Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
It has been almost 40 years since Noma first discovered adenosine triphosphate–sensitive potassium channels in cardiac muscle. Members of this channel family were found in skeletal myocytes, pancreatic β cells, neurons, vascular smooth muscle and endothelium, and lymphatic smooth muscle. Although highly expressed in the heart, cardiac K ATP channels are essentially closed under normal conditions and may play little role in shaping normal action potentials. However, these channels can be opened by severe metabolic stress, such as anoxia, metabolic inhibition, or ischemia, leading to drastic action potential shortening. , The consequent decrease in excitability and contractility is likely to be cardioprotective because of preservation of adenosine triphosphate (ATP), but K ATP activation-dependent shortening of the action potential, and reduction of Ca entry and inhibition of contractility, may in turn lead to arrhythmias and cardiac insufficiency. Thus, if fully activated, cardiac K ATP channels can induce complete cessation of cardiac electrical activity and contractile failure. The K ATP channel may therefore represent a double-edged sword in regulating cardiac excitability. In vascular smooth muscle, in contrast, K ATP channels are physiologically active, with further activation leading to membrane hyperpolarization, resulting in decreased Ca current and vasodilation ; whereas inhibition of K ATP channels will cause membrane depolarization, an increase in Ca current, and vasoconstriction.
Much effort over the first 25 years of research into cardiovascular K ATP channels revealed biophysical and biochemical details of channel regulation in different tissues and the distinct gene products underlying this channel activity. The last 15 years have led to discoveries regarding the role of gene variants in generating cardiovascular pathologies, further illuminated both by the availability of high-resolution imaging of channel structures and genetically modified animals, made possible by revolutions in cryo-electron microscopy (EM) imaging and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) technologies. This chapter focuses on current understanding of the molecular structure and physiologic function of the K ATP channel in heart and blood vessels, and the pathologic consequences of genetic changes in cardiovascular K ATP channels.
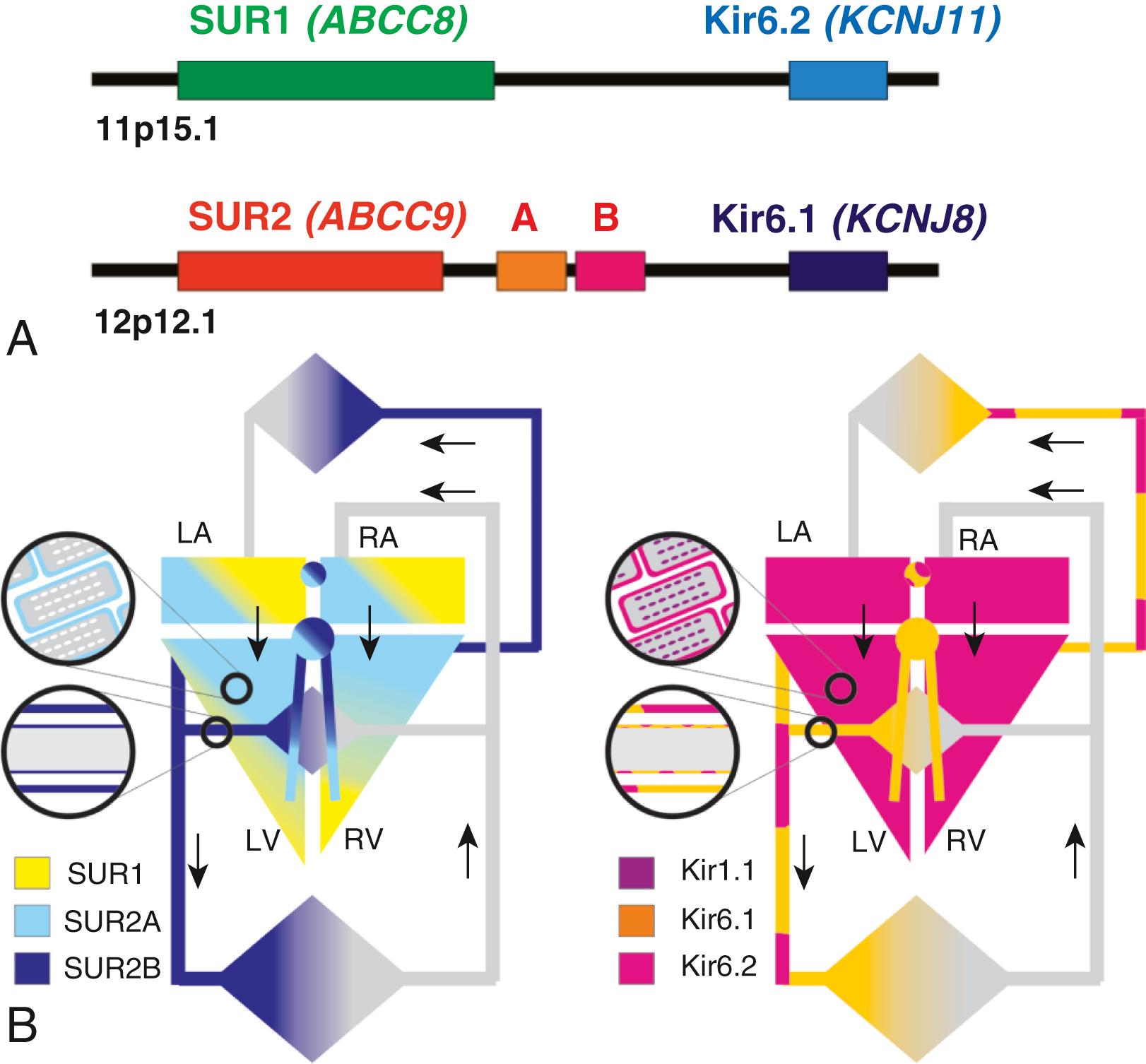
K ATP channels are hetero-octameric complexes of four Kir6 pore-forming subunit members of the inward rectifier channel (Kir) protein family, in association with four regulatory SUR subunits, which are members of the ATP binding cassette (ABC) family of membrane proteins ( Fig. 11.1 ). There are two Kir6-encoding genes, KCNJ8 (Kir6.1) and KCNJ11 (Kir6.2), , and two SUR-encoding genes, ABCC8 (SUR1) and ABCC9 (SUR2) ( Fig. 11.2A ). Alternative RNA splicing can give rise to multiple SUR protein variants; the best characterized are SUR2A and SUR2B (generated by alternate splicing of the C-terminal exon; see Fig. 11.2A ), which confer distinct physiologic and pharmacologic properties on channel complexes. , The ABCC8 and KCNJ11 genes are immediately adjacent to each other on human chromosome 11p15.1, whereas ABCC9 and KCNJ8 are also adjacent to one another on chromosome 12p12.1. , These arrangements implicate both an evolutionary duplication and suggest potential coregulation at the gene level. Consistent with this, as discussed later, the majority of native KATP channel complexes are generated by either one gene pair or the other, even though all subunit combinations can be functionally engineered in recombinant systems.
