Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Genome-wide association studies (GWAS) on neuropsychiatric disorders have been expanding to increasingly large populations and are now including multiethnic groups.
These studies are aimed at identifying specific single nucleotide polymorphisms (SNPs) that may be associated with specific diseases at high frequencies.
Characteristic high-frequency SNPs seem to occur in schizophrenia, bipolar disorder (BD), substance use, major depression disorder (MDD), posttraumatic stress disorder (PTSD), Alzheimer disease (AD), and autism.
Characteristic SNPs found for each disorder occur either in exons or introns.
These SNPs occur in or near genes that encode a variety of proteins, which are receptors, channel proteins, transcription factors, central nervous system developmental proteins, and proteins involved in metabolism, especially fatty acid and carbohydrate metabolism.
Many of these SNPs appear to be unique to each disease type, but there are overlaps such as SNPs involved with calcium channel proteins in both schizophrenia and bipolar disorder.
Several GWAS have uncovered hitherto unknown feedback pathways involved in disease causation and suggest new therapeutic modalities for these diseases.
Psychiatric and substance use disorders are extremely prevalent worldwide ( ). Many of these disorders are highly disabling and associated with many physical comorbidities; they also contribute substantially to global morbidity and mortality ( ). Mental health conditions impair productivity and interpersonal functioning and are associated with significant psychological and financial costs. In view of the seriousness of these conditions, current epidemiologic studies integrating data from multiple levels (e.g., psychosocial, neurobiological, genomic) to identify risk and protective factors are needed to identify points prior to the onset of such disorders in which preventative action could be focused.
Twin and family studies have demonstrated that there are both genetic and environmental components in the inheritance of psychiatric and substance use disorders ( ). Twin studies provide an estimation of a trait’s heritability in a population (i.e., what proportion of phenotypic variation is due to genetic variation underlying the trait). Twin studies accomplish this by comparing trait similarity between monozygotic twins, who share all of their genetic variation, with dizygotic twins, who share (on average) half of their genetic variation. Since measures of heritability are a function of the specific population, the estimates can vary among populations, age, sex, ancestry, and culture.
The search for specific risk and protective loci began with linkage studies, which localized chromosomal regions of interest transmitted by affected individuals in large extended families. While these linkage studies provided the first clues toward many specific chromosomal regions of interest, and in some cases genes of interest, early linkage studies lacked the resolution required to identify specific genetic variants. In addition, the resources required to study large pedigrees of affected individuals made the scaling up of genetic studies of psychiatric illness challenging. Thus the field moved away from large family-based linkage studies and on to candidate gene association studies, which compare a specific candidate gene (or specific candidate loci within genes) among affected and unaffected individuals. Candidate genes or variants were historically chosen based on prior knowledge of their involvement in specific biological pathways or systems, often as a follow-up of promising genes in linkage regions. For example, genes that are part of the dopaminergic system (e.g., dopamine receptor, DRD2 ) are considered candidate genes for several mental illnesses, at least in part because of the role of dopamine in the reward pathway. While the candidate gene strategy has been successful in some studies, this approach was largely limited by the scope of our understanding of human biology, publication biases, and generally small (statistically underpowered) samples typically conducted during the 1990s and 2000s, leading to a large number of nonreplicable genetic association findings ( ).
In part due to the limitations of candidate gene studies, but also due to advances in genotyping technologies that made genome arrays accessible to researchers, the field turned to genome-wide association studies (GWAS) that could be conducted in related or unrelated individuals and, importantly, were hypothesis free. That is, they did not require prior understanding of the biological mechanisms underlying a given disorder but instead scanned the entire genome for variants that differed significantly among individuals with and without a particular disorder or trait of interest.
The basic object of this method in its simplest form is to sequence the genomes of large numbers of individuals (see Chapter 80 ) or to determine chromosomal changes in a group who have a particular disease and a second set of individuals who do not have the disease (control group). These sequences or chromosomes are then analyzed for single nucleotide polymorphisms (SNPs) at specific genomic sites or for chromosomal deletions, duplications, etc. Then the ratio of the number of individuals who have SNPs of chromosomal changes and disease to the number of individuals who have SNPs or chromosomal changes but no disease is computed. This is divided by the ratio of the number of individuals who do not have SNPS but have disease to number of individuals who do not have SNPs and have no disease. This quotient is termed the odds ratio. The statistical significance of the odds ratio is computed using appropriate statistical tests such as the chi-square test. The p value for this computation is usually on the order of 5 × 10 -8 ; p values less than this value indicate statistical significance (i.e., the SNP or chromosomal pattern is unique to the disease). These p values can then be plotted (often as bar graphs) versus the specific SNPs or chromosomal changes identified for the specific disease. In these plots, -log of the p value is plotted on the y -axis, and the corresponding individual genes with significant SNPs or chromosome numbers on which changes occur are plotted on the x -axis. These plots are referred to as Manhattan plots because they resemble the Manhattan skyline in New York City, New York.
