Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
General considerations in the diagnosis, classification, prognosis, and monitoring of hematolymphoid disorders
Summary of the major genetic abnormalities and molecular detection methods in chronic and acute myeloid neoplasms.
Basis of lymphoid antigen receptor gene rearrangements and molecular techniques to assess lymphoid clonality
Concept of risk-adapted therapy for optimized management of childhood acute lymphoblastic leukemias and significance of minimal residual disease assessment
Summary of the major genetic abnormalities and molecular detection methods in the lymphoid neoplasms.
Landscape of genetic abnormalities and clinical relevance in acute myeloid leukemias
Molecular classification of chronic myeloid neoplasms
Chronic myeloid leukemia: paradigm for targeted therapy and response monitoring
Germline susceptibility and risk of developing myeloid neoplasms
Clonal hematopoiesis and clinical significance
Our knowledge, classification, and management of hematolymphoid neoplasms continues to evolve as a result of the rapid pace of new discovery, made possible by new genetic sequencing technologies and the increasing development of rational (“targeted”) therapeutic options. Whereas traditional morphologic (light microscopic) evaluation of glass slides still occupies the centerpiece of pathologic diagnosis, the judicious application of ancillary methods, such as special cytochemistry, immunohistochemistry (IHC), flow cytometry, cytogenetics, fluorescence in situ hybridization (FISH), and molecular genetics, has allowed for more precise, biologically driven characterization. The 2017 update of the World Health Organization (WHO) classification of hematopoietic and lymphoid neoplasms ( ) incorporates a greater proportion of disease-defining or associated genetic abnormalities encompassing both previously known entities and novel provisional categories. The relative ease of sampling sites such as the lymph nodes and bone marrow, as well as the procurement of easily disaggregated viable cell samples for analysis, has driven this paradigm of diagnostic evaluation. Furthermore, improvements in molecular methods have enabled ever more comprehensive utilization of formalin-fixed paraffin-embedded (FFPE) tissue sources. The nature of the hematolymphoid system is also highly conducive to repeated sampling following therapy to assess treatment response or recurrence, which represents another important facet in the management of many patients with these illnesses.
Given the wide array of diagnostic technical options available to hematopathologists, when is molecular testing of greatest necessity or practicality? There are perhaps four major scenarios in which molecular diagnostic analysis of an atypical hematolymphoid process is important. First, molecular investigation can establish a diagnosis in situations wherein morphologic details and results of other laboratory tests are inconclusive for malignancy. The presence of specific genetic abnormalities or the demonstration of tumor genetic clonality of a cell population can establish the presence of a pathologic process. Second, molecular methods are used to subclassify disease entities. To this end, the current WHO classification of hematologic neoplasms includes molecular characterization as a defining or strongly associated feature of most leukemias and lymphomas ( ). Next, specific genetic anomalies in hematolymphoid malignancies have important prognostic or therapeutic value , which in turn can influence the initial treatment of certain tumors to produce an optimal clinical outcome. Finally, being potentially of high analytical sensitivity, molecular techniques can be used to assess patients after the onset of therapy by monitoring for the presence and extent of minimal residual disease (MRD), which is becoming increasingly critical as therapeutic options improve and patients are surviving longer. As current and new molecular technologies accumulate, attention is appropriately shifting to the analysis and incorporation of different types of data (“multiomics”) and leveraging powerful computational approaches to derive even more profound insights into tumor biology and patient-specific treatment (i.e., precision medicine). A major impetus in this regard has been the rapid development and deployment of massively parallel sequencing methods (next-generation sequencing [NGS]), which is simultaneously transforming discovery and diagnostics ( ; ; ; ; ; ).
This chapter focuses on the background, clinical rationale, and pertinent technical considerations underlying the molecular diagnostic evaluation and monitoring of hematopoietic and lymphoid tumors. The intent is to summarize the molecular diagnostic approaches typically employed in hematopathology practice, although it is evident that the rate of discovery and new knowledge in this field are rapidly expanding, requiring continuous attention to the most current literature. Of note, it is apparent that the detection of tumor-specific genetic abnormalities can be achieved by different analytic means—for example, cytogenetics, fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), Sanger sequencing, or NGS. Although at first glance these various methods may seem redundant or duplicative, these laboratory techniques are often complementary, and the choice of methodologies is determined by a number of factors, including the nature of the tumor genetic or genomic alterations, knowledge of assay analytic sensitivities, sample or source-specific limitations (e.g., FFPE tissues), required turn-around time for results, and diagnosis versus posttherapy monitoring, among others. Table 78.1 summarizes the major molecular methods in use in most clinical diagnostic laboratories. When applying and interpreting molecular diagnostic tests, one caveat that cannot be emphasized enough is that the results of these investigations must never be considered in isolation but rather in conjunction with the clinical presentation and morphologic and additional relevant laboratory data concerning the individual patient. Regardless of the remarkable specificity and sensitivity of molecular markers, the evident complexity of hematolymphoid neoplasia mandates such a fully integrated approach to diminish the possibility of serious diagnostic error.
| Method | Analytic Sensitivity (%) | Notes |
|---|---|---|
| Cytogenetics | 5 |
|
| FISH | 1–5 |
|
| DNA microarray | 15–30 |
|
| PCR | 10 –2 –10 –4 |
|
| Sanger sequencing | 15–20 |
|
| NGS | 5-10 |
|
The subclassification of non-Hodgkin lymphomas (NHL) and lymphoid leukemias is established by a combination of characteristic light microscopic tumor cell features, immunophenotypic cell marker profiling (e.g., by flow cytometry and IHC), and the presence of recurrent, nonrandom tumor genetic abnormalities (i.e., structural and numerical genomic variants, somatic gene mutations) ( ). Molecular diagnostic evaluation of abnormal lymphoid proliferations often involves FISH analysis, PCR, and DNA sequencing (including use of targeted NGS panels). A central paradigm in the diagnosis of lymphoid neoplasms is the demonstration of monoclonality, which is highly correlated with the malignant state. In many instances, a monoclonal lymphoid proliferation can be suggested or established with the aid of immunophenotypic studies—for example, by demonstrating light chain restriction in the case of B-cell neoplasms or aberrant antigen expression patterns in the setting of suspicious T-cell malignancies. However, there are often instances in which the foregoing analyses may be insufficient, and the determination of clonality in a lymphoid population by molecular methods remains key to establishing a definitive diagnosis. In these situations, which may include samples with uninformative phenotypic features, small tissue biopsies, or partially degraded specimens such as paraffin-embedded tissues, the molecular assessment of antigen receptor gene rearrangements is often very useful and commonly employed. Standard PCR-based clonality detection methods are often employed to resolve the definitive diagnosis of lymphoid neoplasms with features potentially mimicking benign hyperplasias (e.g., follicular lymphomas [FLs] or extranodal marginal zone B-cell lymphomas) and to aid in the diagnosis of neoplastic T-cell lymphoproliferations. Immunoglobulin and T-cell receptor (TCR) gene PCR evaluation has continued to further evolve with the application of NGS technology.
Chromosome translocation events or aneuploidies identified by FISH techniques also serve to establish the presence of a clonal neoplasm. Recurrent chromosomal translocations occur in many lymphomas and provide the basis for accurate diagnostic characterization and classification ( Table 78.2 ) ( ). In general, chromosome translocations are more prevalent in B-cell NHL and result in the fusion of two genetic loci, commonly involving the IGH locus. As a result of this process, a normally highly regulated proto-oncogene becomes constitutively activated by juxtaposition to the immunoglobulin gene enhancer region, leading to overexpression of the translocated gene locus (e.g., t(8;14)/ MYC-IGH ). In some lymphomas, however, the gene fusion is expressed as a chimeric mRNA transcript and novel oncoprotein (e.g., t(2;5)/ NPM1-ALK ). In either case, interference with normal processes of lymphoid cell growth, homeostasis, or cell death (apoptosis) is central to lymphomagenesis. Lymphoma-associated translocation events are thought in many cases to be linked to aberrant behavior of the recombinase activating gene (RAG) and DNA breakage-repair enzyme systems that are active during the time of normal antigen receptor gene rearrangement or through germinal center processes such as somatic hypermutation (SHM) and class switch recombination (CSR), both of which require the enzyme activation-induced cytidine deaminase (AID) ( ; ). The exact mechanisms underlying translocation events in leukemias and lymphomas are nevertheless incompletely understood but may involve nonrandom spatial proximity of genes during cell cycling, fragile sites such as single-stranded intermediates of DNA prone to breakage and recombination, or other factors ( ; ). A small proportion of lymphoma-associated translocations can be detected by PCR methods using genomic DNA ( Table 78.3 ), but only if substantial breakpoint clustering exists in both partner genes to enable consensus primer strategies and the genomic target distance is amenable to efficient PCR amplification. In many cases, however, PCR detection is not possible or practical because of the substantial and inconsistent breakpoint locations over large genomic regions. Thus, FISH methods (break-apart probe [BAP-FISH], dual-probe FISH [D-FISH]) are most often employed to detect specific rearrangements.
| Advantages | Pitfalls |
|---|---|
|
|
| Abnormality | Prognostic Significance | Notes |
|---|---|---|
| High hyperdiploidy (>52 chromosomes) | Favorable |
|
| t(12;21)/ ETV6-RUNX1 | Favorable |
|
| t(1;19)/ TCF3-PBX1 | Intermediate |
|
| Hypodiploidy (<44 chromosomes) | Unfavorable |
|
| iAMP21 | Unfavorable |
|
| t(9;22)/ BCR-ABL1 | Unfavorable |
|
| BCR-ABL1 -like genetics | Unfavorable |
|
| 11q23/ KMT2A (MLL) gene rearrangements | Unfavorable |
|
| MRD positivity at end-induction therapy | Unfavorable |
|
a Tumor genetic features and early MRD are considered in conjunction with clinical and laboratory findings, including age, presenting blast count, and evidence of extramedullary disease. These risk factors therefore modify and refine traditional parameters for predicting therapeutic response.
Recurrent lymphoma-associated somatic gene mutations represent another class of abnormalities assessable by molecular diagnostic evaluation (see Table 78.2 ) ( ; ; ). Alterations characterized by single base changes in a “hot spot” codon (e.g., MYD88 , BRAF ) can be detected by sensitive allele-specific PCR methods; however, other mutation events can occur over multiple exon regions (e.g., TP53 ), requiring alternate and typically less analytically sensitive approaches, such as Sanger sequencing. NGS methodology has again begun to change the diagnostic landscape in lymphoma by evaluating multiple genes and regions in a single assay with a relatively uniform limit of detection (e.g., 5% variant frequency detection). Targeted NGS panels that can utilize DNA isolated from fixed paraffin-embedded tissues as well as fresh biopsy samples are therefore becoming more commonplace for lymphoma molecular diagnosis. The following sections first describe the molecular basis and methodology for assessing clonal status in lymphoid B-cell and T-cell neoplasms using antigen receptor gene rearrangements followed by descriptions of specific lymphoid malignancies and their associated tumor genetic alterations.
The antigen receptor genes ( IGH , IGK , IGL , TRA , TRD , TRB , TRG ) are rearranged during the early development of immature B and T lymphocytes in the bone marrow and thymus, respectively, to produce a functional immune cell receptor repertoire. The process of DNA rearrangement in these genomic regions involves the splicing and recombination of v ariable (V), d iversity (D) and j oining (J) genes; the immunoglobulin kappa light chain ( IGK ) and T-cell receptor gamma loci ( TRG ) lack D genes but otherwise conform to the same process. These gene rearrangements occur across large segments of the specific genomic loci with looping out of intervening DNA between the rearranged junctions. The rearrangement process requires a complex of specialized proteins involved in site-specific DNA recognition, unwinding, splicing, nuclease activity, and addition of random junctional nucleobases and DNA repair ( ; ). The recombinase activating gene enzymes (RAG1 and 2) are a major component of this process. Enormous diversity exists among the immunoglobulin (IG) and T-cell receptors (TCRs), resulting to a significant degree from the potential for genetic recombination between the numerous V, (D), and J genes at their respective loci. The activity of the enzyme terminal deoxynucleotidyl transferase (TdT) randomly adds a variable number of non-templated (“n”) nucleic acid bases at the junctions of the rearranged V(D)J gene segments, further substantially increasing sequence diversity ( Fig. 78.1A ). An important aspect of the recombination process is that each IG or TCR rearrangement is highly unique at the DNA and peptide level, in large part conferred by the combinatorial nature of the process and associated TdT-mediated junctional nucleotide insertions. This is exemplified by the “CDR3” region of a rearranged immunoglobulin heavy chain ( IGH ) gene, in which the V(D)J junction sequence is highly specific for an individual B lymphocyte. The resulting rearranged V(D)J gene is subsequently joined with a downstream c onstant (C) region gene of the antigen receptor locus to produce a completed coding sequence capable of translation to a functional antigen receptor protein with both extracellular and intracellular domains ( ). Immune cells incapable of producing functional receptor proteins undergo deletion by apoptosis. For B cells in early development stages, the IGH on chromosome 14(q32) is the first to undergo this segmental locus rearrangement followed in turn by the IGK on 2(p12). If the latter attempt is unsuccessful (i.e., nonproductive) at both alleles, the lambda locus ( IGL ) on 22(q11) undergoes rearrangement in order to generate the final tetrameric immunoglobulin molecule, consisting of two identical heavy chains and two identical light chains. For T cells, the TCR delta ( TRD ) on 14(q11) and gamma ( TRG ) on 7(p15) loci initiate this process in thymic T lymphocytes; however, many of these gene rearrangements, particularly involving the TRD locus (which is embedded within the TRA region), fail to produce a functional heterodimeric gamma/delta TCR protein. Thus, rearrangement continues essentially simultaneously at the TCR alpha ( TRA ) and beta ( TRB ) loci, located on chromosomes 14(q11) and 7(q34), respectively, resulting in expression of a TCR alpha/beta protein in the large majority (>90%) of developing T cells. The basic structures of immunoglobulin and TCR proteins are illustrated in Fig. 78.1B . As an important point related to molecular diagnostic analysis, nearly all mature alpha/beta T-cells still retain residual gamma ( TRG ) locus gene rearrangement(s) at the DNA level, which can be utilized as a valuable marker for T-cell lymphoid clonality assessment.
