Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Solid-tissue (predominantly epithelial cell) tumors are mainly caused by genetic lesions of three types: deletion or inactivation of tumor suppressor genes, mutation in or overexpression of oncogenes (i.e., genes encoding proteins that are vital in control of the cell cycle), and hypermethylation of the promoter regions.
These genetic lesions can be detected using the techniques described in Part 8 of this textbook involving real-time polymerase chain reaction, fluorescence in situ hybridization, immunohistochemistry, enzyme-linked immunosorbent assay, and so on.
Detection of genetic lesions in solid-tissue tumors is of great value for the diagnosis of specific types, for classification of the tumor, and for determining prognosis for a patient with a specific type of cancer. A common mutation in several of these cancers is overexpression of the epidermal growth factor receptor (EGFR). Discovery of this lesion allows for implementation of anti-EGFR therapy. However, because ras-p21 is a downstream target of EGFR and because it is commonly mutated in many human cancers, it is necessary to test for oncogenic mutations in th e ras gene. If these are found, the efficacy of anti-EGFR agents is diminished.
Less commonly, oncogenic mutations can be found in downstream targets of ras-p21 , such as BRAF , which makes treatment with anti-EGFR agents less effective.
Many solid-tissue tumors express the same oncogenes, such as BRAF , in melanoma and thyroid cancer, but other cancers have genetic lesions that appear to be specific for that type of cancer, such as RET in medullary thyroid carcinoma.
Although not formally classified as solid-tissue tumors, sarcomas often behave in a manner identical to that of solid-tissue tumors. These cancers have been found to be caused by reciprocal translocations resulting in oncogenic fusion transcripts (accounting for 15%–20% of cases) and by specific oncogenic mutations (e.g., KIT and PDGFRA mutations in gastrointestinal stromal tumors). Both types of genetic alterations are often specific to certain types of sarcomas.
Because the genetic alterations or changes that underlie many familial types of cancers are known, it is possible to screen for these in the children and close relatives of patients known to have a form of familial cancer to detect the presence of these cancers as rapidly as possible.
Next-generation sequencing is currently widely used in the selection of pathway-based therapy and prediction of treatment resistance of many types of cancer.
Cancer is largely a genetically encoded disease. Recent research on cancer molecular genetics, epigenetics, and genomics, as well as their application, has vastly changed the practice of cancer diagnosis, classification, prognosis, and treatment. Application of molecular genetics to solid tumors is a major milestone in diagnostic pathology. Molecular diagnosis was initially introduced for accurate diagnosis of solid tumors based on the unique genetics. Now, the utility of molecular diagnosis is far beyond its original intention, and it is no longer just an adjunct method. It has become an indispensable tool that is widely used to predict clinical outcome, including disease prognosis, selection of optimal therapeutic regimens, and anticipation in response to treatment. It has not only revolutionized the concept and practice of diagnostic pathology for solid tumors, it has also become the basis for personalized medicine.
The principles and method in molecular diagnostics of solid tumors are similar to those used in other fields of molecular diagnosis. Techniques commonly used in cancer molecular pathology include detection or identification of specific sequences of genes (deoxyribonucleic acid [DNA] and/or its transcribed ribonucleic acid [RNA]) or gene product (protein) alterations. They include amplification-based techniques (i.e., polymerase chain reaction [PCR], reverse-transcriptase PCR [RT-PCR], branched DNA testing), hybridization methods (i.e., in situ hybridization, or fluorescence in situ hybridization [FISH]), microarray-based approaches (i.e., comparative genomic hybridization [CGH], DNA, RNA, microRNA [miRNA] microarray), and sequencing. PCR and FISH are among the most commonly used methods, whereas high-throughput techniques, such as array-based tests and next-generation sequencing (NGS) have a promising future but have not been fully established in clinical settings due to the lack of standards. Each method is described in Part 8 of this textbook.
Tissue sources of diagnostic materials of molecular pathology for solid tumor include paraffin-embedded tissue, fresh tissue, and cytologic specimens. Fresh tissue is a preferred choice for preservation of DNA, RNA, or protein. In most cases, only fresh tissue is usable in array-based methods; however, it is usually not readily available or accessible. Thus, paraffin-embedded tissue is used most commonly because of its availability and ease of access. It also allows use of the corresponding hematoxylin and eosin–stained slides for morphologic study. However, exposure of nucleic acid to formalin is associated with the major inherent problems of increased nucleic acid fragmentation and low integrity of DNA, RNA, and protein. Tissue fixation in formalin for longer than 24 hours will likely reduce the yield of high-molecular-weight nucleic acid. Thus, longer formalin fixation, paraffin embedding, and long storage at room temperatures can lead to false-negative results. A second problem is impurity of tumor cells, which usually are mixed with normal cells and stromal cells. Relatively pure tumor cells can be achieved by employing microdissection that isolates the tumor cells, even a single tumor cell, by a manual or instrument-assisted method. Cytologic specimens, including cells from fine-needle aspiration (FNA), urine, and blood, and swabs for molecular pathology, are used most often to assist in accurate diagnosis of superficial solid tumors, such as human epidermal growth factor receptor ( Her ) -2/neu ( ERBB2 ) for breast cancer, human papillomavirus (HPV) testing for cervical cancer, and UroVysion (Abbott Laboratories, Abbott Park, IL) for bladder cancer.
Molecular diagnoses of solid tumors rely on our understanding of the molecular mechanism involved in carcinogenesis of solid tumors. Genetic alterations can be inherited or can result from carcinogenesis, as described in Chapter 77 . In several tumors, polygenic mutations occur. However, often in these cases, some unique or specific genetic alterations lead to carcinogenesis. Those genetic or epigenetic components mainly belong to the following:
Tumor suppressor genes (TSGs) (e.g., Rb , SMAD , adenomatous polyposis coli [ APC ]).
Oncogenes (e.g., EWS , cKIT , Her2/neu , KRAS , BRAF ).
Promoter regions with methylation/inactivation activity.
Copy number alteration (CNA) has also been found to play an important role in tumor genesis.
Application of molecular diagnosis of solid tumors has been used clinically as follows:
Assistance in disease diagnosis and classification: For example, small “blue-cell” tumors encompassing Ewing sarcoma, lymphoma, rhabdomyosarcoma, and so forth, can be diagnosed by identification of specifically altered genes (e.g., Ewing sarcoma–specific gene translocations). In cervical cancer, detection of HPV assists in the diagnosis of neoplastic change on cervical biopsies or smears. Moreover, analysis of microarray data of the tumor detects gene signatures.
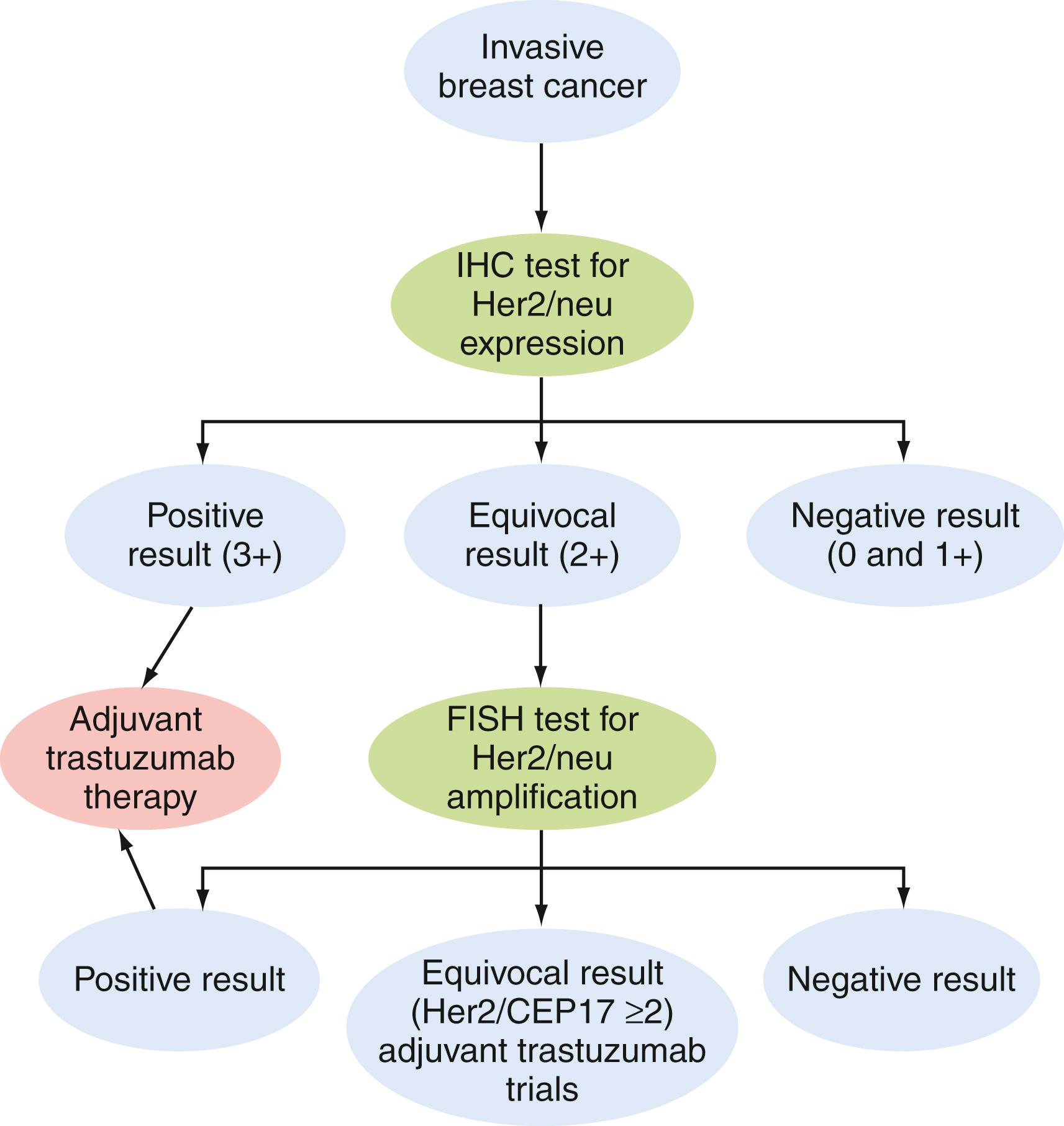
Determination of prognosis: With better understanding of the molecular genetics of solid tumors, a greater number of prognostic markers can be identified. Thus, detection of those markers in some cases has become a routine practice. For example, detection of overexpression/amplification of Her2/neu in breast cancer and 1p19q deletion in brain tumor has been used to predict prognosis or clinical outcome.
Determination of therapeutic options: Many drugs have been developed to target certain genes and gene products (i.e., proteins) and related pathways. Examples include tyrosine kinase inhibitors (TKIs) and monoclonal antibodies to EGFRs in lung and colon cancer. Moreover, only certain groups of patients with unique genetic profiles can respond to these treatments. Thus, providing the right management not only will increase the chances for cure but also will improve patient quality of life by avoiding unnecessary side effects.
Combination of the above: In most cases, detection of molecular markers may provide both diagnostic and prognostic information, such as c KIT mutations and chromosomal abnormalities detected by UroVysion, and amplification of Her2/neu.
This chapter focuses on the clinical application of molecular pathology in cancer diagnosis, prognosis, and predicting treatment response in common solid tumors. The major genetic findings in each tumor type are summarized in Table 79.1 .
