Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Each year nearly 1 million liver cancers are diagnosed across the world, making liver cancer the fifth most common cancer in men and ninth in women , (see Chapter 89 ). Liver cancer is one of the most fatal cancers, being the second leading cause of cancer death in men and sixth leading cause of cancer death in women. The most common liver cancer remains hepatocellular carcinoma (HCC) (see Chapter 89 ), a primary liver malignancy and the third most frequent cause of cancer-related death worldwide. Another category is secondary malignancies, also known as metastatic tumors; common metastatic tumors to the liver include colorectal liver metastases (see Chapter 90 ) and metastatic neuroendocrine tumors (see Chapter 91 ). There are various treatment options available for these lesions, including liver transplant (see Chapter 105 ), surgical resection (see Chapter 101 ), and thermal and nonthermal ablation (see Chapters 94 , 96B , and 96D ), but surgical resection remains the standard of care for selected patients. Many factors must be taken into account when considering which option is viable for a given patient; for example, when considering MWA the histology of the tumor, the size of the lesion, the patient’s comorbidities, the extent of extrahepatic metastasis, and the knowledge of the practicing physician should all be considered. For many patients, resection is not possible.
The thermal and nonthermal ablative techniques, due to their unique physical properties and minimally invasive procedure, have increased the curative ablative treatment options; these techniques can be used when resection is contraindicated. These thermal-based and non–thermal-based techniques are characterized in Table 96C.1 . Microwave ablation (MWA) and radiofrequency ablation (RFA) are the two most common thermal ablation techniques used today, but they differ in how they deliver heat, leading to different amounts of tissue destruction between them. As discussed later in this chapter, comparisons between the two techniques have shown similar long-term results, but MWA has the added benefits of resistance to heat-sink effect, higher intratumor temperatures, faster procedure, and larger ablation volumes.
| TECHNOLOGY | POTENTIAL ADVANTAGES | POTENTIAL DISADVANTAGES |
|---|---|---|
| RFA |
|
|
| MWA |
|
|
| CRYO |
|
|
| IRE |
|
|
MWA achieves heat destruction of tissue through both active and passive heating. The active heating process of microwave energy requires the presence of dielectric molecules, such as water, to function. MWA reaches a much higher operating frequency (2450 MHz) than RFA, which makes it potentially more efficient (rapid temperature) for thermal ablation of solid organs. As a dipole molecule, water is affected by the applied electromagnetic field broadcast by the microwave antenna during the procedure. This is called dielectric permittivity. This property allows for dielectric hysteresis, which induces rotation of the dipole molecules and accounts for the efficient amount of heat generated during microwave ablation. One or more molecules are dipoles with unequal electrical charge distribution and they attempt to reorient continuously at the same rate in the microwave’s oscillating electric field. As a result of the microwave transmission, the water molecules flip back and forth at a billion times a second leading to this vigorous movement to produce friction and heat, which leads to cellular death via coagulation necrosis. An additional mechanism responsible for heat generation in microwave ablation is ionic polarization, which occurs when ions move in response to the applied electric field of the microwave. The displaced ions cause collisions with other ions, converting this kinetic energy into heat. However, this is the lesser of the two mechanisms that generate the efficient heat from microwave ablation. Microwaves emit nonionizing radiation for heating which produces homogeneous heating within the field regardless of the tissue types; this distinguishes MWA from monopolar RFA in terms of mechanism of heating and makes it a clinically superior method for ablation. The passive phase of microwave heating is by conduction of heat beyond the active heating zone and is susceptible to local tissue factors such as heat and current sinking.
The current frequencies of the commercially available microwave ablation devices are at 915 MHz or 2450 MHz. The reported potential benefit of the 915-MHz microwave is that it could penetrate deeper than the 2450-MHz microwave, which may theoretically yield larger ablation zones. However, the energy deposition is influenced by the dielectric properties of the antenna design. Microwave energy can be generated through a magnetron or solid-state amplifier, and the antenna broadcasts the electromagnetic energy to the target tissue. The coaxial cable consists of an inner and outer conductor with the dielectric material placed between the two layers. At its tip, the outer conductor is stopped to expose the inner conductor for broadcasting the microwave energy. This inner conductor is covered in a ceramic pointed tip for insertion into the tissue and microwave energy can pass freely through the ceramic.