![Figure 78.1, Immunoglobulin and T-cell receptor gene rearrangements and determination of clonality in lymphoid proliferations. A, Schematic representation of somatic rearrangements of the immunoglobulin heavy chain ( IGH ) locus (chromosome 14q32) occurring in B-cell development. Selection and rearrangement of IGH diversity (DH) and joining (JH) genes initiates over a large region of intervening DNA followed by the recruitment of a variable region (VH) gene to form a rearranged VDJ coding gene. VH genes can be grouped based on sequence similarities into seven “families.” Substantial immunoglobulin diversity is generated at the nucleotide level because of the large combinatorial selection of the many possible individual V, D, and J genes, as well as by the activity of the enzyme terminal deoxynucleotidyl transferase (TdT), which inserts a random number of “nontemplated” (n) nucleotides at the rearranged junctions. B, The T-cell receptor gamma gene ( TRG ) located at chromosome 7p15 shares similar structural and rearrangement features, with the exception that D genes are not present. The TRG gene is also less complex, having only 11 functional Vγ genes capable of functional rearrangements, along with two sets of J-genes (Jp and Jγ) and two constant (C) regions. C, Detailed schematic of a rearranged IGH gene showing the joined VDJ coding region. The VH gene segment encodes much of the variable component of the immunoglobulin heavy chain peptide. Three relatively conserved framework regions (FR1–3) are identified in the V segment, as well as three more variable sequence “complementary determining regions” (CDR1–3). The CDR3 is composed of the junction between V, D, and J genes, along with inserted nontemplated (n) nucleotides (mediated by the enzyme terminal deoxynucleotidyl transferase [TdT]); this heterogeneous region thus represents a highly unique nucleotide sequence in each B cell. The presence of focal areas of shared sequence homology within the V-gene families (e.g., IGH FR segments) and in the J genes provides the basis for polymerase chain reaction (PCR)–based detection of the nearly all gene rearrangements occurring in a population of B- or T-cell lymphocytes; the placement of such flanking “consensus” oligonucleotide forward and reverse primers is depicted by the arrows . The same strategy is employed to cover gene rearrangements at other antigen receptor genomic loci (e.g., IGK , TRB , TRG ). D, Results of IGH PCR using fluorescently labeled consensus primers and capillary gel electrophoresis (CGE) analysis. In this figure, the y -axis is fluorescence intensity, and the x -axis is fragment size. In a polyclonal B-cell population ( left side of image), a finite range of VDJ rearrangement sizes exists, delimited both by the relative sizes of the recombined V, D, and J genes and the number of inserted junctional n-nucleotides. This fragment size range is normally distributed, and the area contained under each peak represents a “bin” of many similarly sized rearranged IGH fragment sizes. In contrast, a monoclonal B-cell population is characterized by reduction to a homogeneous single- or double-peak rearrangement pattern, as demonstrated in the right side of the image. E and F, IGH gene rearrangement analysis performed by next-generation sequencing (NGS). Polyclonal ( E ) and monoclonal ( F ) patterns are visually similar to those seen by PCR and CGE analysis; however, the NGS data represent a sequence-based distribution. In E , the proportion of distinct IGH VDJ rearrangements is indicated by the relative heights of the color bars. The V-gene family and J-gene rearrangement combinations are displayed across the x -axis representing a different type of distribution relative to CGE size-based data for a polyclonal B-cell population. F reveals a single dominant rearranged IGH population indicative of a monoclonal result. Direct determination of the DNA sequence of every rearranged VDJ sequence is possible by NGS and a sampling of tabular data (including VDJ sequences amplicon lengths, V and J genes used, proportion of total reads, and percent identity to nearest germline V gene) is shown below the graphical output. NGS is currently more expensive than CGE but is very powerful for immunoglobulin or T-cell receptor gene repertoire profiling at the DNA sequence level and allows for evaluation of several specimens in a single experiment (batch processing) with the use of specimen bar code indexing. Figure 78.1, Immunoglobulin and T-cell receptor gene rearrangements and determination of clonality in lymphoid proliferations. A, Schematic representation of somatic rearrangements of the immunoglobulin heavy chain ( IGH ) locus (chromosome 14q32) occurring in B-cell development. Selection and rearrangement of IGH diversity (DH) and joining (JH) genes initiates over a large region of intervening DNA followed by the recruitment of a variable region (VH) gene to form a rearranged VDJ coding gene. VH genes can be grouped based on sequence similarities into seven “families.” Substantial immunoglobulin diversity is generated at the nucleotide level because of the large combinatorial selection of the many possible individual V, D, and J genes, as well as by the activity of the enzyme terminal deoxynucleotidyl transferase (TdT), which inserts a random number of “nontemplated” (n) nucleotides at the rearranged junctions. B, The T-cell receptor gamma gene ( TRG ) located at chromosome 7p15 shares similar structural and rearrangement features, with the exception that D genes are not present. The TRG gene is also less complex, having only 11 functional Vγ genes capable of functional rearrangements, along with two sets of J-genes (Jp and Jγ) and two constant (C) regions. C, Detailed schematic of a rearranged IGH gene showing the joined VDJ coding region. The VH gene segment encodes much of the variable component of the immunoglobulin heavy chain peptide. Three relatively conserved framework regions (FR1–3) are identified in the V segment, as well as three more variable sequence “complementary determining regions” (CDR1–3). The CDR3 is composed of the junction between V, D, and J genes, along with inserted nontemplated (n) nucleotides (mediated by the enzyme terminal deoxynucleotidyl transferase [TdT]); this heterogeneous region thus represents a highly unique nucleotide sequence in each B cell. The presence of focal areas of shared sequence homology within the V-gene families (e.g., IGH FR segments) and in the J genes provides the basis for polymerase chain reaction (PCR)–based detection of the nearly all gene rearrangements occurring in a population of B- or T-cell lymphocytes; the placement of such flanking “consensus” oligonucleotide forward and reverse primers is depicted by the arrows . The same strategy is employed to cover gene rearrangements at other antigen receptor genomic loci (e.g., IGK , TRB , TRG ). D, Results of IGH PCR using fluorescently labeled consensus primers and capillary gel electrophoresis (CGE) analysis. In this figure, the y -axis is fluorescence intensity, and the x -axis is fragment size. In a polyclonal B-cell population ( left side of image), a finite range of VDJ rearrangement sizes exists, delimited both by the relative sizes of the recombined V, D, and J genes and the number of inserted junctional n-nucleotides. This fragment size range is normally distributed, and the area contained under each peak represents a “bin” of many similarly sized rearranged IGH fragment sizes. In contrast, a monoclonal B-cell population is characterized by reduction to a homogeneous single- or double-peak rearrangement pattern, as demonstrated in the right side of the image. E and F, IGH gene rearrangement analysis performed by next-generation sequencing (NGS). Polyclonal ( E ) and monoclonal ( F ) patterns are visually similar to those seen by PCR and CGE analysis; however, the NGS data represent a sequence-based distribution. In E , the proportion of distinct IGH VDJ rearrangements is indicated by the relative heights of the color bars. The V-gene family and J-gene rearrangement combinations are displayed across the x -axis representing a different type of distribution relative to CGE size-based data for a polyclonal B-cell population. F reveals a single dominant rearranged IGH population indicative of a monoclonal result. Direct determination of the DNA sequence of every rearranged VDJ sequence is possible by NGS and a sampling of tabular data (including VDJ sequences amplicon lengths, V and J genes used, proportion of total reads, and percent identity to nearest germline V gene) is shown below the graphical output. NGS is currently more expensive than CGE but is very powerful for immunoglobulin or T-cell receptor gene repertoire profiling at the DNA sequence level and allows for evaluation of several specimens in a single experiment (batch processing) with the use of specimen bar code indexing.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MolecularDiagnosisofHematopoieticNeoplasms/0_3s20B978032367320400078X.jpg)
As a result of the largely random nature of the immunoglobulin and TCR gene rearrangement process and variable junctional nucleotide diversity, individual B and T lymphocytes harbor a myriad of specific gene rearrangement sequences and express correspondingly unique antigen receptor immune proteins on their cell surfaces, enabling a tremendous range of potential antigen recognition and binding affinities. In a polyclonal lymphoid expansion, therefore, a highly diverse and variable distribution of individual antigen receptor gene rearrangements at the DNA level is generally found, whereas a monoclonal population is characterized by a single identical gene rearrangement in each progeny cell. This concept is exploited by several techniques, including DNA-based PCR and capillary or gel fragment sizing, NGS and Southern blot hybridization (SBH) analysis, to distinguish polyclonal (“benign”) from monoclonal (“malignant”) lymphocytic proliferations. The SBH method has largely been supplanted by comprehensive PCR-based approaches for routine lymphoid clonality determination and thus will not be discussed further.
Despite the large spectrum of antigen receptor gene rearrangements encoding this diverse immune repertoire, rearranged V(D)J genes can be reliably detected and analyzed by relatively simple PCR techniques made possible by the presence of relatively conserved DNA sequences within V and J genes that flank the rearrangements ( Fig. 78.1C ). For example, the IGH locus contains approximately 70 to 80 V-region genes that can be grouped into seven families based on partial sequence similarities. These more homologous sequence sites allow the use of “consensus” PCR primers that can bind and amplify the rearranged V(D)J genes in a DNA sample. Most commonly, PCR-based lymphoid clonality assays target the IGH , IGK , TRG , and TRB loci using the consensus primer design strategy. PCR amplification products can be displayed by agarose or polyacrylamide gel electrophoresis, in which polyclonal distributions are seen as broad smears and monoclonal populations are identified as discrete bands; however, current practice employs fluorescently labeled PCR primers and capillary gel electrophoresis (CGE) of the PCR products. CGE enables a high-resolution readout of amplitude (relative PCR product intensity) versus amplicon fragment size distribution ( Fig. 78.1D ). Substantial refinements have been made over time to optimize PCR approaches for lymphoid clonality evaluation, and many standard methods follow the guidelines proposed by the BIOMED-2 consortium ( ; ; ; ; ), which provide recommendations on consensus primer design and primer multiplexing of reactions. In general, PCR techniques have gained wide popularity for clonality determination, principally because of their relative ease of performance and rapidity (see Table 78.3 ). PCR methods are advantageous in that they are fast (3–5 days), relatively inexpensive, and require relatively minimal starting DNA quantity. Moreover, PCR can be performed using DNA extracted from fresh samples and FFPE tissue sources, although certain fixatives (e.g., B5) or agents commonly used in lymph node or bone marrow biopsy processing may cause suboptimal results. Clonality assessment by PCR has a nominal analytical sensitivity of 1% to 5%, although a prominent coexisting polyclonal B or T-cell background can significantly reduce the ability of the technique to detect a coexistent monoclonal lymphocyte population.
Although immunoglobulin and TCR PCR is generally very informative, the technique is occasionally subject to false-negative assay results because it is not possible to design consensus-type primers to capture all possible antigen receptor gene rearrangements in lymphoid cells. For B-cell lymphomas, the issue of SHM of the IGH genes can also be problematic; SHM occurs during the germinal center reaction and is mediated by the enzyme AID ( ). AID activity generates nucleotide base substitutions and corresponding amino acid changes in the V genes and is especially notable in the junctional region of the VDJ rearrangement. This biologic process enhances the binding of specific immunoglobulins for cognate epitope regions of target antigens (known as affinity maturation). In the setting of germinal center–associated or postgerminal center B-cell lymphomas (e.g., follicular and large B-cell lymphomas, myeloma), SHM can result in loss of consensus primer binding recognition, failure of PCR amplification, and inability to detect a monoclonal B-cell population. Nevertheless, the effect of SHM in B-cell lymphomas can be largely overcome by using multiple PCR primer strategies and by including analysis of the IGK locus. In contrast, the problem of analytic false positivity is sometimes encountered when interpreting T-cell (TCR) gene rearrangement results by PCR. This situation is most evident when evaluating the TRG locus. The TRG locus has limited overall V- and J-gene recombination potential, which can occasionally lead to a preferential amplification of certain rearrangements in non-neoplastic T cell proliferations, thus mimicking a clonal population (i.e., “pseudoclonal” result) ( ). Such skewed T-cell populations can be encountered in several situations, including autoimmune disorders, after allogeneic organ transplantation, or as a consequence of immune repertoire contraction with normal aging. Therefore, diagnostic interpretation of T-cell clonality studies by PCR is somewhat more problematic, with the propensity for encountering “equivocal” results in a number of cases. Nonetheless, PCR-based methods provide a rapid and cost-effective approach for interrogating both immunoglobulin and TCR loci, keeping in mind the foregoing caveats that (1) the analytical specificity for identifying monoclonal populations is slightly less than 100%, (2) the potential for false-positive results (especially with T-cell receptor gene rearrangements) can occur, and (3) interpretation is inherently subjective (see Table 78.3 ). It is clear that PCR analysis of antigen receptor gene rearrangements must always be interpreted alongside all relevant clinical, pathologic, and other laboratory data. PCR analysis provides one additional capability: because the monoclonal antigen receptor gene rearrangements in individual lymphoid neoplasms are unique, these highly specific DNA sequences can also be used as tumor-specific markers for very sensitive (e.g., <10 –4 ) detection of MRD in blood or bone marrow after therapy ( ; ).
