Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The rapid development of molecular biologic and genetic techniques has greatly expanded the understanding of cardiac functioning, and these techniques are beginning to be applied clinically.
Cardiac ion channels form the machinery behind the cardiac rhythm; cardiac membrane receptors regulate cardiac function.
Sodium, potassium, and calcium channels are the main ion channel types involved in the cardiac action potential. Many subtypes exist, and their molecular structure is known in some detail, thus allowing a molecular explanation for phenomena such as voltage sensing, ion selectivity, and inactivation.
Muscarinic and adrenergic receptors, both of the G-protein–coupled receptor class, are the main regulators of cardiac function.
Volatile anesthetic agents significantly affect calcium channels and muscarinic receptors.
Powerful genetic analysis techniques are being used to better understand adverse cardiovascular events through molecular approaches. Research using these techniques has begun to explore links between genomics and perioperative adverse cardiovascular events.
Treatment through gene therapy is evolving in cardiovascular medicine, although it currently does not have a prominent role in the perioperative setting.
Excessive systemic inflammation is proposed to be a cause of postoperative organ dysfunction.
No interventions that attenuate systemic inflammation have been proved in large, randomized clinical trials to protect patients from morbidity and mortality.
The past decades have witnessed what may be termed a revolution in the biomedical sciences, as molecular and genetic methodologies suddenly jumped onto the clinical scene. The birth of molecular biology is commonly identified with the description of the structure of deoxyribonucleic acid (DNA) by Watson and Crick in the 1950s. Now, the human genome has been sequenced completely. The development of the polymerase chain reaction, a technique of remarkable simplicity and flexibility, has dramatically increased the speed with which many molecular biology procedures can be performed, and it has allowed the invention of many new techniques. More recent years have seen the development of approaches allowing screening of large amounts of genetic material for changes associated with disease states.
Cardiovascular medicine has benefited from these advances. Not only have the electrophysiologic and pumping functions of the heart been placed on a firm molecular footing, but also the underlying molecular mechanisms have been determined for numerous pathologic cardiac states, thereby allowing progress in therapeutic development. Nothing indicates that the pace of progress in molecular biology is slowing down. If anything, the opposite is the case, and more dramatic advances may be expected in the years to come. Thus techniques such as gene therapy may become effective therapeutic options in cardiac disease.
The cardiac action potential results from the flow of ions through ion channels, which are the membrane-bound proteins that form the structural machinery behind cardiac electrical excitability. In response to changes in electrical potential across the cell membrane, ion channels open and allow the passive flux of ions into or out of the cell along their electrochemical gradients. This flow of charged ions results in a current, which alters the cell membrane potential toward the equilibrium potential (E) for the ion, which is the potential at which the electrochemical gradient for the ion is zero. Depolarization of the cell could, in principle, result from an inward cation current or an outward anion current; for repolarization, the reverse is true. In excitable cells, action potentials are mainly caused by the flow of cation currents. Membrane depolarization results principally from the flow of sodium (Na + ) down its electrochemical gradient (E Na is approximately +50 mV), whereas repolarization results from the outward flux of potassium (K + ) down its electrochemical gradient (E K is approximately −90 mV). Opening and closing of ion channels selective for a single ion result in an individual ionic current. The integrated activity of many different ionic currents, each activated over precisely regulated potential ranges and at different times in the cardiac cycle, results in the cardiac action potential. Ion channels are usually highly (but not uniquely) selective for a single ion, hence the terms K + channels, Na + channels, and so forth. Channels may rectify; that is, pass current in one direction across the membrane more easily than the other. Electrical and chemical stimuli, which lead to opening and closing of the channel, cause a conformational change in the channel molecule (gating) ( Box 6.1 ).