However, an early GWAS conducted by the Wellcome Trust Case Control Consortium quickly showed the field that this new method for novel gene discovery would prove challenging, particularly for complex, multifactorial, and psychiatric traits. A decade of mostly unsuccessful GWAS taught researchers that extremely large sample sizes would be necessary to detect the subtle genetic influences on psychiatric illnesses. Over the past several years, researchers have joined forces in forming large consortia and biobanks to compile the sample sizes needed for gene discovery in psychiatric and behavioral conditions. For example, the Psychiatric Genomics Consortium (PGC), iPSYCH, the UK Biobank, 23andMe, GSCAN, and most recently a US Department of Veterans Affairs effort, the Million Veterans Project (MVP). In the past few years, several landmark studies from these consortia and biobanks have been published in the area of psychiatric and substance use genomics. Additionally, as robust and replicable genomic risk factors for psychiatric disorders emerge, it becomes crucial to understand the biological mechanisms underlying these statistical associations and important interactions with aspects of the psychosocial environment. Also of note, the largest GWAS have disproportionately focused on cohorts of European descent. This European bias is not unique to psychiatric genetics research but systemic within the GWAS literature. Although the proportion of non-Europeans included in analyses has since been increasing, this remains a critical issue that the field must address. In the next section we discuss some of the latest findings in key psychiatric and substance use disorders.
Schizophrenia affects ∼0.6% to 1% of the population worldwide. It is a chronic and debilitating neuropsychiatric syndrome, which can include symptoms such as hallucinations, delusions, disorganized thought and speech, as well as deficits in social interaction and motivation (negative symptoms). Most of the variability in liability to illness is attributable to genetic factors, with ∼80% heritability estimated by twin studies ( ). While rare genetic variants play a role in the underlying liability, most of the currently explained liability is harbored in common variation. The past decade has seen the successes of psychiatric GWAS abound, including the first definitive demonstration of polygenic influences on schizophrenia risk and its shared basis with bipolar disorder, and ever-increasing numbers of robustly associated, replicated SNP associations culminating in the identification of 108 physically distinct risk loci for schizophrenia, a number that has since grown to 145 ( ). This progress can be credited to collaborative enterprise on an unprecedented scale, as exemplified by the PGC, and a philosophy of data sharing that has enabled widespread meta-analysis and replication. Notable findings include dopamine receptor DRD2 and glutamatergic and synaptic plasticity genes (e.g., GRM3, GRIN2A, SRR, GRIA1 ), which are highly relevant to current pharmacologic treatment of schizophrenia and correspond with long-standing etiopathogenic theories. In addition, genes that encode voltage-gated calcium channel subunits ( CACNA1C, CACNB2, and CACNA1I ) have consistently been associated with schizophrenia in GWAS; interestingly, CACNA1C was first robustly associated with bipolar disorder, an illness known to have significant genetic overlap with schizophrenia, as discussed later. We now describe these three classes of proteins further. A summary of these proteins and their functions is presented in Table 73.1 .
| Protein/Chromosomal Changes | Function |
|---|---|
| D2 (dopamine) receptor | Nerve transmission, blocks cAMP pathways. |
| iGluR NMDA receptors: GRIA1 and Grin2A | Nerve conduction, direct channel proteins. |
| Serine racemase (SRR gene) | Catalyzes conversion of L- to D-serine that binds to NMDA receptor. |
| mGluR receptor | Coupled to G0 protein and initiate activation of phospholipase C, IP3, diacylglycerol, Akt. Causes calcium ion fluxes into cells and is involved in depolarization at synapse. Involved with synaptic plasticity. |
| CACNA1C | CaV1.2 calcium channel protein. Channel activity controlled by α-1 subunit. Interacts with calmodulin in its IQ domain. Promotes L-type currents at synapses. |
| CACNB2 | Calcium voltage-gated channel auxiliary subunit β |
| CACNA1I | α-1 subunit of a voltage-gated calcium channel involved with T (transient) currents in rhythmic depolarization. Found in thalamic reticular nucleus (TRN) involved in sleep. |
| Neurexin | Transmembrane synaptic structural protein; also called “handshake” protein because it is involved in holding the axonal and dendritic components of the synapse together. |
| ARC protein (activity-regulated cytoskeleton-associated protein) | Involved in synaptic plasticity mainly at NMDA receptor locations; implicated in learning and memory. |
| Fragile X mental retardation syndrome protein (FMRP) | Involved in synaptic plasticity. CGG repeats in the genome for this protein can cause diminished expression. |
| Chromosomal deletions | 15q13.3 and 1q21.1 |
| Chromosomal changes | 1q21.1, 2p16.3 (neurexin, see text), 3q29, 7q11.2, 15q13.3, 16p11.2, and 22q11.2 |
There are two types of receptors involved in synaptic transmission in neural pathways: ionotropic and metabotropic. The first type are ion channels that respond directly and rapidly to effectors resulting in immediate ion currents that are either excitatory (excitatory postsynaptic potentials [EPSPs]) or inhibitory (inhibitory postsynaptic potentials [IPSPs]), and the second type are more slowly responsive because they require activation of G proteins, which are proteins that become activated by binding guanosine triphosphate (GTP) in place of guanosine diphosphate (GDP) (see later; also see Chapter 77 ). These GTP-activated proteins, in turn, activate downstream targets on signal transduction pathways that ultimately end in the nucleus. These events result in the synthesis of proteins that affect the postsynaptic ion channels resulting in either EPSPs or IPSPs. Dopamine receptors are of the metabolomic type or G protein–coupled receptors (GPCRs).