The evolution of lymphoid clonality analysis is now entering a new phase characterized by combining comprehensive consensus PCR primer design with NGS. By altering the PCR primer design appropriately, lymphoid cell antigen receptor gene rearrangements can be directly analyzed by NGS, which correspondingly replaces the concept of a PCR product fragment size distribution with a DNA sequence distribution. Furthermore, individual sample barcoding (indexing) allows for simultaneous evaluation of multiple samples in the same run. The advent of NGS methodology has brought new opportunities and a profound increase in knowledge and diagnostic capability. Using a similar consensus PCR primer strategy, an amplicon library can be prepared with attached NGS adapters and sample-specific bar codes. Deep sequencing of these PCR products provides specific sequence identities of the many thousands of V(D)J rearrangements within a lymphoid population and enables quantitation of relative species abundance ( Fig. 78.1E and F). NGS applications in this area are also providing an unprecedented opportunity to study epitope mapping, explore lymphocyte subsets, and better understand adaptive or cellular immune responses and repertoires. For IGH locus rearrangements, SHM status can be readily determined ( ; ). To a large extent, these analyses have been made possible by the establishment of high-quality antigen receptor gene specific reference sequence database resources, such as the IMGT Immunogenetics ( http://www.imgt.org ) and IGBLAST ( https://www.ncbi.nlm.nih.gov/igblast ) website repositories. In hematopathology practice, NGS is creating a deeper and richer data set to redefine and explore the concept of “clonality” and antigen receptor diversity in lymphoid proliferations ( ; ; ; ; ; Warren et al., 2013; ). The ability to generate hundreds of thousands to millions of sequence reads from a single sample also provides a powerful advantage for NGS in the direct evaluation of MRD; because each gene rearrangement is unique at the DNA sequence level, an individual lymphoid tumor cell population can be specifically identified and precisely quantified within a large background of other sequences. In step with therapeutic advancements for several lymphoid neoplasms, deep sequencing of immunoglobulin and TCR gene rearrangements is finding increasing utility for MRD detection in patients with B- and T-cell lymphoblastic leukemias, chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), and multiple myeloma (MM) ( ; ; ; ; ; ; ; ).
B-cell lymphoblastic leukemia (precursor B-cell acute lymphoblastic leukemia [B-LBL or B-ALL]) in the pediatric population represents a model linking tumor genetics with therapeutic stratification and clinical outcomes. In childhood B-LBL, a wide spectrum of genomic alterations has been identified, including numerical and structural chromosome alterations, activating chimeric kinase gene fusions, gene amplifications, and pathogenic gene alterations (single nucleotide mutations, insertions and deletions) ( ; ; ; ). These events have distinct prognostic value and help to classify patients according to relapse risk (e.g., standard, low, or high risk) and in turn provide the basis of current risk-adjusted therapy ( ; ). Several similar genetic changes are also found in a subset of adult patients with B-LBL, whereas others are more exclusive to childhood presentations. Recurrent tumor genetic associations in B-LBL are described in more detail later and summarized in Table 78.4 . Genetic abnormalities in T-cell lymphoblastic leukemia/lymphoma (T-LBL) continue to become better defined and include translocations of HOX genes with the TCR gene loci; mutations of NOTCH1 , FBXW7 , PHF6 , or JAK genes; TAL1 oncogene activation; and gene fusions involving ABL1 ( ). Cases of early T-cell precursor (ETP) acute lymphoblastic leukemia have been characterized by gene mutations involving cell signaling (e.g., RAS , FLT3 , JAK1 / 3 ), transcription factors (e.g., RUNX1 , ETV6 ) and epigenetic modification (e.g., EZH2 , SUZ12 ) that unusually overlap with abnormalities observed in myeloid malignancies ( ). Molecular diagnostic evaluation of T-LBL beyond FISH analysis for specific genomic rearrangements is not otherwise widespread in the clinical laboratory and is therefore not further detailed in this section.
| Disease Entity | Common Genetic Abnormalities | Molecular Pathogenesis Hallmarks | Molecular Diagnostic Detection | Key Notes |
|---|---|---|---|---|
| Follicular lymphoma (FL) | Structural variants: t(14;18)/ BCL2-IGH Genetic variants: EZH2 , CREBBP , EP300 , KMT2D ( MLL2 ) |
Overexpression of BCL2 protein; protection from apoptosis |
|
|
| Mantle cell lymphoma (MCL) | Structural variants: t(11;14)/ CCND1-IGH Genetic variants: TP53 , ATM , NOTCH1 , NOTCH2 |
Overexpression of G1-phase cell cycle protein cyclin D1; cell proliferation |
|
|
| Splenic and extranodal marginal zone lymphomas (SMZL, EN-MZL) | SMZL: Structural variants: del7q Genetic variants: TNFAIP3, KLF2, NOTCH2 , MYD88 L265P EN-MZL: Structural variants: +3, +18; del6(q23); t(11;18)/ BIRC3-MALT1 (gastric); t(14;18)/ MALT1-IGH (lung) Genetic variants: KMT2C, CREBBP, KMT2D, TET2, NOTCH1/2 |
Common pathway in EN-MZL is aberrant NF-κB signal transduction; epigenetic modifier alterations and NOTCH pathway disruption also appear significant |
|
|
| Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) | Structural variants: 13/del13q, +12, del11q, del17p Genetic variants: TP53 , SF3B1 , NOTCH1 , ATM , BIRC3 gene Other: SHM status of clonal IGH V gene (i.e., unmutated >98% or mutated ≤98% relative to germline identity) |
Multiple altered pathways, including loss of tumor suppressor activity (e.g., TP53), aberrant B-cell receptor signaling, apoptosis avoidance |
|
|
| Lymphoplasmacytic lymphoma (LPL) | Structural variants: N/A Genetic variants: MYD88 L265P (>90%), CXCR4 (30%) |
Aberrant NF-κB and B-cell receptor signaling |
|
|
| Hairy cell leukemia (HCL) | Structural variants: N/A Genetic variants: BRAF V600E (>95%), MAP2K1 (rare) |
MAPK pathway activation |
|
|
| Diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma (HGBCL) | GCB type: Structural variants: 18q21/ BCL2 (20%–30%), 8q24/ MYC (5%–10%) Genetic variants: KMT2D ( MLL2 ), CREBBP , EP300 , EZH2 ABC type: Structural variants: 3q27/ BCL6 (30%) Genetic variants: CD79A , CD79B , MYD88 , CARD11 , PRDM1 , TNFAIP3 HGBCL (mostly GCB): Structural variants: MYC + BCL2 and/or BCL6 “double hit” |
Cellular proliferation, avoidance of apoptosis, B-cell maturation, aberrant B-cell receptor signaling, epigenetic alterations, immune surveillance escape |
|
|
| Burkitt lymphoma (BL) | Structural variants: t(8;14)/ MYC-IGH and variant MYC translocations Genetic variants: TCF3 , ID3 |
Overexpression of potent early response mitogenic transcriptional factor MYC |
|
|
| Multiple myeloma | Structural variants: Hyperdiploidy (typically odd chromosomes); t(11;14)/ CCND1-IGH t(4;14)/ WHSC1-IGH t(14;16)/ MAF-IGH t(14;20)/ MAFB-IGH del17p del1p gain1q Genetic variants: TP53 , FAM46C , DIS3 , N/KRAS , ATM , BRAF , CCND1 , IRF4 |
Multiple pathways, including deregulated cell proliferation, apoptosis avoidance, abnormal B-cell programming, disrupted transcription or translation |
|
|
| Anaplastic large-cell lymphoma (ALCL) | Structural variants: ALK-positive t(2;5)/ NPM1-ALK and variant ALK translocation partners (e.g., TPM3 , TFG , ATIC ) ALK-negative 6p25.3/ DUSP22 , 3q28/ TP63 |
Constitutive activation and abnormal localization of ALK tyrosine kinase (ALK-positive cases); loss of tumor suppressor function of DUSP22 or TP63 (ALK-negative cases) |
|
|
| Peripheral T-cell lymphoma (PTCL) | Structural variants: t(5;9)/ ITK-SYK CTLA4-CD28 Genetic variants: RHOA , IDH2 , DNMT3A , TET2 , CD28 (mainly seen in AITL and other TFH PTCLs) |
Disordered follicular helper T-cell program, epigenetic modifications, immune dysregulation |
|
|
| T-cell prolymphocytic leukemia (T-PLL) | inv14 or t(14;14)/ TCL1A | Overexpression of TCL1A transcription factor |
|
|
| T-cell large granular lymphocytic leukemia (T-LGLL) | STAT3 , STAT5B gene mutations | Aberrant signaling through JAK-STAT pathway |
|
|
Somatic numerical and recurrent structural genetic variations are identified in approximately half of pediatric patients with B-LBL (see Table 78.4 ) ( ; ). Hyperdiploid chromosome aneuploidy is present in nearly one-third of pediatric B-LBL at diagnosis, defined by the presence of more than 52 tumor cell chromosomes. Hyperdiploidy is typically associated with trisomies of chromosomes 4, 10, and 17, and these patients have a “good” risk profile with a favorable long-term outlook. Hyperdiploid leukemias can be identified by cytogenetics or by flow cytometric assessment of relative DNA content analysis of lymphoblast cell nuclei (DNA index >1.16) coupled with targeted FISH probe analysis (to detect +4, +10, and +17). Conversely, the presence of a hypodiploid chromosome number (<44) defines a rare group of individuals with aggressive disease and early treatment failure; these patients may be considered as early candidates for allogeneic stem cell transplantation. Intrachromosomal amplification of chromosome 21 (“iAMP21”) has also recently been associated with poor outcome, arising from complex break-fusion events (chromothripsis) mainly involving the long (q) arm of the chromosome and leading to amplification of transcription factor genes such as RUNX1 and ERG ( ). Cases of iAMP21 can be identified by FISH analysis of specific loci (e.g., RUNX1 ) or genomic microarray methods to detect the variable somatic copy number alterations.
In approximately 25% of pediatric B-LBL cases, common recurrent chromosome translocations are identified (see Table 78.4 ) ( ; ; ), which include the t(12;21)(p13;q22)/ ETV6-RUNX1 , t(9;22)(p34;q11)/ BCR-ABL1 , and t(1;19)(q23;p13)/ TCF3 - PBX1 and rearrangements of the KMT2A ( MLL ) gene. The t(12;21)/ ETV6-RUNX1 fusion is the most common recurrent gene fusion in pediatric B-LBL (20% of cases) but is very rarely identified in adult patients. ETV6-RUNX1– positive patients have an excellent clinical outcome after chemotherapy, similar to the group with chromosome hyperdiploidy and associated “good prognosis” trisomies, although late relapses in ETV6-RUNX1 B-LBL can be a concern for a subset of these individuals ( ; ). The ETV6 gene, which encodes an Ets family transcription factor, is joined with the RUNX1 transcription factor to form a novel fusion gene on chromosome 12p. The resulting ETV6-RUNX1 oncoprotein disrupts the normal function of core binding factor (CBF) transcriptional activity (similar to the RUNX1-RUNX1T1 in acute myeloid leukemia [AML]), leading to pathogenic effects on normal progenitor cell proliferation and differentiation ( ). A large proportion of ETV6-RUNX1 B-lymphoblastic leukemias also harbor deletions of the remaining ETV6 allele on 12p, as well as additional clonal findings ( ; ). In the majority of t(12;21)/ ETV6-RUNX1 –positive B-LBL cases, the translocation is cytogenetically cryptic, requiring either reverse transcription polymerase chain reaction (RT-PCR) or FISH molecular methods for detection. Breakpoints in the ETV6 gene are generally invariant within intron 5; however, the RUNX1 gene can show heterogeneity both in genomic break-site location, as well as alternative RUNX1 splice variants in the chimeric mRNA transcripts ( ; ). As a result, potentially four ETV6-RUNX1 fusion forms can thus be detected by RT-PCR technique in any given patient; however, the most frequent types involve fusion of ETV6 exon 5 with exons 2 or 3 of RUNX1. FISH technique also readily detects the ETV6-RUNX1 translocation in pediatric B-LBL, and this approach further enables the simultaneous identification of an accompanying deletion of the nontranslocated ETV6 allele, if present.
The t(9;22)(q34;q11)/ BCR-ABL1 abnormality is identified in 3% of B-LBL cases in children and 20% to 25% of adult patients ( )), and is associated with a poor prognosis characterized by therapeutic resistance and high relapse risk (L.K. ; ). Approximately 80% to 90% of pediatric and two-thirds of adult Ph-positive B-LBL produce an e1-a2 BCR-ABL1 fusion mRNA transcript, resulting in a 190-kD oncoprotein. The remaining cases are associated with e13-a2 or e14-a2 (formerly b2-a2 or b3-a2) BCR-ABL1 transcripts and a corresponding p210 chimeric protein. The BCR-ABL1 protein disrupts several normal cell functions and principally results in constitutive deregulation of the ABL1 tyrosine kinase, leading to increased cellular proliferation. BCR-ABL1 is readily transforming in in vivo transduction models of leukemia, but its phenotypic and clinical manifestations (e.g., B-LBL vs chronic myeloid leukemia [CML]) are dependent on the hematopoietic cell context in which the genetic abnormality is acquired (i.e., pluripotent stem cell vs committed lymphoid progenitor). Despite the generally poor prognostic significance for BCR-ABL1 positive B-LBL, chemotherapy regimens now typically include tyrosine kinase inhibitors (TKIs; e.g., imatinib and related agents). Durable responses were initially difficult to achieve, but more patients are attaining longer hematologic remissions, providing a bridge to curative allogeneic hematopoietic stem cell transplantation ( ; L. K. ; ; ). Molecular details of the BCR-ABL1 fusion gene are discussed in more detail in the section on CML and illustrated in Fig. 78.10 . The BCR-ABL1 abnormality can be readily identified at diagnosis by cytogenetics, FISH, or RT-PCR, although quantitative RT-PCR (QRT-PCR) is required for disease burden monitoring after therapy. As with CML, B-LBL patients treated with TKI therapy can develop acquired resistance mutations in the kinase domain of BCR-ABL1 , necessitating ongoing molecular follow-up and vigilance. Deletions involving the IKZF1 gene locus encoding the transcription factor Ikaros have also been identified in many cases of BCR-ABL1 B-LBL, and these alterations represent an important cooperative lesion strongly influencing aggressive behavior in this subset of patients ( ; ; ).
