Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Inborn errors of metabolism (IEM) are inherited disorders characterized by abnormalities in enzyme or other cellular proteins involved in metabolism. These are relatively common problems, often possessing both systemic issues and coexisting movement disorders. Approximately one-third of patients with an IEM have a movement abnormality. , The most common movements described include dystonia, ataxia, and myoclonus. Approached in a different way, about 20% of children with a movement disorder are estimated to have a metabolic etiology. , Recognizing that many IEMs have variable combinations of movement abnormalities, this chapter approaches inborn errors by the metabolic abnormality, rather than a movement defined category.
In general, factors suggesting the presence of IEM include an early age of movement onset; a multisystem involvement; the presence of other neurological signs and symptoms; a progressive, fluctuating, or paroxysmal rather than static course; a positive family history for a similar condition or consanguinity; features including autonomic dysfunction, skin and hair changes; and the presence of specific triggers, for example, infection, fever, fasting, exercise, or high protein intake. , It is also important to recognize that inborn errors not only present in infancy or childhood, but in adolescence as well as occasionally in adulthood. Table 17.1 includes an outline of the disorders covered in this chapter. Lastly, recognizing that some IEM are directly treatable (see Table 17.2 ), it is important that physicians identify their presence as early as possible.
| A. Pediatric neurotransmitter disorders (see Table 17.3 for additional content) |
| Monoamines: Cofactor, synthesizing enzyme, catabolic enzyme, and transporter defects |
| GABA: Catabolic defects |
| B. Storage disorders |
| Mineral accumulation: |
| Copper: Wilson's disease, Huppke-Brendl |
| Manganese: Transporter and storage disorders |
| Iron: PKAN, PLAN, MPAN, BPAN, Neuroferritinopathy Aceruloplasminemia, Kufer-Rakeb syndrome, FAHN, Woodhouse-Sakate, COASY |
| Lysosomal disorders: |
| Gangliosidoses: GM1 and GM2 gangliosidoses |
| Sphingomyelin: Niemann-Pick, Gaucher disease, glucosylceramide: |
| Neuronal ceroid lipofuscinoses |
| CLIN 1-14 |
| C. Leukodystrophies |
| D. Amino acid disorders |
| Homocysteinuria, Hartnup disease, maple syrup urine disease, phenylketonuria |
| E. Organic acidemias |
| Methylmalonic acidemia, propionic acidemia, methyglutaconic, HSD10MD, β-ketothiolase |
| glutaric aciduria, hydroxyglutaric aciduria |
| F. Glycolysis, pyruvate, metabolism, and Krebs cycle disorders and Krebs cycle |
| Triosephosphate isomerase, pyruvate carboxylase, PDH, Ketoglutarate, fumarase deficiency, and Krebs Cycle |
| G. Mitochondrial disorders |
| Leigh syndrome, MELAS, MERRF, MNGIE, NARP, Leber hereditary optic neuropathy plus, Mohr-Tranebjaerg syndrome |
| H. Purine metabolism disorders |
| Lesch-Nyhan disease, adenylosuccinate lyase deficiency, PRPS1 abnormalities |
| I. Creatine metabolism |
| GAMT, AGAT, creatine transporter deficiency |
| J. Congenital disorders of glycosylation |
| K. Cofactor disorders |
| Molybdenum cofactor, sulfite oxidase, cerebral folate, biotin-thiamine responsive BG disease, ataxia with vitamin E deficiency, B12 infantile tremor |
| L. Neuroacanthocytosis disorders |
| Chorea-acanthocytosis, McLeod, abetalipoproteinemia |
| M. Other |
| Aicardi-Goutieres syndrome, Glut1 deficiency |
| Disease (Gene) | Treatment |
|---|---|
| Pediatric neurotransmitter disorders | |
| GTP cyclohydrolase 1 deficiency ( GCH1 ) | Levodopa/carbidopa, dopamine agonists |
| Tyrosine hydroxylase deficiency ( TH ) | Levodopa/carbidopa, dopamine agonists |
| 6-Pyruvoyl-tetrahydropterin synthase deficiency ( 6PTS ) | BH4, levodopa, 5HTP, low phenylalanine diet |
| Sepiapterin reductase deficiency ( SPR ) | Levodopa/carbidopa, 5HTP |
| Dihydropteridine reductase deficiency ( QDPR ) | Levodopa/carbidopa, 5HTP, low phenylalanine diet |
| Mineral accumulation | |
| Wilson disease ( ATP7B ) | d -penicillamine, zinc, trientine, molybdenum |
| Manganese storage ( SLC30A10 & SLC39A14 ) | Chelation (disodium calcium EDTA), iron |
| Lysosomal storage disorders | |
| Gaucher disease ( GBA1 ) | Enzyme replacement therapy |
| Niemann Pick type C ( NPC1 & NPC2 ) | Miglustat substrate reduction therapy |
| Amino acid disorders | |
| Homocystinuria ( MTHFR ) | Vitamin B6, folic acid, hydroxycobalamin, vitamin C, betaine, methionine restriction |
| Hyperphenylalaninemia ( PAH ) | Low phenylalanine diet |
| Nonketotic hyperglycinemia ( GLDC ) | Sodium benzoate, dextromethorphan, ketamine |
| Organic acid disorders | |
| Propionic aciduria ( PCCA & PCCB ) | Low branched chain amino acid diet |
| Glutaric aciduria type 1 ( GCDH ) | Carnitine supplements, lysine/tryptophan restriction diet |
| Pyruvate metabolism | |
| PDH deficiency ( PDHA1 ) | Ketogenic diet, thiamine |
| Creatine metabolism | |
| Creatine deficiency ( GAMT ) | Arginine restriction, creatine, and ornithine supplements |
| Cofactors, Minerals, vitamins | |
| CoQ10 deficiency | CoQ10 supplementation |
| Biotin-responsive BG disease ( SLC19A3 ) | Thiamine and high dose biotin, trigger avoidance |
| Ataxia with vitamin E deficiency ( TTPA ) | Vitamin E |
| B12 infantile tremor syndrome | B12 |
| Cerebral folate deficiency ( FOLR1 ) | Folinic acid |
| Biotinidase deficiency ( BTD ) | Biotin |
| Neuroacanthocytosis syndromes | |
| Abetalipoproteinemia ( MTTP ) | Fat-soluble vitamins A, D, E, K |
| Other | |
| Glucose transport deficiency ( SLC2A1 ) | Ketogenic diet |
The term pediatric neurotransmitter disease has been applied to a broad spectrum of relatively uncommon genetic disorders that affect the synthesis, metabolism, and catabolism of neurotransmitters. The primary neurotransmitters involved in these diseases are the monoamines, which include serotonin and catecholamines (dopamine and norepinephrine), and gamma-aminobutyric acid (GABA).
Monoamine-related neurotransmitter diseases can be divided into separate categories based on the site of abnormality in the metabolic pathway, for example, those affecting: (1) the cofactor tetrahydrobiopterin (BH4); (2) enzymes of monoamine biosynthesis (e.g., tyrosine hydroxylase (TH), tryptophan hydroxylase (TPH), aromatic amino acid decarboxylase (AADC); (3) catabolic enzymes: and (4) transporter defects ( Table 17.3 , Fig. 17.1 ). Despite their differing etiologies, these disorders have many common symptoms, including developmental delay, axial hypotonia, rigidity, movement abnormalities, speech problems, feeding difficulties, autonomic symptoms, and abnormal eye movements, particularly oculogyric crises. Diagnostic studies include: (a) cerebrospinal fluid (CSF) for analysis of monoamines (dopamine, DA; serotonin, 5HT; norepinephrine, NE), neurotransmitter metabolites (homovanillic acid, HVA; 5-hydroxyindoleacetic acid, 5-HIAA; 3-methoxy-4-hydroxylphenylglycol, MHPG; pterins (biopterin and neopterin); (b) quantitative plasma and urine catecholamines; (c) phenylalanine loading tests with and without BH4; (d) genetic investigations.