There are at least five forms of the dopamine receptor that have strong sequence homology to one another but nonetheless differ significantly in sequences in specific domains. In fact, dopamine receptors are located and encoded by different genes. D1 receptor encoding is by the gene 5q31-q34. The genes encoding the D2 and D4 receptors are on chromosome 11, while the gene encoding the D3 receptor is located on chromosome 3 and the D5 receptor on chromosome 4. Activation of each of these receptors results in different regulation of ion channels and ion currents. Each type of dopamine receptor is associated with specific functions (i.e., D1: memory, attention, impulse control, regulation of renal function, locomotion; D2: locomotion, attention, sleep, memory, learning; D3: cognition, impulse control, attention, sleep; D4: cognition, impulse control, attention, sleep; D5: decision making, cognition, attention, renin secretion) ( ). Despite these differences all dopamine receptors are large, almost all α-helical proteins that span the membrane at seven locations, and they contain both amino and carboxyl termini as components of the extracellular domain of the receptor.
There are four G proteins downstream of the dopamine receptor: Gα,0; Gα,i; β; and ϒ. Gα,0 is an activator of downstream targets such as cyclic adenosine monophosphate (cAMP), while Gα,i inhibits this activation. β and ϒ proteins activate phospholipase C and induce the synthesis of inositol triphosphate (IP3), an important downstream target that activates further downstream targets such as induction of calcium ion release and activation of the all-important kinase, Akt (see Chapter 77 ). When activated by binding dopamine, the D2 receptor activates Gi in a signal transduction pathway that ultimately results in the inhibition of adenyl cyclase activity and activation of potassium channels.
Dopamine receptors, like epinephrine receptors (described in Chapter 24 ), exist on both sides of the synaptic cleft (i.e., presynaptic autoreceptors and postsynaptic receptors). The latter, when bound to dopamine in the presence of calcium ions from calcium channels, induce EPSPs or IPSPs. The former, presynaptic autoreceptors, downregulate the amount of dopamine released into the synaptic cleft and are therefore inhibitory.
These receptors are activated by the binding of L-glutamate or NMDA to these receptors. As found for dopamine receptors, glutamate receptors can be ionotropic (iGluR) or metabotropic (mGluR).
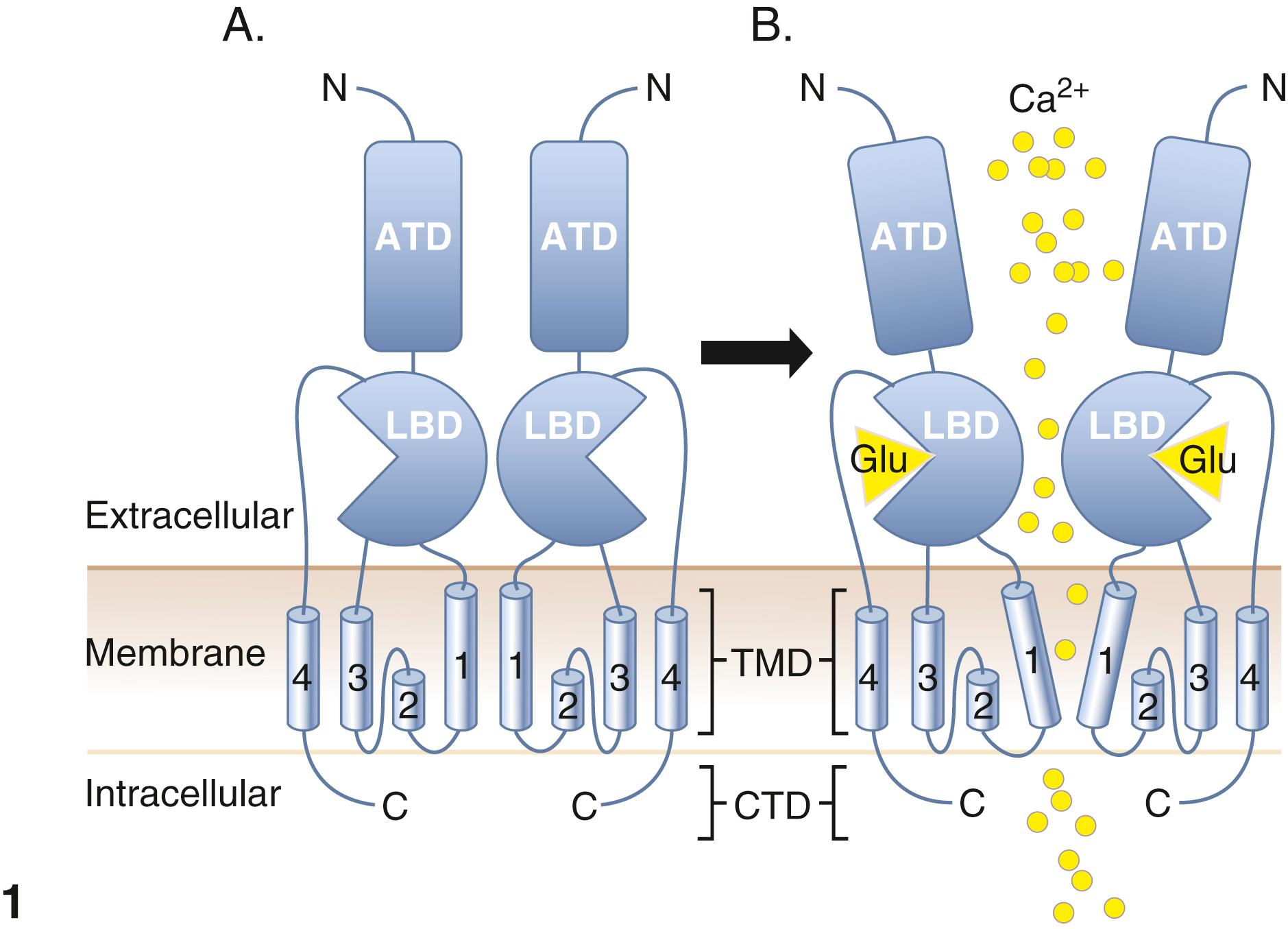