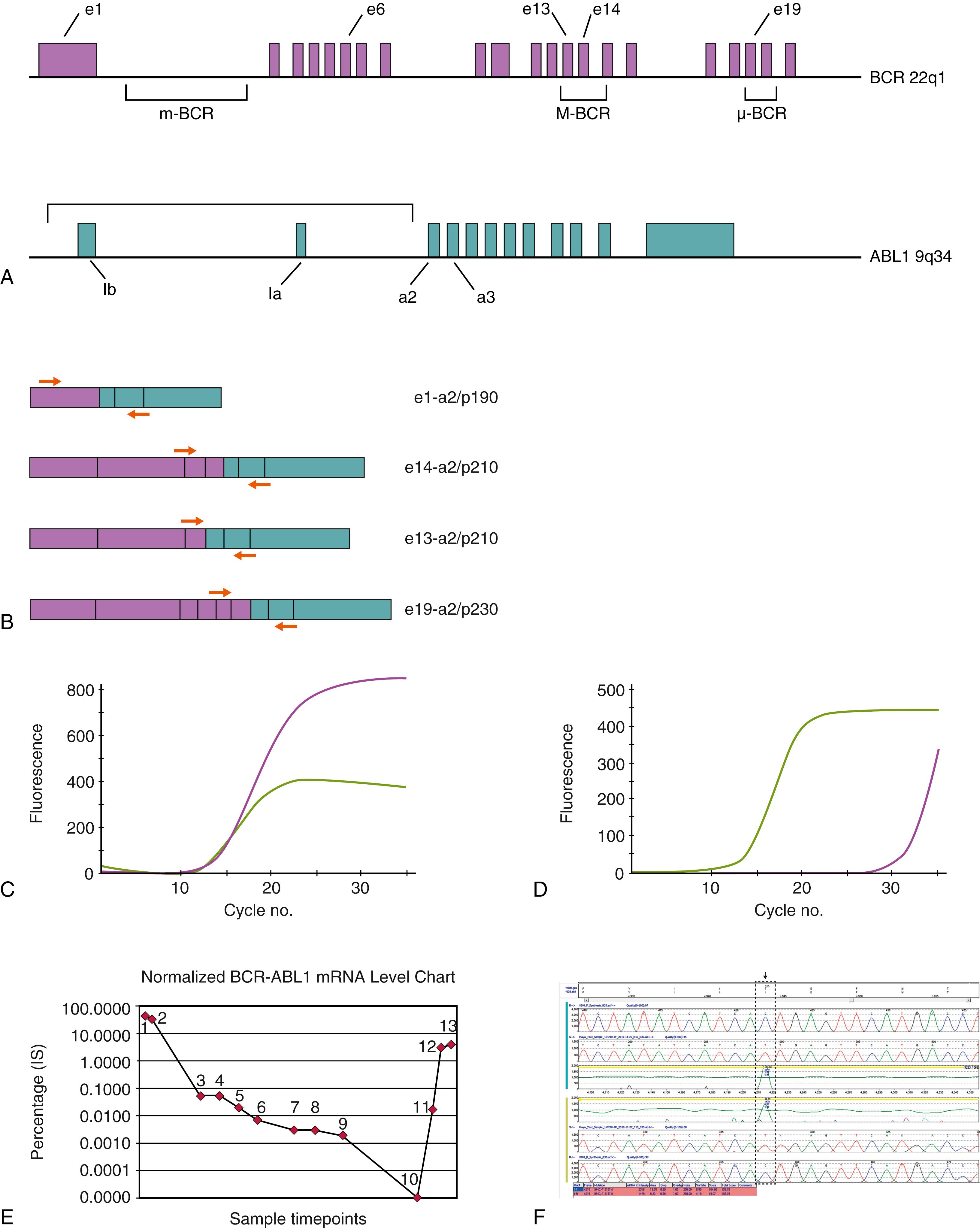
A small proportion of pediatric B-lineage ALL, most commonly with a “pre-B” cell (cytoplasmic IgM positive) immunophenotype, harbors an unbalanced t(1;19)(q23;p13) with the resultant formation of a chimeric TCF3-PBX1 oncogene (the TCF3 gene is also known as E2A ) and corresponding novel leukemogenic protein ( ; ). TCF3 is an important mediator of normal lymphoid cell and myocyte development, whereas PBX1 is a DNA binding protein that is not expressed in normal lymphocytes. Patients with leukemia who have this translocation fusion gene have been linked to more aggressive disease biology, although optimized therapeutic management has now improved the clinical outcome in most cases. A single chimeric TCF3-PBX1 mRNA is produced in nearly all cases, and this transcript can be readily detected by RT-PCR analysis; FISH technique can also be employed to detect the genomic abnormality. Rare occurrences of a balanced t(1;19)(q23;p13) also occur in B-LBL and do not involve the TCF3 and PBX1 genes but rather create novel DAZAP1-MEF2D and reciprocal MEF2D-DAZAP1 gene fusions, which are associated with poor outcome ( ).
The mixed-lineage leukemia gene ( MLL , now designated KMT2A ) is located on chromosome 11(q23) and is rearranged in approximately 5% of childhood leukemias, including the majority of B-LBL cases occurring in infants younger than 1 year of age ( ; ; ). Infantile acute leukemias with KMT2A gene rearrangements present in a clinically aggressive fashion and exhibit a dismal prognosis, particularly when associated with a poor therapeutic steroid response ( ; ; ; ). The KMT2A gene product encodes histone-lysine N-methyltransferase 2A (MLL1 protein), which differentially regulates a set of homeobox or HOX genes that are variably responsible for coordinated skeletal development during embryogenesis and significantly, normal postfetal hematopoietic progenitor cell development ( ; ; ; ; ; ; Yu et al., 1995). In B-LBL, KMT2A becomes juxtaposed with a different genetic locus via chromosome translocation, resulting in the formation of a chimeric gene product. Abnormal MLL1 fusion proteins can promote leukemogenesis by aberrantly deregulating certain HOX gene subsets in hematopoietic stem cells ( ). Numerous (>50) described reciprocal translocation partner genes have been described in KMT2A gene disruption ( ; ; ; ; ). The t(4;11)(q21;q23) is most commonly encountered in infant B-LBL, resulting in the formation of the KMT2A-AFF1 fusion ( ). In this event, the KMT2A gene exhibits substantial genomic breakpoint heterogeneity encompassing a large genomic region containing exons 5 to 11. Likewise, the AFF1 gene (also known as AF4 ) also shows variability of breakpoint location among B-LBL cases. A second translocation, the t(11;19)/ MLLT1 (or ENL )- KMT2A abnormality, is also encountered in infant B-LBL, as well as lymphoblastic leukemia and AML in older children and adults. KMT2A translocation, in particular the t(9;11)/ MLLT3-KMT2A , is also prevalent in the subgroup of therapy-related AML after prior exposure to DNA topoisomerase II inhibiting agents (e.g., etoposide, daunorubicin). Therapy-related AML is associated with a very poor prognosis ( ). Although a single chimeric MLL fusion transcript type is associated with any given translocation event in an individual leukemic tumor, the presence of so many potential rearranging partner genes in KMT2A translocations with associated breakpoint junction heterogeneity poses significant challenges for molecular diagnostic detection. Multiplex RT-PCR approaches have been designed to identify many different KMT2A gene fusions; however, comprehensive molecular evaluation of this gene in childhood leukemias by PCR methods remains problematic because of both biologic and technical complexity, thus limiting clinical sensitivity. FISH technique based on a BAP strategy (with probes spanning either side of the breakpoint cluster region in the KMT2A gene) is very powerful for rapidly identifying genomic disruption at this locus, and this strategy is diagnostically very sensitive for KMT2A rearrangements regardless of the translocation partner; D-FISH can then be employed to define the specific translocation variants in many cases. FISH analysis also discriminates between true 11(q23)/ KMT2A locus rearrangements and other genetic alterations (e.g., deletions) not involving the gene but clustering within the 11(q22-q25) region; this distinction may be difficult to discern by routine cytogenetic analysis. More recently, targeted next-generation RNA sequencing approaches have been employed to successfully detect KMT2A gene fusions ( ).
Interestingly, KMT2A gene rearrangements present in many B-LBL cases occurring in infancy have been traced back to their corresponding neonatal (preleukemic) blood spots, indicating in utero acquisition in the fetal bone marrow ( ; ). This finding has similarly been demonstrated in a subset of older children with ETV6-RUNX1 B-LBL and some other types of acute leukemias in childhood. However, the latency period from initial acquisition of a genetic alteration to potential disease presentation differs between subtypes of genetically defined B-LBL, suggesting that the specific hematopoietic context, with unknown driver gene mutations, environmental influences, or both play important roles in determining whether leukemogenesis proceeds in a given individual. In infantile B-LBL, for example, KMT2A mutation acquired in utero appears necessary and sufficient for postnatal rapid development of B-LBL, whereas this is not similar for other leukemia subtypes. Furthermore, healthy newborn or umbilical cord blood population screening studies have shown an appreciable prevalence (∼0.1%–1%) of leukemia-associated translocations events, albeit at very low quantitative levels (<10 -4 ); these data again point to the requirement for cooperating oncogenic mutations and a conducive background in the generation of overt leukemia. Nevertheless, the realization that at least some childhood leukemias have prenatal origins has sparked interest in identifying risk-modifying factors. For example, recent evidence shows that low penetrance population genetic variations in some genes (e.g., IKZF1, PAX5 ) or polymorphic loci may increase the risk of developing B-LBL ( ; ; ; ).
Data from comprehensive genomic analyses in the era of NGS have further advanced the characterization of childhood B-LBL. A wider array of genomic and genetic alterations that deregulate key cytokine receptor or kinase signaling pathways is now known in B-LBL ( ; ; ). For example, translocations of the IGH gene with the erythropoietin receptor ( EPOR ) or the type 1 cytokine receptor CRLF2 result in overexpression of these proteins leading to autonomous receptor activity. Other genomic rearrangements result in pathogenic activation of tyrosine kinase genes (e.g., ABL1 , ABL2 , PDGFRB , JAK2 ) ( ; ). Single base mutations and small insertion/deletion (indel) type alterations are also evident in a number of genes, including members of the Janus kinase ( JAK ) family. Importantly, many of these alterations converge through the JAK-STAT (signal transducer and activator of transcription) signal transduction pathway. This diverse subset of B-LBL cases nonetheless shares a common gene expression signature similar to BCR-ABL1 -positive (Ph+) B-LBL, leading to their designation as “ BCR-ABL1 –like” leukemias (see Table 78.4 ). Additional genetic deletion or mutation events in critical B-cell transcription factors, notably IKZF1 and PAX5 , are observed in both BCR-ABL1 –positive and BCR-ABL1 –like high-risk B-LBL. The proportion of B-LBL with BCR-ABL1 –like genetic changes increases with age at presentation, with greater frequency seen among older children and young adults ( ); these patients are generally associated with poor outcome. Nevertheless, convergent disruption of known signal transduction pathways in these leukemias is providing new therapeutic opportunities for management using inhibitors of JAK and tyrosine kinases. Comprehensive detection of the complex genomic and genetic aberrations in BCR-ABL1 –like B-LBL is difficult using standard molecular diagnostic methods and instead requires a multimodal strategy including targeted NGS (for gene mutations and small insertion/deletions), FISH (for specific translocations), RT-PCR (for selected expressed gene fusion mRNAs), chromosome arrays (for deletion/duplication analysis), and cytogenetics. CRLF2 gene rearrangement is present in approximately half of BCR-ABL1 –like tumors; genomic or immunophenotypic evaluation of CRLF2 overexpression can detect many of these patients at diagnosis. Targeted gene expression profiling approaches have also been developed to accurately and inexpensively identify the vast majority of BCR-ABL1 –like B-LBL cases ( ), although elucidation of the specific underlying genetic abnormalities with potential for targeted therapy requires more detailed downstream analyses as noted.
In childhood B-LBL, a comprehensive but directed molecular diagnostic testing approach can achieve a high rate of detection of most “favorable-risk” and “high-risk” tumor genetic factors, including the more heterogeneous group of BCR-ABL1 –like cases. The identification of prognostic and predictive molecular markers in pediatric B-LBL has significantly driven the development of risk-adapted practices for managing lower risk and high-risk patients (see Table 78.4 ). This approach is based on the principles of achieving optimal clinical responses while minimizing toxic effects and other late sequelae, such as therapy-related neoplasms. For B-lineage LBL, in particular, initial clinical risk assessment (e.g., age, white blood cell and blast count, extramedullary involvement) is further modified by the presence and type of tumor genetic abnormalities, as outlined earlier. The BCR-ABL1 gene fusion, hypodiploid karyotype, KMT2A rearrangement, BCR-ABL1 –like genotype or phenotype, iAMP21, IKZF1 deletion (often seen in BCR-ABL1 –like disease), or therapeutic induction failure each constitute very high-risk patient subtypes. Conversely, B-LBL cases with hyperdiploidy and “favorable” trisomies or the ETV6-RUNX1 gene fusion are considered good prognostic factors. Early genetic classification (i.e., before the end-induction treatment phase) thereby influences the chemotherapy protocol intensity and duration for a given patient. Most large children’s cancer groups utilize this strategy of tumor genetic risk stratification in slightly different formats. Useful as these parameters have been for more appropriately stratifying patients earlier to receive optimized therapy, a significant subset of children continues to experience treatment failure and leukemic relapse, indicating some enduring lack of precision in the risk assessment models. Better predictive surrogate markers of biologic response and disease clearance have therefore been sought, and MRD detection has emerged as a very powerful method of posttherapeutic prediction, contributing to a more “individualized” approach of risk evaluation. Many large clinical studies have now confirmed the utility of MRD in determining relapse potential in childhood B- and T-lineage B-LBL. Levels of MRD greater than 10 -4 at the end of induction therapy have been shown to be highly correlated with significant risk of relapse compared to MRD negative status and this relationship has been shown to be strongly independent of most clinical and tumor genetic factors in multivariate analyses ( ; ; , ; ; ). MRD can be assessed by flow cytometry techniques or molecular diagnostic methods. Targets for molecular MRD measurements include expressed chimeric fusion gene mRNA transcripts and tumor-specific clonal antigen receptor gene rearrangements.