| Monoamine Neurotransmitter Disorders |
|
| GABA-related neurotransmitter disorders |
|
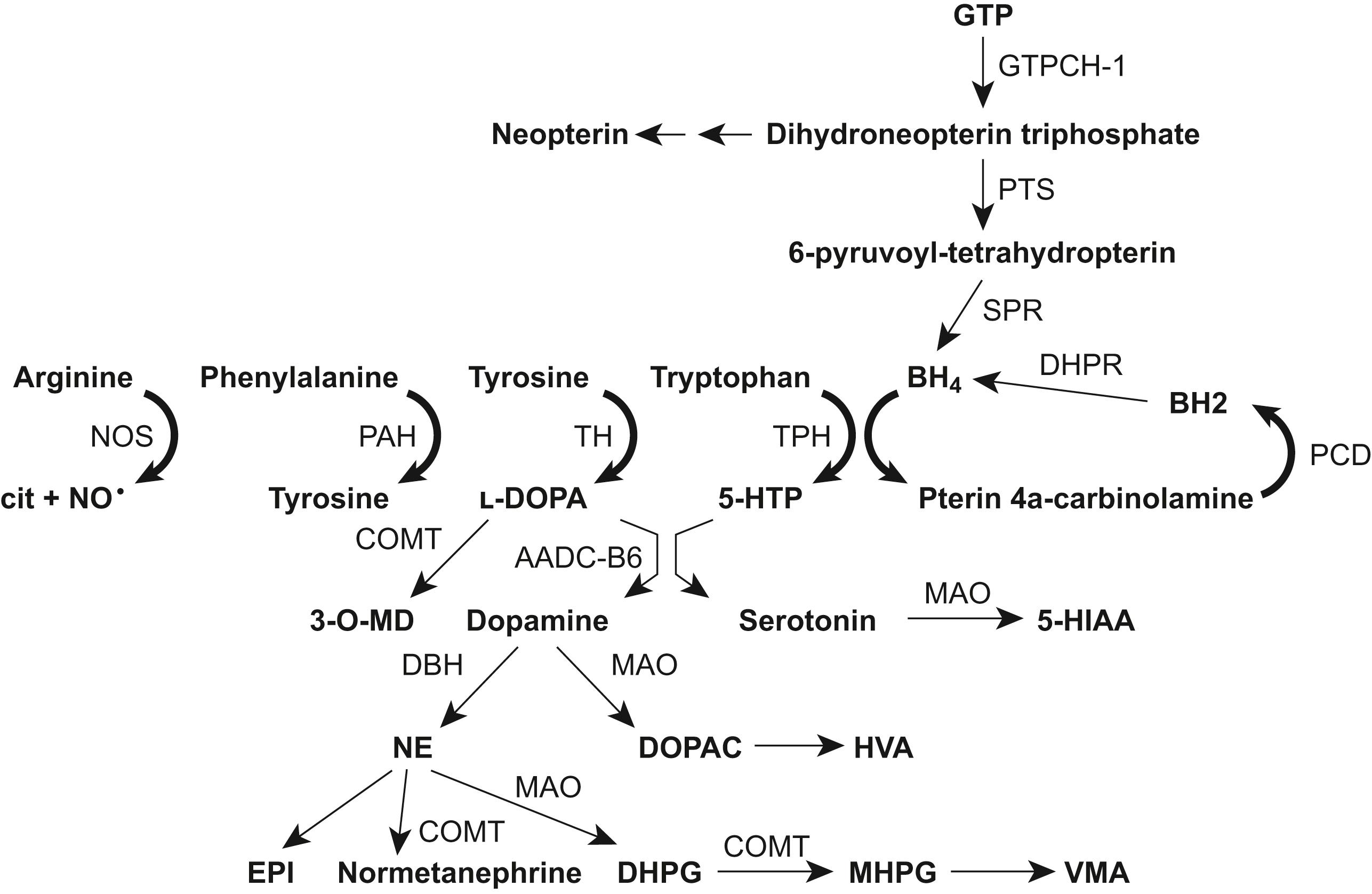
BH4 is an essential cofactor for several neurotransmitter synthesizing enzymes including TH (which catalyzes the conversion of tyrosine to l -dopa), TPH (which catalyzes the conversion of tryptophan to 5-hydroxytryptophan, 5-HTP), and for phenylalanine hydroxylase (PAH, which converts phenylalanine to tyrosine). BH4 itself is synthesized in a multistep pathway starting from guanosine triphosphate (GTP) and, when formed, requires several enzymes to maintain it in its active state ( Fig. 17.2 ).
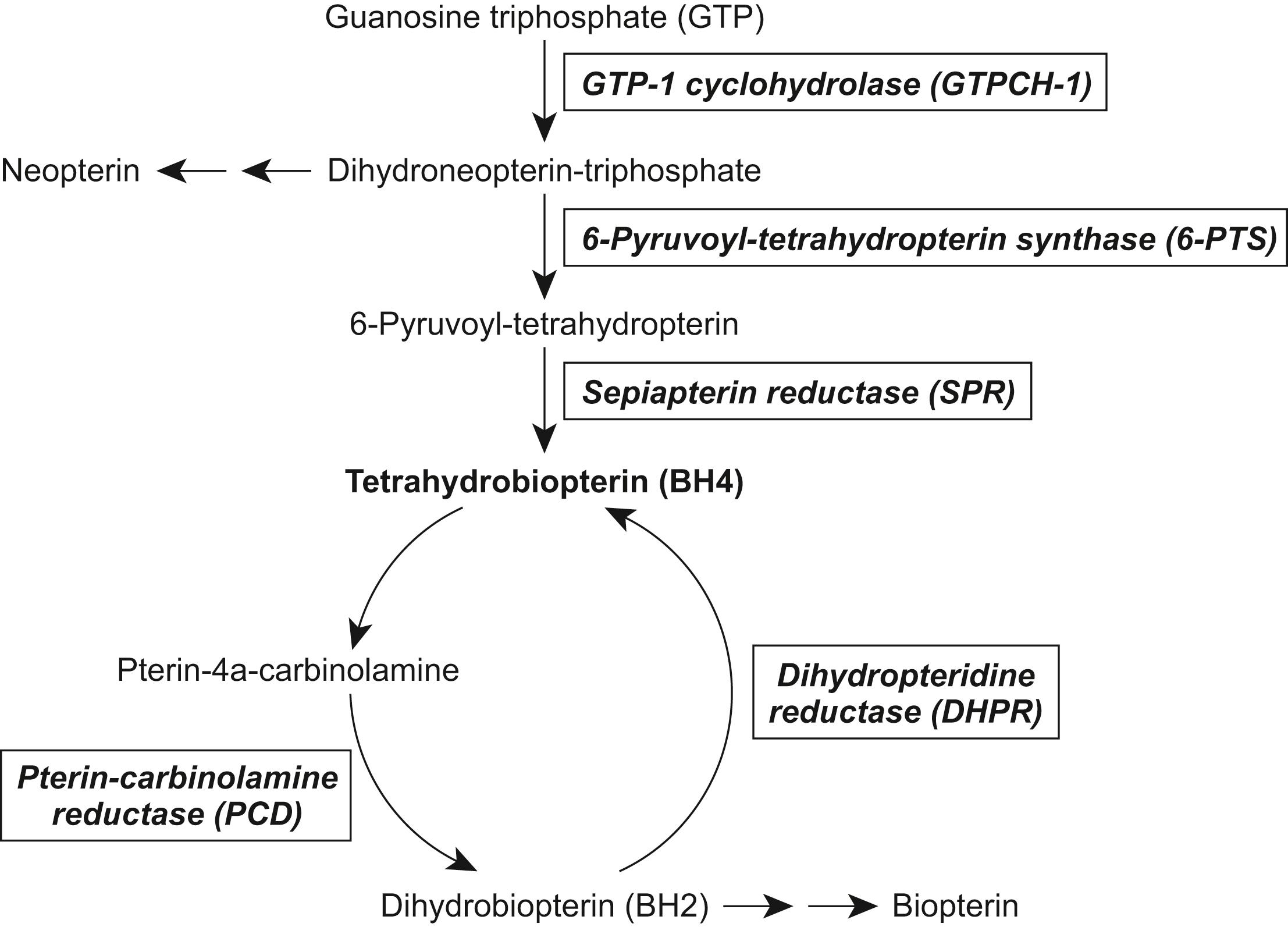
Several enzymatic defects have been identified in BH4 metabolism, for example, deficiencies in (a) the first and rate-limiting synthesizing enzyme GTP cyclohydrolase-1 (GTPCH-1 aka GCH1); in (b) the second and third enzymatic steps, namely, 6-pyruvoyl-tetrahydropterin synthase (6-PTS) and sepiapterin reductase (SPR), respectively; and (c) in the maintenance enzyme dihydropteridine reductase (DHPR). Although one might expect that a defect in BH4 metabolism would be readily detectable based on the presence of hyperphenylalaninemia, which occurs because of a deficiency of PAH activity, this is not always present. Hence, classifications of BH4 metabolism defects are based on presentations with or without hyperphenylalaninemia.
Individuals in this group have defects which include those with autosomal recessively inherited forms of GTPCH-1 (GCH1) deficiency, 6-PTS deficiency, PCD deficiency, and DHPR deficiency. , Since each produces hyperphenylalaninemia and reduced synthesis of monoamines, clinical signs and symptoms tend to overlap.
Early presentation in the neonatal period, presumably due to hyperphenylalaninemia, includes hypotonia, poor suck, diminished movements, and microcephaly. Generally, starting several months later, additional monoaminergic symptoms appear including autonomic symptoms (hypersalivation, temperature instability, excessive diaphoresis, and blood pressure lability), oculogyric crises, swallowing difficulties, variable hypokinetic and hyperkinetic movements, seizures, and cognitive impairment. , , These patients are often identified by neonatal screening for phenylketonuria (PKU). In DHPR deficiency, a secondary reduction in central nervous system (CNS) folate has led to perivascular basal ganglia calcification and multifocal subcortical perivascular demyelination. , Follow-up testing of urine pterins (biopterin and neopterin) and measurement of DHPR activity in blood are necessary to pinpoint the defect.