Large ablative volumes via the 2450-MHz system can be created by increasing the power (wattage) and duration of the microwave energy. The ablative size can be manipulated to personalize each procedure to a specific patient. Physical factors that influence the ablative size include the water content of tissue (e.g., normal liver vs. cirrhotic liver vs. fatty liver) and the type of tissue being ablated (e.g., hepatocellular vs. metastatic colorectal vs. metastatic neuroendocrine). Some mechanical factors include the power output of the generator, the type of cable in use, the design of the antenna, the duration of the electrical current, and the number of antennas being used during the procedure.
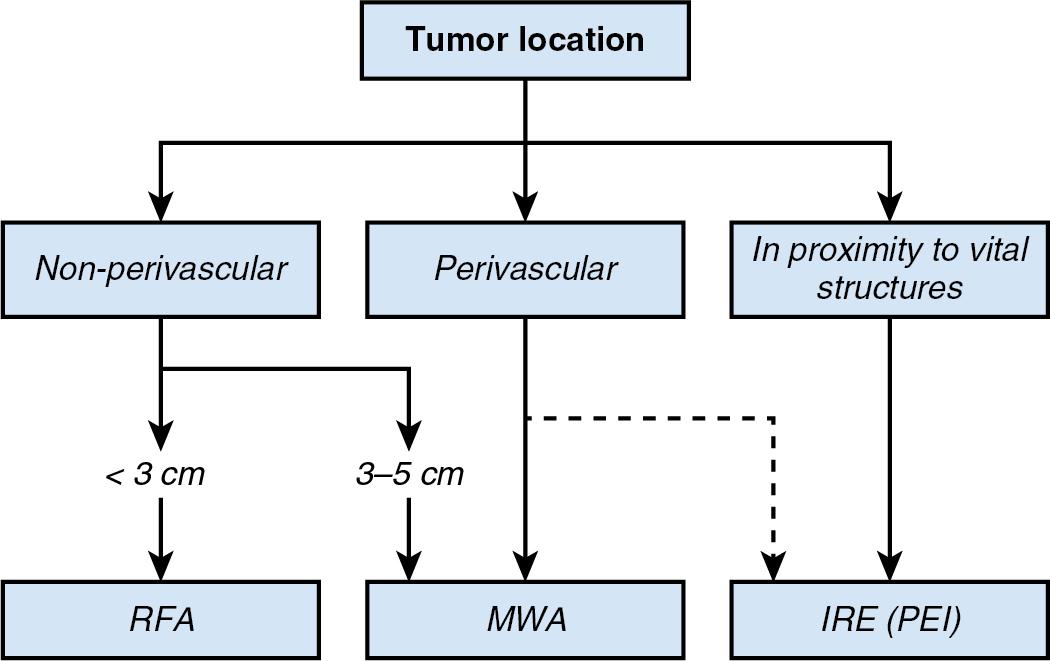
The success of a thermal ablation procedure depends partly on the local tissue surrounding the lesion to be ablated. Intrinsic factors of a given tissue type, such as dielectric permittivity and electric conductivity, will influence the size and shape of the ablation zone formed during the procedure, as well as how energy is deposited in the target tissue by the thermal source, be it RFA, MWA, or irreversible electroporation (IRE). Box 96C.1 summarizes some of the clinical considerations to take into account when using these techniques.
Monopolar RFA is an established technique for the treatment of tumors that are limited in number (3 or less) and size (3 cm or less) and are located 1 cm or more from critical structures and vessels. *
MWA appears to have potential to improve the rate of complete ablation achieved with RFA in tumors that are larger than 2–3 cm or multiple; device-specific safety and efficacy data, including predictability and reproducibility, are warranted.
MWA seems to have potential to overcome the limitations of RFA in the treatment of tumors in perivascular location; device-specific safety and efficacy analyses are warranted.
IRE has demonstrated promise for the treatment of small tumors located in the vicinity of bile ducts and blood vessels; continued efficacy data are warranted given the high demands on targeting of the multiple IRE probes.
More data are needed to define the potential for other energy-based ablation technologies in the specific field of liver tumor treatment.
* Vessels 3 mm or more in caliber are considered relevant for heat-sink effect.