Immunoglobulin or TCR gene rearrangements are unique and specific markers for MRD assessment in the large majority of individual lymphoid malignancies compared with leukemia-specific fusion gene transcripts, which are present in less than one-third of cases. Notably, in B-lineage LBL, “illegitimate” or cross-lineage gene rearrangements also occur frequently at the TCR genes, such that most B-lineage tumors have at least one and as many as two to three unique gene rearrangements available for MRD monitoring. The clonal V(D)J rearrangements in lymphoblastic leukemia cells can be amplified, then Sanger sequenced for the design of tumor-specific PCR primers, and probes that can be used in subsequent targeted QPCR analysis. However, this approach has relatively poor technical reproducibility and is highly labor intensive, significantly limiting routine adoption in many laboratories. Recent developments using NGS technology for deep sequencing of immunoglobulin and TCR genes (see Fig. 78.1E and F) have demonstrated an alternative and very powerful means of detecting tumor-specific clones at very high analytical sensitivity (∼10 -6 ). Using an optimized consensus PCR primer strategy, a library pool of rearranged antigen receptor gene products can be prepared for high throughput NGS ( ; ; ; ; ). Relying on the highly specific nature of V(D)J segmental recombination and the unique CDR3 junctional region sequence in each lymphoid cell, the presence of tumor-specific DNA clonal disease can be directly and specifically queried in a single assay as long as the initial clonal immunoglobulin or TCR gene sequence of the tumor cells was determined at diagnosis. The development of commercial platforms (e.g., Lymphotrack, Invivoscribe; clonoSEQ, Adaptive Biotechnologies) has greatly facilitated clinical molecular laboratory access to this technology and the related bioinformatics software to effectively analyze the large amount of sequence data generated. Flow cytometric protocols can achieve comparable analytic sensitivity to molecular methods and have a potential advantage of being more easily standardized between laboratories, although at the extremes of analytical sensitivity (e.g., 10 -5 to 10 -6 ), NGS methods appear to be more reliable. Incorporation of MRD data as a routine component of risk assessment enables higher risk MRD-positive patients to be rapidly stratified into more intensive therapy arms. Timing of MRD assessment is critical in this regard; in general, the end of induction therapy is considered a significant and highly informative time point in B-LBL, although ongoing serial measurements can further increase the predictive value.
The t(14;18)(q21;q32) abnormality is a common translocation in B-lineage lymphomas, present in the majority of FLs (80%–90%) and in a subset of diffuse large B-cell lymphomas (DLBCLs; 20%–30%). At the molecular level, this translocation results in the juxtaposition of the BCL2 gene located on chromosome 18q21 with the IGH locus at 14q32. As a consequence, BCL2 gene expression becomes subject to the highly active IGH enhancer element on the derivative 14q32, leading to marked overexpression of BCL2 protein. The BCL2 gene product is a prototypic antiapoptotic protein that acts to protect cells from the process of programmed cell death ( ; ). Although regulation of apoptosis is important in normal cell physiologic contexts, aberrant BCL2 overexpression can inappropriately shield neoplastic cells from a variety of otherwise lethal cytotoxic events and promote a milieu favoring acquisition of additional tumor progression-related genetic abnormalities ( ).The partial genomic structure of the BCL2 gene locus is illustrated in Figure 78.2 . The largest proportion (50%–65%) of break-fusion sites in the BCL2 gene occurs in a tightly clustered area of approximately 150 bp in the 3′ noncoding segment of exon 3 known as the major breakpoint region (MBR). Additional breakpoint regions in BCL2 have been described, including the minor cluster (mcr), intermediate cluster (icr), and areas 3′ of the MBR and 5′ of the mcr ( ; ). BCL2 gene rearrangements in FL involving the MBR can be detected by PCR methods using genomic DNA and consensus primers situated near the MBR and in the IGH -JH region (see Fig. 78.2 ) ( ; ; ). Other BCL2 break sites are more difficult to target by PCR because of heterogeneity in distribution. PCR approaches, however, achieve only 60% to 70% detection for BCL2 - IGH rearrangement, particularly when compared with FISH analysis using dual-color BCL2 and IGH locus-specific probes ( ). FISH is a versatile and relatively rapid approach that can be performed on nuclei extracted from fresh or paraffin-embedded tissues and remains the mainstay for identifying BCL2 rearrangements in lymphomas.
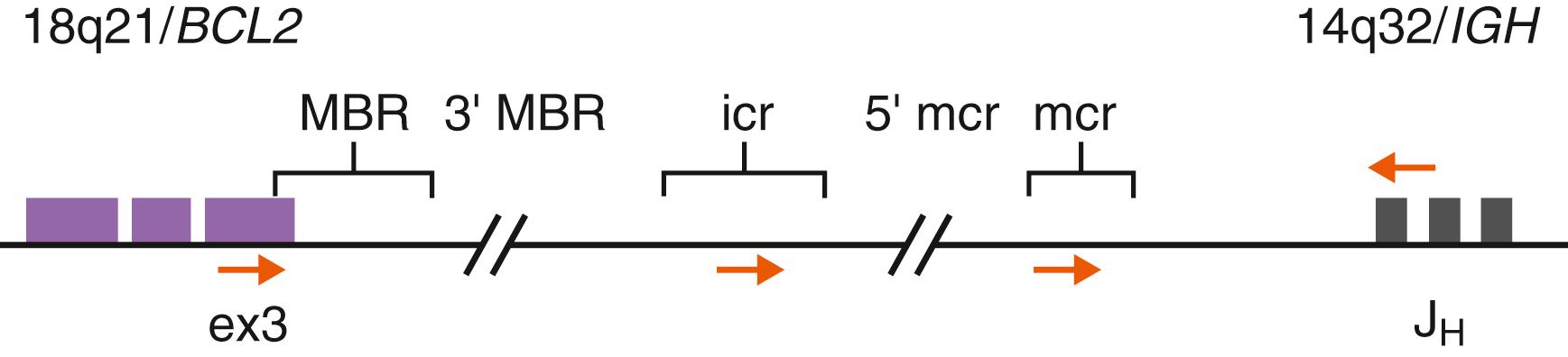
The identification of BCL2 gene rearrangements in neoplastic lymphoid proliferations is useful to establish a diagnosis and for disease subclassification. Cases of atypical or florid but benign follicular hyperplasia can be histologically and phenotypically difficult to differentiate from FL, and in this situation, the presence of the BCL2-IGH abnormality definitively establishes the latter diagnosis. Furthermore, identification of BCL2 gene rearrangement can be used to specifically distinguish low-grade FL from other small B-cell lymphomas with potentially overlapping morphologic patterns, such as mantle cell or marginal zone types exhibiting follicle colonization. The presence of BCL2 gene rearrangements in these contexts is relatively specific for FL and is almost always correlated with BCL2 protein overexpression. Conversely, it is important to note that many B-cell lymphomas and lymphoid leukemias are also associated with deregulated BCL2 protein expression in the absence of structural BCL2 gene translocations (e.g., via gene amplification or possibly epigenetic mechanisms), indicating that the protein expression itself cannot be used for specific lymphoma subtyping ( ). As noted, a small proportion of FLs lacks the t(14;18)/ BCL2-IGH . BCL2 -rearrangement–negative FLs often demonstrate higher cytologic grade (e.g., grade 3) and may be associated with translocations involving the BCL6 proto-oncogene ( ; ). No significant survival differences have been observed between BCL2 -positive and BCL2 -negative FLs, but high-resolution genomics and gene expression profiling studies have suggested the presence of distinct molecular pathways ( , ). Recent genomic studies in FL have also found recurrent mutation events in several genes involved in chromatin modification, including KMT2D ( MLL2 ), CREBBP , EP300 , TNFRSF14 , and EZH2 ( ; ; ; ; ; ; ). These results suggest a central role for epigenetic deregulation in the pathogenesis of FL (see Table 78.4 ). Genomic data have also been evaluated alongside standard risk assessments in improving prognostic models for FL ( ; ).
The t(11;14)(q13;q32) is essentially pathognomonic for MCL in the differential diagnosis of small B-cell neoplasms. This translocation is also detected in a subset of MMs. The t(11;14) places a genomic region on 11q13 called BCL1 in proximity to the JH region of the IGH gene. The gene encoding cyclin D1 ( CCND1 ) is located telomeric to the majority of BCL1 break sites but nonetheless is the consistent target of transcriptional deregulation by the IGH locus as a result of the translocation. Cyclin D1 is an early G1-phase cell cycle protein required for the normal activity of cyclin-dependent kinases 4 and 6 (cdk4, cdk6); these holoenzyme complexes promote progression through the G1/S transition of the cell cycle in proliferating cells through phosphorylation of the retinoblastoma (Rb) protein ( ; ). Cyclin D1 (in contrast to other related proteins cyclins D2 and D3) is not expressed by normal B cells. Aberrant overexpression of the D1-type cyclin is thus thought to play a key role in the pathogenesis of MCL and is supported by the overall “proliferative” transcriptional signature in MCL cells as identified by gene expression profiling studies ( ; ). The presence of a proliferative and antiapoptotic tumor phenotype underlies the relatively poor prognosis of MCL, relative to more indolent small B-lymphocyte neoplasms ( ; ; ).
Successful detection of the t(11;14) or CCND1 genetic rearrangements in MCL depends on the methodology employed. The BCL1 breakpoint region encompasses a large area of 11q13. Approximately half of BCL1 locus breakpoints occur over a small, well-defined site, referred to as the major translocation cluster region (MTC) ( ; ). The MTC is located nearly 120 kb centromeric of the CCND1 gene. Other breakpoints are found either more distal to the MTC or are located in the immediate 5′ region of CCND1 ( ; ). SBH approaches using probes targeting the MTC and other genomic regions have previously mapped BCL1 alterations in up to 70% of MCL cases; however, SBH is impractical for routine diagnostic purposes. DNA-based PCR technique has been successfully used to identify a subset of BCL1-IGH fusions involving the MTC region but only in approximately 30% to 40% of cases ( ). It is thus evident that the large and widely distributed breakpoint region encompassed by the BCL1 locus presents substantial difficulties for common molecular approaches. Break-apart and dual-fusion FISH assays are therefore ideal for resolving t(11;14)/ CCND1-IGH genomic rearrangements in diagnostic specimens, including interphase nuclei obtained from fixed paraffin blocks ( ; ). Cyclin D1 protein overexpression can be readily detected by immunohistochemical techniques in fixed, paraffin-embedded tissues, providing a rapid, widely applicable and complementary method in the diagnosis of MCL, although in situations in which cyclin D1 immunostaining is not successful, FISH for CCND1 locus rearrangement remains of high utility for the identification of MCL. Cyclin D1 overexpression can also be observed in other B-cell neoplasms in the absence of BCL1 / CCND1 rearrangements, including hairy cell leukemia (HCL), the proliferation centers in occasional cases of CLL/small lymphocytic lymphoma (CLL/SLL), and a in subset of large B-cell lymphomas; however, these lymphoid neoplasms can usually be readily distinguished from MCL by morphology and cell marker studies.
Of note, rare cases of classical MCL are recognized that lack the t(11;14)/ CCND1 rearrangement and cyclin D1 overexpression yet exhibit the same morphology, immunophenotype, and gene expression signature ( ). These atypical MCL cases have been associated with translocations involving the CCND2 or CCND3 genes, which are detectable by specific FISH assays ( ; ). SOX11 protein expression is diagnostically helpful in these rare instances because it is present in both classical CCND1 /cyclin D1-positive MCL and CCND1 -negative MCL variant cases ( ). Finally, a subset of primarily leukemic presentations of MCL have been described characterized by the presence of CCND1 rearrangement, somatic IGH -V gene hypermutation, absence of SOX11 expression, lack of bulky adenopathy, and a relatively nonaggressive clinical course ( ; ; ); these “indolent” MCL cases have a distinct gene expression profile relative to classical MCL.