Although one most commonly thinks of the autosomal-dominant form of the disease, that is, dopa-responsive dystonia (DRD), there is an autosomal recessive form of GTPCH deficiency. This latter form is associated with hyperphenylalaninemia, due to a deficiency of hepatic PAH activity, and symptoms as described above. , Treatment with BH4 is usually insufficient and supplementation with l -dopa and 5-HTP is usually required.
This is the most prevalent form of hyperphenylalaninemia not attributed to PAH deficiency. 6-PTS catalyzes dihydroneopterin-triphosphate to form 6-pyruvoyl-tetrahydropterin. Patients have reduced catecholamine and serotonin metabolites and an increased neopterin to biopterin level in the CSF. Two variants have been described, each having elevated levels of phenylalanine in newborn screening tests. , The classic severe form shows persistent delays and progressive neurologic deterioration, with truncal hypotonia, subsequent appendicular hypertonia, bradykinesia, extrapyramidal signs (cogwheel rigidity, choreoathetosis, dystonia), and diurnal fluctuation. Other features may include swallowing difficulties, oculogyric crises, autonomic symptoms, irritability, and seizures. Patients with milder phenotypes have deteriorated following administration of folate antagonists; the latter interferes with the conversion of BH2 to BH4 (see Figs. 17.1 and 17.2 ), via dihydrofolate reductase. A non-CNS “peripheral form” has also been reported having no biochemical abnormalities in the CSF, mild phenylalaninemia, and the potential for normal neurologic development with appropriate treatment. Treatments include the use of neurotransmitter precursors ( l -dopa, 5-HTP), MAO inhibitors, and BH4.
This disorder results from a defect of BH4 regeneration. The disorder is caused by mutations in the QDPR gene. Two forms have been described: the more common neonatal/early infancy form with progressive clinical symptoms like 6-PTS deficiency, and a juvenile-onset form with progressive cognitive deterioration, seizures, long-tract signs, extrapyramidal signs, and cerebellar signs. , CSF shows elevated levels of BH2, normal to elevated levels of biopterin, and reduced concentrations of HVA and 5-HIAA. Early recognition and treatment are important. Basal ganglia calcifications may be reversible with folinic acid supplementation. As described in other hyperphenylalaninemia BH4 defects, treatment is designed to replace CSF dopamine and serotonin and to prevent breakdown of the formed neurotransmitter. The use of BH4 supplementation remains controversial, whereas oral calcium folinate/folinic acid is routinely used.
DRD, also known as DYT5a-PARK-GCH1 or Segawa's disease, is an autosomal-dominant dystonia, caused by a disorder of BH4 metabolism without hyperphenylalaninemia. , More specifically, DRD is due to heterozygous mutations, spanning a 30 kb region with six exons, in the gene for GTP cyclohydrolase 1(GCH-1) located on chromosome 14q22.1–22.2. , , This enzyme is the rate-limiting step in BH4 synthesis. New mutations are frequent, clinical penetrance is incomplete (increased in females), and variable expressivity has occurred in families. Although the spectrum of presentations is wide, parents of patients typically seek treatment in mid-childhood (5–6 years) for the subacute onset of dystonic posturing of leg or foot affecting the gait in a child with normal cognition. Symptoms progressively worsen and about one-quarter develop hyperreflexia and spasticity, leading some to be inappropriately labeled with the diagnosis of cerebral palsy. Diurnal variation occurs in about 50% of cases manifested by progressive worsening dystonia and/or parkinsonism throughout the day and improvement in the morning, after sleep.
Investigators have also identified differences in clinical presentations at older ages: onset around age 8 years presents with retrocollis, torticollis, and possibly oculogyric crisis; and after age 10, presentation with an asymmetric postural tremor; and a more adult presentation with features of parkinsonism, that is, bradykinesia, rigidity, hypomimia, hypophonic speech, and postural instability. , , An unusual case with myoclonus-dystonia syndrome, that is, myoclonic jerks beginning in childhood, has also been described. Rarely, DRD may be associated with tics and tourettism. Older patients have a greater prevalence of obsessive-compulsive disorder and major depressive disorder.
Patients respond dramatically and in a sustained fashion to low-dose levodopa (or a dopamine agonist), making it important to accurately diagnose this disorder. Some DRD patients have also shown a good response to anticholinergics, such as trihexyphenidyl. BH4 may be helpful but is rarely used. Since dopamine synthesis is more affected than serotonin, serotonin reuptake inhibitors are not typically used.
Diagnosis is usually based on clinical symptoms and response to levodopa. A cardinal feature of this disorder is its responsiveness to a therapeutic trial with l -dopa/carbidopa. In unclear cases or to distinguish DRD from other metabolic diseases or forms of parkinsonism, genetic testing of GTPCH-1 (GCH1) or CSF testing, as above, may be helpful. CSF analysis shows markedly decreased HVA, normal or low 5-HIAA, reduced BH4 and neopterin levels, with normal plasma phenylalanine levels. CSF findings may not, however, always be classical. The phenylalanine loading test has been advocated for detection of both affected and nonmanifesting GTPCH-1 gene carriers. , Since individuals with PKU would show similar abnormalities, DRD carriers are distinguished by correction of the loading test after administering biopterin. Nevertheless, in view of limited sensitivity and specificity, the results of phenylalanine loading tests need to be interpreted with caution. , Genetic testing can be done to identify heterozygous mutations of the GTPCH1 (GCH1) gene on chromosome 14q, however, over 100 mutations have been identified. If genetic analyses do not demonstrate mutations in GTPCH-1, it is important to ascertain whether analysis included sequencing of the entire gene. Note: two other genes in which mutations can cause dopa DRD are TH and SPR genes.
Neuroimaging can occasionally be helpful to distinguish DRD from juvenile parkinsonism. The density of presynaptic dopamine terminals in striatum should appear normal in DRD but reduced in juvenile Parkinson's disease (PD), as demonstrated either by use of fluorodopa positron emission tomography (PET) or by single-photon emission tomography with [ 123 I]beta-CIT. Postsynaptic terminal D2 receptor density, using raclopride PET has been variable, but some PET D2 dopamine receptor studies suggest increased binding in both symptomatic and asymptomatic carriers of GTPCH mutations as well as in some juvenile PD cases. ,
This disorder has been reported in several cases. Neurologic symptoms include psychomotor retardation, microcephaly, spasticity, dystonia, oculomotor apraxia, and hypersomnolence. The oral phenylalanine loading test in these patients was abnormal, despite the lack of hyperphenylalaninemia. CSF measurements show reduced monoamines and their metabolites, but normal BH4 and neopterin levels. Treatment of this disorder includes the use of levodopa and 5-HTP in combination with carbidopa to correct central monoamine deficits.
SPR deficiency is an autosomal recessive inherited disorder of BH4 metabolism characterized by signs and symptoms related to monoamine neurotransmitter deficiency, but without hyperphenylalaninemia ( Fig. 17.2 ). SPR: a) catalyzes the final two-step reduction of the intermediate 6-pyruvoyl-tetrahydropterin to BH4; and b) has a role in the salvage and de novo synthetic pathways of tetrahydrobiopterin. Phenylalaninemia is absent, since other reductases in the liver, but not brain, can substitute for this enzyme. Presentation includes progressive psychomotor retardation, axial hypotonia, language delay, dystonia, oculogyric crises, dysarthria, parkinsonian symptoms, weakness, diurnal fluctuation of symptoms, and hyperreflexia. Diagnosis is confirmed by CSF measurements that show low CSF HVA and 5-HIAA, normal to slightly increased neopterin, and elevated total biopterin, dihydrobiopterin (BH2), and sepiapterin. Urine pterins and plasma phenylalanine levels are normal. Several mutations in the SPR gene have been identified. Nonhyperphenylalaninemia BH4 deficiencies can be treated with dopamine and serotonin precursor supplementation. BH4 has not been beneficial. ,
Defects of monoamine biosynthesis have been defined at three sequential enzymatic steps: (a) TH, the rate-limiting step catalyzing the conversion of tyrosine to l -dopa in the formation of dopamine and norepinephrine; (b) ALAAD or AADC, which converts l -dopa to dopamine; and (c) DBH, the enzyme that converts dopamine to norepinephrine (see Fig. 17.1 ).
TH, the rate-limiting enzyme in the biosynthesis of dopamine, catalyzes the conversion of l -tyrosine to l -dihydroxyphenylalanine. TH deficiency is an autosomal recessive disorder secondary to various point mutations mutations in the TH gene. , Presenting phenotypes range from severe onsets in infancy to milder juvenile DRD or juvenile parkinsonism, depending on residual enzyme activity. Symptoms in early onset cases include psychomotor retardation, rigidity, hypokinesia, axial hypotonia, and paroxysmal eye movements. In other presentations, cases have included parkinsonism, gait disorder with stiffness after exercise, dystonia, tremor, and ataxia.