| Tumor Type | Major Molecular Target(s) ∗ | Main Method(s) of Detection | Clinical Application(s) |
|---|---|---|---|
| Solid-Tissue Tumors | |||
| Glioblastoma | EGFR, TP53, 10q, MGMT, mutations in TP53 and PTEN, P16INK4a deletions | RT-PCR, methylation-specific PCR, FISH | Prognosis, treatment response |
| Oligodendroglioma | 1p and 19q deletions, EGFR, TP53 | FISH, RT-PCR, LOH | Diagnosis, prognosis, treatment response |
| Sporadic, nonhereditary breast cancer | Her2/neu , multiplex genes | FISH, IHC, RT-PCR, Oncotype, MammaPrint | Molecular classification, prognosis, treatment response |
| Hereditary breast cancer | BRCA1, BRCA2, EGFR | High-throughput sequencing, multiplex PCR, genetic counseling | Diagnosis, genetic counseling |
| Papillary thyroid cancer | BRAF, RAS, RET, PTC1, PTC3 | FISH, RT-PCR, direct sequencing | Diagnosis, prognosis, therapeutic option |
| Follicular thyroid cancer | PAX8-PPARG | IHC, FISH, RT-PCR | Diagnosis, prognosis |
| Medullary thyroid carcinoma | RET | Sequencing, PCR | Diagnosis, screening, surveillance |
| Non – small cell cancers of the lung | EGFR, ALK, ROS1 | PCR, sequencing, FISH | Prognosis, treatment response |
| Liver (hepatocellular) | EGFR, PIK3CA, p53 , β-catenin, microRNA (miR-122a), multiple genes | FISH, PCR, sequencing, microarray | Diagnosis, prognosis, targeted therapy |
| Gastric cancer, intestinal type | p73 mutations, MSI ( MLH1 and MSH2 ), LOH/mutations of APC genes, and Her2/neu | FISH, PCR, IHC | Diagnosis, prognosis |
| Gastric cancer, diffuse type | CDH1 | FISH, PCR, IHC | Diagnosis, prognosis |
| Colon cancer | Mutated oncogenes: mainly EGFR, KRAS, BRAF , and PI3K. Mutated or deleted antioncogenes: p53, APC, TGFBRI, SMAD2, SMAD4 Microsatellite instability (MSI) genes and CpG island methylator (CIMP) genes |
FISH, PCR, microarray, methylation-specific PCR, sequencing, array technology | Diagnosis, prognosis |
| Pancreatic cancer | Most pancreatic cancers: KRAS mutations | PCR, sequencing | Diagnosis |
| Inactivating mutation or deletion or methylation of genes encoding TP53, SMAD4, p16/CDKN2A | PCR, methylation-specific PCR | Diagnosis (rarely), prognosis | |
| miRNAs (miR-196a, 217, 221, 376a, and 301) | RT-PCR | Diagnosis, prognosis | |
| Renal cell carcinoma (RCC) | Chromosome 3p deletion, von Hippel – Lindau (VHL) gene deletion; PI3K/AKT/MTOR | Sequencing, FISH (for VHL) | Diagnosis |
| Translocation-associated RCC | t(X;17)(p11.2;q25); ASPL-TFE3 and PRCC-TFE3 fusion genes | RT-PCR, FISH, IHC for the C-terminal domain of nuclear TFE3 | Diagnosis, classification |
| RCC, clear cell | Carbonic anhydrase IX Genes of immune response and proangiogenesis |
Gene expression arrays, IHC | |
| Proximal nephron markers: megalin, cubilin, adipophilin | Diagnosis, prognosis, classification | ||
| RCC, papillary | Proximal nephron marker α-methylacyl-coA racemase CDKN2A, CIMP |
Gene expression arrays, IHC | Diagnosis, prognosis, classification |
| RCC, chromophobe | Abundant mitochondria TP53, PTEN, TERT |
Gene expression arrays, IHC | |
| Distal nephron markers: β-defensin, parvalbumin, chloride channel Kb, claudin 7 and 8, and EGF | Diagnosis, prognosis, classification | ||
| RCC, oncocytoma | Abundant mitochondria | Gene expression arrays, IHC | |
| Distal nephron markers: β-defensin, parvalbumin, chloride channel Kb, claudin 7 and 8, EGF | Diagnosis, prognosis, classification | ||
| Bladder cancer | Aneuploidy of chromosomes 3, 7, and 17 and/or deletion of the 9p21 (encoding p16); NMP 22 | FISH | Diagnosis |
| Prostate cancer | TMPRSS2 ( 21q22.3) to transcripts of the ETS family member genes: overexpression of ETS proteins (e.g., ERG, ETV1, ETV4, ETV5) | FISH, ELISA, RT-PCR, gene expression arrays | Diagnosis, classification |
| Cervical cancer | HPV-6 and -18 | Hybrid capture DNA assay, RT-PCR, Southern blot, dot blot | Diagnosis, follow-up of abnormal PAP smears |
| Ovarian Cancer | |||
| Low-grade serous | KRAS, BRAF | PCR, Southern blot | Diagnosis, classification |
| High-grade serous | p53 deletion/mutation; Wnt/β-catenin or PI3K/PTEN signaling pathway defects | PCR, Southern blot | Diagnosis, classification |
| Mucinous | KRAS, BRAF | PCR, Southern blot | Diagnosis, classification |
| Clear cell | PI3K/PTEN mutations | PCR, Southern blot | Diagnosis, classification |
| Endometrioid | Mutations of CTNNB1 (β-catenin) | PCR, Southern blot | Diagnosis, classification |
| Melanoma | CDKN2A, p14, and p16 inactivation; NRAS (G12V) and BRAF oncogenic mutations (V600E) | PCR, sequencing | Diagnosis, classification, therapy |
| Sarcoma | |||
| Sarcoma with fusion genes involving the TET gene: Ewing’s sarcoma | t(11;22)(q24;q12) translocation; fusion of EWSR1 and an ETS family gene, mainly FLI1 | RT-PCR, FISH, Southern blot | Diagnosis Prognosis |
| Sarcoma with fusion genes involving receptor tyrosine kinase: congenital fibrosarcoma | t(12;15)(p13;q25) translocation, resulting in fusion of ETS gene, ETV6 with neurotropin receptor (that has tyrosine kinase activity), NTRK3 gene | RT-PCR, FISH, Southern blot | Diagnosis |
| Sarcoma with fusion genes involving chromatin remodeling: synovial sarcoma (SS) | t(X;18)(p11.2;q11.2) translocation resulting in fusion of SS18(SYT) gene with one of the SSX genes on the X chromosome, creating SS18-SSX1, SS18-SSX2, or SS18-SSX4 chimeric genes Downstream targets of above fusion proteins: CCND1 (cyclin D1) and TLE1 that encodes a transcriptional corepressor |
FISH, RT-PCR IHC |
Diagnosis, classification |
| Sarcoma with fusion genes involving growth factors: dermatofibrosarcoma protuberans (DFSP) and giant cell fibroblastoma (GCF) | t(17;22)(q11;q13.1) translocation resulting in fusion of the COL1A1 gene on chromosome 17 with the PDGFB gene on chromosome 22, leading to PDGFRB overexpression | RT-PCR | Diagnosis, classification |
| Sarcoma with other types of fusion genes: alveolar rhabdomyosarcoma (ARMS) | t(2;13)(q35:q14) translocation that results in fusion of PAX3 with FOXO1A | FISH | Diagnosis |
| Sarcoma with oncogenic mutations: gastrointestinal stromal tumor (GIST) | cKIT/PDGFRA | PCR, sequencing, Southern blot | Diagnosis |
| Sarcoma with no consistent genetic lesions: leiomyosarcoma (LMS) | Loss of 1p12-pter, 2p, 13q14-q21 (targeting the Rb pathway), 10q (targeting PTEN ), 16q. Gains of 17p, 8q, and 5p14 pter. Activation of the PI3K - AKT pathway and mTOR |
FISH | Diagnosis |
| Syndromatic Cancers | |||
| Familial adenomatous polyposis syndrome | Adenomatous polyposis coli (APC) (long arm of chromosome 5) or MutY human homologue (MYH) gene involved in repair of oxidative damage to DNA | PCR, sequencing, Southern blot | Diagnosis |
| Hereditary nonpolyposis colorectal cancer (HNPCC): Lynch syndrome | Microsatellite instability (MSI) in mismatch repair (MMR) genes, including MLH1, MSH2, MSH6, MLH3, and PMS2 | IHC, PCR, sequencing | Diagnosis, classification, genetic counseling |
| Familial juvenile polyposis syndrome | Germline mutations of SMAD4, BMPR1A , and ENG ; also, mutations in kinase, BMPR1A (bone morphogenetic protein receptor type IA), on chromosome 10q22.3. Involvement of TGF-β signal transduction pathway | PCR, sequencing | Diagnosis, classification |
| Peutz-Jeghers syndrome (PJS): melanotic mucocutaneous hyperpigmentation and GI hamartomas, which occur anywhere from the stomach to the anus; multiple polyps in small bowel | STK11 / LKB1 gene | PCR, sequencing | Diagnosis, classification |
| Multiple endocrine neoplasia (MEN) | |||
| MEN type 1: tumors in the parathyroid glands; the stomach, pancreas, and intestinal tract; anterior pituitary gland; endocrine pancreas; and duodenum, and by the presence of other nonendocrine tumors such as hemangioma, ependymoma, and leiomyoma, often at a young age | MEN 1 tumor suppressor gene, MEN 1 | PCR | Diagnosis, classification |
| MEN type 2: medullary thyroid carcinoma (MTC) and associated pheochromocytoma and hyperparathyroidism | RET (chromosome 10q11.2) (encoding a tyrosine kinase) mutations | PCR, sequencing | Diagnosis, classification |
| von Hippel–Lindau syndrome (VHL): neoplasia syndrome characterized by hemangioblastomas in the central nervous system and retina, pheochromocytomas, renal cysts and clear cell renal cell carcinoma, pancreatic cysts and islet cell tumors, endolymphatic sac tumors, and papillary cystadenomas of the epididymis and broad ligament | VHL tumor suppressor gene (three exons) on chromosome 3p25. Encodes VHL protein, critical in regulating hypoxia-inducible factor (HIF-α and -β) | PCR, sequencing | Diagnosis, classification |
| Familial paraganglioma syndromes | Mutation of three genes encoding subunits of mitochondrial succinate dehydrogenase (SDH complex): SDHB at 1p36.1 (PGL4), SDHC at 1q21 (PGL3), and SDHD at 11q23 (PGL1) | PCR | Diagnosis, classification |
| Cowden syndrome (CS); breast, thyroid, and endometrial cancers and other benign conditions, including multiple hamartomas in the colon, lipomas, fibromas | PTEN mutations | PCR | Diagnosis, classification |
| Li-Fraumeni syndrome; soft-tissue sarcomas, breast cancer, osteosarcoma, brain tumors, childhood leukemias, and adrenocortical carcinoma | p53 gene mutations | PCR, IHC | Diagnosis, classification |
| Neurofibromatosis 1 (NF1); neurofibroma and, less commonly, gliomas and other abnormalities (learning disability, vasculopathy, and bony abnormalities) | NF1 , located on chromosome 17q11.2; diminished GTPase activating (GAP) activity activating the ras signal transduction pathway | FISH, direct sequencing, long-range PCR with Southern blot analysis, and/or cytogenetic analysis | Diagnosis, classification |
| Neurofibromatosis (NF2) and schwannomatosis. NF2 involves schwannomas, meningiomas, and ependymomas | NF2 gene (chromosome 22) | Sequencing, mutation scanning, duplication/deletion testing, PCR, quantitative PCR, microarray, comparative genomic hybridization, or combination | Diagnosis, classification |
∗ Full names and functions of molecular targets (i.e., oncogenes and oncoproteins) listed in the second column may be found in the corresponding sections of this chapter describing these targets and the corresponding references.
Primary central nervous system (CNS) gliomas, originating exclusively from brain cells such as astrocytes, oligodendrocytes, and ependymal cells, account for only about 1.35% of all cancers, but rank second among the causes of death from neurologic disease. Glioblastomas are the most common primary CNS tumor and are categorized as World Health Organization (WHO) grades I to IV based on histologic characteristics. With the advent of new treatment modalities, the use of microscopic examination alone is insufficient for the histologic classification and grading of gliomas. Under the 2016 WHO classification schema, molecular and genetic data as layer 4 information supports histologic classification ( ).
Glioblastoma multiforme (GBM) consists of anaplastic malignant astrocytic tumors characterized by predominant microvascular and endothelial proliferation. The current standard of care for GBM is surgical resection, followed by radiation therapy. However, the association of specific molecular genetics not only assists in diagnosis and prognosis but also leads to the development of new adjuvant chemotherapy (e.g., temozolomide) ( ). Glioblastomas (WHO grade IV) may develop de novo (primary glioblastomas) or through progression from low-grade astrocytomas (secondary glioblastomas). The two types show similar histologic features. However, they differ in terms of molecular alterations, with primary glioblastomas showing activation of the EGFR pathway, whereas secondary glioblastomas are more commonly associated with TP53 mutations. Thus, the molecular genetics of these tumors demonstrates that they are distinct diseases and, therefore, may exhibit different prognoses and responsiveness to therapy ( ). Tyrosine kinase inhibitors, such as erlotinib and gefitinib, may offer a therapeutic option for primary tumors in which EGFR signaling is upregulated ( ).
Comprehensive genetic screens of GBM ( ) have confirmed that genetic loss is scattered across the entire genome, affecting numerous chromosomes. Loss of heterozygosity (LOH) on chromosome 10 is the most frequent genetic loss in GBM, occurring in 60% to 80% of cases. Allelic losses on 1p and 7q have also been seen in GBM, but at lower frequencies. Loss of 1p occurs in 6% to 20% of GBMs, and in combination with 19q loss may indicate a better prognosis and improved responsiveness to therapy. However, the combined loss of 1p/19q is a rare event in GBM ( ).
Gains in gene expression have also been demonstrated in GBM in the form of duplication of entire chromosomes, intrachromosomal amplification of specific alleles, or extrachromosomal amplification (often in the form of double minutes) and activating mutations. Many genes have been shown to be amplified in glioma; these include EGFR , CDK4 , SAS , MDM2 , GLI , PDGFRA , MYC , N-MYC , MYCL1 , MET , GADD153 , and cKIT . The most commonly amplified genes in glioblastoma are EGFR on chromosome 7 (in ≈40% of cases), CDK4 , and SAS (in ≈15%).
