Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vasopressin, or in humans and rodents, arginine vasopressin (AVP), is the most important hormone in the regulation of urine concentration. Without the ability to concentrate our urine, we would excrete as much as 170–180 L of urine per day (given an average glomerular filtration rate (GFR) of about 120 mL/min). Other factors have some role, such as the renal sympathetic nervous system and possibly, to a small extent, the renin–angiotensin–aldosterone system, but none of these other systems or regulatory-hormone cascades approaches the importance of AVP. This is illustrated by the fact that mutations that either result in the loss of circulating AVP or affect AVP actions on renal tubules, such as mutations in aquaporin 2 water channel or the V2 receptor, always result in an overt disease state, i.e., diabetes insipidus (discussed further in Chapter 23 ). In this chapter, we detail (1) the synthesis, release, and regulation of AVP; (2) the various subclasses of AVP receptors, their molecular structures, differences in their patterns of expression, regulation, and signaling; (3) the regulation of renal transport including water (greater detail in Chapter 20 ), sodium, potassium, chloride, bicarbonate, calcium, magnesium, and urea and their specific transporters and channels by AVP; and (4) AVP actions on renal hemodynamics including its potential role in blood pressure regulation and underlying mechanisms.
Vasopressin is a peptide hormone that can cause vasoconstriction in arterioles and thus, increase arterial blood pressure, hence the term “vasopressin.” It is also known as the “antidiuretic hormone” (ADH) as it causes antidiuresis (decreases urinary water excretion) by increasing the water reabsorption in the distal nephron.
Mature vasopressin or AVP consists of merely nine amino acid residues, i.e., Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly. The prohormone is synthesized in the cell bodies of magnocellular neurons of the paraventricular and supraventricular nuclei of the hypothalamus, and then packed into membrane-bound neurosecretory granules. The prohormone consists of vasopressin, a glycopeptide, and a vasopressin-associated carrier protein, neurophysin ( Fig. 6.1 ). In the classical neurosecretory pathway, cleavage of this large prohormone occurs during its axional transport in the pituitary stalk to the posterior pituitary. The cleavage results in release, followed by storage, of the final hormonal product, AVP in the axional bulb of the pars nervosa. Upon stimulation for its secretion, AVP is released from these storage lobes into the blood. Recent studies show that in addition to its classical axonal release into the circulation, vasopressin is also released into the extracellular brain compartment, where its concentration has been shown to be ∼100 times higher than plasma concentrations. The dendrites from magnocellular cells are the predominant source of vasopressin released into the brain. This dendritic vasopressin contributes to an increased renal sympathetic outflow.
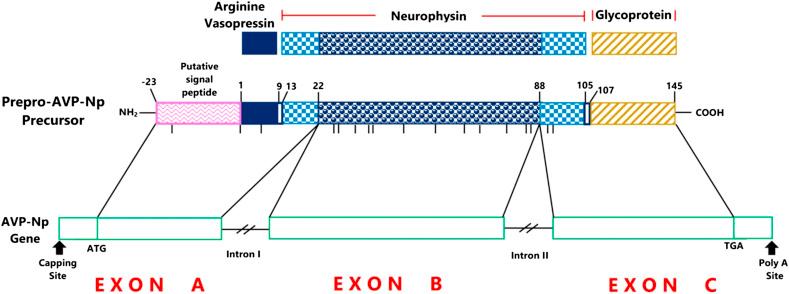
Various kinds of stimuli that result in AVP release are discussed in detail later in this chapter. These stimuli also enhance the biosynthesis, transport, and posttranslational processing of AVP and thus help to maintain the amount of the final hormone product in the neural storage lobe. The entire process of synthesis until storage of AVP takes approximately 1–2 h.
Normal plasma AVP levels in a healthy, hydrated human are approximately 1–2 pg/mL or about 1 pM. AVP circulates as an unbound peptide hormone and is rapidly metabolized by vasopressinase in the kidney and liver, two sites of AVP clearance. The half-life of AVP in rats is reportedly 1–8 min, in humans between 10 and 35 min, and in dogs between 4 and 8 min. Vasopressin is present in significant amounts in urine as there is little tubular reabsorption and degradation of AVP after glomerular filtration. However, urinary AVP levels should not be used as a measure of plasma levels, as its excretion varies with GFR, tubular reabsorption, and luminal degradation.