Diagnosis is confirmed by genetic analysis and biochemical testing showing reduced CSF levels of dopamine, norepinephrine, HVA, and MHPG with normal 5-HIAA, biopterin, and neopterin levels. , The CSF HVA:5-HIAA ratio is < 1 (normal 1.0–3.7). Since TH is primarily expressed in the brain and adrenal gland, enzymatic assessment is generally not feasible. Treatment includes administration of conservative doses of levodopa/carbidopa to minimize l -dopa sensitivity and severe dyskinesias. In general, medication responses for motor symptoms and involuntary movements in individuals who tolerate l -dopa are good. Additional therapeutic recommendations include: the use of an MAO-B inhibitor (selegiline or rasagiline) to prevent breakdown of formed neurotransmitter; dopamine agonists (e.g., pramipexole, ropinirole, rotigotine); and a selective central M1 cholinergic receptor blocker (biperiden). , Deep brain stimulation was beneficial in a single case.
AADC/ALAAD, is a pyridoxal-5-phosphate-dependent enzyme that catalyzes both the formation of dopamine from l -dopa and serotonin from 5-hydroxytryptophan (5-HTP). Deficiency of this enzyme leads to a profound reduction of CSF serotonin and catecholamines. AADC deficiency is associated with mutations in the DDC gene. Although onset and phenotype can be variable, clinical symptoms usually begin in childhood. Major presenting problems include developmental delay, hypotonia, and oculogyric crises. , About 50% also present with movement disorders including arm and leg extension, dystonia, athetosis, myoclonus, torticollis, and bulbar dysfunction. Autonomic symptoms, associated with monoamine neurotransmitter deficiency, have included ptosis, miosis, sweating, temperature instability, hypotension, gastroesophageal reflux, and sleep disturbances. , Milder phenotypes have been reported in siblings with hypersomnolence, fatigability, and dystonia as well as in older individuals. , , Magnetic resonance imaging (MRI) scans may have mild cortical atrophy and EEGs spike or polyspike bursts.
Classic laboratory diagnostic findings include marked CSF reductions of dopamine, HVA, serotonin, 5-HIAA, norepinephrine, and 3-MHPG. Levels of l -dopa and its metabolite, 3- O -methyldopa, are elevated since l -dopa cannot be decarboxylated to dopamine. The CSF pterin profile is normal. Plasma and fibroblast AADC are markedly reduced. A secondary deficiency of AADC, caused by a lack of pyridoxal 5-phosphate, has been reported.
Pharmacological treatments are variable and satisfactory in about 20% of patients. Therapy with monoamine oxidase (MAO) inhibitors and dopamine agonists has improved some symptoms but not signs of developmental delay. Although l -dopa would not be expected to be beneficial, several patients did experience improvement of symptoms, possibly due to a mutation that alters the binding affinity of the protein for l -dopa. High-dose pyridoxal phosphate is also recommended since B6 is a cofactor for AADC. Serotonergic agents have not been of benefit.
In contrast to pharmacotherapy, gene therapy provides a potentially promising new treatment for patients with AADC deficiency. Clinical studies in children and adolescents receiving adeno-associated virus vector harboring DDC, via bilateral intraputaminal infusions, have demonstrated acceptable safety, tolerability, and improvement in motor milestones and cognitive symptoms. , The ultimate success of gene therapy in AADC deficiency treatment will depend on a timely diagnosis and the administration of gene therapy before the onset of neurologic damage.
MAO, encoded by the genes MAOA and MAOB , catalyzes the oxidative deamination of serotonin, epinephrine, and norepinephrine. MAO deficiency is an X-linked disease characterized by clinical manifestations including developmental delay, violent and aggressive behavior, epilepsy, and stereotypic movements. Recent evidence has also suggested that MOA activity in the brain is strongly associated with a greater propensity for aggression.
DBH catalyzes the conversion of dopamine to norepinephrine. Dopamine beta hydroxylase (DBH) deficiency is an autosomal recessive disorder secondary to mutations in the DBH gene. DBH-deficiency leads to undetectable CSF/plasma/urine levels of norepinephrine, epinephrine and their metabolites and increased levels of dopamine in the central and autonomous nervous system and peripheral organs. The key symptom in DBH-deficiency is profound orthostatic hypotension, with surprisingly no clear neurocognitive abnormalities or major sleep disturbances. Typical presenting symptoms are hypotension, hypothermia, and hypoglycemia. DBH-deficiency is a treatable disorder; the metabolic block being able to be bypassed by oral supplementation with L- threo- 3,4-dihydroxyphenylserine (L-DOPS/Droxidopa).
Brain dopamine-serotonin vesicular transport disease is a rare neurological disease caused by mutations in the vesicular monoamine transporter 2 (VMAT2; encoded by SLC18A2 ), Alteration of VMAT2 results in the defective loading of monoamines into synaptic vesicles and, in turn, a functional deficiency.
In the small number of individuals reported, there has been some diversity of symptomatology. Affected individuals commonly had truncal hypotonia and impaired motor skills. Other symptoms included lack of head control, uncontrolled eye movements, abnormal posturing, tremor, dysdiadochokinesia, dysarthria, dystonia and parkinsonism, as well as oropharyngeal and nasal secretions, extreme sweating, fatigue, poor distal circulation, sleep disruptions, and nasal speech. Neuroimaging and CSF neurotransmitter profiles were normal, urine levels of dopamine and norepinephrine were low, and HVA and HIAA were elevated. Treatment with l -dopa caused deterioration in chorea and dystonia, but pramipexole (a dopamine receptor agonist) produced sustained improvement.
Dopamine transporter deficiency syndrome (DTDS), aka infantile parkinsonism-dystonia, is a rare genetic disorder caused by mutations in the SLC6A3 gene encoding the dopamine transporter (DAT). Defective presynaptic uptake of dopamine from the synaptic cleft results in increased synaptic levels, greater catabolism, and diagnostically raised CSF HVA levels. Clinically, DTDS has several phenotypes: a classic infantile form and atypical juvenile and adult variants. The classic variant begins in early infancy with irritability, feeding problems, hypotonia, hyperkinetic movements (chorea, ballismus, dystonia, orolingual dyskinesia), and oculomotor (oculogyric crises, ocular flutter, and eyelid myoclonus) abnormalities. Over the course of years, movements expand to include severe dystonia-parkinsonism. , Patients with atypical variants appear normal during infancy and early childhood, however, subsequently develop hyperkinesia, a variety of movement abnormalities (resting and action tremors, dysarthria, and dystonia), and/or hypokinesia with parkinsonian features (bradykinesia, muscle rigidity, and tremor). , , Most affected patients have been unresponsive to a variety of medications and deep brain stimulation, although some dopamine agonists have produced limited improvement.
Succinic semialdehyde dehydrogenase (SSADH) works in conjunction with GABA transaminase to convert GABA to succinic acid. An SSADH deficiency results in increased concentrations of GABA and γ-hydroxybutyrate (GHB; aka 4-hydroxybutyric acid) ( Fig. 17.3 ). , Diagnosis is based on the detection of massive increases of GHB in the urine, plasma, and CSF. CSF levels of GABA and a GABA peptide, homocarnosine, are also elevated. , The gene for SSADH, an aldehyde dehydrogenase five family member A1 ( ALDH5A1 ), is located on chromosome 6p22. Pathophysiologically, it is unclear whether clinical symptomatology is due to the elevated GHB, GABA, or other metabolic alterations. ,
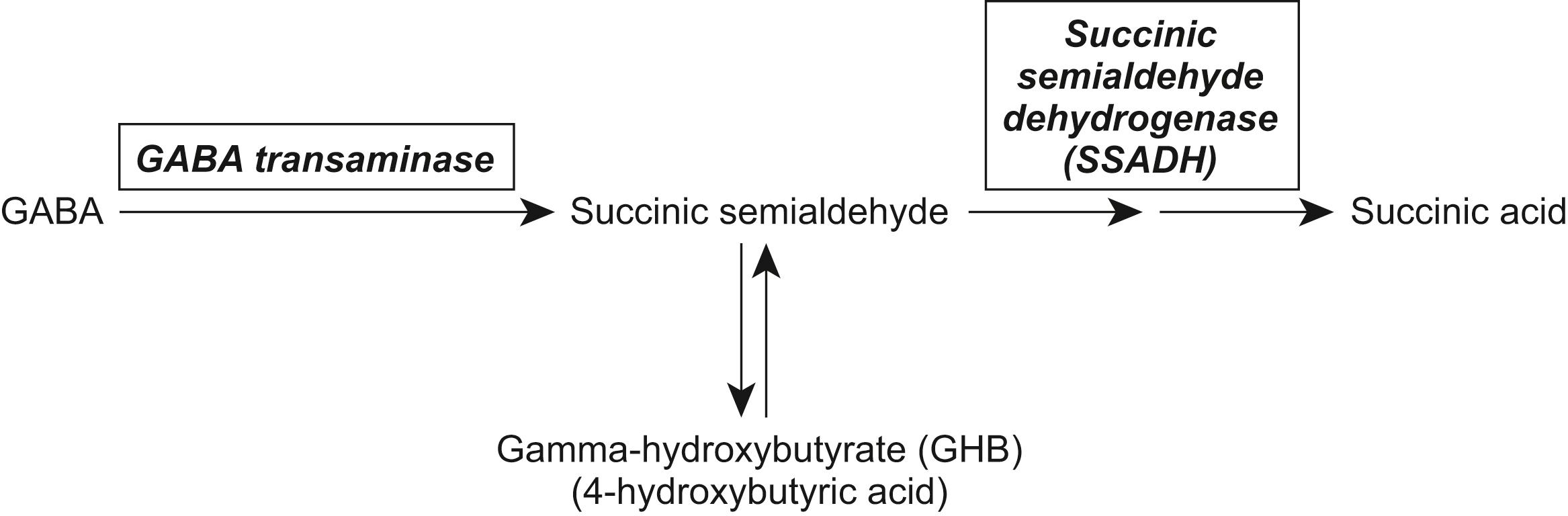
Although SSADH deficiency has a mean age at onset of about 11 months, the diagnosis is often delayed for many years. Clinical features are variable and nonspecific and include intellectual disability, disproportionate language dysfunction, autistic traits, hypotonia, ataxia, aggression, anxiety, hallucinations, hyperactivity, occasionally choreoathetosis, and about half have seizures. , , About 10% have a severe phenotype characterized by developmental regression and a variety of movement disorder manifestations. , MRI imaging has shown cerebral and cerebellar atrophy and T2 hyperintensities in the globus pallidus, cerebellar dentate nucleus, and subthalamic nucleus, although normal in 40% of cases. Magnetoencephalography shows reduction in the gamma frequency band consistent with GABAergic dysfunction and MR spectroscopy shows elevations in the GABA/NAA ratio in all regions. Treatment is symptomatic, with anticonvulsants and medication for behavior problems. Valproate is contraindicated because it may inhibit any residual SSADH activity. Recent advances in clinical application studies have highlighted several therapeutic prospects including mTOR inhibitors, and a putative GHB receptor antagonist.