As discussed in the previous section, microwaves generate heat and subsequent tissue necrosis, based on the generation of an oscillating electric field that causes dipolar molecules, such as water molecules, to flip back and forth generating friction and heat. Not all tissues have the same water content, and some tissues are well perfused by blood vessels containing a rapidly moving stream of blood. Proximity to blood vessels can cause an adverse event called heat sinking that occurs when the electric current is too close to the vessel; the blood flow imparts a cooling effect on the adjacent tissue. Heat sinking can lead to incomplete tumor ablation—the tissue near the vessel will not reach the necessary temperature for ablation that surrounding tissue reaches. Similarly, close proximity to the blood vessel will also cause current sinking— the vessel causes a distortion of the electric current, which can alter ablation performance. RFA suffers from both of these adverse events due to its method of heat delivery; RFA produces circulating electrical currents that dissipate heat to the surrounding tissue. Heat deposition depends on the electric conductivity of the tissue, which is in turn influenced by water content. Thus MWA is often a better choice for the patient. MWA is still affected by these adverse effects, but much less so; the microwaves generated rely primarily on the dielectric permittivity of the target tissue, and these microwaves will transmit through any tissue, including nearby vasculature or tissue with variable water content, keeping the electric field relatively constant throughout. , Clinicians should still bear these adverse events in mind during the procedure, though, as ablation zone size and shape can never be assumed and must be checked to ensure ablation success.
In addition, dehydration and consequent carbonization can occur when using RFA to treat a given lesion. This will lead to a significantly decreased final ablation volume; the treated tissue, without any remaining water, will block the necessary complete electric circuit from forming and less heat will be generated. At the same time, if the tissue to be ablated is heated too quickly to above 100°C, the intracellular components can vaporize and carbonize; the gas will act as an insulator and prevent proper heat dissipation to the surrounding tissue. Both components will impede complete ablation of the desired lesion. Microwave ablation is not limited by these factors, because, as mentioned, microwaves transmit throughout the tissue more evenly, producing a consistently more uniform ablation.
For these reasons, MWA has gained traction as an excellent option for tumors in close proximity to vital structures. As can be seen in Table 96C.2 , authors have compared MWA success rates for tumors near blood vessels, the diaphragm, and in subcapsular locations with less risky locations. Based on the studies listed, we have noted no significant difference in ablation success and recurrence rates for HCC tumors in these various locations when treated with MWA. This reinforces the utility of MWA for liver tumors in challenging locations, although some authors did report increased complications when performing ablations in difficult to access areas. ,
| STUDY | NO. OF PATIENTS | HISTOLOGY | WAS ABLATION SUCCESS REPORTED AND DEFINED? | OVERALL SURVIVAL (Y/N) MEDIAN | LOCAL RECURRENCE | LIVER RECURRENCE | MORTALITY | COMPLICATIONS |
|---|---|---|---|---|---|---|---|---|
| An et al., 2020 | 489 | 489 HCC | Yes, 95.2% in challenging locations vs. 94.9% in nonchallenging locations | At 5 years: Adjacent vital structures 70.5%, gallbladder 76%, hepatic hilar regions 62.9%, major vessel 78.3%, diaphragm 92.8%, capsule 85.3%, nonchallenging location 89.7% |