The structure of oxytocin, another nonapeptide hormone, is very similar to that of vasopressin with a substitution of two amino acids, i.e., isoleucine (Ile) in place of phenylalanine (Phe), and leucine (Leu) in place of arginine (Arg). This may explain why oxytocin is slightly diuretic. Besides the similarities in their structure, oxytocin and vasopressin are located on the same chromosome (separated by 1500 genes), and both hormones can cause uterine contractions.
There are various factors that trigger vasopressin production and secretion into the circulation. Preeminently, is a change in extracellular fluid osmolality, detected by the presence of “osmoreceptors” located in the hypothalamus. Osmoreceptors sense changes in extracellular fluid osmolality caused by a gain or loss of water and send a signal for the increase or decrease of AVP secretion. An increase in osmolality increases the rate of AVP secretion, while reduced osmolality inhibits AVP secretion.
The other major factor regulating AVP secretion is a change in blood volume and pressure detected by baroreceptors. A decrease in extracellular volume, due to conditions such as hemorrhage or diarrhea, is sensed by the baroreceptors in the cardiac atria, aorta, and carotid sinus. The signal is carried by neurons of the vagus and glossopharyngeal nerves to the central nervous system (CNS), resulting in the stimulation of AVP secretion. However, a decrease in plasma volume (hypovolemia) alone cannot cause a rise in plasma AVP until it is accompanied by a steep fall in blood pressure, to trigger arterial baroreceptors and AVP secretion. Thus, a decrease in cardiovascular pressure leads to an increase in AVP secretion and vice versa. The increased levels of circulating AVP results in water conservation through its antidiuretic actions on the kidney, thereby maintaining extracellular volume, and hence, blood pressure. Thus it can be predicted that diseases associated with decreased input from the arterial baroreceptors, such as congestive heart failure, would have elevated levels of AVP secretion. During a severe decrease in extracellular volume and pressure, for example due to hemorrhage, strong signals sent by baroreceptors result in secretion of AVP which far exceeds that which is required for its antidiuretic actions on kidney. Under these circumstances, AVP directly acts on the vascular smooth muscle cells as a vasoconstrictor, which helps to maintain cardiovascular pressure independently of its slow antidiuretic action. Thus, excessive increases in AVP levels are an adaptive reflex in response to the prompt need for blood pressure maintenance under a severe fall in extracellular volume and cardiovascular pressure.
The subfornical organs of the lamina terminalis, a part of the brain, have been recognized as a crucial site for the physiological regulation of vasopressin secretion. Circulating hormones such as angiotensin II and relaxin, as well as plasma hypertonicity are among factors that have been suggested to exert their actions on the lamina terminus to regulate vasopressin secretion. Angiotensin II is the first blood-borne peptide hormone discovered to exert action on the lamina terminalis and increase vasopressin release. This action of angiotensin II was attenuated when the efferent neural connections of the subfornical organ were disrupted, suggesting that this is the main site of action of circulating angiotensin II to induce vasopressin release. A second hormone which may stimulate vasopressin release is relaxin. When injected systemically or directly into the brain, relaxin has been shown to act as both a dipsogenic agent and a stimulant for vasopressin release.
Sex steroids have also been shown to regulate the secretion of vasopressin. In this regard, estrogen and testosterone have both been shown to affect central neuropeptide transmission. In rodents, estrogen, in particular, has been shown to stimulate the expression of the AVP gene, resulting in a massive increase in AVP peptide in the bed nucleus of the stria terminalis. This estrogen-stimulated or testosterone-stimulated upregulation of vasopressin may play a key role in maintaining or regulating olfactory memory.
The inappropriate or sustained secretion of AVP by the hypothalamic-pituitary system leads to a condition known as syndrome of inappropriate antidiuretic hormone (SIADH secretion), a relatively common cause of hyponatremia. Elevated AVP secretion leads to water retention and extracellular fluid expansion, which is compensated for by increased urinary Na + excretion. The combination of water retention and Na + excretion leads to hyponatremia. Bartter and Schwartz first characterized SIADH as “hypotonic hyponatremia,” urine osmolality greater than appropriate for the concomitant plasma osmolality, increased natriuresis, absence of edema or volume depletion, and normal renal and adrenal function. There are various conditions associated with inappropriately high levels of circulating AVP, e.g., cancer that induces ectopic AVP production, HIV/AIDS, pulmonary disease, endocrine disease, neurologic disease, trauma, and surgery. This condition is expanded upon in Chapter 23 .