Wilson's disease is inherited as an autosomal recessive trait with mutations in the ATP7B gene, which encodes for the copper-transporting P-type ATPase protein, ATP7B. , This protein, located in the trans-Golgi network, is responsible for excretion of copper out of the hepatocyte and the incorporation of copper into ceruloplasmin ( Fig. 17.4 ). Multiple ATP7B mutations have been described, and there is extensive phenotypic:genotypic heterogeneity. Abnormalities in the ATP7B protein lead to failure to excrete copper in the bile and the subsequent accumulation in liver, brain, cornea, kidney, bones, and blood. Intestinal absorption of copper is normal, and serum levels of ceruloplasmin, an α-2-globulin that binds and transports copper molecules, are nearly always reduced. It has been suggested that quantitative susceptibility mapping using 3T MRI could be a useful tool in evaluating metal accumulation in deep gray matter nuclei. Increased excretion of copper in the urine is insufficient to prevent copper accumulation. The accumulation of other metals, has also been speculated to have a contributing pathogenic role, for example, dysfunction of ceruloplasmin leading to iron accumulation and liver dysfunction.
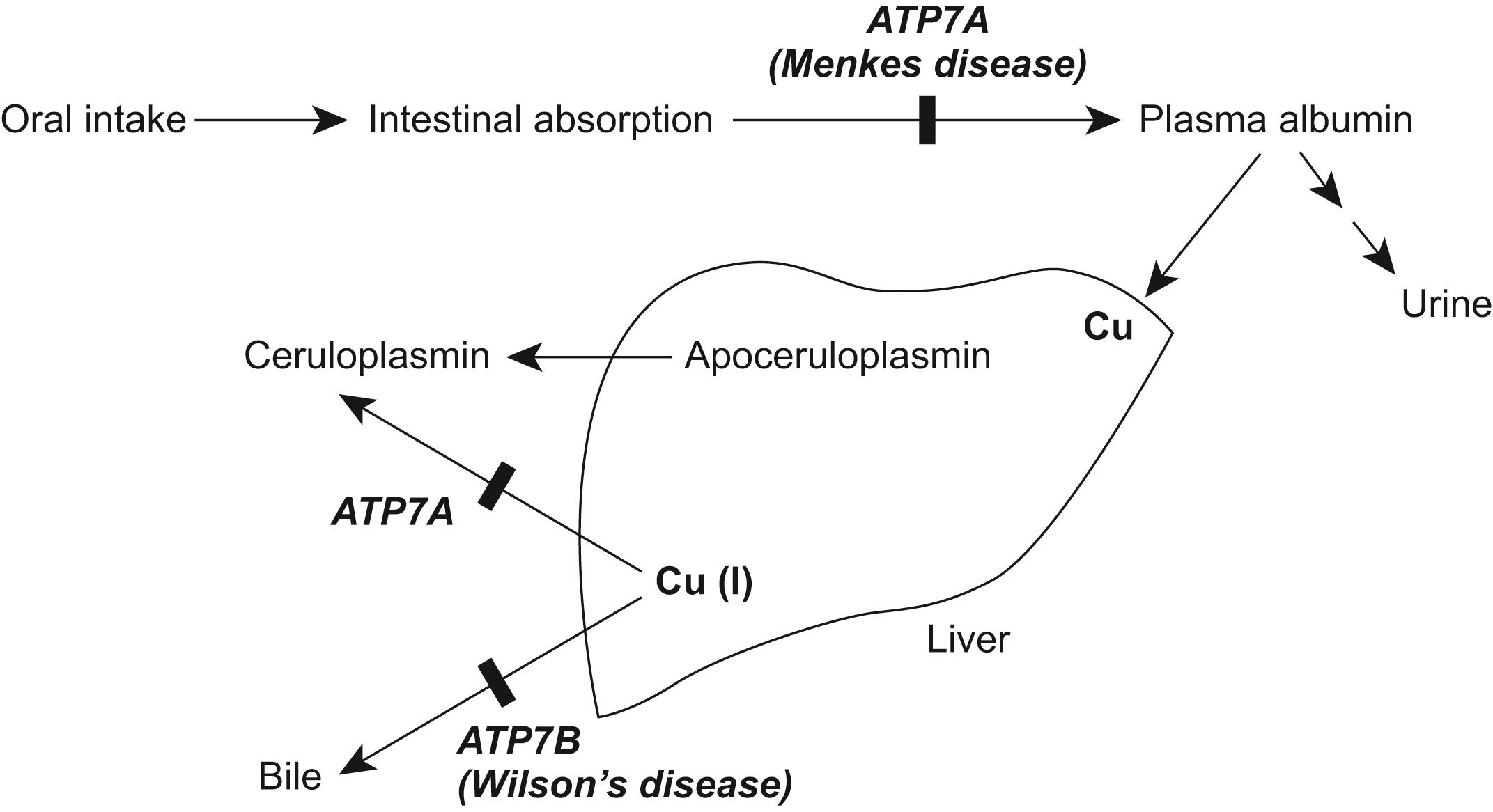
Clinically, there are two distinct initial clinical presentations; hepatic and neurologic. The typical hepatic presentation begins about age 12 years, although early childhood and late adulthood cases have been reported. Hepatic dysfunction includes early asymptomatic hepatomegaly, hepatitis, and cirrhosis with later decompensation. The neurologic presentation includes three variants: (1) a juvenile, generalized dystonic type with subacute onset and progression; (2) a pseudosclerotic or Westphal form having a juvenile-adulthood onset, and presenting with dysarthria, tremor, and cerebellar symptoms; and (3) a parkinsonian akinetic-rigid form. , , In general, the mean age of onset for neurological symptoms is about 19 years, although symptoms have been reported in a six-year-old. Clinical presentation tends to be insidious and variable and include alterations in speech, drooling, pharyngeal dysmotility, a resting, postural, or kinetic tremor, motor function difficulties, and mental changes. Psychiatric symptoms precede the neurologic abnormalities in about 20%, with problems ranging from subtle changes in personality and behavior to frank psychosis. , The aforementioned have a greater correlation with neurological symptoms than with the existence of hepatic symptomatology. Tremor, although common in Wilson's disease, has variable characteristics and may be unilateral or bilateral; appear at rest or during postural maintenance, with movement, or in any combination of the above. When presenting as an action tremor, it is typically coarse and irregular and when elicited with arms forward and flexed may have the classical “wing-beating” or proximal quality. Dystonia can be generalized, segmental, focal, or multifocal. As the disease progresses, dystonia of the head, body, and face (facial grimacing, sardonic smile or risus sardonicus, blepharospasm, and tongue dyskinesia) are common. Abnormal oculomotor vertical smooth pursuit is frequently observed. The Kaiser-Fleischer ring, a yellow-brown deposition of copper in Descemet's membrane of the cornea, is best observed by slit-lamp examination.