Marginal zone lymphomas (MZLs) include nodal and extranodal presentations, with the latter encompassing splenic and mucosa-associated lymphoid tissue (MALT) subtypes. Although they share similar morphologic and phenotypic features, MZL at certain sites harbor relatively distinct genetic findings, most notably seen among the extranodal MZL (EN-MZL) of MALT type (see Table 78.4 ). Common to MZL are certain recurrent numerical or structural chromosomal alterations, although these are not disease-specific associations. These alterations include +3, +18, and deletions of 6q23 with loss of the TNFAIP3 gene locus ( ; ; ). Splenic MZLs (SMZLs) are often associated with del7(q) ( ), as well as acquired mutations in the KLF2 and NOTCH2 genes in 10% to 40% of cases ( ; ; ). NOTCH2 alterations are associated with an adverse prognosis in SMZL. A small number of SMZL (≤10%) may have the MYD88 Leu265Pro somatic alteration, discussed in more detail with lymphoplasmacytic lymphoma (LPL). The EN-MZLs, also referred to as MALT lymphomas, are uncommon and biologically unusual lymphoid tumors. In addition to neoplastic growth in many diverse extranodal tissue sites (e.g., stomach, lung, salivary gland, thyroid), these tumors are often characterized by the presence of predisposing infectious or autoimmune conditions and a preceding or coexistent reactive lymphoid component. Gastric EN-MZL is a prototypic example: a significant subset of these lymphomas arises in the presence of Helicobacter pylori infection, which appears to play a key role in early disease pathogenesis ( ; ). In addition to underlying infectious or inflammatory conditions, several recurrent genetic abnormalities have been more distinctly associated with EN-MZL in various tissue sites ( ; ; ; ; ). The t(11;18)(q21;q21)/API2-MALT1 abnormality is characterized by fusion of an inhibitor-of-apoptosis gene ( ). BIRC3-MALT1 –positive tumors have a predilection for gastric and lung sites, with infrequent occurrences elsewhere. A second translocation event occurring in EN-MZL is characterized by the t(14;18)(q21;q32)-derived MALT1-IGH gene fusion ( ). Notably, the MALT1 gene is situated slightly centromeric to the BCL2 locus on chromosome 18q, and as such, these two t(14;18)-associated gene fusions are indistinguishable by standard cytogenetics; however, FISH analysis can provide specific delineation. MALT1-IGH –positive lymphomas are distributed in orbital, parotid, lung, and cutaneous sites and are very uncommonly encountered in the stomach. Other rare translocations described sporadically in EN-MZL are the t(1;14)(p22;q32)/ BCL10-IGH and t(3;14)(p14.1;q32)/ FOXP1-IGH abnormalities (Streubel et al., 2005). Despite the diversity of translocation-associated abnormalities and apparent variability in tissue distribution in EN-MZL, a common pathogenesis links these events. Overexpression of MALT1 or BCL10 proteins or expression of a chimeric MALT1 oncoprotein (e.g., from the BIRC3-MALT1 gene fusion) each results in constitutive activation of the nuclear factor kappa B (NF-κB) signal transduction pathway, leading to altered effects on cell growth, immunity, and apoptosis regulation ( ; , ). NGS-based investigations are also beginning to shed additional perspective on genetic and epigenetic alterations in MALT lymphomas, revealing common defects in pathways involving chromatin modification and NOTCH signaling, as well as NF-κB regulation ( ).
Detection of specific translocation events or numerical chromosome alterations can be accomplished using FISH techniques ( ) and can be useful in some cases to establish the diagnosis of MZL; these situations might include morphologically challenging biopsies with prominent reactive or hyperplastic features, or when distinguishing MZL from a different subtype of low-grade B-cell lymphoma (e.g., MALT1 + MZL versus BCL2 + FL). RT-PCR analysis for the BIRC3-MALT1 transcript has been described as an alternative method for detecting the t(11;18) ( ); however, owing to substantial breakpoint heterogeneity in both genes, comprehensive primer design can be problematic and obtaining sufficient high-quality RNA may be a limiting factor in small paraffin-embedded biopsies. Detection of the BIRC3-MALT1 genetic lesion in gastric EN-MZL is potentially important for therapeutic management. Broad-spectrum antibiotic eradication of H. pylori can induce tumor regression in a significant subset of translocation-negative gastric EN-MZL, whereas the presence of the BIRC3-MALT1 gene fusion implicates a distinct pathogenesis that is refractory to this non-cytotoxic option ( , ; ). PCR methods to identify a monoclonal immunoglobulin gene rearrangement in biopsied tissue are often used to support a diagnosis of gastric or other EN-MZL. Follow-up immunoglobulin PCR for assessing residual disease is also sometimes employed in patients with treated gastric EN-MZL; however, the significance of detecting clonal B cells by PCR technique in posttreatment gastric biopsies has been controversial, with some reports suggesting prolonged persistence of monoclonality after histologic eradication of lymphoma and others paradoxically showing the presence of clonotypic B-cell populations in benign gastric lymphoid proliferations ( ; ; ). These studies suggest that the reduction in PCR-detectable clonotypic B cells generally lags behind the histologic normalization of gastric tissue in successfully treated patients and follow-up PCR studies should be interpreted with caution.
CLL and its nodal manifestation small lymphocytic lymphoma (CLL/SLL) are among the most common B-cell malignancies and typically are considered to be indolent entities. Yet, advances in the past 2 decades have revealed distinct prognostic subgroups in CLL/SLL, in turn identifying a proportion of patients at risk for early progressive disease and adverse outcome ( ; ; ; ; ). This area of malignant hematology is illustrative of the convergence of phenotypic, cytogenetic, and molecular genetic tumor features to produce a more refined approach for outcome prediction. Well-known phenotypic adverse factors in CLL include high expression of cell surface CD38 antigen and the overexpression of the signaling kinase ZAP-70. Several recurrent cytogenetic anomalies have been identified in CLL/SLL (see Table 78.4 ), none of which are disease specific. These abnormalities include deletions of chromosome 13 or 13q, deletions of 11q, deletions of 17p, and trisomy 12. The presence of isolated 13q- has been associated with relatively favorable outcome. In contrast, 11q- and 17p- alterations confer a more aggressive disease course with early requirement for chemotherapy ( ). In the latter two situations, minimally deleted regions of these chromosomal loci include the ATM and TP53 genes, respectively. These CLL prognostic cytogenetic changes are most reliably identified with targeted DNA probes using FISH technique. In most cases of CLL with loss of 17p, the remaining allele shows mutation of TP53 , indicating biallelic loss of tumor suppressor p53 activity ( ; ; ; ). Although 17p- CLL cases account for 10% or less of all treatment-naive patients, the prevalence is higher in those with advanced disease or therapy resistance; these individuals exhibit an aggressive disease course requiring earlier therapeutic intervention. TP53 mutated CLL patients are also poorly responsive to commonly used purine nucleoside analog chemotherapeutic agents and require alternative treatment regimens. TP53 gene mutations can be evaluated by the clinical molecular laboratory using PCR and Sanger sequencing (typically of exons 4–9); recommended guidelines for TP53 mutation analysis have also been described ( ). The SHM status of the clonal IGH V-region is also an important prognostic marker in this disease. CLL/SLL cases with mutated IGH V genes (defined as >2% nucleotide deviation from the corresponding germline V-gene DNA sequence) are associated with significantly better outcome compared with cases with unmutated status (≤2% divergent from germline) ( ; ; ; ). Mutational analysis of IGH V genes involves PCR of the complete VDJ clonal rearrangement (i.e., encompassing the VH-FR1 though JH region, including the CDR3 sequence), with precise V-gene family delineation by Sanger sequencing, or increasingly now by NGS methods (see Figs. 78.1E and F) ( ; ). The determination of VH gene identity and SHM status has been greatly facilitated by standardized recommendations and the establishment of comprehensive antigen receptor gene reference databases (e.g., ImmunoGenetics, http://imgt.cines.fr , IGBLAST, https://www.ncbi.nlm.nih.gov/igblast ) ( ; ; ).
NGS studies of CLL/SLL using targeted gene panels, whole-exome sequencing (WES), or whole-genome sequencing (WGS) have also identified several recurrent and prognostically relevant genetic alterations (see Table 78.4 ), including acquired mutations in SF3B1 , NOTCH1 , BIRC3 , POT1 , and MYD88 genes, as well as known genetic changes in TP53 and ATM ( ; ; ; ; ). Utilizing the wide and complex array of genetic and phenotypic prognostic parameters in CLL/SLL for optimizing patient management is challenging, but it has become increasingly apparent that specific prognostic subgroups are strongly associated with outcome or specific therapy considerations ( ; ; ; ; ; ; ). IGH V-gene SHM status appears to be an initial dichotomization for general patient prognostic categories, with additional genetic alterations further refining disease biologic features and course. More complexity also continues to emerge, with the discovery of “stereotyped” (i.e., constrained receptor motif) classes of rearranged IGH V genes (e.g., VH3–21), some of which can exhibit prognostic features either independently or in concert with coexisting adverse or favorable factors ( ; ). The role and understanding of ancillary genetic testing in CLL is being further modified by the presence of newer classes of non-cytotoxic therapeutic agents, such as the Bruton tyrosine kinase (BTK) inhibitor ibrutinib, PI3 kinase delta inhibitors, BCL2 antagonists (e.g., venetoclax), and newer types of therapeutic monoclonal antibodies ( ; ). To summarize, current standard molecular and cytogenetic approaches to evaluate CLL/SLL tend to include a targeted FISH panel, IGH V-gene SHM analysis, and TP53 mutation screening. The application of gene-specific NGS panels for evaluating additional genetic alterations is increasing, but the clinical relevance of these findings requires further validation. Finally, because more patients are being managed using nonchemotherapeutic agents, treatment-resistant mutations can arise in CLL subclones, as evidenced by acquired BTK and PLCG2 gene alterations in patients receiving ibrutinib treatment ( ; ; ; ); these drug resistance variants can also be assessed using PCR and sequencing-based methods. With more patients achieving durable responses from improved risk stratification and newer treatment options, the need for MRD monitoring has emerged. Tracking of clonal CLL cells can be accomplished using flow cytometry, but NGS-based IGH V-region deep sequencing (see Figs. 78.1E and F) is also being increasingly utilized for this purpose ( ; ).
LPL is characterized by a small lymphocytic and variably prominent plasmacytic component and by its association with the clinical syndrome of Waldenstrom macroglobulinemia ( ). Morphologic distinction from other small B-lymphocyte lymphomas with plasmacytic features (e.g., some MZLs) can be challenging and the typical elevation of IgM monoclonal serum protein may also occur in the setting of other B-cell neoplasms. LPL is strongly associated with the presence of a recurrent somatic mutation in the MYD88 gene, which results in an amino acid substitution of leucine to proline at codon 265 (i.e., p.Leu265Pro or L265P) ( ; ; ). This genetic alteration is identified in more than 90% of LPL cases but is also found in approximately 10% of splenic MZL cases, one-third of nodal DLBCL (mostly of ABC type) cases, and at high prevalence in DLBCL occurring in extranodal sites ( ; Hamadeh et al., Cook, 2015; ; ; ; ; ; ). MYD88 L265P also occurs in approximately 50% of patients with IgM monoclonal gammopathy of uncertain significance (MGUS) but is absent in true cases of IgM-positive MM ( ); this finding suggests a relationship between IgM MGUS patients with MYD88 mutation and the development of LPL ( ). In the context of low-grade B-cell lymphomas, the presence of lymphoplasmacytic differentiation, characteristic histopathologic features in the bone marrow, IgM monoclonal paraproteinemia, and presence of MYD88 L265P are together essentially diagnostic of LPL ( ); however, the small proportion of patients with splenic MZL who also harbor MYD88 L265P can pose a challenge in some diagnostic situations ( ). Notably, IgG or IgA expressing lymphomas with lymphoplasmacytic features are less frequently associated with MYD88 mutation ( ; ).
Molecular detection of MYD88 L265P mutation can be accomplished in several ways. PCR of exon 5 followed by Sanger sequencing can be performed to directly identify the single base change but has limited analytic sensitivity (15%–20%). Because of the patchy and often fibrotic nature of bone marrow infiltrates, more sensitive methods such as allele-specific or QPCR ( ) and droplet digital PCR ( ) are more desirable for formalin-fixed paraffin-embedded tissues, as well as peripheral blood samples, which typically have a low number of circulating lymphoma cells ( Fig. 78.3A ). In approximately one-third of patients with LPL with MYD88 L265P, a second somatic genetic alteration can be detected in the chemokine gene CXCR4 . C-terminal truncation or frameshift alterations in CXCR4 can be identified by PCR and Sanger sequencing ( ); Ser338∗ is a commonly observed CXCR4 mutation ( Fig. 78.3B ). Co-occurrence of CXCR4 mutations with MYD88 L265P in LPL is associated with several adverse features, including decreased blood counts, higher lymphoma burden, and impaired response to targeted ibrutinib therapy ( ; ; ; ; ). CXCR4 mutation does not appear to occur independently of MYD88 L265P in LPL; furthermore, when present together, these genetic alterations are relatively specific for the diagnosis of LPL in the setting of low-grade B-cell lymphoma. Current evaluation of LPL thus now includes MYD88 L265P and CXCR4 mutation analyses by molecular techniques.
HCL is often readily diagnosed based on its cytologic features, pattern of bone marrow infiltration, and characteristic immunophenotype ( ). Occasionally however, HCL can be difficult to distinguish from other small B-cell neoplasms, particularly splenic MZL. In these difficult cases, molecular analysis for BRAF Val600Glu mutation (i.e., V600E) can be a useful and relatively specific diagnostic adjunct. As discussed in Chapter 77 , BRAF is a member of the RAS/RAF/MEK/ERK mitogen-activated protein kinase (MAPK) pathway, and aberrant activation by acquired mutation results in deregulated signal transduction ( ; ). BRAF V600E is identified in more than 95% of patients with HCL and is not significantly observed in those with other low-grade B-cell neoplasms ( ). Of note, BRAF V600E mutation is also present in approximately 50% to 60% of patients with Langerhans cell histiocytosis ( ) and in most patients with the rare Erdheim-Chester disease ( ). In addition to providing a molecular marker for diagnostic subclassification of HCL, the presence of BRAF mutation provides a potential targeted therapy option using the small-molecule inhibitor vemurafenib in patients who respond inadequately to standard cladribine or pentostatin treatment. The BRAF V600E alteration can be detected by PCR and Sanger sequencing; however, HCL is often associated with significant reticulin fibrosis in the bone marrow, and “dry tap” aspirate samples are not uncommon, thus necessitating more highly sensitive methods for mutation detection. Allele-specific or QPCR methods can be used for this purpose but require careful optimization to achieve specific mutant allele nucleotide discrimination relative to wild type ( ; ). In fixed paraffinized bone marrow core biopsies, the use of a BRAF V600E-specific antibody is a simple and often informative addition to a HCL diagnostic IHC panel ( ; ). In a small number of HCL cases associated with VH4-34 IGH clonal gene rearrangements, BRAF V600E mutation is not present, and it is similarly absent in cases of so-called of HCL variant ( ; ); these rare cases may harbor acquired alterations in the MAP2K1 gene, further highlighting the importance of MAPK pathway deregulation in HCL.