Other molecular mechanisms of gliomagenesis include loss of the DNA repair enzyme, O(6)-methyl guanine DNA methyltransferase (MGMT), which specifically removes promutagenic alkyl groups from the O6-position of guanine in DNA ( ). Expression of MGMT protects normal cells from carcinogens; however, it can also protect cancer cells from chemotherapeutic alkylating agents. This has been implicated as an important mechanism of drug resistance because it reduces the cytotoxicity of alkylating chemotherapeutic agents. Loss of MGMT expression may be caused by methylation of promoter CpG islands, which have been detected in 75% of secondary GBMs—much more frequently than in primary GBMs (36%). Immunohistochemical staining for MGMT does not offer a reliable way to stratify GBM ( ); therefore, PCR assay is necessary. TP53 is one of the more commonly studied TSGs in GBM. Loss of normal TP53 function due to mutation occurs more frequently in secondary GBM. Thus, the major molecular targets of glioblastomas are EGFR, TP53, 10q LOH, and deletion of the MGMT gene. These can be detected using RT-PCR, methylation-specific PCR, and FISH. The main clinical applications are related to prognosis and treatment response. These findings are summarized in Table 79.1 and, in greater detail, in Table 79.2 .
| Primary GBM | Secondary GBM | |
|---|---|---|
| LOH 10q | 70% | 63% |
| EGFR amplification | 36% | 8% |
| P16INK4a deletion | 31% | 19% |
| TP53 mutation | 28% | 65% |
| PTEN mutation | 25% | 4% |
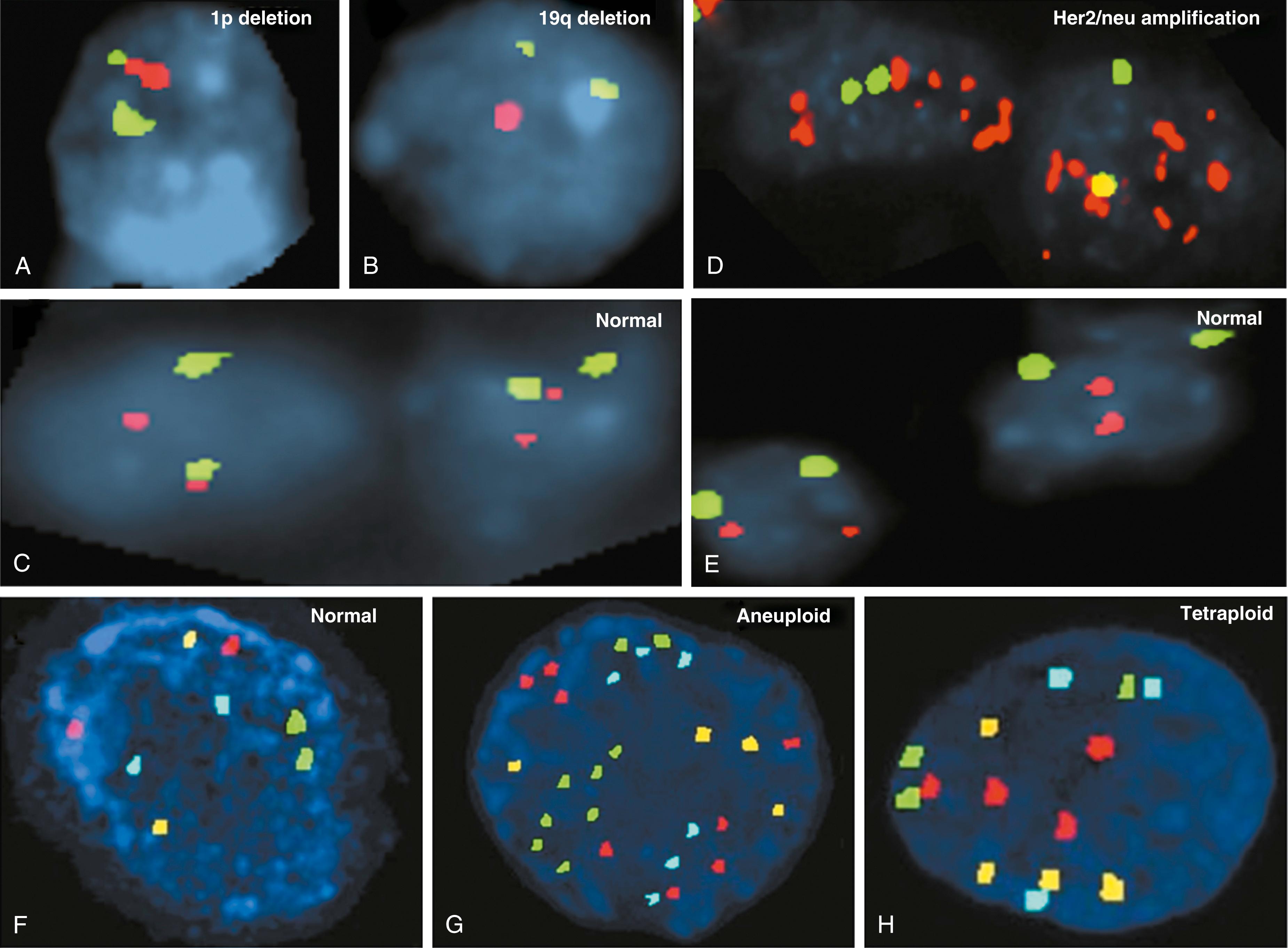
Oligodendroglioma is an infiltrating glioma of the cerebral cortex diagnostically characterized by a triad of uniformly round to ovoid nuclei, perinuclear halos, and an even distribution of cells, together with a delicate chicken wire type of vasculature. However, in a significant number of these lesions, the microscopic morphology is not so clear-cut, and distinction from other diffuse glial lesions may be difficult. Combined loss of 1p and 19q ( Fig. 79.1A to 79.1C ) is typical in oligodendroglioma ( ), and loss of 19q occurs in astrocytoma and mixed oligoastrocytoma ( ). The incidence of 1p/19q loss varies from 50% to 80% in oligodendroglioma in different studies and is 1% to 10% in other gliomas, which indicates its utility in differentiating the diagnosis. Recent studies suggest that the combined loss of 1p/19q may follow a (1;19)(q10;p10) translocation, with subsequent loss of the derivative chromosome der(1;19)(q10;p10) ( ). Identification of 1p/19q loss is associated with two unique features of tumor biology with clinical indications: first, they grow slowly, even those that are anaplastic in nature; second, they correlate with better prognosis and response to chemotherapy ( ). In terms of treatment response, initial studies suggested that 1p/19q loss is a marker of response to PCV (procarbazine, lomustine/CCNU, and vincristine) or temozolomide chemotherapy ( ). Thus, the 1p/19q status has become an important part of diagnosis, prognosis, and predicted therapeutic response of oligodendrogliomas. In addition to 1p/19q alteration, LOH mutations in p53 and p16 may be associated with poor survival or tumor progression ( ).
Nearly all 1p/19q codeleted oligodendrogliomas are also mutated on isocitrate dehydrogenases (IDHs), IDH1 or IDH2 ( ). IDH1 has been found to be mutated in a vast majority of astrocytic, oligodendroglial, and oligoastrocytic gliomas (WHO grades II to III) as well as in secondary glioblastomas (WHO grade IV). However, IDH1 mutation is very rare in primary glioblastoma and is not involved in pilocytic astrocytomas. IDH1 and IDH2 are homologous, NADP + -dependent cytoplasmic and mitochondrial enzymes, respectively. The role of these enzymes is the conversion of isocitrate to α-ketoglutarate with the simultaneous reduction of NADP + to NADPH. The most common mutation is a heterozygous point mutation with substitution of arginine by histidine at residue 132 (R132H), located in the substrate-binding site. This IDH1-R132 mutation has a reported frequency of 50% to 93% ( ; ). IDH2 gene mutations affecting the amino acid R172 are much less common than the IDH1 isoform, 3% to 5%, yet have been identified in a small subset of gliomas that lack the typical IDH1 mutation with predominance in oligodendrogliomas ( ). IDH1 and IDH2 genes appear to behave dominantly and are mutually exclusive. IDH1 mutation has been shown to be a strong, independent positive prognostic biomarker in diffuse gliomas, glioblastomas, and oligodendroglioma ( ). IDH mutation was also identified as a marker for response to temozolomide in low-grade gliomas ( ).
Rhabdoid tumors are highly malignant (WHO grade IV) embryonal CNS tumors of infants and very young children showing rhabdoid features. Similar to renal and other extrarenal rhabdoid tumors, over 90% of atypical teratoid/ rhabdoid tumors (AT/RT) demonstrate loss of all or part of chromosome 22, particularly involving 22q11.2. INI1 (hSNF5/SMARCB1/BAF47), a putative suppressor gene, is mapped to the 22q11.2 region. INI1 deletions and/or mutation have been detected in the majority of AT/RT cases. Nearly all AT/RTs demonstrate absence of the nuclear immunohistochemical expression of INII/BAF47 protein. FISH for monosomy 22, 22q and deletion or the INI1 gene are commonly utilized as adjunct molecular studies in the diagnosis of AT/RTs and other pediatric embryonal tumors ( ).
Breast cancer is the most common cancer in women and the second most common cause of cancer death in women in the United States. Until recently, breast cancer was characterized according to tumor type (ductal vs. lobular carcinoma), histologic grade (I to III), steroid hormone receptors (estrogen receptor [ER] and progesterone receptor [PR]), and Her2/neu status (positive vs. negative), along with metastasis to lymph nodes and distant organs, for its prognosis and treatment. Gene expression profiling has also been utilized as an adjunct for the management of breast cancer. Gene panel study and germline testing using NGS have been introduced to search for actionable mutations and identify genetic predisposition for counseling and screening.
Various genetic, epigenetic, and genomic changes have been associated with breast cancer. Traditionally, ER and PR status is used as a prognostic and predictive marker for breast cancer. Her2/neu (detailed in Chapter 77 ) status has been added in the past decade as a breast cancer prognostic and predictive marker. The FISH method has been used primarily to determine the copy number of the Her2/neu gene (see Fig. 79.1D and 79.1E ) for the purpose of selecting Her2-targeted therapies such as trastuzumab and lapatinib in both adjuvant and neoadjuvant settings. Enumeration of 20 interphase nuclei from tumor cells on a given case is reported as the ratio of average Her2/neu copy number to that of chromosome enumeration probe 17 for centromere, CEP17. Specimens with amplification showed a Her2/neu :CEP17 signal ratio ≥2.0 as abnormal and a ratio <2.0 as normal. A three-color FISH assay has been commercialized for assessment of standalone prognosis in ER-positive and ER-negative stage I breast cancers ( ). In addition, HercepTest (Dako Corp, Carpinteria, CA), an immunohistochemistry (IHC) test for Her2/neu using a polyclonal antibody, was the first approved by the U.S. Food and Drug Administration (FDA). Subsequently, Pathway (Ventana Medical Systems, Tucson, AZ) produced a monoclonal antibody (CB11) for Her2/neu for the same response indication, which has also been FDA-approved and is used in combination with FISH ( Fig. 79.2 ). IHC is widely used to measure prognostic factors, including ER, PR, Her2/neu , and the proliferation marker, Ki-67, and to predict response to hormonal and Her2-targeted therapies ( ).
Concerns associated with IHC-based assays for multiple markers include the nonlinear nature of IHC staining, the different subcellular localizations of different markers, and the impact of different slide-scoring thresholds for different antibodies. The introduction of whole-genome profiling technologies has greatly expanded our knowledge of the genomic pathways associated with the development and progression of breast cancer ( ). These research results have led to the development of several commercialized tests with multigenes/proteins as prognostic and predictive tools for clinical application using IHC, FISH, RT-PCR, and genomic microarray technologies with integrated bioinformatic/statistical algorithms designed to calculate disease recurrence and patient survival ( ).
Molecular classification of breast cancer has been proposed, based on gene expression profiling, to include luminal-like, normal-like, Her2-positive, and basal-like subtypes of invasive breast cancer ( ). It is important to note that all of the luminal groups of breast cancers are ER positive, and nearly two-thirds are of low or intermediate histologic grade, whereas 95% of all ( ) basal-like cancers are ER negative and 91% of these tumors are of high grade ( ). Studies of clinical outcomes based on molecular subtypes have shown that luminal A is more sensitive to antiestrogen than is luminal B. Her2-positive responds to trastuzumab, an anti–Her2 antibody, whereas basal is more aggressive (but is especially sensitive to anthracycline-based neoadjuvant chemotherapy) than are the luminal breast cancers ( ). Basal-like and Her2-positive subgroups are associated with the highest rates of pathologic complete response to neoadjuvant multiagent chemotherapy ( ).