Another potentially life-threatening disorder associated with dysregulation of AVP is exercise-associated hyponatremia (EAH). The primary cause of EAH is inappropriate vascular water retention leading to dilutional hyponatremia. EAH results from the combination of a high or excessively high fluid intake in the setting of a prolonged or sustained cardiovascular activity, which seems to be associated with modest elevations in plasma AVP levels. The mechanism(s) responsible for nonosmotic stimulation of vasopressin secretion in EAH remain to be determined. However, possible factors are pain and hypotension, which commonly occur in marathon runners. In addition, the release of muscle-derived interleukin-6 during rhabdomyolysis as shown in human subjects may also stimulate secretion of AVP. Rhabdomyolysis has been observed in other clinical conditions in which SIADH may accompany inflammatory stress. Finally, prophylactic use of nonsteroidal prostaglandin inhibitors as a means to alleviate pain or inflammation due to exercise may also be detrimental and lead to increased fluid salt and water retention. Prostaglandins antagonize the actions of AVP with regard to cyclic AMP (cAMP) generation and urine concentration.
Three main G-protein-coupled vasopressin receptors have been cloned and characterized. These are as follows: (1) type 1a (V1a, gene symbol Avpr1a); (2) type 1b (V1b, gene symbol Avpr1b); and (3) type 2 (V2, gene symbol Avpr2) receptors. However, besides these three receptors, vasopressin also has equal affinity with oxytocin for oxytocin receptors (OT, gene symbol Oxr1), a related G-protein-coupled receptor (GPCR), and may exert some of its action via OT signaling. In addition to these classical receptors, a dual angiotensin II/vasopressin receptor was reported in 1998 using pharmacological approaches. This receptor was localized to the outer medullary thick ascending limb (TAL) and inner medullary collecting duct (CD), and reportedly has a role in hypertension. However, examination of the protein sequence using available databases suggests it has no membrane-spanning regions, and thus, is unlikely to function as GPCR.
In 1992 Morel et al. cloned the complementary DNA (cDNA) encoding the hepatic AVP receptor type 1a (V1a) gene from the rat liver. V1a cDNA is 1354 base pairs in length and encodes a 394-amino-acid protein with a molecular weight (M r ) = 44,202. Subsequently, the structural organization of the rat V1a receptor gene was reported; the gene spans 3.8 Kb and consists of two coding exons separated by a noncoding intron of 1.8 Kb. The first exon encodes six transmembrane (TM) domains and the second exon encodes the last TM domain. Two different transcription initiation sites were reported, i.e., at −243 and −237 base pairs upstream of the initiation codon (ATG). The region between −296 and −222 (relative to initiation codon) was found to exhibit promoter activity.
The human V1a receptor cDNA was cloned by Thibonnier and associates. They reported a 1472 nucleotide sequence encoding a 418-amino acid protein. Subsequently, they reported its structure, sequence, and chromosomal localization (chromosome 1). The gene has two coding exons and a 2.2 Kb intron located before the seventh TM domain, as in the case of the rat V1a gene. For the human V1a receptor gene, the transcription initiation site is at 1973 bp upstream of the initiation codon. There are untranslated regions of 2 and 1 Kb, respectively, at the 5′ and 3′ ends of the gene.
The human V1b receptor was cloned from the pituitary gland. It is a 5.2-Kb transcript as detected by Northern blot analysis. The deduced 424-amino acid sequence (relative molecular mass is 47,034) has seven TM domains. The human V1b receptor, encoding a 424-amino-acid sequence, has quite high homology with the other vasopressin receptor subtypes, i.e., V1a (45%) and V2 (35%), and also with oxytocin receptors (45%).
The V1b receptor was also cloned and characterized from mouse and rat pituitary. The rat V1b receptor gene contains three exons and two introns. The first short intron (161 bp) is located in the 5′-untranslated region from bp +124 to +285 (+1 was assigned to the proximal transcriptional start point). Similar to the other vasopressin receptor subtypes, the second intron was found to be present at the end of the sixth TM domain. Two major putative transcription initiation sites have been mapped: (1) the proximal site, which is assigned +1, and (2) a distal site located at −31.
