Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lysosomal storage diseases (LSDs) are rare disorders caused by genetically transmitted lysosomal enzyme deficiencies. The resulting lysosomal accumulation of uncleaved substrates impairs cell function and causes cell death. Although some LSDs have been known for more than 100 years, the concept of lysosomal storage did not crystallize until the 1960s, after lysosomes were discovered by De Duve and the electron microscope became available. LSDs involve all organ systems but most severely the brain and the skeleton. They usually begin early in life and are inexorably progressive, and most are fatal in childhood. For these reasons, they are an important problem in genetics, pediatrics, and child neurology.
Lysosomes contain enzymes that biodegrade diverse molecules. Lysosomal enzymes are glycoproteins that are active at an acid pH. They are produced in the endoplasmic reticulum and are brought into lysosomes using a specific receptor-mediated mechanism. Most lysosomal enzymes cleave sugar chains that are attached to glycolipids, glycoproteins, and other compounds. Lysosomal biogenesis and function are controlled by transcription factor EB (TFEB), which is regulated by the mechanistic (or mammalian) target of rapamycin complex 1 (mTORC1).
Primary lysosomes are round to oval organelles measuring 0.2 to 0.8 microns. They have a phospholipid bilayer membrane in which are embedded 100 glycosylated proteins and contain approximately 60 enzymes (phosphatases, nucleases, glycosidases, proteases, peptidases, sulfatases, phospholipases) that are active near pH 4.5. Acidification of lysosomes is essential for their function and is regulated by vacuolar ATPase, which generates energy by hydrolyzing ATP and uses this energy to pump protons across the lysosomal membrane into the lysosomal lumen. The lysosomal hydrolases are capable of hydrolyzing most biomolecules (mostly damaged or nonfunctional compounds) produced by cells or brought into cells by endocytosis. They obtain their substrates by merging with endosomes, phagosomes, and autophagosomes.
Lysosomal enzymes are glycoproteins. They are synthesized in the rough endoplasmic reticulum and are glycosylated in the smooth endoplasmic reticulum. As they travel through the Golgi, a mannose-6-phosphate (M6P) molecule is attached. By binding to complementary M6P receptors located on the Golgi membrane, the M6P marker enables lysosomal enzymes to home into parts of the Golgi that bud off to form lysosomes. The M6P marker also helps internalize lysosomal enzymes from the extracellular space, including those that are administered for treatment. The M6P attachment to the lysosomal enzyme protein is catalyzed by the sequential action of N-acetylglucosamine-phosphotransferase (GlcNAc), which adds N-acetylglucosamine-1 phosphate to the mannose residue on the lysosomal enzyme, and αN-acetylglucosaminidase, which removes the acetylglucosamine to expose the M6P marker. Two lysosomal enzymes, glucocerebrosidase and acid phosphatase, are located in the lysosomal membrane and do not require the M6P marker. Proteolytic processing and other modifications, which begin in the prelysosomal compartments and end when the enzymes are in the lysosomes, trim and modify the lysosomal enzyme proteins, stabilize their molecules, and protect them from internal proteolytic digestion.
Most enzymes that are involved in the LSDs cleave sugar chains that are attached to glycoproteins (glycoproteinoses) and glycolipids (sphingolipidoses), disaccharide chains of glycosaminoglycans (mucopolysaccharidoses [MPS]), and glycogen (Pompe disease). Farber disease and Wolman disease are the only LSDs in which the catalytic actions of the respective enzymes are directed against lipid molecules. Some of the neuronal ceroid lipofuscinoses are caused by defects involving proteases.