Several commercially available assays have been developed to predict the risk for recurrence. Prosigna Breast Cancer Assay, developed by NanoString, is based on the prediction analysis of the microarray-50 (PAM50) gene expression signature. The PAM50 gene signature measures the expression levels of 50 genes in a surgically resected breast cancer sample to classify a tumor as one of four intrinsic subtypes (Luminal A, Luminal B, HER2-enriched, and Basal-like). This test outputs a risk of recurrence (ROR) score in these intrinsic subtypes. Prosigna uses multiplexed gene-specific fluorescently labeled probe pairs to measure gene expression in frozen or formalin-fixed paraffin-embedded (FFPE) tissues with equivalent ease and efficiency ( ). Clinical trials demonstrated its utility in prognostic information of distant recurrence in hormone receptor–positive, postmenopausal breast cancer patients treated with endocrine therapy ( ) and in node-positive patients ( ). The other two gene microarray-based clinical tests are the OncotypeDx and MammaPrint assays ( Fig. 79.3 ) ( ; ). Oncotype Dx (Genomic Health, Inc., Redwood City, CA) is a 21-gene multiplex prognostic and predictive RT-PCR assay performed on primary FFPE breast cancer samples. The original 16 informative genes ( ) that calculate the recurrence score (RS) were discovered on archived FFPE samples by transcriptional profiling and then were converted to the FFPE RT-PCR assay ( ). OncotypeDx determines the 10-year risk for disease recurrence in patients with ER-positive, lymph node–negative tumors by using a continuous variable algorithm and assigning a tripartite RS (17, low risk; 18–30, intermediate risk; >30, high risk). Of the multiple pathways assessed by this assay, the proliferation and ER pathways are the most influential in RS calculation, followed by the Her2 pathway. High relative levels of ER messenger RNA (mRNA) and low levels of Ki-67 proliferation gene mRNA have a low RS. Low levels of ER mRNA and high levels of Ki-67 mRNA have a high RS. The other 14 informative mRNA levels play their greatest roles in determining the RS in tumors with intermediate ER and Ki-67 mRNA levels. It should also be noted that OncotypeDx is best suited for detecting breast cancers with a low potential for recurrence. The MammaPrint assay (Agendia BV, Amsterdam, The Netherlands) was the first fully commercialized microarray-based multigene assay for breast cancer. This test is offered as a prognostic test for lymph node–negative breast cancer in women younger than 61 years of age with an ER-positive or ER-negative tumor. The prospective RASTER trial showed that MammaPrint testing could spare from chemotherapy 94 of 295 patients (32%) for whom chemotherapy would have been recommended by a standard (non–gene-based) decision tool ( ). The clinical utility of the assay supported the test to be the first assay approved by the FDA’s new in vitro diagnostic multivariate index assay classification. Like Prosigna and OncotypeDx, both fresh-frozen and FFPE tissues can be used for analysis. The 70 genes that make up the MammaPrint assay are focused primarily on proliferation. Additional genes are associated with invasion, metastasis, stromal integrity, and angiogenesis. Of note, the OncotypeDx and MammaPrint assays have only one gene in common—the SCUBE2 gene, which is a member of the ER pathway. Unlike the OncotypeDx test, which features a continuous RS result, the MammaPrint test uses a dichotomous “high risk versus low risk” result format. The MammaPrint test does not include ER, PR, or Her2 in the 70-gene microarray.

Multigene testing procedures such as disease classifiers and prognostic and predictive markers have been introduced with the use of slide-based methods (e.g., IHC, FISH) or molecular platform–based methods such as quantitative multiplex PCR and genomic array profiling. Morphologically based mRNA extraction performed with the use of tissue macrodissection or microdissection is recommended for analysis as it is highly enriched for invasive carcinoma and is not diluted with cells from benign tissues and in situ carcinoma areas ( ). The heterogeneous expression of important mRNAs—such as ER, Her2, and Ki-67, often reflected in the varying histologic grades seen in larger tumors—can influence the predictive accuracy of transcriptional profiling measurements. One caveat is that, although the number of genes that can be simultaneously assessed by multiplex qRT-PCR is significantly greater than that for IHC, multiplex qRT-PCR requires a more complex statistical evaluation of the gene expression profiles. Nevertheless, the RT-PCR technique has been used to predict overall prognosis and response to both hormonal and cytotoxic therapies.
High-throughput NGS has made it possible to sequence large numbers of genes to identify actionable mutations in the tumor. The diagnostic yield of breast NGS has been studied but is difficult to define and measure ( ). Sequencing of 46 cancer-related genes in 415 breast cancer samples (including some primary-metastatic pairs) revealed somatic nonsynonymous mutations in 220 of 354 patients (62.1%) ( ). A systematic multinational study of NGS profiling in breast cancer is underway ( ).
Hereditary breast cancer (HBC) accounts for approximately 10% of all breast cancers. Positive family history is the strongest risk factor, as it is present in about 20% of breast cancer cases. Population-based studies indicate that 15% of familial risk can be attributed to BRCA gene mutations and that a further 10% involve TP53 , phosphate and tensin homologue ( PTEN ), serine/threonine kinase 11 ( STK11 ), cadherin 1 ( CDH1 ) and ATM . The rest may be explained by a polygenic model. A significant number of HBC-prone families are characterized by the more common hereditary breast-ovarian cancer syndrome. The BRCA genes operate as TSGs, and germline mutations affecting one allele of BRCA1 or BRCA2 confer susceptibility to breast and ovarian cancers. The cumulative risk for developing breast cancer by 70 years of age approaches 50% to 70% in BRCA1 -mutation carriers; for ovarian cancer, the risk is 30% to 40% ( ). For BRCA2- mutation carriers, the risk is 40% to 50% for breast cancer and 10% to 15% for ovarian cancer. However, individuals who carry BRCA2 mutations also have an increased risk for other cancers, including those of the male breast (≈75–fold relative risk), pancreas (fourfold to eightfold), and prostate (twofold to fourfold) ( ). The spontaneous instability of chromosome structure and number is a hallmark of BRCA -deficient cells and arises from the distinct cellular functions performed by the BRCA1 and BRCA2 proteins in DNA repair and mitotic control. Since the discovery of BRCA genes and the development of methods for mutation screening, identification of BRCA -mutation carriers during the clinical management of familial cancer cases has become widespread. Gene expression profiling has revealed that tumors from patients who are BRCA1 -mutated segregate within the basal subgroup of breast cancers ( ) of the five different molecular groups, as discussed earlier. Tumors from BRCA1 -mutation carriers are likely to stain positive for basal cytokeratins 5/6 and 14, and to exhibit negative estrogen receptor staining. These breast carcinomas also commonly stain for EGFR. BRCA1 -associated tumors are commonly of high grade and are p53 -mutated. They have a tendency to occur in younger women and carry a poor prognosis ( ).
Thyroid cancer is the most common endocrine neoplastic condition. The two most frequent types of thyroid malignancy derived from follicular cells are papillary and follicular carcinomas, which constitute approximately 80% and 15%, respectively, of all thyroid cancers. These follicular cell–derived tumors are well differentiated, in contrast to poorly differentiated and anaplastic carcinomas, which constitute about 2% of cases. Another malignancy of the thyroid is medullary carcinoma, which originates from parafollicular C cells and accounts for approximately 3% of cases. Several genetic alterations in various thyroid tumors have been well documented, including translocations and point mutations. The genetic alteration in thyroid cancer involves BRAF , RAS , RET , and PAX8 .
Recent studies have characterized papillary thyroid carcinoma (PTC) into two classes: “BRAF-V600E-like” and “RAS-like.” These mutations are mutually exclusive ( ).
RAF, as noted earlier (see Chapter 77 ), is a direct target of ras-p21. The BRAF gene mutation is the most commonly known genetic alteration in papillary carcinoma, found in approximately 45% of these tumors ( ). A majority of mutations involve T1799A transversion mutation in exon 15 of the gene, which causes amino acid change from valine to glutamic acid at amino acid residue 600 (V600E), leading to constitutive activation of BRAF kinase, subsequent phosphorylation of MEK and ERK protein kinases (see Chapter 77 ), and downstream effectors of the mitogen-activated protein kinase (MAPK) pathway, as is summarized in Chapter 77 (see Fig. 77.1 ). It is believed that BRAF-V600E-like tumors carry profound downregulation of expression of thyroid differentiation markers ( ). Other mechanisms of BRAF activation, although rare, include K601E point mutation, small in-frame insertions or deletions surrounding codon 600, and AKAP9-BRAF rearrangement, which is more commonly associated with radiation exposure papillary carcinoma. BRAF V600E mutation is highly prevalent in papillary carcinoma with classical histology and in the tall cell variant while tumors that harbor the K601E BRAF mutation typically have the follicular variant of papillary carcinoma histology ( ) and are associated with a low recurrence risk ( ). The BRAF V600E mutation has been correlated with aggressive features such as extrathyroid extension, advanced tumor stage at presentation, recurrence, and lymph node involvement and/or distant spread ( ), conferring an intermediate risk of disease recurrence ( ). This mutation also occurs in 20% to 40% of poorly differentiated thyroid carcinomas and 30% to 40% of anaplastic thyroid carcinomas. Many of these carcinomas also reveal areas of well-differentiated papillary cancer, and BRAF V600E is present in both tumor components, which suggests that this mutation is an early event that predisposes the tumor to dedifferentiation ( ). Cancers with these mutations have decreased ability to trap radioiodine, which leads to treatment failure and more aggressive tumor behavior ( ).
Detection of the BRAF mutation can be achieved by direct sequencing, colorimetric mutation detection assay based on shifted terminal assay ( ), real-time PCR, and allele-specific SYBR green PCR on an FNA sample, which is virtually diagnostic of papillary carcinoma. The BRAF mutation represents an attractive therapeutic target for papillary carcinoma with the advent of various BRAF inhibitors (e.g., BAY43-9006) ( ; ).
Human HRAS , KRAS , and NRAS genes encode the ras-p21 proteins, which are highly conserved in sequence from yeast to humans and are strongly homologous in amino acid sequence to one another. As described in Chapter 77 , the ras-p21 protein is a G-protein, that is, a guanosine triphosphate (GTP)–binding protein that is activated when GTP binds in place of guanosine diphosphate (GDP). The exchange is promoted by the SOS or GNEF (guanine nucleotide exchange factor) that, in turn, is activated when a tyrosine kinase–containing receptor binds to its growth factor. The activated tyrosine kinase binds to the growth factor receptor–bound (grb) adapter protein that binds simultaneously to SOS-GNEF, activating it to bind to ras-p21 thereby promoting GTP-GDP exchange. To be active, ras-p21 must bind to the inner cell membrane in a covalent link between a farnesyl moiety in the membrane and the thio group of Cys 186. In its active form, ras-p21 binds directly to raf, thereby activating the raf-MEK-MAP kinase (or ERK) pathway as described in Chapter 77 . Simultaneously, it activates phosphatidylinositol-3-hydroxy kinase (PI3K), which activates AKT. Unlike BRAF V600E-driven tumors, RAS-like tumors typically preserve the expression of thyroid differentiation genes ( ). Point mutations involving several specific sites (codons 12, 13, and 61) in NRAS and HRAS are more common in thyroid cancer. They are found in 10% to 20% of cases of papillary carcinoma, 40% to 50% of follicular carcinomas, and 20% to 40% of poorly differentiated and anaplastic carcinomas. Among papillary carcinomas, virtually all tumors that harbor a RAS mutation grow, forming neoplastic follicles and no papillary structures. Therefore, they are diagnosed as the follicular variant of papillary carcinoma ( ).
RAS mutations are also found in 20% to 40% of benign follicular adenomas ( ). The finding of this mutation in benign adenomas as well as in follicular-patterned carcinomas suggests that RAS-positive follicular adenomas may serve as a precursor for RAS-positive follicular carcinomas and the follicular variant of papillary carcinomas. Furthermore, RAS mutations may predispose well-differentiated cancers to dedifferentiation, resulting in anaplastic tumors. With regard to the rare but important cribriform morular variant of papillary carcinoma, nuclear β-catenin accumulation is essentially a defining feature that is diagnostically invaluable.
The RET/PTC gene rearrangement is another prominent genetic alteration that plays a role in the pathogenesis of up to 20% of sporadic PTCs ( ). The RET gene, located on chromosome 10q11.2, encodes the tyrosine kinase receptor consisting of an extracellular ligand-binding domain for the glial-derived neurotrophic factor family of growth factors, a cysteine-rich region, a transmembrane domain, and an intracellular tyrosine kinase domain. RET is highly expressed in parafollicular C cells and at very low levels in thyroid follicular cells ( ). RET/PTC rearrangements are a very early event in thyroid cancer development; this would explain their high prevalence in occult or microscopic PTC. Twelve forms of RET/PTC rearrangements have been reported to date, linking the 3′ portion of the RET gene with the 5′ portion of various different genes of the PTC family ( ), the two most common of which are PTC1 ( H4 ) and PTC3 ( ELE1 , ARA70 , NCOR4 ), which account for up to 70% and 30% of rearrangements in sporadic PTC, respectively ( ). In particular , RET/PTC rearrangements are more frequent in individuals exposed to ionizing radiation (50%–80%) ( ), with the exception of ELKS-RET (ELKS is a polypeptide sequence that is rich in glutamic acid [E], leucine [L], lysine [K], and serine [S]) and HOOK3RET fusion (HOOK3 encodes the protein Hook homolog 3). RET/PTC rearrangements are also more frequent in children (40%–70%) ( ) as compared with the general population (15%–30%). Papillary carcinomas with RET/PTC gene rearrangements typically present in younger individuals and exhibit a high rate of lymph node metastasis with classical papillary histology and lower stage at presentation, particularly in cases harboring RET/PTC1 ( ). In tumors arising after radiation exposure, RET/PTC1 was found to be associated with classic papillary histology, whereas the RET/PTC3 type was more common among solid variants ( ).
Activated RET kinase has been explored as a target of therapy using TKIs such as ZD6474 ( ). The distribution of RET/PTC rearrangements within each tumor may vary from involving almost all neoplastic cells (clonal RET/PTC ) to being detected only in a small fraction of tumor cells (nonclonal RET/PTC ) ( ). This heterogeneity is a potential problem in RET receptor–targeted therapy.
Most of the frequent genetic alterations in follicular carcinoma are RAS point mutations and PAX8-PPARG rearrangements, altering the PI3K/ AKT signal pathway. Several studies have reported the utility of molecular testing in diagnosing this type of thyroid cancer preoperatively on FNA specimens.