Diffuse large B-cell lymphoma (DLBCL) is the most common type of aggressive lymphoma and is biologically and clinically heterogeneous ( ; ). DLBCL is broadly subclassified according to germinal center B-cell (GCB) or activated B-cell (ABC) “cell or origin” (COO) phenotypes, which were originally identified by unbiased gene expression profiling (GEP) studies ( ). IHC approaches such as the Hans algorithm are often used to define GCB or ABC types; more recently, targeted GEP techniques have been developed as an alternative to IHC in the clinical setting. COO status in DLBCL can generally identify subsets of patients with better or poorer outcomes on rituximab + CHOP (R-CHOP)–based immunochemotherapy; however, substantial variability in tumor aggressiveness and outcomes still exists both within and between COO subgroups. Recurrent genetic alterations are well known in DLBCL (see Table 78.4 ) and frequently include gene rearrangements involving MYC , BCL2 , or BCL6 loci, most often in conjunction with immunoglobulin gene (e.g., IGH ) translocation events. BCL2 (18q21) and BCL6 (3q26) gene rearrangements each occur in approximately 20% to 30% of cases of DLBCL ( ; ). BCL2 locus abnormalities in DLBCL further appear linked to cell of origin classification. The presence of the t(14;18)/ BCL2-IGH is correlated with a GCB phenotype in DLBCL and poorer outcome with standard therapy ( ). More comprehensive genetic analysis has indicated that genomic gains at the BCL2 locus (e.g., gene amplification) without structural rearrangements are associated instead with ABC phenotype DLBCL, and these cases have an adverse prognosis ( ; ). BCL6 rearrangements also occur more commonly in the ABC group of DLBCL. These associations suggest that specific genomic alterations may underlie distinct biologic and phenotypic subgroups of DLBCL, and this notion has been confirmed to some extent by a large body of research literature ( ; ; ; ; ; ; , ; ; ; ). For example, acquired genetic abnormalities in the B-cell receptor (BCR) signaling pathway (e.g., CD79A , CD79B ), and the NF-κB signaling pathway (e.g., CARD11 , MYD88 ) are associated with ABC type DLBCL, whereas alterations in other genes involved in epigenetic modulation (e.g., EZH2 , KMT2D , CREBBP , EP300 ) characterize GCB tumors. These genetic alterations in turn define major pathways of oncogenesis in DLBCL, including aberrant signal transduction, apoptosis avoidance, immune surveillance evasion, and alterations in tumor-associated microenvironment. Some genetic alterations, such as MYD88 L265P, show much greater prevalence in extranodal DLBCL presentations ( ; ; ; ). TP53 gene mutations are also present in a subset of DLBCL and are associated with adverse prognosis ( ; ).
MYC gene rearrangement occurs in 5% to 10% of de novo DLBCL cases and has been associated in most, but not all, studies with adverse prognostic outcome. MYC translocations typically occur with the IGH or less so IGK and IGL genes, although a small number of cases involve nonimmunoglobulin partners. The net pathogenic effect is constitutive activation of the MYC gene via the strong IGH enhancer element, leading to unchecked Myc expression and cellular proliferation. Aberrant Myc overexpression induces cellular apoptosis in normal cells, so tumor cells concurrently activate additional mechanisms to prolong cell survival (e.g., BCL2 family upregulation, TP53 inactivation) in the face of proliferative stress. The presence of MYC gene rearrangement with coexisting BCL2 and/or BCL6 gene rearrangements is of major clinical relevance in large B-cell lymphomas ( ; ; ; ; ; ). This pattern of “double- (or triple-) hit” gene rearrangements is observed in a subset (5%–15%) of mainly GCB lymphomas with “high-grade” or blastic nuclear features, although some cases can present as morphologically typical DLBCL. These B-cell lymphomas are highly aggressive and respond poorly to standard R-CHOP treatment, requiring instead dose-intensive alternative immunochemotherapy regimens. Some studies have suggested that the adverse association of MYC rearrangement is mainly confined to cases with immunoglobulin gene (IG) partners, suggesting that lymphomas with nonimmunoglobulin (non-IG) gene reciprocal translocations do not appear to behave as aggressively; however, this is controversial, with recent reports finding either no difference in outcomes or a worse prognosis only when MYC translocation is present along with additional rearrangements of BCL2 and/or BCL6 genes ( ; ; ).
The complexity of DLBCL as defined in terms of GEP subtypes, recurrent genetic rearrangements, and gene-level somatic mutations creates a major challenge for molecular diagnostic assessment. Currently, the COO subtype is determined by IHC or less commonly targeted GEP ( , ). The mainstay of DLBCL evaluation involves FISH analysis to identify structural rearrangements of MYC , BCL2 , BCL6 , and IGH (or IGK , IGL ) genes using break-apart and dual fusion probes. A reflex testing strategy is very effective in this regard, beginning, for example, with MYC BAP assessment followed by IGH/MYC , BCL2 , and BCL6 analysis only if MYC is rearranged. This approach establishes prognostic categories according to MYC -normal, MYC -rearranged, or double- or triple-hit ( MYC with BCL2 and/or BCL6 ) status, the latter defining high grade B-cell lymphoma. The role of recurrent tumor genetic sequence alterations in DLBCL continues to be better defined, but it is increasingly apparent that more precise classification and individualized management of these patients will require this information, provided by way of clinically validated targeted NGS gene panels. It is thus apparent that evaluating any single genetic abnormality in DLBCL is increasingly insufficient and more comprehensive diagnostic profiling will be required. A better understanding DLBCL pathogenesis is also more relevant in light of targeted or novel therapies including the BTK inhibitor ibrutinib, the BCL2 antagonist venetoclax, and others.
Burkitt lymphoma (BL) is a relatively rare yet distinct subtype of aggressive B-cell lymphoma that is diagnosed by its signature morphologic features, germinal center phenotype (with absence of BCL2 expression), and variable association with Epstein-Barr virus (EBV) ( ). In this lymphoma, the MYC gene on chromosome 8 translocates to IGH on chromosome 14 or immunoglobulin light chain genes, resulting in its overexpression; these translocations are also a hallmark, being present in 100% of cases. Endemic BL (i.e., sub-Saharan Africa) is highly associated with EBV. In this setting, the MYC gene is translocated to the JH region of the IGH locus, likely as a result of aberrant AID activity occurring during the SMH process ( ; ). Sporadic BL is infrequently associated with EBV and the translocation event with MYC involves the IGH switch region. BL is far more commonly encountered in the pediatric age population; in contrast, significant morphologic overlap exists between BL and DLBCL or high grade B-cell lymphomas in older adults, suggesting caution when diagnosing BL in the latter cases ( ). In general, a diagnosis of true BL is less likely to be encountered in adults given the greater incidence of DLBCL and high-grade B-cell lymphomas with double-hit genetics. Therapy and prognosis are likewise critical considerations because BL requires intensive treatment on B-lymphoblastic leukemia type protocols, in contrast to R-CHOP chemotherapy in DLBCL, or alternative dose-adjusted intensive regimens for high-grade lymphoma. Recurrent tumor-associated mutations in TCF3 and ID3 genes have more recently emerged as relatively specific for BL based on results of research studies using NGS ( ; ). TCF3 is a helix–loop–helix domain transcription factor that is negatively regulated by ID3. Alteration of these genes affects signaling by the BCR complex, creating a “tonic” state of cellular activation. In difficult differential diagnostic situations, identification of TCF3 and/or ID3 tumor genetic changes can therefore be helpful to establish the classification of BL, in conjunction with results from standard break-apart and dual fusion probe FISH evaluation for the presence of MYC , BCL2 , BCL6 , and immunoglobulin gene rearrangements.
Plasma cell or MM is a relatively common hematologic malignancy with complexity in disease presentation and distribution ( ). Significant improvements in patient survival have been achieved with a combination of nonchemotherapeutic drugs and hematopoietic stem cell transplantation (most commonly autologous). Tumor-associated genetic abnormalities in MM form the basis of risk stratification for these patients and broadly are grouped by structural and numerical chromosomal lesions (see Table 78.4 ) ( ; ; ). Cytogenetic hyperdiploidy is a common finding in MM and is unusually represented by trisomic gains in odd-numbered chromosomes. Structural variants are characterized by genomic translocations with the IGH locus, including the t(4;14), t(14;16), t(14;20), and t(11;14) events, which involve the WHSC1 (aliases NSD2 , MMSET ), MAF , MAFB and CCND1 (cyclin D1) genes, respectively. Recurrent gains or losses of chromosome segments are also identified in MM, including 1q amplification, del1p, del13q, and del17p, the last of these being associated with loss of the TP53 tumor suppressor locus. The prognostic framework for MM is based largely on these abnormalities: hyperdiploid MM and cases with CCND1 rearrangements are associated with relatively favorable outcomes, whereas genomic rearrangements or alterations involving MAF / MAFB , WHSC1 , del17p, and 1p or 1q loci are associated with adverse outcome ( ; ). MYC gene rearrangements also occur in a subset of MM patients, associated with increased risk of progression ( ; ). The range of structural and numerical changes in MM is optimally evaluated in the diagnostic laboratory using a panel of FISH probes for enumeration and locus-specific interrogation of target genomic regions. NGS profiling of MM has also revealed recurrent mutations in a number of genes, including NRAS , KRAS , TP53 , BRAF , IRF4 , FAM46C , and DIS3 , among others ( ; , , ). Comprehensive genomic profiling has identified key affected pathways, including MEK-ERK, cell cycle, NF-κB, and epigenetic modification ( ). These studies have also revealed a more intricate relationship of certain acquired genetic mutations with subgroups of structural or numerical chromosome abnormalities in MM. Some genes (e.g., FAM46C ) encode proteins whose functions are inadequately understood at the present time. Others, such as alterations in proteasome complex genes (e.g., PSMB5 ) or cereblon ( CRBN ), an E3 ubiquitin ligase cofactor, have been associated with acquired resistance to common myeloma therapeutics, such as bortezomib and lenalidomide ( ; ). These genetic alterations may affect multiple aspects of MM pathogenesis, such as drug susceptibility or resistance and disease progression. Although the utility of clinical NGS testing to detect gene mutations remains to be determined in relation to the current well-established karyotypic stratification, an intriguing finding is that some recurrently altered genetic targets also reside in regions affected by structural variation (e.g., FAM46C on 1p, DIS3 on 13q, TP53 on 17p), suggesting a role for tumor suppressor loss in both initiation and progression of disease. Furthermore, insights from genomic analysis of MM have identified the relative tumor mutation burden and presence of neutral mutation evolution as important prognostic factors for outcome ( ; ).
Anaplastic large-cell lymphoma (ALCL) is a unique subtype of peripheral T-cell NHL that may present as a primary cutaneous disease or, more commonly, as a systemic form involving lymph nodes, visceral organs, and skin ( ; ; ; ; ). A rare presentation occurs in breast capsule tissue in patients with silicone prosthetic implants ( ; ). A phenotypic hallmark in nearly all cases of ALCL is strong uniform expression of the CD30 (Ki-1) antigen, an activation marker and member of the tumor necrosis factor family. Many cases of ALCL either show minimal T-cell–associated markers, and some exhibit a “null” cell phenotype, but these cases can usually be confirmed as being of T-cell lineage based on T-cell receptor gene rearrangement studies. A significant subset of systemic ALCL is characterized by translocations involving the anaplastic lymphoma kinase ( ALK ) gene located on chromosome 2p23 (see Table 78.4 ). ALK encodes a tyrosine kinase that is not normally expressed in lymphoid cells. Approximately 90% of ALK -rearranged ALCL harbor the t(2;5)(p23;q35) abnormality, leading to a fusion of the ALK gene with the NPM1 gene ( ; ; ). Most genomic translocations in lymphomas aberrantly induce expression of a critically regulated gene (e.g., BCL2 , MYC ) under the promoter or enhancer element of the genetic partner. In ALCL, the NPM1-ALK chimeric fusion produces a novel oncoprotein that drives ALK overexpression in the neoplastic T cells. As a result of the NPM1-ALK gene fusion, the tyrosine kinase activity and intracellular location of ALK protein become deregulated, contributing to lymphomagenesis through increased target protein phosphorylation and signal transduction ( ; ; ). Research data have shown that ALK activation is associated with downregulation of T cell–associated protein expression, NF-κB target gene activation, functional inactivation of normal p53 tumor suppression, and immunosuppressive effects mediated through STAT3 signaling ( ; Werner et al., 2017; ). In addition to NPM1-ALK , several less common variant genetic rearrangements of ALK are also described in ALCL, including the t(1;2)(q25;p23)/ TPM3-ALK , t(2;3)(p23;q35)/ TFG-ALK, and inv(2)(p23q35)/ ATIC-ALK abnormalities, all similarly resulting in ALK gene deregulation (reviewed in ). Notably, the abnormal cellular sublocalization of constitutive ALK kinase activity is mediated by the fusion partner protein moiety. Thus, NPM1-ALK oncoproteins are situated in both a nuclear and cytosolic pattern, whereas TPM3-ALK products show diffuse cytoplasmic distribution, in accordance with the usual site of the TPM3 gene product tropomyosin-3. Several clinical studies have demonstrated a relatively favorable prognosis for ALK -positive ALCL, regardless of the underlying type of translocation ( ). ALK -positive ALCL is more prevalently seen in younger patients (e.g., younger than 50 years) and in the pediatric population. Of note, the primary cutaneous form of ALCL is not commonly associated with ALK gene abnormalities or aberrant ALK expression and has a very favorable outcome. A second group of systemic ALCL is now recognized by absence of ALK gene disruption ( ALK -negative ALCL) but instead with rearrangements of the DUSP22/IRF4 gene locus (6q25.3) or the p53-related TP63 gene (3q28) (see Table 78.4 ). DUSP22 and TP63 ALCL share similar morphologic features and expression of CD30 antigen as ALK -positive cases. Most significant, ALK -negative ALCL with DUSP22 gene rearrangement has a relatively favorable prognosis similar to ALK -positive counterparts, whereas TP63 gene rearrangement identifies a subset of ALCL with very poor outcome ( ; ; ; ; ; ). In part, the better outcomes for DUSP22 rearranged ALCL may be related to the presence of an immunogenically primed state, establishing a more robust host response ( ).