The human V2 receptor gene was cloned and shown to be localized to the long arm of the X-chromosome at the q28-qter region. V2R cDNA predicts a protein with 341 amino acids with a calculated M r of 40,285 in the absence of any posttranscriptional modification. Also in 1992, Morel, Lolait, and colleagues cloned the rat V2R gene from kidney, and reported that, in humans, the gene appeared on the X chromosome near a locus for nephrogenic diabetes insipidus (NDI). The human V2R gene consists of three exons separated by two noncoding intronic sequences. The first intron is 360-bp long and interrupts a codon corresponding to the ninth amino acid of the receptor sequence. The second intron separates the sixth and seventh TM domains. The human V2 receptor gene has 35% homology with the human β1 adrenergic receptor and its ligand-binding properties are similar to those of V1 receptors and the oxytocin receptor.
Table 6.1 describes the location and the principal effects of vasopressin receptors. The V1a receptor is expressed in a variety of tissues including the brain, vasculature, and kidney. In the brain, it is found in the septum, cerebral cortex, hippocampus, and hypothalamus. V1a receptors are also localized on the smooth muscle cells of vessels of the systemic, splanchnic, renal, and coronary circulation. In the kidney, V1a has been localized in renal tubules and vasculature of the kidney cortex and medulla. Terada and associates examined mRNA expression of V1a in microdissected tubular and vascular segments and found high expression in the glomerulus, initial cortical CD, cortical CD (CCD), outer medullary CD (OMCD), inner medullary CD (IMCD), and arcuate artery. Small but detectable signals were found in proximal convoluted and straight tubules, inner medullary thin limbs, and medullary TAL. The presence of V1a in the TAL is somewhat controversial. Binding studies in isolated medullary TAL cells have suggested that a “V1a-like receptor” is present in this segment, as a V1a antagonist was able to bind to this site. Furthermore, vasopressin has been demonstrated to trigger a transient increase in intracellular Ca 2+ , possibly via a V1 receptor-mediated signaling pathway, in the cortical TAL cells of the rabbit. In the CD cells, V1a receptor protein has been localized at the luminal membrane in the rat kidney using immune-based approaches. Subsequently, Tashima et al. have demonstrated V1a receptor mRNA and protein in microdissected cortical CD. They reported that this receptor is present primarily in the principal and intercalated cells of the CD. Using transcriptomics of microdissected rat kidney tubules, Lee and associates found modest V1aR (Avpr1a) transcript in the distal convoluted tubule, connecting tubule, and CCD, with essentially no expression in the TAL or OMCD and IMCD.
| Receptors | Tissues | Principal Effects | Intracellular Signaling |
|---|---|---|---|
| Avpr1a (V1a) | Vascular smooth muscle, liver, kidney, platelets, brain | Vascular tone, memory | Gq/11, phosphoinositide pathway (activate phospholipase C) |
| Avpr1b (V1b, V3R) | Pituitary, kidney, uterus, thymus, heart, breast, lung, liver | Neurotransmitter, ACTH release, vascular tone | Gq/11, phosphoinositide pathway (activate phospholipase C) |
| Avpr2 (V2) | Renal connecting tubule, collecting duct and thick ascending limb | Water permeability, potentially sodium transport | Gs, cAMP |
| Oxtr (OT) | Uterus, mammary gland | Vascular tone | Gq/11, phosphoinositide pathway (activate phospholipase C) |
| Endothelium |
In contrast to the V1a receptor, the V1b receptor has a limited tissue distribution. It was initially defined as a pituitary-specific subtype in rat and human; indeed, its mRNA was markedly abundant in pituitary glands. Nonetheless, V1b receptors have been reportedly localized in the adrenal glands, kidneys, and pancreas. However, it appears to have a much weaker expression in the kidney, with specific cell types not well defined. Deep-sequencing did not detect V1b (Avpr1b) transcript in any renal tubule segment dissected from rat.
The V2 receptor is expressed primarily in the kidney and mediates vasopressin-stimulated antidiuresis. In general, by immunohistochemistry, it has been localized to the distal tubule (connecting tubule through CD) and also in the TAL in mice and rats. In agreement, transcriptomics (deep sequencing) revealed a significant level of V2R transcript from the medullary TAL through the IMCD. In the CCD through IMCD, expression was about 300–500 RPKM (reads per kilobase per million), while expression in the TAL was about 15–35 RPKM. The distal convoluted tubule (DCT) was very low (around 10). Other segments (proximal tubule and thin limbs) were <3. In comparison, the V1a transcript was about 50 RPKM in the CCD.