— | — | — | Challenging locations: hemoperitoneum (1), cholangiectasis with jaundice (3), liver abscess (2) Nonchallenging location: large pleural effusion (2) |
| Dou, 2017 | 406 | 406 HCC | Defined, not reported | Vessel group: 98% at 1 year, 82% at 3 years, 46.9% at 5 years Safe group: 98.1% at 1 year, 73.7% at 3 years, 48.2% at 5 years (After PSM) |
Vessel group: 6.5% at 1 year, 10.8% at 3 years, 10.8% at 5 years Safe group: 9% at 1 year, 14.3% at 3 years, 14.3% at 5 years (After PSM, LTP) |
— | — | Vessel group: pleural effusion (2), tumor seeding (2), abscess (1), thrombosis (1), hemorrhage (2) Safe group: pleural effusion (2), tumor seeding (1), abscess (1), hemorrhage (1) |
| Li et al., 2015 | 155 | 97 HCC, 58 metastases | Yes, 92.2% study group, 94% control group Also 97.1% HCC, 87.5% metastatic tumors |
— | 17.6% study group, 12.8% control group. 8.9% HCC, 25% metastatic tumors *Recurrence not defined |
— | — | Study group: fever (12.2%), abdominal pain (20.4%), pleural effusion (6.1%), nausea and vomiting (14.3%) Control group: fever (2.8%), abdominal pain (3.8%), pleural effusion (4.7%), nausea and vomiting (1.9%) |
| Liu, 2017 | 463 | 463 HCC | Yes, 95.5% subcapsular tumors, 98.3% non-subcapsular | Subcapsular group: 95.7% at 1 year, 90.1% at 2 years, 82.9% at 3 years, 71.1% at 4 years Non-subcapsular group: 98.5% at 1 year, 92.8% at 2 years, 83.2% at 3 years, 73.6% at 4 years |
5.4% for subcapsular group, 6.3% for non-subcapsular (LTP here both new lesions and incomplete ablation growth) |
0% procedure related; 14.7% subcapsular group, 10.4% non-subcapsular group on follow-up | Subcapsular: pleural fluid aspiration (4), peritoneal seeding (1), moderate right upper quadrant pain (30) Non-subcapsular: pleural fluid aspiration (2), abdominal wall seeding (1), moderate right upper quadrant pain (17) |
|
| Zhi-Yu, 2017 | 189 | 189 HCC | Yes, 98.2% | — | 11.1% at 1 year, 18.1% at 2 years, 19.1% at 3 years, 19.9% at 4 years (LTP) |
— | — | Pain, postablation syndrome, minimal asymptomatic perihepatic fluid or blood collection |
A wide array of MWA systems are available for use ( Table 96C.3 ). On an individual basis, they may use a single probe or multiple probes, use gas-cooling or water-cooling systems, and use different ablation frequencies. , Many different manufacturers worldwide have produced their own ablation systems, and there are currently many different competing models available. There are limited comparisons available due to the widely varying nature of tumors present and although authors have recently compared systems for individual indications, no clear consensus is present yet. In 2015 Leung et al. found a 4% local recurrence rate for ablations performed with a 915 MHz system and a 12.6% local recurrence rate for ablations performed with a 2.4 GHz system, but the results may have been affected by the fact that the 2.4 GHz system was used for patients with larger tumors, more noncolorectal metastases, and less pretreatment chemotherapy. However, Vogl et al. performed a similar comparison and found a complete ablation rate of 90.2% with 915 MHz and 95.5% with 2.4 GHz, as well as an overall survival of 82.98% at 4 years with 915 MHz, against 92.91% overall survival at 4 years with the 2.4 GHz system. In addition, multiple authors have established the use of the Emprint 2.45 GHz Ablation System for MWA and found it to be safe and effective for a variety of liver tumors, with complete ablation rates ranging from 83% to 100%. Hopefully in future years additional comparisons will be performed following the ablation standards established by North and Martin in 2014 and specific indications for particular MWA frequencies can be set.