Genomic breakpoints in cases of t(2;5)-positive ALCL lie consistently within the same intron regions in both NPM1 and ALK genes, resulting in the generation of a single, unique NPM1-ALK chimeric mRNA. As a result, RT-PCR technique can be used to detect the fusion transcript with high specificity and sensitivity ( ; ; ). In addition, long-distance DNA PCR has also been successfully employed to identify the genomic NPM1-ALK abnormality ( ; ). The latter approach requires high molecular weight DNA but obviates the need for RNA isolation and reverse transcription. FISH methodology is nevertheless the most widely employed diagnostic modality to detect ALK gene rearrangements ( ; ; ). Essentially all translocation-associated alterations can be detected using a BAP method directed to the ALK locus; elucidation of the specific translocation gene partner is not required because of its limited clinical value. As with other gene overexpression phenomena in the NHLs, ALK -positive ALCL can also be readily diagnosed using monoclonal antibodies against the ALK portion of the oncoprotein. The immunohistochemical staining pattern of the abnormal ALK protein, as suggested previously, also correlates to some extent with the causative translocation event, reflecting the contribution of the fusion partner segment to cellular localization ( ; ; ). The subset of ALK -negative ALCL with DUSP22 or TP63 gene rearrangements can be reliably detected by specific FISH probe techniques. The presence of ALK , DUSP22 , or TP63 gene rearrangements provides important prognostic information in ALCL and can also be helpful in the differential diagnosis between ALCL and classical Hodgkin lymphoma.
As a footnote on ALK -positive lymphomas, rare presentations of an aggressive large B-cell lymphoma with t(2;17)(p23;q23)/ CLTC-ALK or t(2;5)/ NPM1-ALK gene fusions have been described, in association with IgA heavy chain expression and plasmablastic cytologic features ( ; ; ). These tumors are also recognized by the presence of plasmacytic cell markers but lack of CD20 expression and are CD30 negative. The CLTC gene encodes clathrin, and the chimeric CLTC-ALK protein has a unique granular membranous cellular immunohistochemical pattern, corresponding to ALK localization in clathrin-coated pits.
The genomic pathology of peripheral T-cell lymphoma (PTCL) is becoming better understood in the era of NGS and comprehensive tumor genetic profiling ( ; ; ; ). One example is that of angioimmunoblastic T-cell lymphoma (AITL) and related entities under the subgroup of lymphomas of T-follicular helper (TFH) cell origin ( ). NGS studies have identified certain recurrent acquired genetic changes associated with TFH lymphomas, including mutations involving IDH2 (particularly IDH2 Arg172), RHOA , CD28 , DNMT3A , and TET2 genes ( ; ; ; ; ). Gene fusions such as the t(5;9)/ ITK-SYK and CTLA4-CD28 occur in small subsets of PTCL (including some cases of AITL). Molecular diagnostic evaluation of PTCL (excluding ALCL) currently does not require extensive studies beyond TCR gene rearrangement PCR analysis to establish monoclonality in some cases. However, the presence of recurrent gene mutations or chimeric gene fusion abnormalities suggests that a focused NGS approach may be of value for diagnosis and subclassification. Furthermore, the availability of selective inhibitors of mutated IDH2 (e.g., enasidenib) may provide a potential therapeutic option for these patients in future. Despite significant gains in our understanding of PTCL molecular pathogenesis (most evidently for ALCL and TFH lymphoma), the wider category of PTCL-unspecified remains genetically complex, requiring ongoing research efforts to improve outcomes for these patients.
T-cell large granular lymphocytic leukemia (T-LGLL) and T-cell prolymphocytic leukemia (T-PLL) are examples of chronic T-cell lymphoproliferative neoplasms with characteristic tumor genetic alterations. Acquired mutations of STAT3 (present in approximately one-third of cases) and less frequently STAT5B (<5% of cases) occur in T-LGLL ( ; ; ; ; ; , ). STAT3 gene mutations are also observed in a subset of chronic lymphoproliferative disorders of natural killer (NK) cells. Mutations in these genes are considered to deregulate signaling through the JAK-STAT pathway. STAT3 and STAT5B alterations can be identified by PCR and Sanger sequencing, allele-specific PCR, or as part of a targeted NGS panel. The latter testing approach may be of particular value given that many cases of T-LGLL are associated with unexplained peripheral blood cytopenias (mainly neutropenia), raising the differential diagnostic consideration of a myelodysplastic syndrome (MDS). NGS results can help to delineate a possible T-LGLL process from MDS provided that STAT3 and STAT5B are included in the NGS panel design. The presence of STAT gene mutations may be useful to distinguish T-LGLL from reactive granular lymphocyte expansions in some patients, especially when TCR gene rearrangement analysis is equivocal.
T-PLL is a well-known lymphoid neoplasm with heterogeneous clinical presentation and course, and is associated with recurrent rearrangements of the TCL1A gene ( ). T-PLL often demonstrates cutaneous involvement in addition to peripheral blood, bone marrow, and spleen ( ). TCL1A is deregulated in 80% of T-PLL cases as a result of an inv14(q11q32) or t(14;14)(q11;q32) event ( ). TCL1A is a proto-oncogene and after translocation to the TCR-α locus ( TRA ) on 14(q11) becomes activated, leading to overexpression of the TCL1A transcription factor, which is normally not present in mature T cells (but is expressed in a subset of B lymphocytes). TCL1A gene rearrangement is not identified in other T-cell neoplasms and is thus a relatively specific genetic marker for T-PLL ( ). FISH analysis is most often employed to detect 14q translocation or inversion abnormalities affecting the TCL1A locus, although IHC is a rapid alternative to demonstrate the overexpressed TCL1A protein (e.g., in paraffin-embedded bone marrow biopsy specimens). Rare patients with T-PLL lack TCL1A rearrangements but instead have alternate t(X;14)(q28;q11) or t(X;7)(q28;q35) events ( ; ), resulting in translocation of the TCL1A -related gene MTCP1 to the TRA or TCR-β ( TRB ) loci, respectively. These cases are more challenging to identify without specific FISH probes or karyotype studies. The finding of TCL1A rearrangement by FISH analysis or TCL1A overexpression by IHC is valuable in distinguishing T-PLL from other mature T-cell neoplasms with overlapping morphologic or immunophenotypic features.
The importance of genetic markers is reflected in the present WHO classification, in which AMLs are increasingly defined by their characteristic genetic abnormalities in relation to morphologic and immunophenotypic features, prognosis, and therapeutic implications. The identification of specific genetic markers can thus establish the diagnosis of AML subclassification, provide important prognostic information, guide therapeutic intervention, and reveal insights into tumor biology ( ; ; ).
Cytogenetic studies of large cohorts of patients with AML have shown that balanced gene rearrangements underlie the disease pathogenesis in 30% to 50% of pediatric and younger AML patients and a minority of older AML patients ( ). These changes had been recognized in the WHO classification under the category of “AML with recurrent genetic abnormalities” ( Box 78.1 ). Although the diagnosis of AML routinely requires at least 20% myeloblasts in the peripheral blood or bone marrow, detection of t(8;21)(q22;q22.1); RUNX1-RUNX1T1, inv(16)(p13.1q22)/t(16;16)(p13.1;q22); CBFB-MYH11 , or t(15;17)(q24.1;q21.2); PML-RARA warrants a diagnosis of AML regardless of blast count. Therapy-related myeloid neoplasms may also harbor these balanced translocations or inversion and should be diagnosed as therapy-related myeloid neoplasms with the genetic abnormalities noted. At the time of AML diagnosis, cytogenetic analysis is a crucial component of the work-up not only because it defines AML subtypes in the context of patient’s clinical and therapeutic history and molecular findings but also because it serves as the cornerstone for AML risk stratification.
AML with t(8;21)(q22;q22.1); RUNX1-RUNX1T1
AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11
Acute promyelocytic leukemia with PML-RARA
AML with t(9;11)(p21.3;q23.3); KMT2A-MLLT3
AML with t(6;9)(p23;q34.1); DEK-NUP214
AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2, MECOM
AML (megakaryoblastic) with t(1;22)(p13.3;q13.1); RBM15-MKL1
AML with BCR-ABL1 : provisional
AML with gene mutations
AML with mutated NPM1
AML with biallelic mutation of CEBPA
AML with mutated RUNX1 : provisional
AML with Myelodysplasia-Related Changes
Therapy-Related Myeloid Neoplasms
AML, Not Otherwise Specified
Myeloid Sarcoma
Myeloid Proliferation Associated with Down syndrome
The prognosis of patients with AML is determined by multiple factors, including patient demographics and clinical and treatment variables as well as a major impact from genetic abnormalities. AML had historically been assigned into “favorable,” “intermediate,” or “adverse” prognosis types based on cytogenetic findings. However, in the past decade, the molecular landscape of AML has been unveiled with the advent of high-throughput sequencing. Recurrent somatic mutations were observed in several common functional pathways: signaling and kinase pathways, NPM1 , transcription factors, epigenetic modifiers, spliceosome complex, cohesin complex, and tumor suppressors ( Table 78.5 ). Although a normal karyotype is seen in about 40% to 50% of patients with AML, somatic mutations are seen in 90% to 95% of patients with AML with a median of three or four mutations per case ( ; Cancer Genome Atlas Research et al., 2013; ; ). These findings shed light on the genetic basis of AML, particularly in the cytogenetic intermediate-risk group with normal karyotype or chromosomal gains or losses. Because AML is clinically and genetically heterogeneous with co-occurrence of competing clones and genetic abnormalities impacting different yet interacting functional pathways, and naturally comes with dynamic clonal evolution, a major challenge for today’s medical practitioners is to accurately decipher the clinical impact encrypted by the various combinations of genetic abnormalities and translate them into clinical gain for individual patients. Aided by numerous correlative clinical studies, progress has been made to this end, as reflected in the 2017 European LeukemiaNet (ELN) recommendation and 2020 National Comprehensive Cancer Network (NCCN) guideline in which mutational status further refined the cytogenetic-based risk stratification system, particularly in the intermediate cytogenetic risk group ( Table 78.6 ) ( ; ). Recent years have also witnessed meaningful advancement in targeted therapy in AML, and the U.S. Food and Drug Administration (FDA) has approved FLT3 inhibitors midostaurin and gilteritinib, IDH1 inhibitor ivosidenib, and IDH2 inhibitor enasidenib for adult AML patients harboring these respective mutations. The field of genomics for AML and other myeloid neoplasms is rapidly evolving, and at the time of AML diagnosis, multiple clinically relevant molecular markers such as FLT3, NPM1, CEBPA, KIT, RUNX1, ASXL1, TP53, IDH1, and IDH2 should be evaluated (most efficiently by NGS) along with cytogenetics for comprehensive prognostic assessment and possible targeted therapy.
| Function or Pathway | Genes |
|---|---|
| Epigenetic modifier | DNMT3A |
| DNA methylation and hydroxymethylation | TET2 |
| IDH1 | |
| IDH2 | |
| WT1 | |
| Epigenetic modifier | EZH2 |
| Chromatin histone acetylation and methylation | ASXL1 |
| MLL/KMT2A | |
| BCOR | |
| Spliceosome complex | SRSF2 |
| U2AF1 | |
| SF3B1 | |
| ZRSR2 | |
| Signaling and kinase pathway | FLT3 |
| KRAS | |
| NRAS | |
| KIT | |
| PTPN11 | |
| NF1 | |
| CBL | |
| Transcription factors | CEBPA |
| RUNX1 | |
| GATA2 | |
| ETV6 | |
| BCOR | |
| Tumor suppressor | TP53 |
| PHF6 | |
| Cohesin complex | RAD21 |
| STAG1 | |
| STAG2 | |
| SMC1A | |
| SMC3 | |
| Nucleophosmin | NPM1 |
| Poorly defined | SETBP1 |
| Risk Category | Genetic Abnormality |
|---|---|
| Favorable | PML-RARA |
| t(8;21)(q22;q22.1); RUNX1-RUNX1T | |
| inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11 | |
| Mutated NPM1 without FLT3 -ITD or with FLT3 -ITD low | |
| Biallelic mutated CEBPA | |
| Intermediate | Mutated NPM1 and FLT3 -ITD high |
| Wild-type NPM1 without FLT3 -ITD or with FLT3 -ITD low (without adverse-risk genetic lesions) | |
| t(9;11)(p21.3;q23.3); MLLT3-KMT2A | |
| Cytogenetic abnormalities not classified as favorable or adverse | |
| Adverse | t(6;9)(p23;q34.1); DEK-NUP214 |
| t(v;11q23.3); KMT2A rearranged | |
| t(9;22)(q34.1;q11.2); BCR-ABL1 | |
| inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2,MECOM(EVI1 ) | |
| -5 or del(5q); -7; -17/abn(17p) | |
| Complex karyotype, monosomal karyotype | |
| Wild-type NPM1 and FLT3 -ITD high | |
| Mutated RUNX1 in the absence of favorable risk AML subtypes | |
| Mutated ASXL1 in the absence of favorable risk AML subtypes | |
| Mutated TP53 |
This section focuses on the common AML-related genetic abnormalities that are currently considered to be the most clinically relevant because of frequency of occurrence and impact on prognosis and therapeutic intervention.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here