A systematic exploration of the human proteome using antibody-based proteomics indicated pronounced staining for the V2 receptor gene product in the parathyroid and moderate expression in the exocrine pancreas, nonkeratinized squamous epithelia, smooth muscle, salivary glands, breast, and Leydig cells. However, further studies using an alternate approach is necessary before firm conclusions can be drawn about extrarenal expression of the V2 receptor.
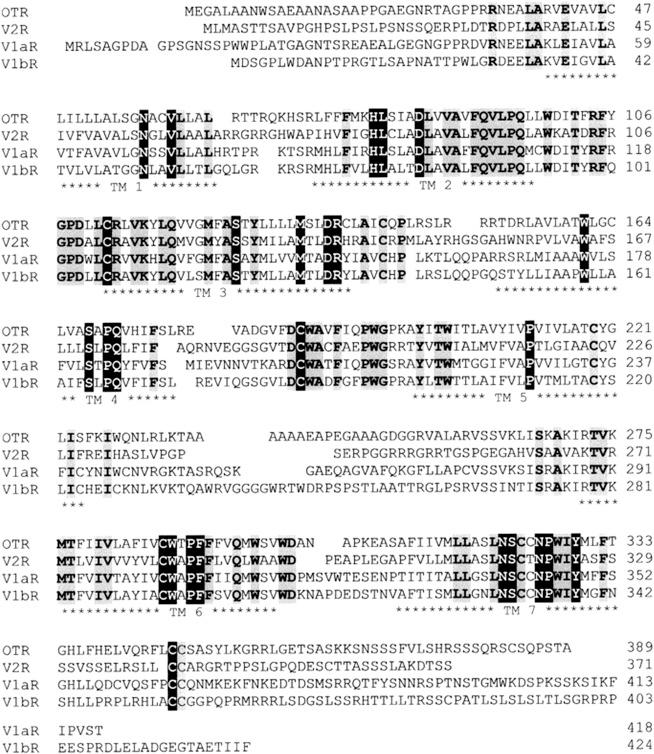
Molecular cloning has confirmed that vasopressin receptor subtypes are members of the GPCR superfamily, consisting of seven hydrophobic TM alpha-helices joined by alternating intracellular and extracellular loops, an extracellular N-terminal domain, and a cytoplasmic C-terminal domain ( Fig. 6.2 ). The vasopressin receptor subtypes, including the oxytocin receptor, display a high degree of sequence identity, showing about 102 invariant amino acids among the 370–420 amino acids in the human receptors ( Fig. 6.3 ). These receptor subtypes exhibit structural features which are characteristic of most of the GPCRs, such as the presence of a disulfide bridge between two highly conserved cysteine residues in the second and third extracellular domains, glycosylation on asparagine residues present in the extracellular domains, and two relatively well-conserved cysteine residues within the C-terminal receptor domain, which have been shown to be palmitoylated in other GPCRs. The disulfide bond appears to be required for the correct folding of vasopressin receptors.
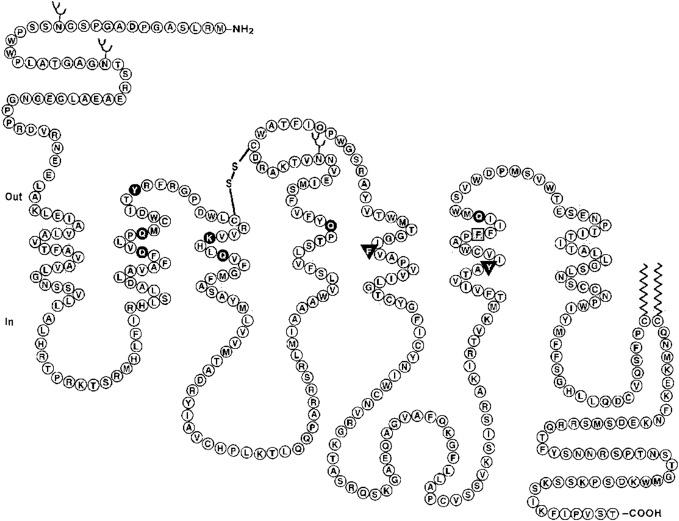
Receptor-bound AVP initiates a cascade of intracellular signaling events. The ligand-binding site on the vasopressin receptors is proposed to be in a pocketlike structure formed by the arrangement of the seven TM domains. Furthermore, mutagenesis experiments suggest that the agonist-binding site is located in a narrow cleft encircled by TM domains (primarily TM II–VII), about 15 Å away from the extracellular surface.