| SYSTEM | DEVICE MANUFACTURER | GENERATOR AND ANTENNA | MAXIMUM GENERATOR POWER (W) | MAXIMUM ANTENNA POWER (W) | MAXIMUM SELECTABLE ABLATION TIME (MIN) | PROPOSED MAXIMUM ABLATION TIME (MIN) | FREQUENCY (HZ) | WATER COOLED? | NO. OF ANTENNAS |
|---|---|---|---|---|---|---|---|---|---|
| A | AngioDynamics | Soleror, Acculis Sulis Vp MTA and Accu2i | 180 | 140 | 6 | 6 | 2.45 × 10 9 | Yes | 1 |
| B | HS Hospital Service | HS Amica-Gen and APK14150T19V4 | 140 | 100 | 25 | 10 | 2.45 × 10 9 | Yes | 1 |
| C | Medtronic | Evident MWA Generator and VT1720 | 60 per device | 45 per antenna | 10 | 10 | 915 × 10 6 | Yes | 1-3 |
| D | Medwaves | Avecure Microwave Generator and 14-15-LH-35 | 40 | 32 (modulated) | 15 | 15 | (902-928) × 10 6 (modulated) | No | 1 |
| E | Perseon | MicroThermX and SynchroWave Antenna | 180 | 60 per antenna | — | — | 915 × 10 6 | Yes | 1-3 |
| F | Ethicon | NEUWAVE Certus | 140 | — | — | — | 2.45 × 10 9 | No | 1-3 |
| G | Vision Medical (formerly Forsea) | MTC-3C | 150 | — | — | — | 2.45 × 10 9 | Yes | 1-2 |
| H | Canyon Medical | KY-2000 | 100 | — | — | — |
|
Yes | Multiple |
| I | Alfresa Pharma | Microtaze | 70 | — | — | — | 2.45 × 10 9 | Yes | — |
| J | Medtronic | Emprint Ablation System | 100 | — | 10 | — | 2.45 × 10 9 | Yes | 1 |
| K | Eco Medical | ECO-100A1 | 120 | — | — | — | 2.45 × 10 9 | Yes | — |
| L | Qi Ya Medical Treatment Facility Limited Company | Model III | 120 | — | — | — | 2.45 × 10 9 | Yes | — |
Three approaches can be used for MWA: open, laparoscopic, and percutaneous. The underlying principles of any microwave ablative therapy stay the same, but the ability to accommodate and individualize each procedure in accordance with the patient’s unique needs is essential to MWA success. The approach chosen should reflect the tumor biology, tumor histology, size of tumor, and segments involved; it is important to target the skill level of the practitioner and tailor the choice to the patient’s needs. The goal of any MWA should be complete ablation in greater than 95% of all tumors. Terms such as cytoreduction, partial ablation, and debulking do not reflect accepted concepts in oncology and are poor substitutes, indicative of bad technique and poor patient selection. Patients should not be “owned” by a physician; the technique used must always be in the patient’s best interest regardless of the MWA access.
Surgical resection remains the optimal management for selected patients, based on histology and disease extent. A surgical MWA approach is primarily used when tumor morphology requires multiple ablation therapies; when tumors are located near the dome of the liver, for which percutaneous ablation might cause pneumothorax or damage to the diaphragm; or when the tumor is located near the visceral organs such as the gallbladder, colon, or stomach. If MWA alone is the sole goal in a patient’s care, a laparoscopic approach should be performed in most cases. Lesions located anteriorly and not adjacent to a major pedicle can be ablated percutaneously using ultrasound (US) guidance and computed tomography (CT) confirmation. If the lesion is located deep in the liver at the dome, next to a major pedicle, or adjacent to other structures such as the diaphragm or colon, ablation should be performed surgically, either laparoscopically or open, based on the patient’s past surgical history, body habitus, and level of the surgeon’s laparoscopic ultrasound skill. If an open approach is used, it is best done through a subcostal or midline laparotomy.
Regardless of the approach, the patient is usually positioned supine or in a lateral position on the table. The key to a successful ablation session is adequate exposure of the liver in order to perform a methodic evaluation of the liver by intraoperative US. All eight segments of the liver must be evaluated to ensure no lesions are overlooked (see Chapter 24 ). After all lesions have been identified, the plane of the needle track should be evaluated to ensure that it does not cross a portal or major hepatic vein pedicle. The type of antenna(s) to deploy should be decided on before the operation. All of the 915 MHz systems require at least 2 to 3 probes placed in parallel in order to obtain a similar ablation as a single antenna of the 2450 MHz systems when treating a lesion greater than 2.5 to 3.9 cm in size. Different amounts of power are deposited by the antenna at different frequencies, which leads to different ablation volumes created depending on the antenna used. Considering desired ablation size may also aid in deciding which frequency to use. It is important to make sure that there are not any vital structures within 1.0 to 1.5 cm near the microwave field ( Fig. 96C.1 ). MWA times can vary from 5 minutes to 45 minutes. The deepest lesion is usually treated first; in a staged procedure, the most difficult lesion is treated first.
The need to perform overlapping ablations should be decided before the first ablation. Given the artifact and distortion that occurs during the first ablation, subsequent US imaging can lead to inaccurate second and third needle placements to complete an overlapping ablation. The three-dimensional ablation zone is conceptually difficult to grasp for practitioners, considering that intraoperative US provides only a two-dimensional view.