iGluR receptors, of which GRIA1 and Grin2A are members, are ion channels consisting of dimeric protein receptors with their amino termini containing the ligand (e.g., glutamate) binding domain on the extracellular surface connected to four transmembrane domains and relatively short intracytosolic carboxyl terminal domains. This complex is illustrated in Panel 1A of Figure 73.1 . Upon binding to the appropriate ligand, each of the two dimers is displaced such that they line an open cation channel allowing for an influx of a cation such as sodium, potassium, or calcium, as illustrated in panel 1B of Figure 73.1 . This influx leads to depolarization and action potentials. The fastest action potentials with this system are achieved with NMDA receptors in the retina and parts of the central and peripheral nervous systems. Signaling is regulated by transport proteins that clear the synapse of the ligand.
A strong ligand that binds to the NMDA receptor is D-serine, which is produced by the action of serine racemase on L-serine in the presence of vitamin B 6 , pyridoxal phosphate. Serine racemase is encoded by the SRR gene.
mGluR exists as 12 splice variants encoded by eight genes ( ). The 12 splice variants can be classified into three groups based on sequence homology, specific agonist binding, and downstream targets of the activated receptor protein. Thus mGluR1a and mGluR5a are placed in group 1. Both receptors are coupled to Gα protein and are activated when two glutamate molecules bind to each of two neighboring receptors. Activated mGluR1a initiates a series of signal transduction pathways (see Panel 2 of Fig. 73.1 ). These pathways result in activation of calcium mobilization from intracellular organelles or in activation of pathways concerned with cell survival (antiapoptosis) and growth.
Panel 2 of Figure 73.1 shows that activated receptor activates Gα protein that is involved probably through activation of the serine-threonine kinase, raf, that in turn activates MEK that activates MAP kinase, which, when activated, crosses the nuclear membrane and activates transcription factors such as fos that ultimately induces transcription of cell growth genes. This pathway is discussed more extensively in Chapter 77 .
G protein activation also results in activation of an important downstream target protein, phosphoinositide-3-hydroxy kinase (PI3K), that results in the synthesis of 1,4,5-triphosphoinositide (IP3). This molecule is critical for mobilization of calcium ions from intracellular stores shown for the endoplasmic reticulum (ER) in Figure 73.1B (left lower portion of the figure). The released calcium activates an important calcium binding protein, calmodulin, discussed later, which in turn activates calmodulin kinase II that is involved in long-term potentiation.
In a second activation pathway (see Panel 2 of Fig. 73.1 ) the scaffold protein HOMER1 binds to the carboxyl terminal end of the intracytoplasmic domain of mGluR at a specific amino acid sequence. This results in the attachment of a second scaffold protein, SHANK, that induces release of calcium ions from the rough ER (RER). Both HOMER and SHANK proteins are of importance in bipolar disorders, as discussed later.