The diagnosis of Wilson's disease is made via a combination of studies, including serum ceruloplasmin, 24-hour urine copper, serum “free” copper, slit-lamp examination, and liver biopsy with histologic assessment and determination of copper content. However, as emphasized in the literature, laboratory-based chemical parameters of copper metabolism can represent both deviations from the norm as well as the presence of other copper metabolism disorders. For example, ceruloplasmin, an α2-glycoprotein acute-phase reactant, measurement at a level of 20 mg/dL or lower has a good sensitivity; however, false positives (low levels) may occur in severe malnutrition, protein losing conditions, acute liver failure, and aceruloplasminemia. Similarly, false negatives (higher levels) can occur in affected individuals who have hepatitis, other inflammatory disorders, or are using oral contraceptives. In addition, copper metabolism abnormalities are seen in a variety of other disorders including Menkes disease, occipital horn syndrome, Indian childhood cirrhosis, ceruloplasmin deficiency, MEDNIK syndrome, Huppke-Brendl syndrome, and copper chaperone CCS deficiency. Although numerous mutations have been identified, the detection of biallelic (compound heterozygous or homozygous) pathogenic variations in the ATP7B gene is important in the diagnosis of Wilson's disease.
MRI abnormalities include increased signal intensity on T2-weighted images of the tectal plate, basal ganglia, thalamus, and brainstem and central pontine myelinolysis-like changes. Less common findings include the “face of the panda” located in the midbrain. Pathologically, copper accumulates extensively in the basal ganglia, where it can cause necrosis, as well as diffusely in the cortex and adjacent white matter. Although MRI findings can improve with copper chelation therapy, brain content of copper may remain elevated and prominent neuropathological features persist. The severity of neuropathological findings has been correlated with the content of cerebral copper.
Treatment for Wilson's disease is divided into an acute decoppering therapy and lifelong maintenance treatment. , , Existing questions have made it difficult to select the most appropriate decoppering compound and randomized control trials are lacking. Penicillamine is a powerful copper chelating agent, but because of potential side effects, including sudden and possibly permanent worsening of neurologic symptoms, its use is controversial. , , Ammonium tetrathiomolybdate has four sulfur groups, which allows it to form a tripartite complex with copper and protein. Clinical experience with this drug is limited and its formulation may not be stable. Trientine (triethylene tetramine dihydrochloride) is not beneficial for the acute treatment of patients with neurologic symptoms because it does not mobilize copper from the brain. It is useful, however, as maintenance therapy and in the treatment of patients with pure hepatic disease. Zinc, which induces the synthesis of metallothionein in the intestine, has a slower onset of action and is typically used as maintenance therapy or in case of early diagnosis.
Serum parameters of copper metabolism, urinary copper levels, and liver function tests should be monitored in patients receiving chronic therapy to prevent iatrogenic copper deficiency. The latter has caused both hematological and neurological (polyneuropathy and myelopathy) problems. , Initiation of therapy in presymptomatic patients is recommended and may prevent the development of neurologic, hepatic, and/or psychiatric problems. Recovery of existing neurologic problems typically does not begin until after about 6 months of treatment. Although radiographic improvement on brain MRI may be seen for up to 4 years, disabilities that persist for longer than 2 years after initiation of therapy tend to be permanent. Orthotopic liver transplantation may be lifesaving for patients with fulminant liver failure; however, this procedure is controversial for individuals with severe neurological symptoms. , Deep brain stimulation may be an effective treatment. Gene therapy approaches are potentially effective strategies. ,
Huppke–Brendel syndrome is an autosomal recessive defect of the SLC33A1 gene which codes for an acetyl-CoA transporter (AT-1). This mutation leads to a dysfunction of posttranslational ceruloplasmin modification resulting in a low serum ceruloplasmin and copper levels. Clinically this syndrome begins in infancy with congenital cataracts, sensorineural hearing loss, and severe developmental delay. MRI shows cerebellar hypoplasia and hypomyelination. All affected individuals died between age 10 months and 6 years.
Manganese (Mn) is a trace metal with an essential cofactor role for multiple enzymes, including neurotransmitters. , Mn deposition in the brain can occur secondary to excess environmental exposure (miners, contaminated water), as well as to inherited defects in Mn transport and metabolism. Patients with severe environmental Mn exposure are at risk for an extrapyramidal syndrome termed manganism, with rigidity, bradykinesia, and dystonia. In addition, accumulation in brain and other organs can also result from inherited genetic defects involving either of the two Mn transporters; efflux transporter (SLC30A10) and uptake transporter (SLC39A14) , —both cause basal ganglia toxicity which can lead to a progressive dystonia-parkinsonism syndrome.
Symptoms beginning in childhood (age 2–14 years) include polycythemia, chronic liver disease, progressive dystonia, spasticity, and an unusual high-stepping “cock-walk” gait. Other symptoms include loss of milestones, motor and cognitive impairment, behavioral changes, dysarthria, tremor, and bradykinesia. Late-onset forms in adulthood typically have an akinetic-rigid parkinsonism with early postural instability. Laboratory findings include increased serum Mn concentrations, polycythemia, reduced iron stores with low ferritin, and high total iron binding capacity. Brain MRI contains T1-weighted hyperintensities in the basal ganglia, and white matter. Polycythemia and liver cirrhosis only occur in the SLC30A10 mutation variant.
Symptoms usually begin early and cause a rapidly progressive dystonia, parkinsonism, dysarthria, bulbar dysfunction, and T1 hyperintensities in the globus pallidus.
Treatment for Mn accumulation, due to either genetic abnormality, includes lifetime chelation therapy with intravenous disodium calcium EDTA (increases urinary excretion of Mn), iron supplementation (competes with and displaces Mn), and the avoidance of foods high in Mn (nuts, dark chocolate, pumpkin, sesame, cloves, and sunflower seeds). Treatment, if started early, generally leads to a good neurological improvement.
Neurodegeneration with brain iron accumulation (NBIA) is a group of rare disorders associated with iron deposition in the brain. Initially labeled Hallervorden–Spatz syndrome (HSS), this heterogeneous group of conditions has been associated with at least 10 different NBIA genes ( ATP13A2 , C19Orf12 , COASY , CP , DCAF17 , FA2H , FTL , PANK2 , PLA2G6 , and WDR45) ( Table 17.4 ). Clinical differentiation between different types of NBIAs is often exceedingly difficult because of the pleiotropy of the causative genes. , In addition, there are patients with phenotypes compatible with NBIA and undiscovered genotypes. Several pathways are involved in NBIA syndromes: iron and lipid metabolism, mitochondrial dynamics, and autophagy. , Based on their prevalence, the two major subtypes are pantothenate kinase–associated neurodegeneration (PKAN) and PLA2G6-associated neurodegeneration (PLAN).