A RAS mutation is found in 40% to 50% of conventional follicular carcinomas and in 20% to 40% of adenomas. It is interesting to note that the most common ras mutations, instead of occurring at codon 12, as in most other cancers, occur at codon 61 for NRAS and HRAS ( ). These mutations are associated with tumor dedifferentiation, a less favorable prognosis, and metastasis to bone ( ). Furthermore, RAS mutations are potentially transformative as indicated by a 20% to 40% prevalence in poorly differentiated and undifferentiated carcinoma.
The PAX8-PPARG gene rearrangement is a result of the translocation t(2;3)(q13;p25). It leads to a fusion between the PAX8 gene, which encodes a thyroid-specific paired domain transcription factor, and the PPARG gene. It occurs in 35% of conventional follicular carcinomas and has a lower prevalence in oncocytic (Hürthle cell) carcinomas ( ). This translocation may also be seen in up to 13% of follicular adenomas and approximately 5% of follicular variants of papillary carcinoma. Tumors harboring PAX8-PPARG rearrangements tend to present in a younger age group, are smaller, and exhibit solid or nested patterns and more frequent vascular invasion. This rearrangement results in overexpression of the PPARG protein that is detectable on IHC ( ). This molecular overlap between follicular adenoma, follicular carcinoma, and follicular variant of papillary thyroid carcinoma thus likely reflects a biological continuum.
Medullary thyroid carcinoma (MTC) is a sporadic malignancy in a majority of cases (75%), with the remainder spanning three familial syndromes, including multiple endocrine neoplasia (MEN 2A and MEN 2B) and familial MTC (FMTC). In MTC, RET is activated by point mutations in contrast to its activation by chromosomal rearrangement in PTC. In sporadic medullary carcinomas, somatic mutations of RET are found in 20% to 80% of cases without a germline mutation. Germline mutations in RET are found in almost all patients with familial forms of medullary carcinoma; this correlates with the aggressiveness of MTC. However, it must be noted that sporadic tumors may also harbor RET mutations (30%–66%) as well as HRAS or KRAS mutations (up to 25%).
Thyroid-specific gene panels using microarray or NGS have also been developed ( ). The tumor profile data, including telomerase reverse transcriptase (TERT) gene promoter mutations may improve individualized patient management.
Lung cancer remains the most common cause of cancer-related death among men and women worldwide. Epithelial tumors of the lung are of four principal types: squamous cell carcinoma, adenocarcinoma, large cell carcinoma, and small cell carcinoma. Clinically, the most important distinction is noted between small cell carcinoma and the others, which can be grouped together as non–small cell carcinoma. Small cell cancers respond to chemotherapy, whereas non–small cell lung cancers (NSCLCs) are removed surgically if possible. However, with the rapid advancements in the understanding of NSCLCs and the corresponding growth in available molecularly targeted therapy, recognition of specific molecular alterations is required for optimal patient care.
EGFR mutations have been identified in many human cancers, such as lung, breast, head and neck, colorectal, pancreatic, and bladder cancers; glioma; squamous cell carcinoma; Wilms tumor; and certain NSCLCs (adenocarcinoma). EGFR is a membranous oncoprotein that induces cell proliferation upon activation. Binding of a ligand to a receptor activates its tyrosine kinase activity, which phosphorylates several substrates on a number of signal transduction pathways, leading to increased signaling activity and activation of downstream effectors. This results in uncontrolled cell proliferation, tumor invasion, angiogenesis, and resistance to normal apoptotic signals (see Chapter 77 ). Increased EGFR signaling can be the result of EGFR gene amplification, protein overexpression, or specific activation mutations in the EGFR gene, as described in detail in Chapter 77 .
It has been shown that a subset of patients with NSCLC (10%–40%) ( ) have specific activating mutations in the EGFR gene ( Fig. 79.4 ) that are associated with increased sensitivity to TKIs, such as gefitinib (Iressa) or erlotinib (Tarceva), targeting EGFR in lung cancer. Common activating EGFR mutations occur most often in exons 18 to 21 and cluster in two major hot spots. The prototype mutation L858R in exon 21 (40% of EGFR mutations) encoding the tyrosine kinase domain and small in-frame deletions in exon 19 (>50%) are reportedly most often constituting up to 90% and are associated with responses to EGFR TKI therapy. Mutations in exons 18 and 20 account for the remaining 10% of EGFR mutations in NSCLC ( ). Recent guidelines suggest that routine EGFR assays should include EGFR exon 19 for deletions and insertions, EGFR exon 18 for E709 and G719 mutations; exon 20 for S768I, T790M, and insertions; and exon 21 for L858R, T854, and L861Q mutations. Clinical EGFR mutation testing should be able to detect all individual mutations that have been reported with a frequency of at least 1% of EGFR-mutated lung adenocarcinomas ( ). Studies have reported that EGFR gene mutations are more common among females, Asians, and nonsmokers with adenocarcinoma; these are the same groups that have the highest response rates to TKIs ( ). However, recent guidelines ( ) suggest that EGFR molecular testing should be done in all patients with lung adenocarcinoma component. T790M mutation detection is required by 2018 guidelines reversion for relapse cases ( ).
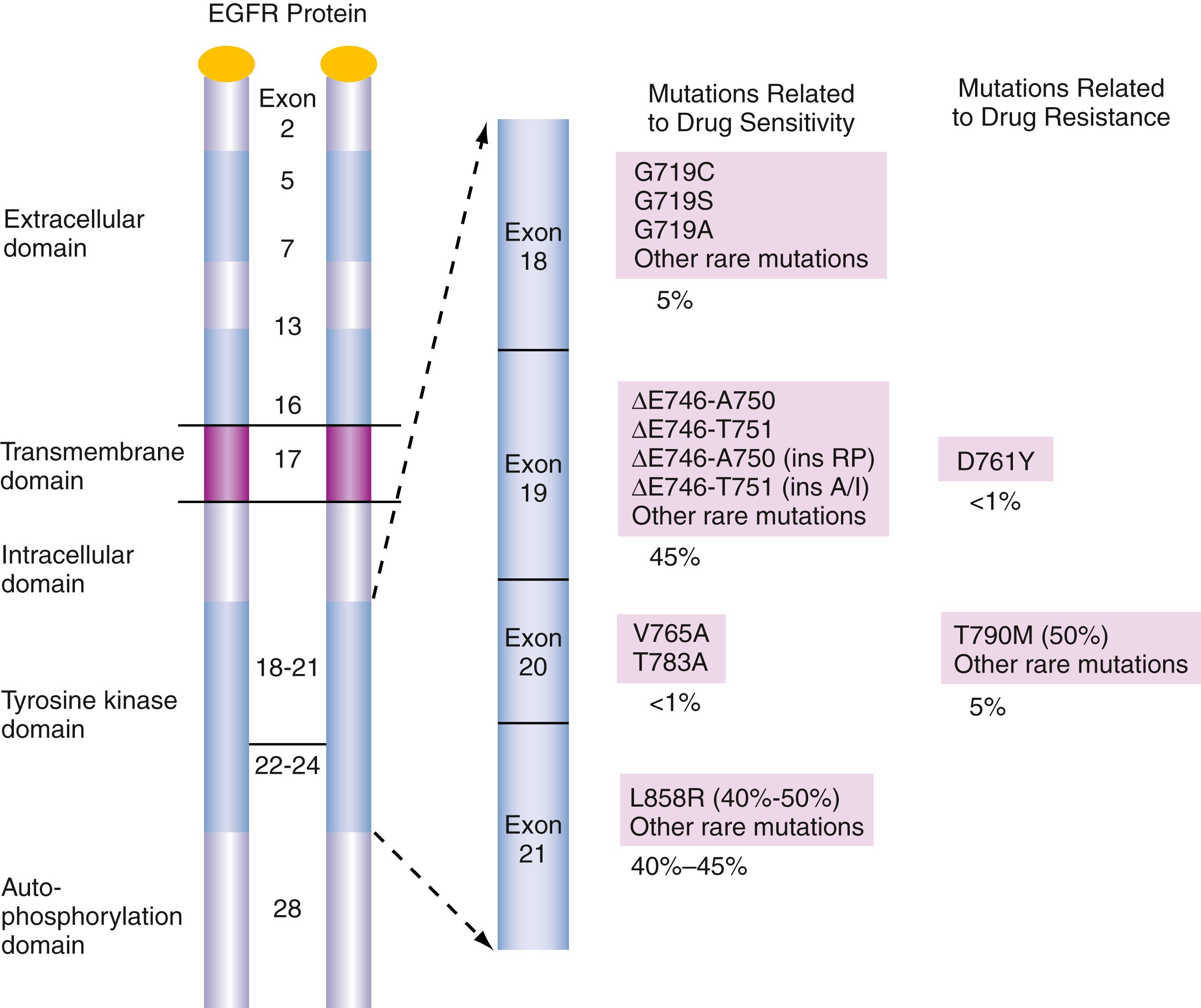
Immunohistochemistry for total EGFR and EGFR copy number analysis (i.e., FISH or chromogenic in situ hybridization) are not recommended for selection of EGFR TKI therapy according to recent guidelines. Recent studies have demonstrated that in patients with EGFR mutations, the EGFR locus is often concurrently amplified.
ALK gene chromosomal rearrangement is found in another ∼7% of lung adenocarcinomas, most commonly in the form of an intrachromosomal inversion leading to the EML4-ALK fusion product associated with ALK protein overexpression. This mutation is present more frequently in lung adenocarcinomas from younger patients with either no or only light smoking history ( ; ). However, recent guidelines suggest that ALK molecular testing should be done in all patients with a lung adenocarcinoma component. Some studies have reported associations with solid, mucinous cribriform and/or signet ring histology ( ; ). The translocation is infrequent in pure squamous cell carcinoma but has been reported in adenosquamous carcinoma ( ). Patients with this tumor type are responsive to therapy with the multitargeted TKI crizotinib ( ). FISH using break-apart probes is currently considered the gold standard for detection of ALK rearrangements ( ). Polysomy (multiple copies) at the ALK locus is common in lung adenocarcinoma and, when present, confirms that FISH has been performed in a tumor cell population. ALK immunohistochemistry has been included as an alternative to FISH by 2018 updated guidelines ( ). However, current evidence suggests that it does not predict response/resistance to targeted therapies. RT-PCR is not recommended as an alternative to FISH for selecting patients for ALK inhibitor therapy ( ).
Recent recommendations for EGFR and ALK testings are as follows: EGFR and ALK testing is not recommended in lung cancers that lack any adenocarcinoma component, such as “pure” squamous cell carcinomas, “pure” small cell carcinomas, or large cell carcinomas lacking any IHC evidence of adenocarcinoma differentiation. EGFR and ALK testing are the most important uses of the diagnostic sample after a diagnosis of adenocarcinoma is established. If specimens are insufficient for molecular testing, patients may need to undergo another invasive diagnostic procedure before they can be treated. NGS enables the use of small specimens for multiple molecular marker detection, avoiding the risks associated with obtaining surgical biopsies. Cell-free circulating DNA can be used for T790M detection in relapse ( ). EGFR and ALK results should be available within 2 weeks (10 working days) of receiving the specimen in the testing laboratory ( ).
Mutations in KRAS , frequently in codons 12 and 13, have been reported in up to 30% of cases of lung adenocarcinoma ( ). These mutations are usually found in cancers of smokers and are more common in adenocarcinoma (30%–50%) than in NSCLC (15%–20%). Conversely, oncogenic mutations in the KRAS gene were reportedly associated with resistance to EGFR-TKIs in metastatic colorectal cancer. Thus, testing for KRAS mutations as a negative predictor of response to EGFR TKI has become part of molecular diagnostic algorithms for lung adenocarcinoma in many centers. However, more recent data suggest that EGFR wild-type tumors have less favorable outcomes if they are treated with EGFR TKI than if they are treated with conventional platinum-based chemotherapy. Therefore, KRAS mutation testing is not recommended as a sole determinant of anti-EGFR TKI therapy ( ).
Lung adenocarcinomas contain a number of other less common alterations that may lead to treatment with targeted inhibitors. These include RET (∼2% of lung adenocarcinomas) ( ), increased copies of MET ( ), and sequence-altering mutations in ERBB2 ( HER2 ), BRAF , and PIK3CA ( ) as well as chromosomal rearrangements involving ROS1 (∼2% of lung adenocarcinomas; may respond to treatment with crizotinib). In fact, ROS1 mutation detection has been strongly recommended for all lung cancer patients regardless of clinical characteristics according to the updated guidelines. According to the updated guidelines from Association for Molecular Pathology (AMP), the College of American Pathologists (CAP) and the International Association for the Study of Lung Cancer (IASLC), multiplexed genetic sequencing panels (e.g., NGS testing with detection of BRAF , ERBB2 , MET , RET , and KRAS gene alterations) are preferred over multiple single-gene tests to identify other treatment options beyond EGFR , ALK , and ROS1 ( ).
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third most common cause of cancer death worldwide. Major risk factors include chronic viral infection (hepatitis B virus [HBV] and hepatitis C virus [HCV]), alcoholic/nonalcoholic liver disease, environmental carcinogens (e.g., aflatoxin B1), and inherited genetic disorders (e.g., Wilson disease, hemochromatosis, α 1 -antitrypsin deficiency, tyrosinemia) ( ; ; ; ). The development of HCC is a multistep process, comprising hyperplastic change, dysplasia, early HCC, and well-developed HCC in the setting of chronic hepatitis or cirrhosis ( ; ). A small percentage of HCC develops in patients with nonalcoholic steatohepatitis (∼14%), with 37% with no cirrhosis ( ).