IP3 is also synthesized from the action of phospholipase C on the phosphoinositol ester of a diglyceride yielding IP3 and diacylglycerol (DAG) that activates protein kinase C (PKC; not shown in Fig. 73.1 ). PKC can activate MEK and MAPK in the presence of raf, although it is not clear that this occurs in the mGluR pathway.
Besides cell growth pathways, as in the MAPK pathway, there are other pathways that block apoptosis. As can be seen in Panel 2 of Figure 73.1 , PI3K activates the protein Akt, also known as protein kinase B, that is both a cell growth-inducing pathway and an antiapoptosis protein. Phosphorylated Akt blocks the protein BAD from promoting apoptosis and, at the same time, promotes growth by activating mTOR protein (target of rapamycin protein, discussed in Chapter 24 ). In addition, phosphorylated Akt activates the protein IKK that in turn activates the protein NFκB that crosses into the nucleus and activates transcription of growth proteins.
It should be noted that there appear to be two endpoints for these G protein–induced pathways. The first is mobilization of calcium ions from stores in organelles. It must be remembered that these pathways occur in dendritic neurons at whose membranes EPSPs and IPSPs occur. Mobilization of calcium ions into the cytosol would have a tendency to lower the transmembrane potential in the dendrite likely leading to EPSPs and nerve conduction. Second is transcription of proteins involved in cell growth and survival, including inhibition of pathways involved in apoptosis. These pathways, such as MAPK- and BAD-induced pathways, assure survival of neurons, which cannot be replaced.
In contrast, mGluR2 and R3 (Grm3) in group 2 activate Gi and inhibit synthesis of cAMP (see earlier). Group 3, the largest group, contains mGluR4, R6, R7A, and R8A, which likewise activate Gi.
The overall structure of mGluR is similar to that of the dopamine receptor since it is a GPCR and has seven transmembrane domains with its amino terminus and the ligand-binding site located extracellularly. However, it is considered to be an atypical GPCR since it has a large extracellular domain ligand binding site (most GPCR have small ligand binding domains). In addition the receptors are paired such that one glutamate binds to the ligand binding domain of each of the monomers in the pair; in this process groups of sulfhydryl groups of cysteine residues become paired as disulfides upon ligand binding.
Importantly, glutamate neural pathways are involved in synaptic plasticity. This is the ability of synapses to increase or decrease their activities (e.g., number of EPSPs or IPSPs over time). This phenomenon is thought to be important in learning and memory. Changes in plasticity can result from changes in the number of postsynaptic receptors, alteration of the number of neurotransmitter quanta released into the synapse, and changes in the affinities of postsynaptic receptors for the neurotransmitter. Plasticity is also dependent on postsynaptic release of calcium ions.
Ion channels are formed by multisubunit proteins that form transmembrane pores or channels, which can allow ions to pass through these pores. In the resting state, these pores are closed. At specific changes in the transmembrane voltage, the subunits change their disposition and conformation such as to allow ions to flow through the channels. Depending on the type of ion that passes through the pores, the cell may become depolarized or hyperpolarized as, for example, the EPSPs and IPSPs in the postsynaptic dendritic membrane at synapses discussed earlier. These channels are referred to as voltage-gated channels.
Voltage-dependent calcium channels mediate the entry of calcium ions into cells and calcium release from cellular organelles in a variety of calcium-dependent processes, such as muscle contraction, hormone or neurotransmitter release, and gene expression. Calcium channel proteins are composed of α-1, β, α-2/δ, and ϒ subunits. Channel activity is controlled mainly by the α-1 subunit, while the other subunits help to regulate this activity. Calcium channels differ from one another principally with respect to α isoforms (i.e., α-1A, B, C, D, E, and S). CACNA1A encodes the α-1A isoform of calcium channel protein that occurs almost exclusively in neuronal tissue.
Mutations in the CACNA1A gene are associated with multiple neurologic disorders, many of which are episodic, including familial hemiplegic migraine, ataxia, and epilepsy. This gene has been found to have (CAG)n-repeats in open reading frames, which encode polyglutamine, which has been associated with spinocerebellar ataxia.
The CACNA1C (α-1C) gene encodes the CaV1.2 channel protein, which is expressed usually in clusters of eight in smooth muscle, pancreas, fibroblasts, and neurons. It is also present in the myocardium where it mediates L (long-lasting)–type currents and helps define the shape of the action potential in cardiac muscle.
This protein is tightly regulated by calcium ions. Increase in intracellular concentration of calcium ions induces increased CaV1.2 channel–associated calcium ion influx into the cell. However, the influx of calcium ions itself induces a change in the structure of this channel protein such that it blocks further entry of calcium ions. These effects are governed by the interaction of the protein calmodulin with the carboxyl terminal segment of the CaV1.2 protein in its so-called IQ domain, an 11–amino acid residue segment. This event seems to induce interactions between the CaV1.2 units in the eight-member complex that allow simultaneous opening and reflex closing of these channels.