To further improve MWA technique, authors have developed mathematical models to aid in guidance of MWA. Gao et al. used their model to significantly improve their complete ablation rates for CT-guided MWA from 88.3% in the control group to 97.7% in the model group. Similarly, Mbalisike et al. reported the usage of a robotic guidance system for MWA in 2015, with fewer needle insertions per procedure, fewer needle readjustments, and quicker insertion time present in procedures with the robotic guidance compared with manual.
Further, authors experimented with different imaging modalities during procedures. 1.5T magnetic resonance imaging (MRI) guidance for MWA had a complete ablation rate of 100% in a recent study and 98% in another, with an overall survival of 41.6 months. , A study comparing the use of ordinary US-guided MWA versus contrast-enhanced US-guided MWA found significantly reduced local recurrence of 8% in contrast-enhanced US compared with 16% in ordinary US. In addition, fewer complications, such as pain, intraabdominal hemorrhage, and infection, were noted in contrast-enhanced US MWA. As evidenced by this work, further refinement of MWA protocol can continue to improve outcomes.
Defining the success of the MWA is paramount. Well-established quality parameters were published by North and Martin et al. and agreed on in a multi-institutional review of all clinical papers. They are defined as follows:
Ablation success. Defined as complete eradication of the tumor using high-quality cross-section contrast-enhanced imaging (CT or MRI) within 4 weeks of ablation, specifically disappearance of any intratumoral contrast enhancement as described in modified RECIST criteria (see Chapters 14 , 15 , and 96B ).
Local recurrence after ablation. After confirmation of ablation success (i.e., >4 weeks), local recurrence is defined as evidence for viable tumor at or within 1.0 cm of a prior ablation site for which ablation success was documented. Confirmed by multislice, multiphase dynamic imaging (see Chapters 14 and 15 ).
Nonlocal hepatic recurrence. Evidence for viable intrahepatic tumor more than 1.0 cm from any prior ablation site at any time interval after ablation.
Indications for ablations and morbidity. The number of patients and the number of tumors with the segments involved should be reported with at least 90-day morbidity follow-up on all ablation cases regardless of access. Morbidity should be reported per established surgical morbidity definitions.
Rate of complete ablation of liver tumors, ablation recurrence defined as recurrent disease within 1 cm of ablation site, hepatic recurrence at nonablated sites, and morbidity and mortality associated with the procedure should be reported in all clinical MWA studies regardless of access. It is also important to be able to define success shortly after the procedure is performed so any potential corrections can be made. Success can be defined on immediate or 24-hour post-CT scan that demonstrates a zone of ablation encompassing both the tumor and a rim of normal liver tissue approximately 1 cm beyond the tumor in each dimension.
In the intervening 25+ years since Seki et al. first reported the application of microwave ablation for hepatocellular tumors, use of MWA has expanded to encompass both primary and metastatic liver tumors, as well as symptomatic hepatic hemangiomas, all of which are discussed below. The utility of MWA has grown such that substantial work has compared its efficacy with other more established treatment methods, such as surgical resection and RFA, as authors look to define the role of MWA in today’s surgical practice.
MWA continues to be used successfully for the treatment of hepatocellular carcinoma ( Table 96C.4 ). In recent years many studies have recorded their experience with MWA, with complete ablation rates as high as 100% for tumors less than 5 cm in diameter and 91.7% for tumors greater than 5 cm in diameter. Ma et al. reported a local recurrence rate of 12.9% for MWA of HCC tumors, despite the fact that their study included 72 tumors ≥5 cm in diameter, with a local recurrence rate of 19.7%, raising their overall local recurrence rate. Other authors reported even lower local recurrence rates for HCC; Yin et al. reported a local intrasegmental recurrence rate of 7.3% for CT-guided ablation of HCC tumors with an average diameter of 2.98 cm.