The genomic abnormality of HCC is heterogeneous, largely as the result of various molecular mechanisms of carcinogenesis related to different causes and multifactorial processes ( ). Two major mutually exclusive subtypes of HCC are classified by CTNNB1 (the gene encoding β-catanine) and p53 mutations. Tumors with CTNNB1 mutations are featured with nuclear β-catenin staining and downregulation of the interleukin (IL)-6/JAK-STAT pathway. They are larger well-differentiated tumors with microtrabecular and pseudoglandular architecture. The tumors are cholestatic and are not associated with significant inflammatory infiltrate. Tumors with p53 mutations are related to activation of the PI3K/AKT pathway. They are poorly differentiated, highly proliferative, massive tumors with macrotrabecular architecture and pleomorphic histology, often with sarcomatous and multinucleated components. They have a high propensity for both macrovascular and microvascular invasion. In addition, somatic mutations with TERT are frequent genetic alterations in dysplastic nodules and HCC ( ; ).
Detailed molecular findings in various subtypes of HCC include the following. (1) Scirrhous HCC has been found to express the tuberous sclerosis genes, TSC1 and TSC2 , that promote epithelial to mesenchymal transition (transforming growth factor-β, VIM) and PI3/AKT pathway activation. CTNNB1 is not activated. (2) Steatohepatitic HCC is associated with IL-6/JAK-STAT involvement. It is also featured with lack of Wnt/β-catenin pathway activation. (3) A macrotrabecular massive variant of HCC has been found to express Tp53 mutations, FGF19 amplifications, ATM mutations, angiogenesis activation consisting of upregulation of the ANGPT2 gene, and vascular endothelial growth factor (VEGF) A expression. (4) Carcinosarcomas have been found to have overexpressed or mutant Tp53 involvement, which is found in both the carcinoma and sarcoma parts, PI3K involvement in the carcinoma part and FGFR3 involvement in the sarcoma sections. Activation of protein kinase A resulting from deletion on Chr 19 is reported in (5) fibrolamellar HCC, which leads to fusion between the DNAJB1 gene and the PRKACA gene ( ). Homologous recombination of telomeres has been involved in the pathogenesis of (6) chromophobe HCC. Wnt/β-catenin and transforming growth factor-β signaling are reported to be significantly activated in mixed (7) HCC–cholangiocarcinoma (CCA). (8) A stem cell subclass of HCC is associated with the MYC, mTOR, and NOTCH signaling pathways. Upregulation of the insulin-like growth factor 2 pathway and genes implicated in liver progenitor cells ( PROX1 , HNF-1β , FOXA1 , FOXA3 ) may also be involved ( ; ; ).
In addition, differential expression of miRNA in HCC has been reported to have prognostic value and pathogenesis significance ( ; ). The only curative therapy for HCC is surgical resection or liver transplantation. PD-L1 tumoral expression is observed in 16% of HCC from recent reports ( ). However, the identification of accurate predictive markers and/or immune classifiers to select ideal candidates for immunotherapy is still under investigation ( ).
Using genome-wide expression profiling analysis six, five, eight, and nine samples were analyzed for HBV, HCV, AC, and NAFLD HCC, respectively. The regulatory networks for HBV HCC revealed E2F1 as activated UR, regulating genes involved in cell cycle and DNA replication, and HNF4A and HNF1A as inhibited UR. In HCV HCC, interferon-γ, involved in cellular movement and signaling, was activated, while IL1RN, mitogen-activated protein kinase 1 involved in IL-22 signaling and immune response, was inhibited. In alcohol consumption HCC, ERBB2 involved in inflammatory response and cellular movement was activated, whereas HNF4A and NUPR1 were inhibited. For HCC derived from nonalcoholic fatty liver disease, miR-1249-5p was activated, and NUPR1 involved in cell cycle and apoptosis was inhibited. The prognostic value of representative genes identified in the regulatory networks for HBV HCC can be further validated by an independent HBV HCC data set established in our laboratory with survival data ( ).
A subtype of HCC usually occurs in children and young adults in the noncirrhotic liver. Histologically, it is identified by a proliferation of large hepatocytes with abundant granular eosinophilic cytoplasm and round nuclei with prominent, centrally placed nucleoli. Recent studies have identified activation of protein kinase A as the defining molecular feature of this tumor, the vast majority of which demonstrate fusion of the DNAJB1 and PRKACA genes (Graham et al., 2015). A separate genetic defect in the regulatory subunit of the protein kinase A complex, PRKAR1A, was also recently identified as the underlying genetic defect in a small subset of cases, some of which are associated with the Carney complex.
Hepatic adenoma (HA) is an uncommon, benign, hepatic neoplasm that typically occurs in women of childbearing age, often with a history of long-term use of oral contraceptive drugs. Molecular studies have revealed that HAs bear several recurrent mutations that involve unique molecular pathways that are distinct from hepatocellular carcinoma. In a recent review, hepatic adenoma has been reclassified from the four groups that were originally proposed by the French Bordeaux collaborative group into 6 groups, based on the molecular characteristics, as determined by IHC markers and their correlation with histologic features. These six subtypes are (1) HAs with inactivating mutations of hepatocyte nuclear factor 1a (HNF1A; HCA-H), either somatic or germline steatosis; (2) HAs with activating mutations of β-catenin gene (HCA-B) in exon 3; (3) HAs with activating mutations of β-catenin gene (HCA-B) in exons 7 and 8; (4) HAs with inflammatory features and associated with activation of JAK/STAT pathway—these neoplasms may also harbor β-catenin mutations (HCA-I); (5) HAs with activation of sonic hedgehog signaling (sh-HCA); and (6) unclassified HAs that have no specific gene mutations or unique morphologic features (HCA-U) ( ; ).
TCF1 (transcription factor 7, also known as HNF1A ) is a tumor suppressor gene involved in liver tumorigenesis. It is located on the long arm of chromosome 12. It encodes transcription factor HNF1, which, in the liver, is implicated in hepatocyte differentiation and is required for expression of certain liver-specific genes, including albumin, b-fibrinogen, and α 1 -antitrypsin ( ). The French Bordeaux group showed that HAs with biallelic inactivating mutations in the TCF1 ( HNF1A ) gene constituted a homogenous, morphologically distinct group that comprises 35% to 40% of all HAs, and classified the group as HNF1A-mutated hepatic adenomas ( HA-H ). In most of this subtype of HAs (about 85%), mutations are somatic in origin. However, in a few cases, one mutation is somatic and the other is germline in origin. Furthermore, heterozygous germline mutations in the TCF1 gene have been associated with occurrence of a rare autosomal-dominant condition, maturity-onset diabetes mellitus of youth, type 3 (MODY3), which presents in early adulthood (usually younger than 25 years of age). HNF1A mutation increases lipogenesis by promotion of fatty acid synthesis and downregulation of liver-type fatty acid–binding protein 1 (L-FABP), leading to diffuse intralesional steatosis ( ).
The β-catenin gene ( CTNNB1 ) is a component gene of the Wnt/β-catenin pathway, which is important in hepatocellular development and physiology. In normal hepatocytes, activation of the β-catenin gene is transient, followed by rapid degradation of the β-catenin protein, which is facilitated by a set of genes, including axins , glycogen S-kinase 3 ( GSK3 ), and adenomatosis polyposis coli ( APC ). β-Catenin mutations in exon 3 are associated with strong nuclear expression of β-catenin; exons 7 and 8 mutations show weak activation of β-catenin. Decreased degradation, sustained activation, and nuclear accumulation of β-catenin protein could be due to either a mutation of the β-catenin gene or mutations in the axin , APC , or GSK3 genes. Activating β-catenin mutation is reported in 15% to 19% of HA cases ( ). β-Catenin mutations have also been seen in 20% to 34% of well-differentiated HCCs ( ). Mutations bearing high expression are more frequently associated with malignant transformation ( ).
Some hepatic adenomas show sustained activation of IL-6 receptor signaling because of somatic gain-of-function mutations of the IL-6 signal transducer gene ( IL6ST ), which encodes glycoprotein-130 (gp130); gp130 is a component of the IL-6 receptor ( ). This activation of IL-6 promotes the signal transducer and activation of the JAK/STAT3 signaling pathway and induces an acute-phase inflammatory response within neoplastic hepatocytes. This is manifested as overexpression of acute-phase reactants, such as serum amyloid A and C-reactive protein (CRP), and inflammatory cell infiltration of the tumor. Such HAs are referred to as inflammatory HA (HA-I) and comprise 30% to 35% of all HAs ( ). Somatic mutations of the IL6ST gene are seen in about 60% of the HA-I subtype. About 10% of HA-I cases show coexistent β-catenin mutations. Historically, the HA-I subtype was referred to as telangiectatic focal nodular hyperplasia and was thought to belong to the focal nodular hyperplasia (FNH) family. In certain studies, the telangiectatic focal nodular hyperplasias are monoclonal neoplasms that are morphologically and biologically closer to HAs and included within the subgroup HA-I in the new classification scheme ( ; ). This classification is clinically relevant because it identifies a subset of HAs, specifically HA-B, with increased potential for malignant transformation ( ; ).
Furthermore, it helps in identifying cases for which genetic counseling is recommended in the subset of patients with hepatic adenomatosis that shows a steatotic morphology resembling HNF1A-mutated adenomas. This familial form of hepatic adenomatosis involves germline mutations of HNF1A and is associated with maturity-onset diabetes mellitus of youth, type 3 (MODY3). Hence, the rationale for genetic counseling is to search for germline mutations of HNF1A in the family. Reclassification of telangiectatic FNH as HA-I, based on molecular genetics and proteomic profiling, has added to the morphologic and molecular heterogeneity of HAs ( ).
Cholangiocarcinomas are tumors arising from the biliary tract that can be intrahepatic or extrahepatic. Based on histology, they are divided into large-duct and small-duct type (also referred to in the literature as cholangiolar and, occasionally, cholangiocellular ). Small-duct type tumor is characterized by low-grade cytologic atypia, anastomosing cords, and glands resembling cholangioles or canals of Hering. Immunohistochemically, CD56 (also known as neural cell adhesion molecule [NCAM]), N-cadherin, and CRP expression have been thought of as combined hepatocellular-choriocarcinoma with stem cell features or distinct liver tumor. However, a recent study of identified cholangiolocellular carcinoma based on genomic profiling (IDH1/2 mutations, FGFR2 fusions, chromatin-remodeling gene mutations such as ARID1A, PBRM1, and copy number alterations) found that this condition is a subtype of cholangiocarcinoma ( ). In fact, IHC and molecular features of cholangiolocellular carcinoma are similar to well-differentiated intrahepatic cholangiocarcinoma.
Stomach cancer represents 1.6% of all new cancer cases in the U.S. (Surveillance, Epidemiology, and Ends Results). The average annual percent change (AAPC) from 2012 to 2016 is significantly decreased by 1.7%. A majority of gastric cancers (GCs) are adenocarcinomas, historically classified into two major groups by Lauren’s classification: intestinal type with glandular pattern, and diffuse or poorly differentiated type with a signet ring cell morphology in some cases ( ). These two types of GCs have a distinct molecular basis of carcinogenesis, and clinical and genetic characteristics. The main causes of the intestinal type include dietary habits, environmental factors, and Helicobacter pylori infection ( ). Gastric cancer risk is also associated with hereditary tumor syndromes, such as hereditary diffuse-type gastric cancer syndrome, hereditary nonpolyposis colorectal cancer (HNPCC), Li-Fraumeni syndrome, and Peutz-Jeghers syndrome (PJS; i.e., familial adenomatous polyposis [FAP] and juvenile polyposis) ( ; ).
The development of intestinal-type GC is a multistep process that proceeds from gastritis (chronic or atrophic) and related changes (e.g., pernicious anemia) to intestinal metaplasia, dysplasia, and, eventually, carcinoma ( ; ). These processes are accompanied by a series of genetic alterations. The molecular alterations occur in the early stage of carcinogenesis, including molecules leading to genetic instability, inactivation of TSGs by methylation of CpG, telomerase activation, and p53 gene loss and mutation. Elevated telomerase activity was observed in precancerous lesions ( ). The inactivation mutation of the TSG p53 (see Chapter 77 ) is also seen in 38% to 71% of GCs ( ; ; ). Mutation in p73 , a member of the p53 family, is indicated in the development of GC associated with H. pylori infection in mouse model. A high level of microsatellite instability (MSI-H) is frequently seen in intestinal-type GC, largely caused by hypermethylation of the promoter regions of mismatch repair genes (most commonly, MLH1 and MSH2 ) or gene mutations (in a small percentage of GCs) ( ; ; ). The CpG island methylator phenotype, originally found in colorectal cancer, has been detected in 24% to 47% of GCs ( ; ; ). The oncogene product Her2/neu is frequently amplified in intestinal-type GC ( ).