A single-nucleotide polymorphism in the third intron of the Cav1.2 gene (SNP rs1006737) is associated with cardiac arrhythmias, bipolar disorder, and schizophrenia ( ; ; ). Also, a CACNA1C risk allele has been further associated with patients diagnosed with bipolar disorder that is not generally present in their unaffected relatives or healthy controls.
CACNB2 is a calcium voltage-gated channel auxiliary subunit β (see earlier). Polymorphisms in the gene encoding this channel protein are associated with cardiac arrhythmias (e.g., Brugada syndrome), autism, attention deficit-hyperactivity disorder (ADHD), bipolar disorder, major depressive disorder, and schizophrenia ( ).
CACNA1I is the α-1 subunit of a voltage-gated calcium channel. It is the channel associated with T (transient) calcium currents that occur in repolarized or hyperpolarized cells that lead to depolarization. It is this channel that occurs in the sinoatrial (SA) node in the heart and is a pacemaker channel. In the central nervous system (CNS) CACNA1I channels occur in neurons of the thalamic reticular nucleus (TRN) involved in sleep. Abnormalities of sleep and different patterns of neuronal activity in the thalamus have been found in patients with schizophrenia, a finding that suggests thalamocortical dysfunction ( ).
We have recently undertaken the largest GWAS of admixed African individuals to date, with a combined sample size of 6152 schizophrenia and schizoaffective disorder cases and 3918 screened controls from the Genomic Psychiatry Cohort (GPC). Meta-analysis of African ancestry results with PGC-SCZ2 (Psychiatric Genomics Consortium–Study of Schizophrenia) summary statistics yielded 94 associated loci, of which 11 were not among the 108 previously reported, and 7 were newly genome-wide significant (Bigdeli et al., 2017).
In addition to the aforementioned common variant studies, another major class of genomic risk factors has been uncovered using the same technology that underlies GWAS—namely, copy number variants (CNVs), including both deletions and duplications. An increased burden of CNVs genome-wide was first reported in 2008.
Several large CNVs were reported, most notably deletions on both 15q13.3 and 1q21.1. Subsequently, large-scale “mega-analysis” in the PGC confirmed this and showed a more or less specific increase in burden in genes with synaptic function as well as those implicated in animal models of illness. Furthermore, a number of specific loci showed significant enrichment in CNVs, including 1q21.1, 2p16.3 (neurexin1), 3q29, 7q11.2, 15q13.3, 16p11.2, and 22q11.2 ( ).
The involvement of neurexin 1 is significant since this protein is involved in synapse formation. It is expressed as a one-pass transmembrane protein on the presynaptic membrane and is vital for connections made between nerve endings at the synapse. It binds to the protein neuroligin in the synaptic cleft. Its intracytoplasmic domain is involved in exocytosis and is presumably involved in neurotransmitter release. Neurexins in general regulate signaling across the synapse and therefore control the interactions of neural networks at synapses. Besides being implicated as being involved with schizophrenia, they have further been implicated in other cognitive disorders such as Tourette syndrome and autism.
Rare variant studies utilizing both whole-genome sequencing (WGS) or whole-exome sequencing (WES) are in a relatively early phase, in part due to the inherently lower power to detect significant effects for lower frequency variants. Currently, there are a number of large-scale case control studies ongoing in both European and non-European ancestry populations. An early report utilizing WES showed that cases are enriched for rare (<1 in 10,000) disruptive mutations in coding regions. This study confirmed the involvement of gene sets previously implicated in GWAS and CNV studies. Furthermore, it additionally implicated gene sets, including the voltage-gated calcium ion channel and the ARC (activity-regulated cytoskeleton-associated) protein and targets of the fragile X mental retardation protein (FMRP) ( ). Both of these proteins are involved in synaptic plasticity. ARC protein expression seems to occur at NMDA receptor locations and has been implicated in learning and memory. FMRP is encoded on the X chromosome and has been found to be required for formation of synapses. Absence or low expression of this protein causes the so-called fragile X mental retardation syndrome. Individuals with this condition are found to have intellectual disabilities, a long and narrow face, large ears, flexible fingers, and (in males) enlarged testicles. Many of those affected have features of autism (see below), including problems with social interactions and delayed speech. The cause of the low protein expression is the occurrence of more than 200 repeats of CGG on the gene encoding this protein (normal is up to 40 repeats). Overall, the results of these more recent cytogenetic and rare variant studies suggest that abnormalities in synaptic proteins may contribute importantly to the occurrence of schizophrenia.
Adequately powered GWAS of the beneficial and adverse effects of psychotropic medications are limited by the considerable difficulty and expense of conducting longitudinal drug trials, which require valid and reliable symptom assessment over the course of an adequate trial. This is usually defined as 6 weeks of treatment at a therapeutic dose. The largest GWAS of antipsychotic efficacy to date examined the landmark CATIE (Clinical Antipsychotic Trials of Intervention Effectiveness) study ( ), which included 738 patients.