Mutational analysis techniques have also revealed several domains that impart G-protein-coupling selectivity and are important for the activation and function of the receptors. The conserved aspartate (Asp 97 ) residue in TM II is found to be functionally important for the activation of V1a vasopressin receptor. Also, the conserved Pro 322 situated on TM VII in the human V2 receptor is probably necessary to allow the relative movements within the helical bundle that are required for receptor activation. This highly conserved residue has also been found to have a key role in the vasopressin receptor activation process. The highly conserved triplet Asp-Arg-Tyr (Asp-Arg-His in human V2R) located at the N-terminal of intracellular loop 2 is also required for efficient G-protein activation. The importance of this conserved triplet is also true for various other receptors. In the human V2 receptor, an Arg137 to His137 mutation was found to abolish coupling to G-proteins and result in phenotypic NDI. By analogous replacement of the intracellular loops between V1a and V2 receptors, it has been demonstrated that intracellular loop 3 of the V2 receptor has the key role in correct recognition and activation of Gs G-protein while intracellular loop 2 of the V1a receptor is critically involved in selective activation of Gq/G11 G-protein.
Sites for N-linked glycosylation are present in the extracellular segments of all three receptor subtypes. In V1a, two N-linked glycosylation sites are present: one at the N-terminus and the other in the extracellular loop 2. Whereas both V1b and V2 receptor have only one N-linked glycosylation present at the extracellular N-terminus. The glycosylation at the extracellular sites has been suggested to increase the stability of the protein. Furthermore, both Cys residues at the C-terminus of the V2 receptor and equivalent sites (three) in the V1 receptors have been shown to undergo palmitoylation. The palmitoylation had been shown to impart stability to the V2 receptor at the cell membrane. Furthermore, there are 8 threonine and 17 serine residues in the third cytoplasmic loop and C-terminal region of the human V1a receptor, which could be sites for regulatory phosphorylation.
Loss-of-function mutations in the gene encoding the V2 receptor causes a severe disturbance in water homeostasis known as X-linked NDI. In this disorder, patients are unable to concentrate their urine despite increased serum vasopressin levels. Over 180 mutations in the V2 receptor gene have been described. These mutations may disrupt V2-receptor signaling, and make the CD principal cells insensitive to plasma vasopressin levels. The step at which V2-receptor signaling is disrupted depends on the site and type of mutation. Based on the fate of the cellular processes, Robben and associates grouped a number of V2-receptor mutations into five different classes ( Table 6.2 ). In addition, functional mutations in the aquaporin 2 (AQP2) gene, for the major apical water channel of the CD principal cell, can also result in congenital NDI. The topic of NDI will be examined in greater detail in Chapter 23 .
| Nucleotide | Amino Acid | Functionality | Conserved (Location) | Class |
|---|---|---|---|---|
| 492T>C | L44P | F | Y (tmdl) | II |
| 488T>A | I46K | F | N (tmd1) | II |
| 548T>C | L62P | ? | Y (tmdl) | II? |
| 545–553 del | Δ62-64 | G | N (tmdl) | II |
| 574G>A | W71X | N (ICL 1) | I | |
| 612C>A | A84D | A | N (tmd2) | II |
| 614G>A | D85N | F | Y (tmd2) | III |
| 623G>A | V88M | ? | Y (tmd2) | II |
| 692T>C | W99R | A | Y (EGL1) | II, IV |
| 671C>T | R104C | F | Y (ECL1) | II |
| 674T>G | F105V | A | Y (ECL1) | IV |
| 698C>T | R113W | F | Y (tmd3) | II, IV |
| 749A>T | I130F | F | N (tmd3) | II |
| 771G>A | R137H | G | Y (1CL2) | II, III, V |
| 860T>A | S167T | F | N (tmd4) | II |
| 861C>T | S167L | A | N (tmd4) | II |
| 902C>T | R181C | A | N (tmd4) | IV |
| 914G>T | G185C | A | N (ECL3) | IV |
| 963G>A | G201D | F | Y (ECL3) | II, IV |
| 965C>T | R202C | A | N (ECL3) | IV |
| 966–967 del | ΔR202 | A | N (ECL3) | IV |
| 972C>A | T204N | A | N (ECL3) | II |
| 975A>G | Y205C | A | Y (ECL3) | II |
| 978T>A | V206D | A | N (tmd5) | II |
| 1431C>T | P322S | F | Y (tmd7) | III, IV |
| 1476C>T | R337X | A | N (C-tail) | I |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here