The molecular pathogenesis of diffuse-type GC is less clear. H. pylori infection seems not to be related to the diffuse type of GC according to epidemiologic studies. It can be part of a hereditary gastric cancer predisposition syndrome ( ). The unique molecular abnormality of diffuse-type GC distinct from intestinal-type GC is deficiency in cell-cell adhesion caused by loss or downregulation of the E-cadherin gene ( CDH1 ) as a result of genetic or epigenetic alterations. The abnormality in CDH1 can be found in the early stages of diffuse GC development. Decreased CDH1 expression is detected in 50% of diffuse-type GCs, and more than 70% of those somatic CDH1 mutations consist of complete or partial deletion of exons ( ; ). The unique molecular genetics of GC might be useful for diagnosis in that CDH1 mutations can be detected by PCR on paraffin-embedded tissue. Detection of the germline mutation in CDH1 may help in identification of carriers of asymptomatic mutations involved in the GC family syndrome and may offer an opportunity for prophylactic total gastrectomy in patients carrying the CDH1 mutation ( ). Other frequently altered genes or gene products in diffuse-type GC include the met proto-oncogene, encoding the hepatocyte growth factor receptor, and the SC-1 antigen, an apoptosis receptor ( ; ).
Currently, the most valuable predictive and prognostic factor for GC is clinical staging independent of cancer type, but it does not reflect the heterogeneity of tumor biology. Molecular biomarkers have been investigated as alternatives or supplements to the current system and have shown very promising results in predicting prognosis and therapeutic response. For example, microsatellite instability (MSI) results in an accumulation of genetic abnormalities during the early stages of gastric carcinogenesis ( ). MSI-H is shown in only 4% of stomach carcinomas, especially in well-differentiated types in elderly patients. It is usually associated with intestinal-type GC, commonly seen in the distal stomach or antrum, and is associated with less frequent local lymph node metastasis. However, whether patients with MSI-H GC have more favorable long-term survival than those with MSH-L GC (novel tumor bands with one marker)/microsatellite stable [MSS], no novel tumor bands) remains controversial ( ; ). Expression in tumor tissue of caudal-type homeobox transcription factor 2 ( CDX2 ), in combination with normal E-cadherin and nonexpression of the transmembrane protein mucin 1 ( MUC1 ), is a favorable prognostic factor for patients with GC ( ; ).
Abnormal methylation is present in multiple TSGs, such as p16 , CDH1 ( E-cadherin ), hMLH1 , RAR-beta , RUNX3 , MGMT (O6-methylguanine methyltransferase), TSP1 (thrombospondin-1), HLTF (helicase-like transcription factor), and RIZ1 (retinoblastoma protein-interacting zinc finger gene-1). A 12-gene study has shown that methylation changes are more frequently observed in stages III/IV cancers compared with stages I/II cancers ( ). Overexpression of growth factors, such as EGF/TGF-α and EGFR, is associated with cancer progression and often indicative of poor prognosis ( ). VEGF overexpression enhances tumor angiogenesis; thus, it is associated with lymph node metastasis, hepatic metastasis, and shorter survival time.
In addition, mutation or abnormal expression of p53 may predict a reduced cumulative survival and is associated with lymph node metastasis and lower chemosensitivity ( ; ; ).
An alternative system, proposed by the WHO, divides GC into papillary, tubular, mucinous (colloid), and poorly cohesive carcinomas ( ). These classification systems have little clinical utility, making the development of robust classifiers that can guide patient therapy an urgent priority. Recently, The Cancer Genome Atlas ( ) project proposed a molecular classification dividing GC into four subtypes: (1) Epstein-Barr virus–associated tumors (∼9%, which display recurrent PI3KCA mutations, extreme DNA hypermethylation, and amplification of JAK2, CD274 [also known as PD-L1] and PDCD1LG2 [also known as PD-L2], EBV-CIMP, CDKN2A silencing, and immune cell signaling); (2) microsatellite unstable tumors (∼22%), which show elevated mutation rates, including mutations of genes encoding targetable oncogenic signaling proteins; (3) genomically stable tumors (∼20%), which are enriched in the diffuse histologic variant and have mutations of RHOA or fusions involving RHO-family GTPase-activating proteins, CLDN18-ARHGAP fusion, or mutation in the cell adhesion gene CDH1; and (4) tumors with chromosomal instability (∼50%), which show intestinal histology, TP53 mutation, RTK-RAS activation, marked aneuploidy, and focal amplification of receptor tyrosine kinases ( ). Notably, the chromosomal instability tumors were predominant (65%) in the gastroesophageal junction and the gastric cardia, whereas the EBV-positive tumors (62%) were primarily noted in the fundus and body of the stomach ( ).
Importantly, these molecular subtypes showed distinct salient genomic features, providing a guide to targeted agents that should be evaluated in clinical trials for distinct populations of GC patients. Through existing testing for MSI and EBV and the use of emerging genomic assays that query focused gene sets for mutations and amplifications, the classification system developed through this study can be applied to new GC cases.
The ToGA trial, an international multicenter phase III clinical study involving 24 countries globally, has shown that the anti-HER2/neu humanized monoclonal antibody trastuzumab (Herceptin) is effective in prolonging survival in patients with HER2/neu-positive adenocarcinoma of the stomach and the gastroesophageal junction ( ; ). Molecular therapy targeting HER2/neu is now approved for patients with inoperable locally advanced, recurrent, or metastatic adenocarcinoma of the stomach or esophagogastric junction if the tumor shows unequivocal HER2/neu overexpression by IHC (score 3+) or amplification by FISH or chromogenic in situ hybridization (CISH). Therefore, HER2 testing in gastric carcinoma is warranted for determination of treatment eligibility.
The prognosis of GC is relatively poor. Treatment of GC, especially late-stage tumor, usually requires a multidisciplinary approach that includes chemotherapy in combination with surgical resection. TCGA studies has developed a genetic profile of somatic gene alteration in GC using GNS ( ; ). With such high-throughput technology, potential novel biomarkers—that is, genes (genomics), mRNA (transcriptomics), proteins (proteomics), and metabolites (metabolomics)—are able to be discovered.
Colorectal cancer (CRC) is the third most common malignancy worldwide ( ). Prognostication of newly diagnosed CRC predominantly relies on stage or anatomic extent of disease based on the International Union Against Cancer and the American Joint Committee on Cancer staging classifications. However, CRC should be regarded as a heterogeneous, multipathway disease—an observation substantiated by the fact that histologically identical tumors may have neither similar prognosis nor similar response to therapy ( ). Mutations and/or epigenetic alterations in TSGs (e.g., APC , DCC , SMAD-2 , SMAD-4 , p53 ) and oncogenes (e.g., KRAS , p53 ) are molecular determinants during the development of sporadic CRC, which was first summarized in the adenoma-carcinoma sequence described by . Based on genetic background, sporadic CRCs can be subclassified into two major types: (1) chromosomal unstable and (2) microsatellite unstable, plus an additional group with CpG island methylator phenotypes. Various genetic syndromes are known to predispose to colorectal carcinoma and to have specific inherited gene defects. These molecular markers are currently being investigated as a means of improving the identification of patients likely to have poor clinical outcomes and, therefore, possibly benefiting from adjuvant treatment ( ; ; ).
EGFR (a receptor tyrosine kinase) overexpression is detected in up to 80% of CRCs ( ). Tumors with a high level of EGFR expression usually have a poor prognosis ( ; ). These data have led to the clinical use of EGFR blockade in the treatment of CRCs with anti-EGFR monoclonal antibodies such as cetuximab or panitumumab (see Chapter 77 ). Cetuximab is a mouse-human hybrid monoclonal antibody approved by the FDA in 2004 as second-line treatment of CRC. Panitumumab is a fully human immunoglobulin G 2 monoclonal antibody approved by the FDA in 2007 as third-line monoclonal antibody therapy of refractory CRC. Both drugs are expensive and have significant side effects. Thus, it is imperative to use a biomarker to predicate the therapeutic response. However, use of EGFR as a biomarker to predict response to EGFR monoclonal antibodies has had limited success. Only detection of increased EGFR copy number by FISH has predictive value ( ), and it has been proven that EGFR expression levels detected by IHC are not as useful. In contrast to EGFR mutation in lung cancer, EGFR mutation in CRC is a rare event that occurs in fewer than 1% of cases ( ). Current studies are focused on finding the downstream effectors of the EGFR pathway that may serve as biomarkers for predicting the effectiveness of anti-EGFR therapy.
As summarized earlier in this chapter and in Chapter 77 Ras-p21 proteins are guanosine triphosphate (GTP)-coupled proteins that are important in many receptor tyrosine kinases that utilize signaling on the Ras/Raf/Erk/Map kinase signal transduction pathway. Mutations that result in single amino acid substitutions at critical positions in the amino acid sequence of this protein, such as Gly 12, Gly 13, and Gln 61, occur in the Ras protein and cause its constitutive activation. This leads to a series of oncogenic events that activate downstream signaling pathways, promoting tumor initiation and tumor growth and progression, including local invasion, angiogenesis, metastasis, and even immune response ( ; ).
The KRAS mutation occurs in at least 35% to 45% of cases of CRC and, in some series, in up to 75% of cases. Mutations of this gene occur almost exclusively in three hot spots (codons 12, 13, and 61) and rarely in others, including codon 146 ( ; ). Because KRAS is the downstream effector of EGFR and is frequently mutated in CRC, the mutation status of KRAS has become an important predictive marker for the effectiveness of cetuximab or panitumumab on metastatic CRCs. The American Society of Clinical Oncology (ASCO) has recommended that patients with diagnosed metastatic colon cancer who are candidates for anti-EGFR therapy should be tested for KRAS mutations in a Clinical Laboratory Improvement Amendments (CLIA)-accredited laboratory. If negative for KRAS mutations, these patients are deemed suitable for treatment with anti-EGFR antibody (cetuximab or panitumumab) therapy. This recommendation is based on the results of phase II and phase III clinical trials. Retrospective subset analyses of these trial data suggest that patients who have KRAS mutations detected in codon 12 or 13 do not benefit from this therapy. This was further confirmed by five randomized, controlled trials of cetuximab or panitumumab response in relation to KRAS mutational status (no mutation [wild type] and mutated [abnormal]) and another five single-arm retrospective studies undertaken to evaluate tumor response according to KRAS mutational status.
According to the CAP Perspectives on Emerging Technology (POET) guideline published in 2009, acceptable samples for KRAS mutation include fresh, frozen, and FFPE tissues. Acceptable assay types are RT-PCR and direct sequencing analysis. However, other methods, such as pyrosequencing or microarray (chip)–based tests, have also been developed ( ; ). As of the time of this writing, no type of KRAS testing has been approved by the FDA. Although recommended testing is limited to codons 12 and 13 of exon 2, which make up the vast majority of cases with KRAS mutations, many laboratories and commercial kits also include testing for other mutations, such as for codons 61 and 146.
The 2016 National Comprehensive Cancer Network (NCCN) guidelines state that patients with any known KRAS or NRAS mutation should not receive an EGFR inhibitor. This opinion is shared by ASCO ( ).
BRAF is an immediate downstream effector of RAS in the Ras/Raf/Map kinase pathway (see Fig. 79.1 ). Mutation of BRAF has been identified in approximately 10% of CRCs ( ). A vast majority of the point mutations are V600E, a mutational hot spot at nucleotide 1796 within exon 15, accounting for a T:A transversion mutation and a valine-to-glutamic acid substitution. All point mutations occur within the kinase domain of B-Raf protein, leading to constitutive activation of B-Raf kinase ( ). The BRAF mutation is almost mutually exclusive with KRAS mutations. Unlike the KRAS mutation, BRAF mutations frequently occur in female patients older than 75 years of age with proximal lesions. These tumors exhibit MSI with higher frequency than those in other patients with colon cancer ( ), which appears to be due to epigenetic effects involving hypermethylation of the promoter regions of mismatch repair genes. This finding helps in differentiating between sporadic MSI-H tumors with somatic mutations in the V600E hot spot in BRAF and Lynch-associated cancers with MLH1 or MSH2 mutations.
BRAF mutations in tumors have been characteristically associated with a worse survival than in those without mutations and with resistance to treatment with anti-EGFR monoclonal antibodies, cetuximab, or panitumumab for metastatic CRCs. Currently, molecular testing for BRAF mutation V600E has been performed in many clinical laboratories as a therapeutic indicator for anti-EGFR treatment in combination with KRAS mutation analysis ( ).
PI3KCA is a membrane lipid kinase that phosphorylates the 3-hydroxyl group of phosphatidylinositol. This critical enzyme, which binds directly to ras-p21 protein, can be activated by EGFR-mediated signaling in parallel with the K-ras/B-raf/MAP kinase pathway. Activation of PI3KCA counteracts PTEN, an antioncogene protein, and promotes AKT1 phosphorylation, stimulating cell proliferation, survival, and angiogenesis in cooperation with other pathways. The PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit α) gene is mutated in about 20% of CRCs ( ). The mutation of PIK3CA usually occurs in the hot spots in exon 9 (E542K, E545K) and exon 20 (H1047R), both of which are oncogenic in CRC cellular models. Unlike the BRAF mutation, PIK3CA and KRAS mutations may coexist. In addition to their association with poor prognosis (i.e., local recurrence and increased mortality of CRCs), PIK3CA mutations can independently predict resistance to anti-EGFR therapy with panitumumab or cetuximab for metastatic CRCs ( ; ; ). Testing for combinations of the PIK3CA/PTEN and KRAS mutations can detect up to 70% of patients who are unlikely to respond to anti-EGFR therapy, although more validation is required for clinical practice ( ).