Lysosomal biogenesis and function are controlled by Transcription Factor EB (TFEB), a cytosolic protein encoded by the TFEB gene on chromosome 6. TFEB activity, in turn, is regulated mTORC1, a kinase complex that controls the state of phosphorylation of TFEB in response to environmental cues such as fluctuations in amino acid levels. Under conditions of starvation and cellular stress, when amino acid levels are low, TFEB is activated by dephosphorylation. Activated TFEB is translocated from the cytosol into the nucleus and enhances the transcription of a set of genes that encode lysosomal enzyme and membrane proteins, resulting in increased levels of lysosomal enzymes and increased numbers of lysosomes. In resting, nutrient-rich cells, TFEB is phosphorylated and is retained in the cytosol. The interaction of TFEB and mTORC1 occurs at the lysosomal surface. Thus, lysosomes are able to adapt to environmental changes and regulate their own biogenesis and function. TFEB holds promise as a potential therapeutic target for LSDs and other conditions characterized by accumulation of toxic products such as Alzheimer disease, Parkinson disease, and Huntington disease. TFEB overexpression has been shown to be beneficial in animal models of these diseases.
Another gene that is under TFEB transcriptional control is GRN ( PGRN ), which encodes progranulin (PGRN), a glycoprotein that is involved in the regulation of inflammation, wound healing, oncogenesis, and multiple other processes. PGRN is secreted into the extracellular space where it is cleaved into granulin peptides. Extracellular PGRN is endocytosed and carried into lysosomes by sortilin or bound to prosaposin. In lysosomes, PGRN is similarly processed into granulin peptides. PGRN and granulin upregulate lysosomal genes, including two lysosomal enzymes, cathepsin D (CTSD) and glucocerebrosidase (GBA). Heterozygous mutations in GRN cause frontotemporal lobar degeneration with TDP-43 inclusions, and homozygous mutations cause childhood-onset neuronal ceroid lipofuscinosis (NCL), which is due to deficiency of CTSD. GBA mutations cause Gaucher disease and are a known risk factor for Parkinson disease (PD). Thus, lysosomal dysfunction appears to be a link between LSDs and other neurodegenerative diseases.
