs have been sequenced, and, like IPMNs, many of the same genes targeted in ductal adenocarcinomas (KRAS , p16 / CDKN2A , TP53) are also targeted in MCNs (see Table 35.2 ). , Somatic mutations in KRAS oncogenic hotspots are common in MCNs, with mutation prevalence that correlates with degree of dysplasia (approximately 30% KRAS mutation prevalence in MCNs with low-grade dysplasia vs ∼80% KRAS mutation prevalence in MCNs with high-grade dysplasia or with an associated invasive carcinoma). , , KRAS mutations are also identified in areas of MCNs that are lined by flat, nonmucinous epithelium. Somatic mutation of TP53 as well as p53 protein overexpression are limited to MCNs with high-grade dysplasia. , Loss of Smad4 protein expression, indicating somatic mutation in the SMAD4 gene, has been reported primarily in invasive carcinomas associated with MCNs; this loss of Smad4 expression occurs in only 14% of MCN-associated invasive carcinomas, a far lower prevalence than in invasive carcinomas not associated with MCNs. , The p16/CKDN2A gene is also infrequently altered in MCNs: somatic mutation has been reported in a neoplasm with high-grade dysplasia, and hypermethylation of the p16/CKDN2A promoter occurs in approximately 15% of MCNs. , ,
These findings suggest a stepwise genetic progression in MCNs from low-grade dysplasia to invasive carcinoma, in which KRAS mutation is an early event and loss of Smad4 is a late event. The dysplasia can progress at different rates in different cyst locules, and so the sampling of one locule cannot be used to define the degree of dysplasia in all locules ( Fig. 35.4 ).
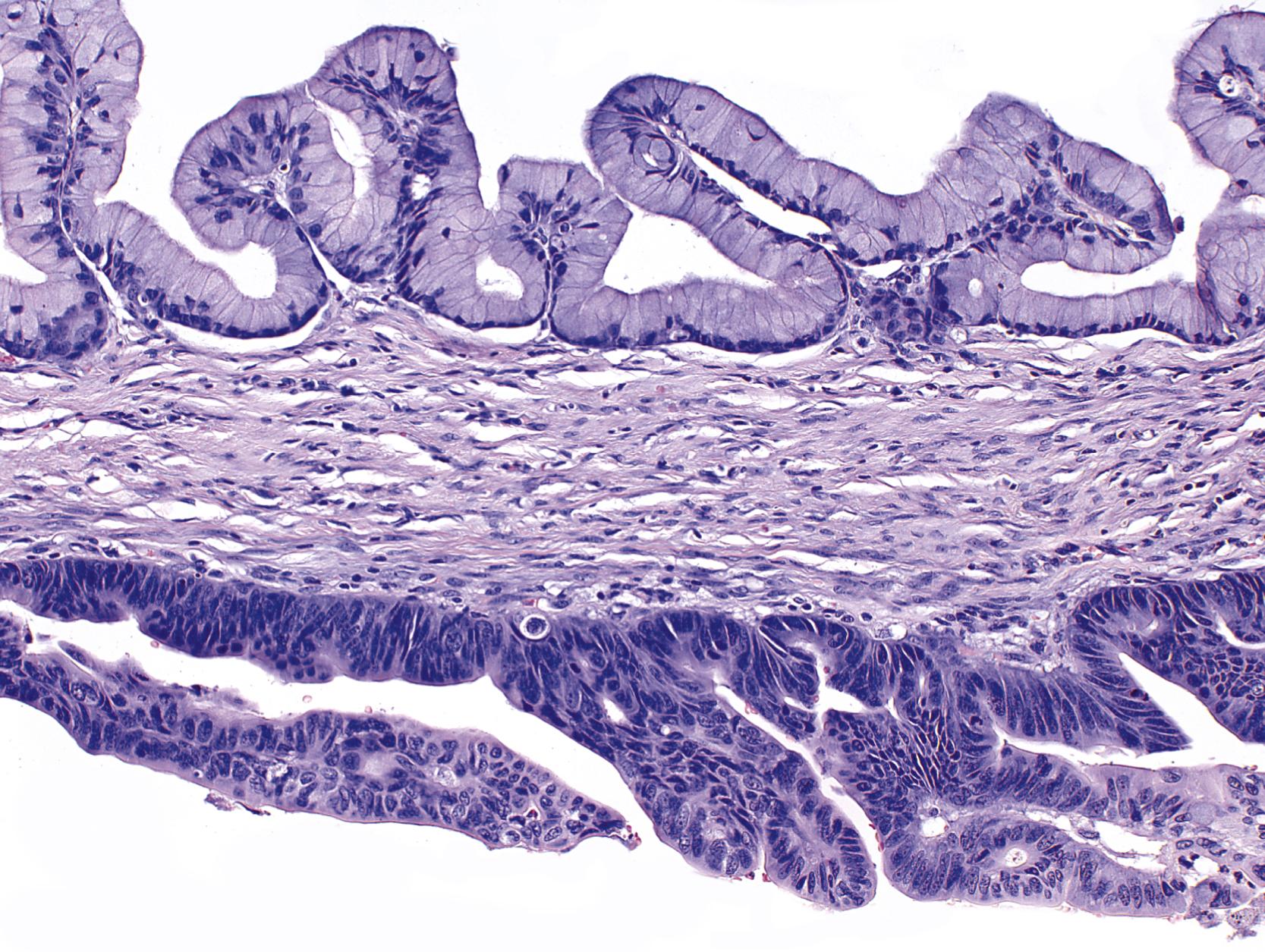
In addition to mutations in genes frequently altered in ductal adenocarcinoma, MCNs also share alterations in genes unique to mucin-producing cyst-forming neoplasms of the pancreas (see Table 35.2 ). Like IPMNs, MCNs often contain alterations in RNF43 : approximately 40% of MCNs harbor somatic mutations in RNF43 , and these mutations are enriched for nonsense substitutions. PIK3CA can also be somatically altered in MCNs.
Molecular studies have also provided insight into the biphasic nature of MCNs. Gene expression studies suggest different expression profiles in the epithelial and stromal components, with activation of the Notch pathway ( JAG1 and HES1 ) in the epithelial component and activation of estrogen metabolism ( STAR and ESR ) and markers of primordial germ cells in the stromal component. , The genetic or epigenetic basis for these differences remains unclear. In exceedingly rare mixed malignant neoplasms with both epithelial and high-grade “sarcomatous” components, studies of loss of heterozygosity suggest a monoclonal origin for the two components with subsequent genetic and morphological divergence.
As in other pancreatic neoplasms, immunohistochemical labeling for p53 and Smad4 can facilitate identification of neoplastic cells in MCNs, though the prevalence of alterations in these genes is far lower in MCNs than in ductal adenocarcinoma. Like IPMNs, identification of mutations in DNA from cyst fluid (including mutations KRAS and RNF43 ) represents a promising preoperative diagnostic test to determine the likelihood of a preinvasive mucin-producing cyst. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here