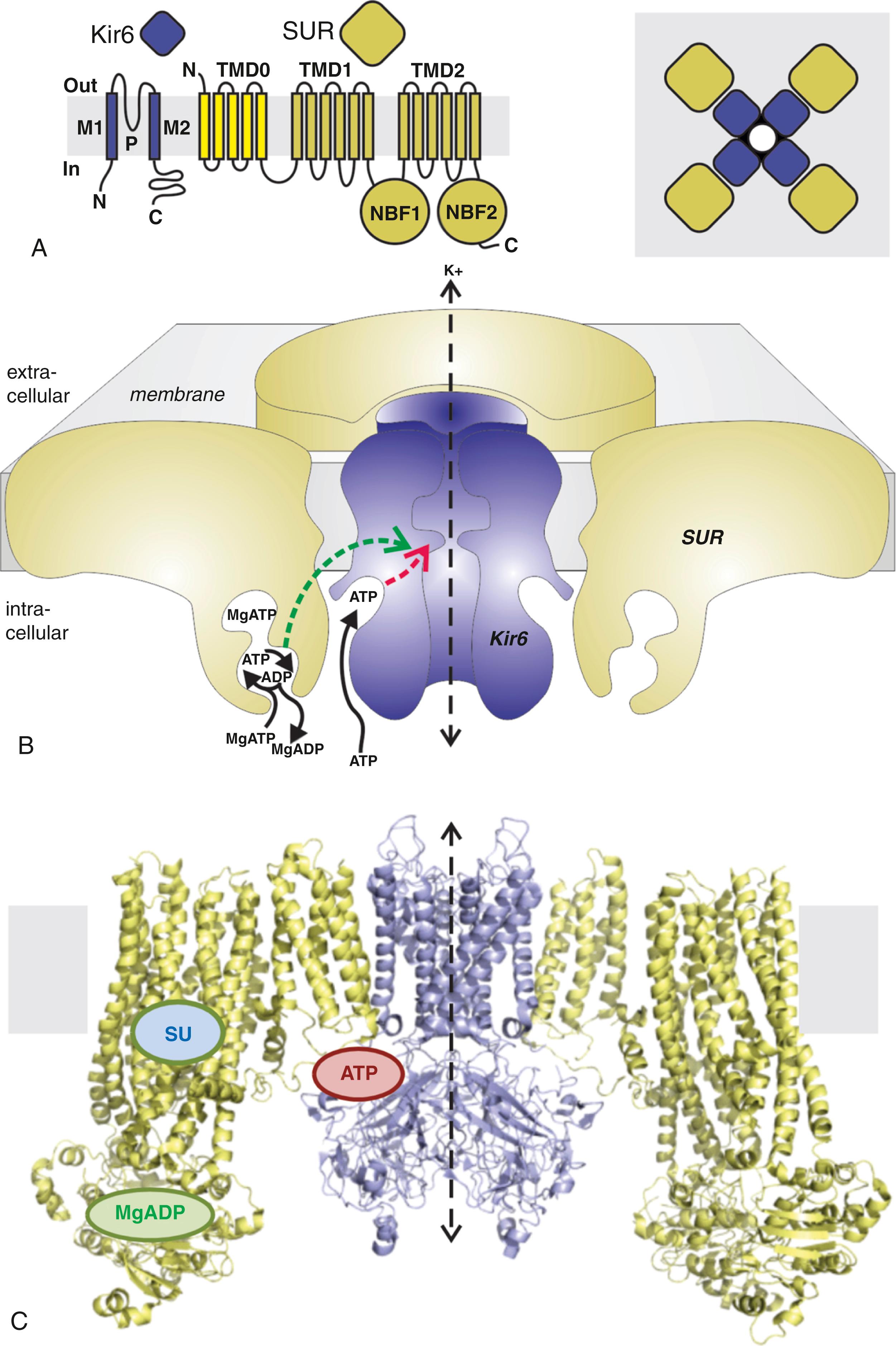
Kir6 subunits maintain the conserved architecture of other Kir channels, with two transmembrane helices (M1, M2) bridged by an extracellular loop that generates the narrow portion of the pore and controls ion selectivity (see Fig. 11.1A ), and harboring the inhibitory ATP binding site. As with other ABCC family members, SURs contain two six-helix transmembrane domains, TMD1 and TMD2, and contain an additional N-terminal five-helix TMD0 domain (see Fig. 11.1A ), which is critical for Kir6.x trafficking and gating. SURs contain one nucleotide binding fold (NBF1) between TMD1 and TMD2, and a second (NBF2) after TMD2 in the cytoplasmic loops (see Fig. 11.1A ). These NBFs, which pair up as head-to-tail dimers (see Fig. 11.1C ) to generate two nucleotide binding sites (NBDs) at the interface between them, are responsible for channel activation by Mg-nucleotides. A flurry of structural studies of SUR1/Kir6.2 complexes using Cryo-EM have recently confirmed K ATP complexes to be formed by intimate packing of 4 SUR and 4 Kir6.x subunits (see Fig. 11.1C ). Very similar structures were independently reported at approximately 6 Å resolution by two groups in 2017, , and subsequently refined structures have since been reported at near-atomic 3.6 Å resolution. In these structures, the binding of glibenclamide, repaglinide, and carbamazepine, three distinct K ATP channel inhibitors, is well resolved at essentially the same location within the SUR core (see Fig. 11.1C ).