Loss of PTEN has been found to co-occur with KRAS , BRAF , and PIK3CA mutations ( ; ; ; ). The recorded frequency of loss of PTEN expression varies from 19% to 36%, with some studies reporting an effect on response rate and survival, whereas others found an effect only on progression-free or overall survival. Moreover, data on the loss of PTEN expression are not concordant in primary and metastatic tissues ( ). It has been suggested that loss of PTEN expression, as determined by immunohistochemistry, is associated with lack of benefit from cetuximab in metastatic colorectal cancer ( ; ; ; ). Two recent retrospective studies reported an association between PIK3CA mutations and better outcomes among patients taking aspirin after a diagnosis of colorectal carcinoma ( and ).
The APC gene, a tumor necrosis factor-stimulated gene (TSG), encodes a 2843-amino acid protein and is located on chromosome 5q21-22, regulating Wnt/β-catenin signaling ( ). Mutations of this gene can be point mutations or small deletions or insertions. The latter cause frameshifts that generate stop codons. Germline mutations of this gene are responsible for FAP, whereas somatic mutations occur in the majority of sporadic CRCs. Somatic mutations of this gene may initiate and promote carcinogenesis of sporadic CRCs, and can be found in early neoplastic stages, even in dysplastic cryptic foci ( ; ; ). CRCs with APC mutation usually are those with chromosomal instability or MSS ( ). The APC gene can serve as a biomarker for early detection or as a risk factor for familial polyposis syndrome. Detection methods include various types of PCR, LOH, and others ( ). Screening for APC mutations may detect patients with relatively early CRC by purified DNA from routinely collected stool samples ( ).
p53 (or TP53) is a tumor suppressor that regulates the cell cycle and is involved in apoptosis and DNA repair. This protein is discussed at length in Chapter 76, Chapter 77 . It is located on the short arm of chromosome 17 (17p13.1) (Isobe et al., 1986). Mutation or homozygous deletion of TP53 is found in 40% to 50% of CRCs. Loss of wild-type p53 activity is thought to be a major predictor of failure to respond to radiotherapy and chemotherapy in various human cancers, including CRCs, and predicts lower survival ( ; ). Although TP53 mutation analysis has shown promising prognostic value, the ASCO Tumor Markers Expert Panel does not currently recommend its use in routine practice.
TGFBR1, SMAD2, and SMAD4 are proteins involved in the transforming growth factor-β (TGF-β) signaling pathway (see Chapter 77 ). SMAD proteins are the principal components mediating the TGF-β signaling pathway, which negatively regulates the growth of epithelial cells ( ). It has been found that germline allele-specific expression (ASE) of the gene encoding the TGF-β type I receptor (TGFBR1) occurs in 10% to 20% of CRCs in both familial (dominantly inherited and segregated) and sporadic forms ( ). The presence of multiple-mutated TGF-β has been linked with poor prognosis in a subset of CRC patients. Approximately 50% to 60% of CRCs have been found to harbor SMAD4 mutations ( ); in contrast, only a few have been found to have SMAD2 mutations. Both SMAD2 and SMAD4 are mapped to chromosome 18q21 ( ; ). Most mutations of SMAD2 and SMAD4 occur in their so-called MH2 domain. Inactivation of the SMAD4 gene through germline mutation and loss of the unaffected wild-type allele are responsible for familial juvenile polyposis, whereas inactivation of the SMAD4 gene through homozygous deletion or intragenic mutation occurs frequently in CRC and pancreatic cancer. SMAD4 mutation occurs at a later stage of colorectal carcinogenesis and is an indicator of advanced phenotypes. Loss or low levels of SMAD4 expression in CRCs are found to be associated with poor prognosis following surgery and 5-fluorouracil (5-FU)-based adjuvant therapy. However, the predictive value of SMAD4 mutation/inactivation is controversial ( ; ). Chromosomal loss at 18q encompassing both SMAD2 and SMAD4 has been reported in up to 70% of CRCs ( ). Patients with locally advanced stage II or stage III disease appear to demonstrate a significantly poorer prognosis with loss of 18q ( ).
MSI is involved in approximately 75% to 85% of CRCs, characterized by aneuploidy, allelic losses, amplifications, translocations, and mutations of APC , KRAS , and TP53 ( ). Although the prognosis of patients with MSI CRC is stage and grade dependent, tumors with identical morphologic features display considerable heterogeneity in terms of clinical outcome. The remaining 15% to 20% of CRCs have MSI-H characterized by inactivation of mismatch repair ( MMR ) genes ( ), as discussed in Chapter 77 . Sporadic MSI-H cases have been shown to arise predominantly through promoter hypermethylation and silencing of the hMLH1 gene in contrast to a hereditary form of MSI-H CRC, HNPCC, which typically shows germline mutations in one of the DNA MMR genes ( ). Tumors with MSI-H tend to be more proximally located, poorly differentiated, and of mucinous histologic type, and to show considerable lymphocytic infiltration; they have been linked with TGFBRII and BRAF mutations ( ). Patients with colon cancers demonstrating MSI-H have a stage-independent improved survival compared with patients with MSS tumors in most, although not all, studies. However, they do not benefit from treatment with 5-FU in randomized adjuvant therapy trials ( ). In stage II patients, when the tumor is MSI-H, no additional treatment is necessary because, based on trial, further chemotherapy or radiation treatment will not be beneficial and can potentially be harmful for this group of patients. Recently MSI is also defined as a predictor for a positive response to immunotherapy. Pembrolizumab is an anti–PD-1 (programmed cell death protein 1) immune checkpoint inhibitor that induces an objective response in 40% of patients with treatment-refractory metastatic, microsatellite unstable colorectal cancers. On the other hand, immunoexpression of PD-1 ligands on tumor cells does not seem to be a predictive marker of response to PD-1 blockade ( ).
Approximately 3% of colorectal cancers show amplification of HER2 ( ). Preclinical models of HER2-amplified colorectal tumors have shown that the combination of an antibody and TKI leads to sustained tumor shrinkage. Both HER2-amplified and HER2-mutated cancers can show de novo and acquired resistance to EGFR inhibitors, resulting in persistent signaling in the presence of an EGFR inhibitor ( ).
The CpG island methylator phenotype ( CIMP + ), or simultaneous hypermethylation of the promoter regions of a number of TSGs, is one of the principal mechanisms of colorectal carcinogenesis, independent of chromosomal instability or MSI. CIMP + is found in approximately 20% of patients with CRCs. These patients usually are female and of more advanced age, with a history of cigarette smoking. These cancers occur predominantly in the proximal colon and are associated with MSI, wild-type TP53 , BRAF mutation, and β-catenin activation ( ; ). The association between CIMP status and prognosis is controversial, however, and later studies suggest that the occurrence of CIMP appears to be an independent predictor of a low colon cancer–specific mortality and survival advantage and a high responsive rate to 5-FU–based treatment ( ; ). Additional studies, including randomized clinical trials, are required to confirm the value of CIMP + and associated molecular features for the prediction of clinical outcomes of CRCs.
Approximately 5% to 10% of CRCs are hereditary, predominantly in a form of autosomal-dominant transmission. Genetic polyposis syndrome accounts for less than 0.5% of all CRCs, whereas nonpolyposis forms of hereditary CRC are seen in a much higher percentage in overall hereditary CRCs ( ; ). The next section offers a brief introduction of the genetics of common hereditary colonic cancer syndromes.
FAP is a rare disease characterized by numerous colorectal polyps (usually more than 100) ( ). Diagnosis of FAP is based on clinical and endoscopic findings. Classic FAP is an autosomal-dominant inheritable disease, with nearly 100% penetrance. It is mainly attributable to the mutation/deletion of the APC gene, located at the long arm of chromosome 5. So far, more than 500 different germline APC mutations have been identified in FAP families ( ). About one-third of patients are found to harbor de novo mutations. However, 5% to 30% of FAP patients show no APC mutations on current genetic testing. Among those patients with APC -negative FAP, biallelic mutations in a different gene, the MutY human homologue ( MYH ) gene, may be found. The MYH gene encodes a member of the base excision repair pathway that is involved in repairing oxidative damage to DNA. Those APC -negative FAP patients may have a family history compatible with recessive inheritance ( ; ), indicating that this may be a different disease. Genetic testing may be helpful in screening and diagnosis of atypical FAP cases and in the management of affected patients. PCR-based methods have been developed to detect APC germline mutations ( ).
HNPCC is an autosomal-dominant genetic condition characterized by high predisposition to CRC and other cancers outside of the colorectum, including the genitourinary and female reproduction systems, the upper gastrointestinal tract, hepatobiliary tract, brain, and skin. Approximately 3% of all diagnosed cases of CRC belong to HNPCC, which makes it the most common hereditary CRC-predisposing syndrome ( ; ). It has been found to have lifetime risks of approximately 80% for CRC and 40% to 60% for endometrial disease. Colorectal cancer develops in younger patients (average 45 years of age); two-thirds of these cancers occur in the proximal colon. Histologically, HNPCC is not associated with a large number of colorectal polyps, as seen in FAP; rather, it forms a large nonpolypoid malignant lesion. Compared with sporadic CRC of equivalent staging, patients with HNPCC may have a better response to chemotherapy and better clinical outcomes ( ).
The molecular basis for the development of HNPCC is due to MSI, which is a consequence of inherited or germline mutations of DNA MMR genes. Among the nine MMR genes discovered, mutations in MLH1 , MSH2 , MSH6 , MLH3 , and PMS2 are associated with the development of HNPCC ( ). The significance of mutations in two other MMR genes, TGFBR2 and PMS1 , is less clear ( Table 79.3 ). Among those identifiable mutations associated with HNPCC, 90% occur in MLH1 or MSH2 ( ), 7% to 10% in MSH6 , and 1% to 2% in PMS2 or MLH3 ( ). Most germline mutations in MSH2 lead to complete loss of gene expression and usually are accompanied by loss of expression of MSH6 . CRC with MLH1 mutation is frequently caused by missense mutations. Mutations of MMR genes cause abnormal mismatch repair function, failing to correct a larger number of insertions and deletions, eventually leading to frameshift within the microsatellites or MSI ( ; ). However, approximately 50% of all HNPCCs have no identifiable mutations in MMR genes according to current methods of detection ( ).
| MMR Gene | OMIM Name | Chromosomal Locus | % of HNPCC with Mutation |
|---|---|---|---|
| MLH1 | HNPCC2 | 3p21 | 50 |
| MSH2 | HNPCC1 | 2p16 | 40 |
| MSH6 | HNPCC5 | 2p15 | ≈7–10 |
| PMS2 | HNPCC4 | 7p22 | 1–2 |
| MLH3 | HNPCC7 | 14q24.3 | 1 |
| PMS1a | HNPCC3 | 7p22 | Unclear |
| TGFBR2 | HNPCC6 | 3p22 | Case report |
Two rare but distinct variants of HNPCC are known: (1) Muir-Torre syndrome, resulting from mutations in the MSH2 gene and showing coexisting sebaceous skin tumors with small and large intestinal, gastric, kidney, endometrial, and ovarian cancer ( ); and (2) Turcot syndrome, resulting from mutations in the MLH1 and PMS2 genes and showing coexistent glioblastoma with CRCs ( ).
The diagnosis of HNPCC is based mainly on clinical information and familial history. Genetic testing has been introduced for diagnostic purposes. A testing kit for germline mutations is commercially available for blood samples. MMR dysfunction can be analyzed by immunohistochemistry on paraffin-embedded tissues. PCR and sequencing are methods commonly used to detect MSI. According to the Bethesda Guidelines, five microsatellite markers are to be tested. Of these, three are dinucleotide repeats, and two mononucleotide repeats. MSI-H is defined as LOH in two or more of five markers in tumor tissue compared with a normal tissue control. However, although it is a sensitive test, MSI testing is not specific for HNPCC, as HNPCC can be MSI-low and MSI-stable in some cases, and sporadic CRCs may also show MSI-H. Thus, genetic testing may be useful only after patients at risk for HNPCC are identified by the Amsterdam or Bethesda clinical criteria ( ; ; ).
Familial juvenile polyposis syndrome (JPS) is a rare disorder inherited in an autosomal-dominant fashion in at least 30% of patients. The incidence of JPS is approximately 1 per 100,000 births, and it is the most common of the hamartomatous syndromes ( ).
Germline mutations of SMAD4 , BMPR1A , and ENG have all been described in patients with JPS. SMAD4 is located on chromosome 18q21.1 and usually is found to be mutated in approximately 15% of cases of JPS ( ). BMPR1A (bone morphogenetic protein receptor type 1A), located on chromosome 10q22.3, is a serine threonine kinase that is a mediator of the bone morphogenetic protein intracellular signaling pathway ( ). It has been identified as mutated in about 25% of affected patients with JPS. Both genes mentioned previously encode proteins that are involved in the TGF-β signaling pathway, which is an important modulator of many cellular processes (e.g., cell growth, apoptosis, growth arrest) ( ).
The lifetime risk for CRC is about 70%, and the risk for pancreatic, gastric, and duodenal carcinoma is increased in these patients ( ). Molecular screening of individuals at risk within a JPS family for a known genetic mutation would allow clinicians to make the diagnosis at an earlier age and would lead to closer endoscopic screening and follow-up. Individuals without the mutation would need less frequent endoscopic screening and perhaps endoscopy only after 50 years of age, as is provided in the normal population. Therefore, genetic testing can help to define the recommended interval for screening and surveillance; it is hoped that it will help to prevent malignancy by early polypectomy and heightened surveillance ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here