| Pantothenate kinase–associated neurodegeneration (PKAN); pantothenate kinase 2 (PANK2) |
PLA2G6-associated neurodegeneration (PLAN); PLA2G6
|
| Mitochondrial membrane protein-associated neurodegeneration (MPAN); C19orf12 |
| Beta-propeller-associated neurodegeneration (BPAN); WDR45 |
| Hereditary Ferritinopathy (Neuroferritinopathy, NF); FTL |
| Hereditary aceruloplasminemia; CP |
| Kufor–Rakeb syndrome (KRS): ATP13A2 |
| Fatty acid hydroxylase-associated neurodegeneration (FAHN); FA2H |
| Woodhouse–Sakati syndrome: DCAF17
COASY protein-associated neurodegeneration (CoPAN): COASY |
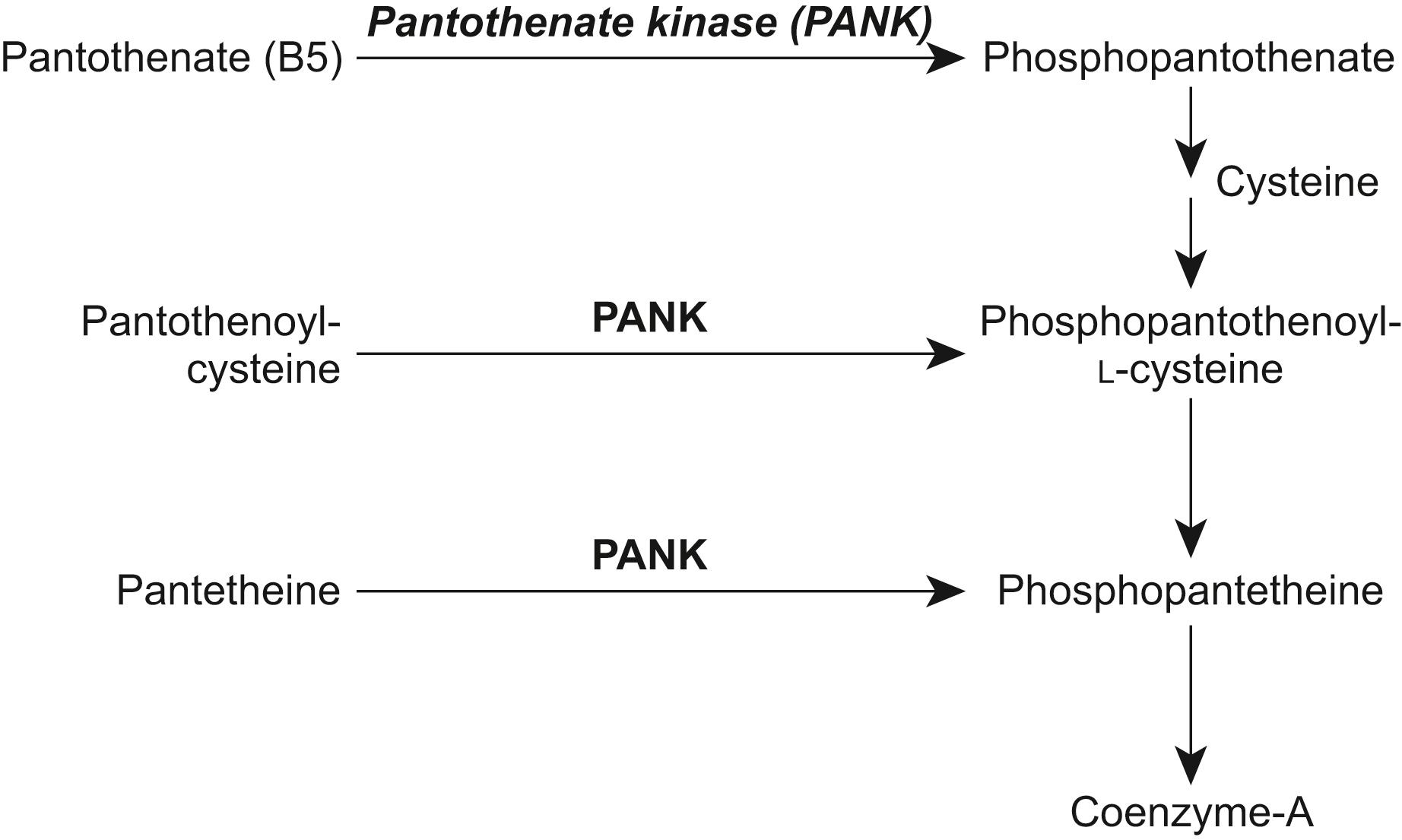
PKAN is a rare autosomal recessive disorder due to a mutation in the gene for pantothenate kinase 2 (PANK2 ), a regulatory enzyme in the synthetic pathway for coenzyme A. The enzyme PANK2 catalyzes the cytosolic phosphorylation of pantothenate (vitamin B5), N-pantothenoylcysteine, and pantetheine. ( Fig. 17.5 ). Pathologically it is associated with abnormal iron deposition and high concentrations of lipofuscin and neuromelanin in the substantia nigra pars reticulata and the internal segment of the globus pallidus. , The definitive mechanism by which basal ganglia iron uptake is increased in PKAN is unknown, recognizing that systemic and CSF iron levels, as well as plasma ferritin, transferrin, and ceruloplasmin, are normal. Patients homozygous for null mutations in PANK2 have a significantly earlier age of disease onset and progress more rapidly.
PKAN has variable presentations and has been divided into (a) a classic early-onset form, containing 75% of cases, with presentation before age 6 years and a rapidly progressive course, and (b) “atypical forms,” which present at a mean age of 14 years. , In the classical form, onset is in early childhood with progressive motor difficulties, personality changes, cognitive decline, dysarthria, and spasticity. Abnormal motor function is typically present but may be delayed for several years. Dystonia is the most common movement disorder (orobuccolingual, truncal, and limb), but rigidity, choreoathetosis, and a resting or action tremor may also be present. Ophthalmologic abnormalities include pigmentary retinal degeneration in about two-thirds of patients, alteration of vertical and saccadic pursuits, progressive loss of peripheral visual fields, and blindness. Patients also develop cognitive dysfunction and seizures may be present. Acanthocytosis has been reported in about 8% of classic patients. The course of the early-onset form is variable and is divided into a rapidly and a slowly progressive type. The rapidly progressive early-onset type has a short transition from spasticity to severe movements with opisthotonus, and death within 1–2 years. In contrast, the more prevalent slowly progressive early-onset type advances at a nonuniform rate with dystonia and spasticity leading to being wheelchair-bound by the teens and death occurring within 20 years.
In the “atypical” late-onset childhood form, patients often have speech defects (palilalia, tachylalia, and dysarthria) as an early sign and develop significant psychiatric symptoms (personality changes, impulsivity, obsessive-compulsive disorder, aggression, episodic outbursts, and depression) and even motor and phonic tics. , , Although affected individuals are often described as clumsy, motor involvement (parkinsonism, bradykinesia, dystonia, tremor) is a later feature, and spasticity ultimately limits ambulation. Freezing during ambulation, especially when turning or encountering irregular surfaces, has been described. Subclinical retinal changes may be present. In general, the course of atypical PKAN is less severe and more slowly progressive than the early-onset types.
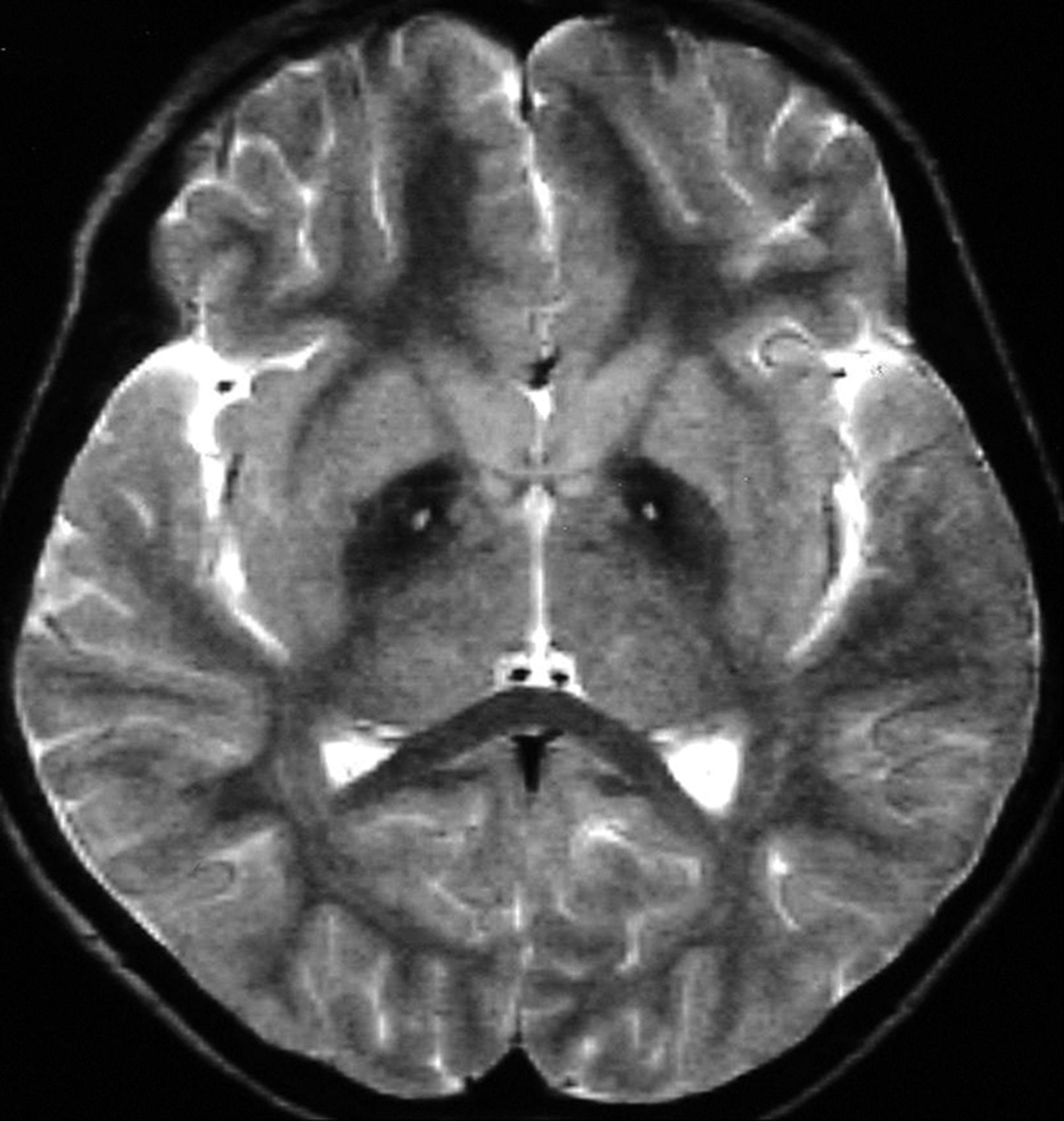
The diagnosis of PKAN depends on the presence of obligate features: onset in the first 2 decades, progressive course, extrapyramidal symptoms, and classic MRI findings showing hypointense T2-weighted and proton density signal in the globus pallidus and substantia nigra ( Fig. 17.6 ). The presence of a hyperintense area within the hypodense areas, named the “eye of the tiger sign,” is considered almost pathognomonic for PKAN. Hyperdense lesions may be present in presymptomatic patients, but as the disease progresses hypodensities appear and ultimately predominate. , In patients with PKAN, 7-Tesla imaging has identified elevated concentrations of iron in the globus pallidus, substantia nigra, and internal capsule; not seen in those with heterozygous mutations. Other findings include abnormal cytosomes in lymphocytes, and sea-blue histiocytes in bone marrow—the latter findings being typical of ceroid-lipofuscin accumulation.