In these studies, responses to both positive and negative symptoms of schizophrenia were determined. Positive symptoms include hallucinations, delusions, and racing thoughts; negative symptoms include apathy, lack of emotion, poor or nonexistent social functioning, disorganized thoughts, and difficulty in concentrating or following instructions. Only one SNP was genome-wide significant (on 4p15) and associated with positive symptom response to ziprasidone. However, two other SNPs exhibited low q values and were therefore deemed of interest. A SNP in ANKS1B (a postsynaptic scaffold protein involved in long-term potentiation) was associated with olanzapine effects on negative symptoms, while a SNP in CNTNAP5 (a neurexin protein; see earlier) was associated with risperidone effects on negative symptoms.
An analysis of metabolic side effects, which can be associated with significant morbidity and mortality, was also performed in the CATIE study ( ). This reported two genome-wide significant findings: two SNPs in GPR98 (a large adhesion GPCR protein with 6300 amino acids) were associated with elevated hemoglobin A 1c (HbA 1c ) in olanzapine use, and a SNP in MEIS2 (a transcription regulator protein) was associated with increased hip circumference in risperidone use. A number of other SNPs were suggestively significant in analyses of measures that also included total cholesterol, high-density lipoprotein (HDL), and triglyceride levels. It is hoped that the availability of genomic data in large-scale registry-based cohorts will make it possible to conduct naturalistic pharmacogenomic studies of antipsychotic effects.
Similar to schizophrenia, BD has been shown to have a prevalence of 1% to 2% and heritability of >70% ( ). It is diagnosed on the basis of episodes of both mania and depression. In addition, about one-third of patients have psychotic symptoms, which can be very similar to those seen in schizophrenia. Indeed, the diagnostic distinction between the two disorders has been a subject of much discussion for more than a century. Furthermore, there are significant distinctions between different subgroups of BD (i.e., BD1 and BD2, schizoaffective disorder, and manic depression) that tend to complicate genomic analysis (Ikeda et al., 2018a, 2018b; ).
However, the total sample size of the PGC-Bipolar Disorder Working Group has enlarged significantly since and is currently comprised of 20,352 cases and 31,358 controls. This study reported a total of 30 genome-wide significant loci, 20 of which had previously not been reported. In addition, recent large-scale studies were performed on Japanese and trans-European Japanese populations (2964 bipolar patients and 61,887 controls) (Ikeda et al., 2018a, 2018b). Gene sets that were significantly associated included ion channels, neurotransmitter transporters, synaptic components, nerve transmission, transcription and regulation of the cell cycle, neuronal stability, and immune and energy metabolism components ( ). The most prominent genes and their encoded proteins that were found thus far to be significantly associated with bipolar disorder are summarized in Table 73.2 and are discussed later.
| Protein | Function |
|---|---|
| CHANNEL PROTEINS | |
| CACNA1C | Calcium channel protein (see Table 73.1 ). |
| PROTEINS CRITICAL FOR NEURAL/SYNAPSE DEVELOPMENT AND NERVE TRANSMISSION | |
| ODZ4-Teneurins | Sequence conserved one-pass transmembrane glycoproteins expressed in neural pattern formation and development. |
| SHANK2 | Important protein in synaptogenesis. It links mGlu receptors to NMDA receptors by binding to attachment protein PSD-95 that binds to mGlur and to HOMER1 scaffold protein, that attaches to the NMDA receptor. For other scaffold proteins, see ANK3. |
| ADCY2 | G protein–coupled protein that has strong adenyl cyclase activity and transduces signaling via cAMP. Vital for functioning of neocortex, hippocampus, amygdala, and corpus striatum. |
| ZNF804A | Zinc finger protein involved in neurite outgrowth, dendritic branching, and synapse formation. Vital for differentiation of oligodendroglia. |
| NCAN (NeuroCAN) | Modulates neuronal adhesion, migration, and axon guidance. Also associated with abnormalities in lipid metabolism. See also FADS below. |
| PROTEINS CRITICAL FOR TRANSCRIPTION AND REGULATION OF THE CELL CYCLE | |
| POU3F2 | CNS transcription factor that binds to DNA at ATGCAAAT sequences and regulates mammalian neurogenesis by regulating their diverse patterns of gene expression. |
| DDN (Dendrin) | Transcription factor and RNA polymerase. |
| NEK4 | Regulates entry of cells into senescence. |
| GNL3 | GTP-binding protein involved with cell cycle and cell differentiation. |
| Mad1L1 | Mitotic spindle assembly check point protein. |
| ERBB-2 (Neu/HER2) | Growth factor that transmits mitotic signaling via ras-p21. |
| PROTEINS INVOLVED IN NEURONAL STABILITY | |
| ANK3/Ankrin | Scaffolding protein that binds neuronal cell membrane and spectrin that itself binds to the cell membrane. Thus this is a membrane anchoring protein. See also TRANK1 below. |
| SYNE1 | Encodes the protein Nesprin-1α that links membrane proteins and receptors to the cytoskeleton. |
| PROTEINS WITH OTHER FUNCTIONS (MAINLY IMMUNE AND ENERGY METABOLISM) | |
| ITIH3, ITIH4 | Alpha trypsin inhibitors with three heavy and one light chain called bikunin. Important in modulating the neural inflammatory response. |
| FADS (fatty acid desaturase) | Enzyme that regulates levels of unsaturated fatty acids in lipid metabolism. |
| DGKH (diacylglycerol kinase eta) | Major enzyme in lithium-sensitive phosphatidyl inositol pathway. |
| TRANK1 | Structural protein of unknown function. It contains a tetratricopeptide sequence (tandem repeats of 34 amino acids) and contains an ankyrin domain. |
Interestingly, the calcium channel protein CACNA1A (discussed earlier, as being a major protein implicated in schizophrenia) overlaps with bipolar disorder pointing to a possible common ion channel protein involved in both diseases.