Ion selectivity
Rectification (passing current more easily in one direction than the other)
Gating (mechanism for opening and closing the channel):
activation (opening)
inactivation (closing)
The rapid upstroke of the cardiac action potential (phase 0) is caused by the flow of a large inward Na + current (I Na ) ( Box 6.2 ). I Na is activated by depolarization of the sarcolemma to a threshold potential of −65 to −70 mV. I Na activation, and hence the action potential, is an all-or-nothing response. Subthreshold depolarizations have only local effects on the membrane. After the threshold for activation of fast Na + channels is exceeded, Na + channels open (ie, I Na activates), and Na + ions enter the cell down their electrochemical gradient. This action results in displacement of the membrane potential toward the equilibrium potential for Na + ions, approximately +50 mV. I Na activation is transient, lasting at most 1 to 2 ms because, simultaneous with activation, a second, slightly slower conformational change in the channel molecule occurs: inactivation, which closes the ion pore in the face of continued membrane depolarization. The channel cannot open again until it has recovered from inactivation (ie, regained its resting conformation), a process that requires repolarization to the resting potential for a defined period. Thus the channels cycle through three states: (1) resting (and available for activation), (2) open, and (3) inactivated. While the channel is inactivated, it is absolutely refractory to repeated stimulation.
Phase 0 (rapid upstroke): primarily Na + channel opening
Phase 1 (early rapid repolarization): inactivation of Na + current, opening of K + channels
Phase 2 (plateau phase): balance between K + and Ca 2+ currents
Phase 3 (final rapid repolarizations): activation of Ca 2+ channels
Phase 4 (diastolic depolarization): balance between Na + and K + currents
Ca 2+ , Calcium; K + , potassium; Na + , sodium.
The early rapid repolarization phase of the action potential, which follows immediately after phase 0, results both from rapid inactivation of the majority of the Na + current and from activation of a transient outward current (ITO), carried mainly by K + ions.
The action potential plateau and final rapid repolarization are mediated by a balance between the slow inward current and outward, predominantly K + , current. During the plateau phase, membrane conductance to all ions falls, and very little current flows. Phase 3, regenerative rapid repolarization, results from time-dependent inactivation of L-type Ca 2+ current and increasing outward current through delayed rectifier K + channels. The net membrane current becomes outward, and the cell repolarizes.
Phase 4 diastolic depolarization, or normal automaticity, is a normal feature of cardiac cells in the sinus and atrioventricular nodes (AVN), but subsidiary pacemaker activity is also observed in the His-Purkinje system and in some specialized atrial and ventricular myocardial cells. Pacemaker discharge from the sinus node normally predominates because the rate of diastolic depolarization in the sinoatrial node is faster than in other pacemaker tissues.