There is no specific treatment for PKAN. Theoretically, in patients with residual PANK2 activity the downstream delivery of phosphopantothenate or products in the coenzyme A pathway might be therapeutic. A trial with pantethine did not significantly improve motor function. Iron chelation therapy with desferrioxamine has not been effective, but response was better with deferiprone. , Therapy for movement disorders and spasticity is symptomatic. Oral baclofen has been helpful in early stages, but intrathecal infusion has provided only limited benefit. In 23 PKAN patients, deep brain stimulation improved dystonia and quality of life. Pallidotomy and thalamotomy have produced limited and transient benefit.
The PLA2G6 (phospholipase A2 group VI) gene encodes for a calcium-independent PLA2, which plays a major role in phospholipid remodeling. Functionally, the enzyme catalyzes the hydrolysis of glycerophospholipids and generates a free fatty acid and lysophospholipid, playing a role in membrane homeostasis through the regulation of phospholipids. PLAN represents a second major NBIA phenotype and has at least three separate, but overlapping, phenotypes including (1) infantile neuroaxonal dystrophy (INAD); (2) atypical neuroaxonal dystrophy; and (3) PLA2G6-related dystonia parkinsonism. MRIs in this group may overlap with that seen in PKAN.
INAD is an autosomal recessive disorder, manifesting between 6 months and 3 years, with a gait disturbance and truncal hypotonia. Onset may occur following an intercurrent infection. Progressive symptoms include motor and sensory impairment, spastic tetraplegia, hyperreflexia, and visual impairment associated with optic atrophy. Seizures are a late manifestation. This is a rapidly progressing disorder with death at the end of the first decade. Electromyography shows denervation and nerve conduction studies a distal axonal sensorimotor neuropathy. MRI demonstrates cerebellar atrophy, claval hypertrophy, and brain iron accumulation within the globus pallidus, dentate nucleus, and substantia nigra.
Atypical form of NAD has an older age at onset with presenting symptoms including gait impairment, ataxia, speech difficulties, and autistic features. , The clinical course is relatively stable in early childhood with neurological deterioration in mid-childhood consisting of extrapyramidal manifestations (primarily dystonia), dysarthria, and in later stages spastic tetraplegia. Previously described Karak syndrome is now considered an atypical NAD. MRI shows prominent brain iron accumulation with or without cerebellar atrophy.
PLA2G6-related dystonia Parkinsonism is a rare disorder with a broad range of presentations, mostly occurring in early adulthood. Childhood presentations have initially overlapped with atypical NAD, including development of a movement disorder manifested by dystonia, parkinsonism, and choreoathetosis. MRIs have shown nonspecific changes including cerebral atrophy. Symptoms may temporarily respond to l -dopa.
Mitochondrial membrane protein-associated neurodegeneration (MPAN) is a form of neurodegeneration with brain iron accumulation caused by pathogenic variants in the C19Orf12 gene. Although MPAN mutations involve autosomal recessive biallelic C19orf12 variants, autosomal dominant forms have been described with clinically indistinguishable phenotypes. , C19orf12 expression is primarily in the brain, blood cells, and adipocytes with derived proteins located within the mitochondria. The precise function of C19orf12 proteins remains unclear, although a role in lipid metabolism, is proposed. At autopsy, neuropathologic analysis has shown a complex pattern of alpha-synucleinopathy and tauopathy.
Clinically, the mean age of symptom onset is 10 years, with a range of 3–30 years. Frequent symptoms include spasticity, cognitive decline, dysarthria, optic atrophy, tremor, dystonia, psychiatric symptoms, dysphagia, and parkinsonism. , About half have a motor axonal neuropathy with muscle atrophy, fasciculations, and EMG changes. Neuroimaging shows brain iron accumulation in the globus pallidus and substantia nigra, hyperintense streaking of the internal medullary lamina of the globus pallidus, and white matter lesions. The parkinsonism may improve with l -dopa but some patients develop l -dopa-related dyskinesia.
Beta-propeller protein-associated neurodegeneration (BPAN) is due to heterozygous or hemizygous germline mutations in the X-chromosome gene WDR45 . WDR45 belongs to a family of WD40 proteins that interact with phosphoinositides and promote protein–protein interactions with a role in cell cycle control, translational regulation, signal transduction and autophagy. , The name beta-propeller is derived from WDR45's seven-bladed tertiary structure. BPAN is an X-linked dominant neurodegenerative disease and most cases have occurred in females. Typical clinical presentation is in children. Affected children have global developmental delay, epilepsy (multiple seizure types), Rett-like behaviors, stereotypies, dysfunctional sleep, and ocular defects. BPAN with Rett-like features is associated with more epileptic seizures and less deceleration of head growth and breathing irregularities than classic Rett cases with MECP2 variants. Progressive regression occurs in adolescence with the appearance of dystonia, parkinsonian features (bradykinesia, freezing of gait, rigidity), progressive dyskinesia, and dementia. Early MRI imaging may be normal, but substantia nigra swelling and dentate nucleus T2 hyperintensity are early manifestations. Iron deposition in the globus pallidus and substantia nigra generally appear with increasing symptoms. On T1-weighted imaging, the substantia nigra/cerebral peduncles have a distinctive hyperintense “halo.” Parkinsonian symptoms have improved with l -dopa, but medication-induced dyskinesia is common.
Neuroferritinopathy is an autosomal-dominant disorder characterized by mutations in the ferritin light-chain gene ( FTL) , which codes for ferritin light polypeptide, on chromosome 19q13. Ferritin plays an important role as the major iron sequestration and storage protein. It is composed of both light (FTL) and heavy chain subunits that possess complementary iron-handling functions. Along with hereditary aceruloplasminemia (HA), it is classified as an adult onset NBIA syndrome. The mean age of onset is 40 years, and presentations have been highly variable. In some it presents as a movement disorder with possible tremor, cerebellar signs, chorea, dystonia, prominent oromandibular dyskinesias, and parkinsonism. , Others have neurocognitive features that include dementia, depression, emotional lability, and acute psychosis. , Patients have a low serum ferritin level, and the MRI shows abnormal hypointense T2 signal intensity in the globus pallidus and putamen, even early in the disease. With disease progression, hypointensity expands into the substania nigra, dentate nucleus, and cortex.
HA is a rare autosomal recessive disorder caused by mutations in the gene encoding ceruloplasmin gene (CP ). More than 70 mutations have been reported, and this disorder is often confused with Wilson's disease; both having extremely low levels of ceruloplasmin and overlapping imaging results. In contrast to Wilson's disease, however, aceruloplasminemia is a disorder of iron metabolism due to its activity as an iron oxidase and its absence resulting in iron accumulation within the brain, liver, and pancreas. Clinical manifestations usually occur in adulthood (fifth–sixth decade) with adult-onset refractory anemia, diabetes mellitus, retinal degeneration, and neuropsychiatric symptoms. Progressive neurological problems include dystonia, dysarthria, bradykinesia, rigidity, cerebellar ataxia, and deterioration of cognition and behavior. A few cases with seizures have been described. Neurological symptoms are thought to be secondary to massive iron overload within the basal ganglia, thalamus, and dentate nucleus. Anemia is associated with low serum iron and transferrin saturation, but elevated serum ferritin is usually present. MRI shows hypointensities in the basal ganglia, dentate, and thalamus. Attempts to treat with iron chelators have been disappointing.
Kufor–Rakeb syndrome (KRS) is a rare autosomal recessive disorder associated with various mutations in the ATP13A2 gene. Mutations in this gene alter the production of a transmembrane protein that regulates intracellular iron metabolism and protects cells against cytotoxicity due to iron accumulation. Clinical symptoms are heterogeneous but often begin in adolescence with parkinsonism, that initially responds to l -dopa. Eye movement abnormalities include slow saccades and supranuclear vertical gaze palsy. Pyramidal signs, including spasticity, increased deep tendon reflexes, and Babinski signs, are common. Cognitive decline has a variable severity and action myoclonus and seizures have been reported. Like other NBIAs, iron accumulation in the basal ganglia has been reported.
Fatty acid hydroxylase-associated neurodegeneration (FAHN) is associated with mutations in the fatty acid two hydroxylase ( FA2H) gene. FA2H produces hydroxylated ceramides and participates in myelin formation. , Why there is iron accumulation remains unclear. Clinically the disorder is characterized by the childhood onset of gait abnormalities, spastic quadriparesis, ataxia, dystonia, and ocular abnormalities. Cognitive impairment and seizures may also be present Mutations also cause a form of hereditary spastic paraplegia and leukodystrophy. , MRI findings, in addition to iron deposition in the globus pallidus, may also include white matter abnormalities, a thin corpus callosum, and supratentorial atrophy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here