ODZ gene-encoded teneurins are transmembrane proteins that may interact with signal transduction molecules and are crucial in the regulation of neuronal and synaptic connectivity during brain development. They have been found to be involved with organization of the development and differentiation of oligodendrocytes and the myelination of neuronal axons during brain maturation. The common variant rs12576775 in an intron of gene ODZ4 has been associated with bipolar disorder in large studies. This gene has a minor allele frequency (10% across all ethnic populations with the exception of European populations; in European individuals the minor allele occurs in about 20% of individuals) ( , ; ).
SHANK2 is a scaffold protein involved in signal transduction involving mGluR-induced calcium mobilization (see earlier, including Figure 73.1B ). It is also involved in cross-linking mGluR and iGluR.
ADCY2(5p15.31) is a key enzyme in cAMP signaling pathway. There are two genome-wide significant SNPs located in ADCY2. The ADCY2 gene on chromosome 5p15.31 is expressed in the brain and encodes a cell membrane–bound enzyme for the synthesis of the second-messenger molecule, cAMP, which is involved in nerve conduction. ADCY2 plays a key role in cAMP-dependent GPCR pathways and is regulated by PKC and raf kinase (see preceding section and Chapter 77 ). Disturbed neurotransmission in cAMP pathways is considered to be important in the pathogenesis of psychiatric illnesses ( ; ).
The human ZNF804A gene is located on chromosome 2q32.1 and codes for a protein consisting of 1210 amino acids, containing a zinc finger domain, which binds to and regulates the activities of transcription factors. This protein is expressed in the brain and regulates genes involved in neurodevelopmental processes, cell adhesion, neurite outgrowth, dendritic branching and synapse formation, and differentiation of oligodendrocytes and proliferation of oligodendrocyte progenitors. It has been found that a single nucleotide polymorphism rs1344706 that is strongly associated with bipolar disease occurs in an intronic region of the ZNF804A gene (substitution of A for C). This results in expression of altered messenger ribonucleic acid (mRNA) that encodes a protein that has altered affinity for nuclear proteins, including transcription factors, in the CNS that results in disrupting myelination and reduction of white matter integrity ( ; ).
NCAN (NeuroCAN) is a large gene located on chromosome 19p13.11. The SNP rs1064395, which is found in an intronic region of the gene, has been associated with bipolar disorder in one study. Rs1064395 affects only one of five alternative transcripts of the gene. This gene has significant differences in allele frequencies across all ethnic populations. The overall frequency of the A allele (or disease-associated allele) is about 23%. It is the major allele in 51% of African populations, 12% in Asia, and 15% in Europe. Postmortem brain studies in bipolar patients show increased cortical folding in some brain regions of patients with the A allele compared with healthy controls.
The gene NCAN codes for a large secreted neurocan protein that is found predominantly in the extracellular space, the lumen of the Golgi apparatus, and lysosomal cavities. There are 741 known variants in the gene, but coding variants have not been associated with any mendelian disorder. Neurocan protein modulates neuronal adhesion, migration, and axon guidance and has been associated with abnormalities in lipid metabolism ( , ; ).
These proteins have known functions related to transcription and regulation of the cell cycle, but the relationship of these functions to bipolar disorder is not clear. As with other proteins that have been identified as being involved with this disease, some of the SNPs in the genes coding for these proteins occur in introns or in intergenic domains of the genome rather than with direct gene expression.
POU3F2 on chromosome 6q16 is a transcription factor that binds to specific deoxyribonucleic acid (DNA) sequences (ATGCAAAT) and regulates expression of neurogenic proteins. It occurs downstream of MIR2113 (microRNA 2113) gene. In the spanning intronic region, the genome-wide significant SNP in this region (rs12202969) appears to be associated with neocortex development in mice and with a cognitive phenotype and speed of information processing ( ; ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here