K ATP channel regulation is complex and involves metabolites, hormones, and neurotransmitters and transcriptional mechanisms. Micromolar ATP inhibits channels by direct interaction with Kir6 subunits, and because the cellular ATP concentration is relatively high (i.e., millimolar) in physiologic conditions, ATP inhibition is usually sufficient to maintain cardiac K ATP channels in predominantly closed states (see Fig. 11.1B ). However, ATP inhibition is overridden by MgATP and MgADP interacting with SUR subunit NBFs, and ATP sensitivity is also reduced by membrane phosphoinositides, such as phosphatidylinositol-4,5-bisphosphate (PIP 2 ), and by long-chain acyl-CoA esters (LC-CoAs), and metabolic derivatives of free fatty acids, which act on Kir6.2 to antagonize ATP inhibition and increase channel open probability. Negative coupling between PIP 2 activation and ATP sensitivity of K ATP channels means that ATP sensitivity is not a fixed parameter, and it may change dynamically with changes in membrane composition. Consistent with an electrostatic interaction between the negatively charged head group and a region of the Kir6.2 protein close to the membrane, a number of positively charged amino acid residues in the slide helix region directly interact with PIP 2 in the membrane. , , PIP 2 interaction with Kir6.1 has been less extensively studied; Kir6.1 channels are activated by PIP 2 , suggesting conservation of the fundamental determinants of phospholipid binding and gating. In addition, other metabolic factors, including pH, nitric oxide (NO), eicosanoids, hydrogen sulfide (H 2 S), and hormones and neurotransmitters, can also affect K ATP channel activity.
K ATP channels are uniquely endowed with sensitivity to many pharmacologic agents that interact with the SUR subunits. Multiple K ATP channel openers (KCOs) (e.g., nicorandil, cromakalim, pinacidil, and diazoxide) act on SURx subunits to activate the channel. SUR1-containing channels can be strongly activated by diazoxide, but not by pinacidil or cromakalim, whereas channels containing SUR2A respond potently to both pinacidil and cromakalim, but not to diazoxide. , , Channels containing SUR2B are sensitive to diazoxide, cromakalim, and pinacidil. Because nucleotide binding and hydrolysis at NBDs are important for the binding and action of these KCOs, the different KCO sensitivities of SUR1 and SUR2 subtypes may partially result from differences in nucleotide sensitivity. , These openers all require the presence of hydrolysable ATP, , which suggests that they act to stabilize or enhance ATP hydrolysis at the NBFs. They effectively act to decrease sensitivity to inhibitory ATP, leading to increased channel opening at a given level of cytosolic ATP. All SUR isoforms can be inhibited by sulfonylureas such as tolbutamide and glibenclamide, although SUR1-containing channels are more sensitive to sulfonylureas than SUR2-containing channels, underlying the widespread use of sulfonylureas to treat diabetes, where they act to trigger insulin secretion via interactions with SUR1-dependent K ATP channels in pancreatic β cells.
Given that any pair of SURx:Kir6.x tetramers can coassemble in heterologous expression , and that multiple SUR or Kir6 isoforms can coexist within even a single octameric channel, the molecular makeup of the channel in specific cell types can be complex (see Fig. 11.2B ) and may change under different physiologic or pathologic conditions.
Each of Kir6.1 and Kir6.2 and SUR2A, SUR2B, and SUR1 have been identified in the heart. , , SUR1 and Kir6.2 are major constituents of the murine atrial myocyte sarcolemmal K ATP , whereas SUR2A and Kir6.2 generate ventricular K ATP . In humans, both SUR1 and SUR2A subunits probably contribute to sarcolemmal channels in both atrial and ventricular myocytes (see Fig. 11.2B ). Within subregions of the heart there may be further subunit combinations: K ATP channel currents have been detected throughout the pacemaking and conduction systems, but smaller K ATP single-channel conductances in rabbit sinoatrial (SA) node and mouse conduction system cells , suggest that Kir6.1 may be involved in addition to Kir6.2. K ATP channels in these cell types also respond to the relatively SUR2-specific openers cromakalim and pinacidil, suggesting a role for SUR2 in nodal K ATP channels.
Single-channel studies of vascular myocyte K ATP have reported predominantly low conductance (20–50 pS), but these channels tend to be inactive in isolated vascular smooth muscle membrane patches and require nucleotide diphosphates (ADP, UDP, and GDP) in the presence of Mg 2+ to open, leading to their functional designation as nucleotide-dependent K + -channels, or K NDP channels. Heterologously expressed Kir6.1/SUR2B channels recapitulate many of the biophysical properties of native VSM K ATP /K NDP , , and at the whole-cell level, K ATP conductance disappears in Kir6.1 −/− animals. Thus the Kir6.1/SUR2B channel represents the predominant VSM K ATP 67 (see Fig. 11.2B ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here