The preceding sections focus on the electrical events that underlie cardiac electrical excitability and on the identification of cardiac ionic currents on the basis of their biophysical properties. This section reviews the molecular structures behind these electrical phenomena. The first step in understanding the molecular physiology of cardiac electrical excitability is to identify the ion channel proteins responsible for the ionic currents.
The presence of four homologous domains in voltage-gated Na + and Ca 2+ channels suggests that basic ion channel architecture consists of a transmembrane pore surrounded by the four homologous domains arranged symmetrically (see Fig. 6.1 ).
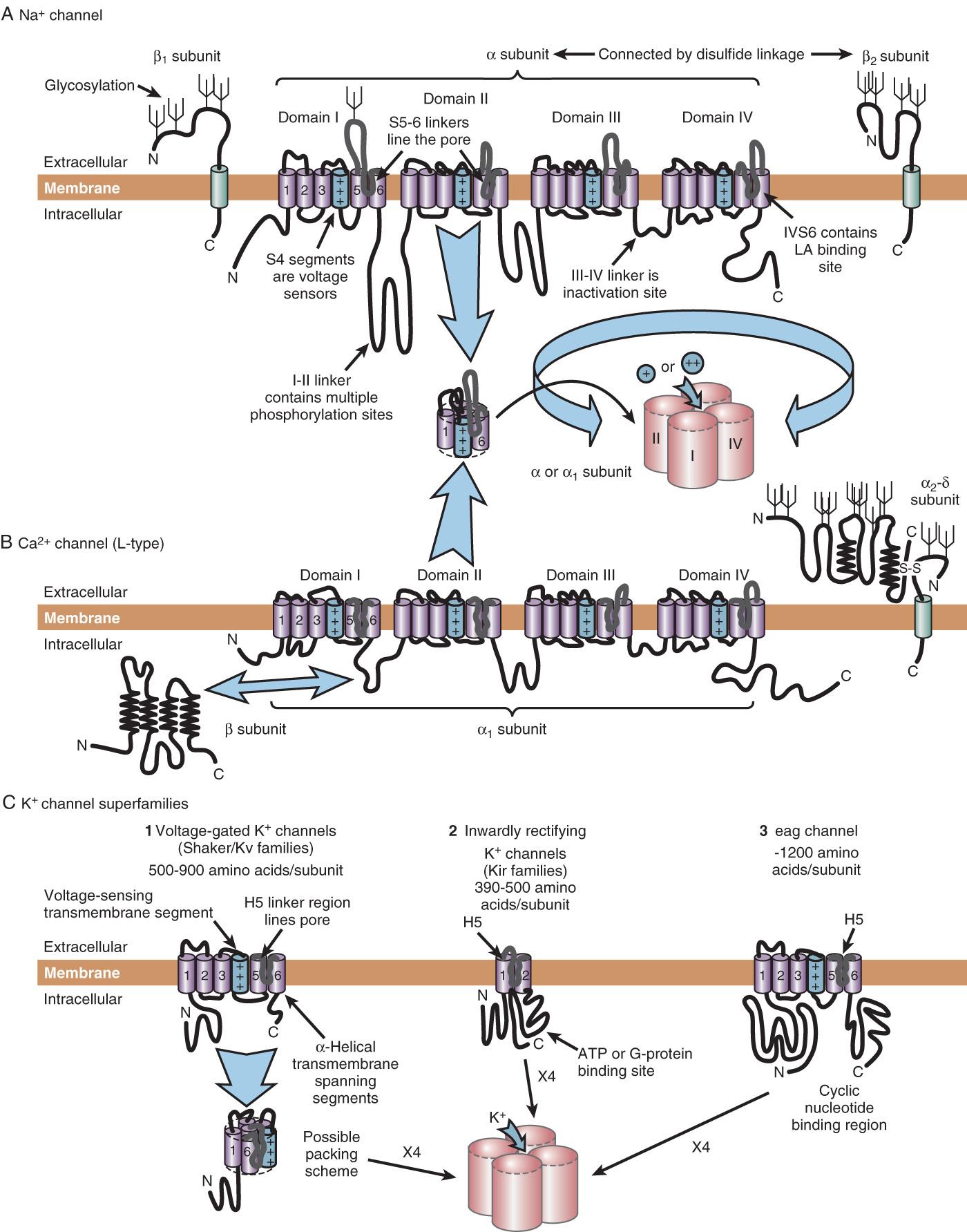
Drug therapy of cardiac arrhythmias would ideally be targeted at an individual ionic current, thereby tailoring the cardiac action potential in such a way that abnormal excitability is reduced but normal rhythmicity is unaffected. This goal remains unrealized. The prototype antiarrhythmic agents (eg, disopyramide and quinidine) have diverse effects on cardiac excitability and, similar to agents introduced more recently, frequently exhibit significant proarrhythmic activity with potentially fatal consequences. In the Cardiac Arrhythmia Suppression Trial (CAST), the mortality rate among asymptomatic patients after myocardial infarction (MI) was approximately doubled by treatment with the potent Na + channel-blocking agents encainide and flecainide, an effect likely attributable to slowing of conduction velocity with a consequent increase in fatal reentrant arrhythmias. Drugs that prolong action potential duration all block I Kr , and it is not clear that this therapeutic goal will result in arrhythmia control without induction of clinically significant proarrhythmia. The only drugs currently available that definitely prolong life by reducing fatal arrhythmias are β-blockers, and these agents have no channel-blocking effects.
Elucidation of the molecular mechanisms of the cardiac action potential is beginning to have a direct impact on patient management. This is most obvious in patients with inherited genetic abnormalities of ion channels that lead to cardiac sudden death. Two groups of diseases illustrate this point: long QT syndrome (LQTS) and Brugada syndrome. An understanding of the molecular mechanism of cardiac electrical excitability is also starting to lead to the emergence of gene therapies and stem cell therapies that may in the future allow manipulation of cardiac rhythm and function.
Receptors are membrane proteins that transduce signals from the outside to the inside of the cell. When a ligand —a hormone carried in blood, a neurotransmitter released from a nerve ending, or a local messenger released from neighboring cells—binds to the receptor, it induces a conformational change in the receptor molecule. This process changes the configuration of the intracellular segment of the receptor and results in activation of intracellular systems, with various potential effects ranging from enhanced phosphorylation and changes in intracellular (second) messenger concentrations to activation of ion channels.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here