| STUDY | NO. OF PATIENTS | HISTOLOGY | WAS ABLATION SUCCESS REPORTED AND DEFINED? | OVERALL SURVIVAL (Y/N) MEDIAN | LOCAL RECURRENCE (WITHIN 1 CM OF ABLATION) | LIVER RECURRENCE | MORTALITY | COMPLICATIONS |
|---|---|---|---|---|---|---|---|---|
| Baker, 2017 | 219 | Hepatocellular carcinoma (219) | Yes, 97.1% | Yes, 14.8 months | 13.9% | 34.8% | Overall 38.5%, 30-day mortality 1.8% | Pulmonary (13.7%), renal (14.2%), cardiac/circulatory (3.7%), gastrointestinal (9.6%), infectious (0.9%), other (5.2%) |
| Kapoor et al., 2020 | 53 | 100 lesions: hepatocellular carcinoma (76), CRCLM (18), neuroendocrine metastasis (5), cholangiocarcinoma (3), GIST (1), prostate cancer metastasis (1) | Yes, 83% | Yes, 24.7 months | — | 30% (16/53) in different liver segment, 17% in same liver segment | 0% | Pneumothorax (2), partial portal vein thrombosis (3) |
| Ma et al., 2017 | 433 | Hepatocellular carcinoma (433) | Yes, 100% for tumors <5 cm, 91.7% for tumors >5 cm | Yes, 43 months | 12.9% | 58.9% | 1-year (18.5%), 2-year (40.8%), 3-year (58.6%) | Renal insufficiency (6), hepatic encephalopathy (1), adrenal crisis (1), ablation lesion infection (2), ascites (3), pleural effusion (5), hyperbilirubinemia (5) |
| Perrodin et al., 2019 | 23 | 8 Neuroendocrine tumors, 4 breast cancer, 3 sarcoma, 2 non–small cell lung cancer, 6 other | Yes, 97.5% | 18 months | 10% | 29% | — | Major complications (0%), minor complications (12%) |
| Qin et al., 2019 | 137 | 137 CRCLM | Yes, 97.81% of patients | 98.1% at 1 year, 90.6% at 2 years, 85.9% at 3 years | 16.06% | — | 0% | Major complications (3.65%), minor complication (8.03%) |
| Ryu, 2019 | 459 (421 after exclusions) | Hepatocellular carcinoma (421) | Not defined, but reported 97.9% | Yes, 5.5 years | 8.3% | 78.9% | 0% | Major: wound infections (8), ascites (5), pleural effusion (4), intra-abdominal abscesses (2), intraabdominal hemorrhages (2) |
| Wang et al., 2016 | 221 | Hepatocellular carcinoma (221) | Yes, first procedure (91%), second procedure (3.6%) | Yes, 41 months | Out of 209 patients with complete ablation, 16% | Out of 209 patients with complete ablation, 57% | 0% |
|
| Yin et al., 2017 | 220 | Hepatocellular carcinoma (220) | Yes, 92.82% | Yes, 95.45% at 1 year, 89.09% at 2 years | 7.3% | 15.5% | 0% | Hepatalgia (136), transient abnormal liver function (110), fever (68), mild liver subcapsular hemorrhage (4), biloma (2) |
| Yu et al., 2015 | 1249 | Hepatocellular carcinoma (928), cholangiocarcinoma (14), metastases (307) | No | Yes, 92.7% at 1 year, 81.1% at 2 years, 70.6% at 3 years, 58.9% at 4 years, 51.7% at 5 years |
|
— | — | — |
| Zaidi et al., 2016 | 53 | CRCLM (20), neuroendocrine (10), primary (9), breast (4), ovarian (2), leiomyosarcoma (2), other (6) | Not defined, reported incomplete ablation in 1 of 149 lesions | — | 0.7% | 13% | — | Small bowel obstruction (1), portal vein and superior mesenteric vein thrombus (1), deep vein thrombus and asymptomatic segmental pulmonary embolism (1), asymptomatic segmental pulmonary embolism (1), postoperative cardiac dysrhythmia (1), postoperative urinary retention (1) |
| Zhou et al., 2017 | 295 | 295 CRCLM | Yes, 96.6% | 33 months | 8.8% | 35.9% | — | Pleural effusion (6), transient fever (136), abdominal pain (63), nausea (25) |
As MWA has grown in prominence, authors have delineated success rates for tumors of varying sizes. Wang et al. reported in 2016 that complete ablation rates at first MWA session decrease as tumors increased in size from ≤3 cm (94.9%) to 3 to 5 cm (93.2%) to greater than 5 cm (81.8%). They also reported that tumor sizes ≤3 cm and 3 to 5 cm have a significant positive correlation with overall survival when compared with tumors greater than 5 cm.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here