The most common cause of lysosomal enzyme deficiency is mutation of the gene coding for the lysosomal enzyme. Less frequent causes include failure of addition of the M6P marker that targets lysosomal proteins to lysosomes, the absence of various cofactors (saposins, protective protein, GM 2 activator), errors of posttranslational modification of enzyme proteins, defects of lysosomal transport, and drug-induced lysosomal dysfunction.
Lysosomal enzymes are encoded by nuclear genes. Most LSDs are due to mutations in these genes that result in the absence of the enzyme protein or in a catalytically inactive or unstable polypeptide. Less frequently, enzyme deficiency and storage arise by other mechanisms described in the following section ( Box 8.1 ).
Mutations in lysosomal enzyme genes
No addition of the mannose-6-phosphate marker
Absence of saposins, protective protein, GM 2 activator
Errors of posttranslational modification
Drug-induced lysosomal dysfunction
Defects of lysosomal transport
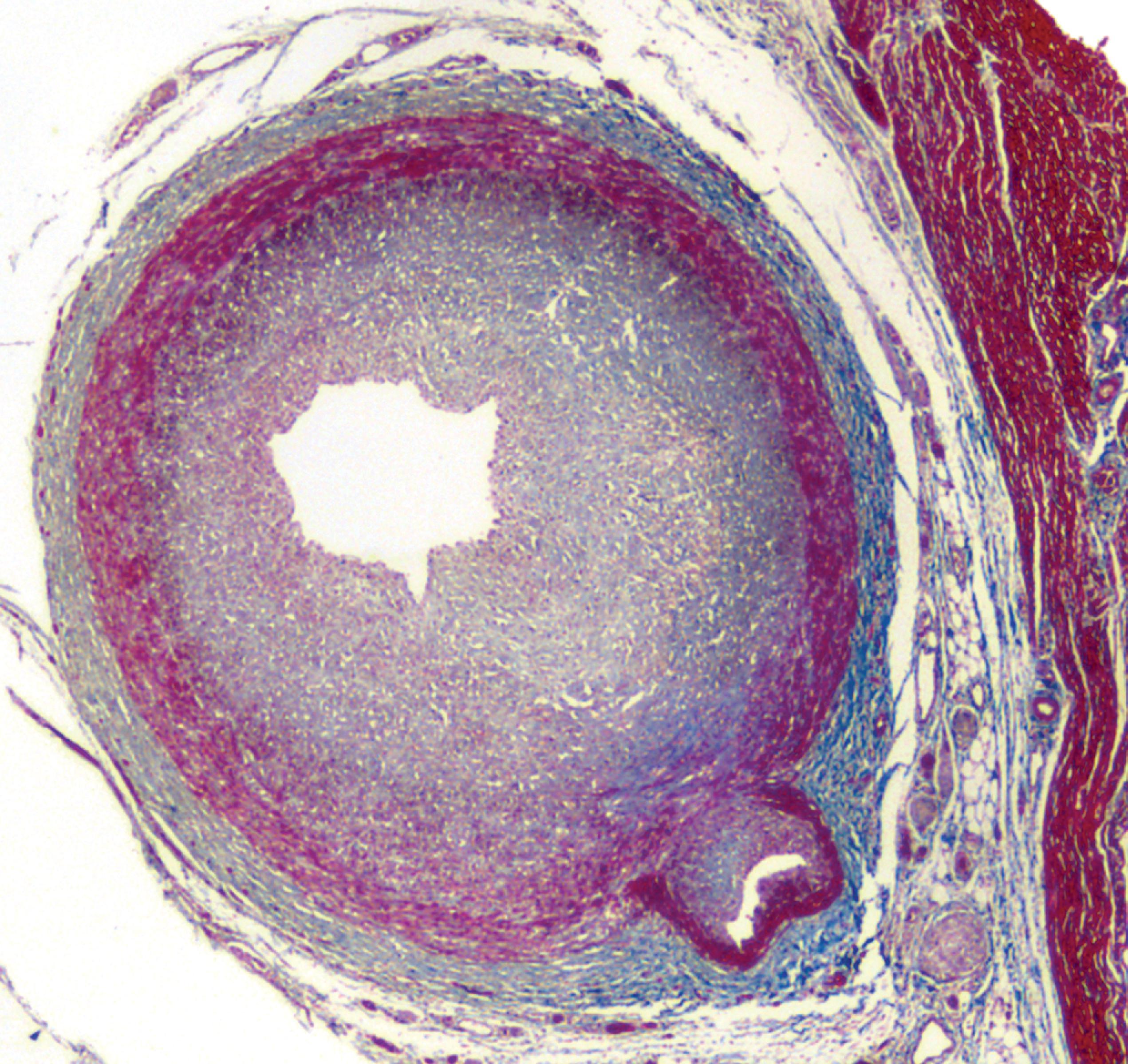
Failure of addition of the M6P marker (due to deficiency of GlcNAc) results in the inability to segregate lysosomal enzymes into lysosomes. The enzymes then leak into the cytosol and are inactivated by the slight alkaline pH. This leads to the deficiency of multiple lysosomal enzymes and the accumulation of diverse substrates in many cells and organs. GlcNAc deficiency causes I-cell disease, a severe LSD with psychomotor retardation and a Hurler phenotype (see the section on Mucopolysaccharidoses further on) and a milder variant, pseudo-Hurler polydystrophy. The name of this condition, I (inclusion)-cell disease, describes the storage vacuoles that develop in cultured fibroblasts and in vivo ( Fig. 8.1 ).

Sphingolipid activator proteins (saposins) bind substrate molecules and link up with lysosomal enzymes. In the absence of these activators, substrate is not presented to the lysosomal enzyme. There are four saposins: A, B, C, and D, all derived from a single precursor by different pathways of proteolytic processing. Mutations of all saposins have been described and cause rare variants of GM 2 gangliosidosis, metachromatic leukodystrophy, and Gaucher disease. Combined deficiency of β-galactosidase and sialidase (galactosialidosis) is caused by the absence of a protective 32-kDa lysosomal protein that assembles these two enzymes into a functional complex.
Multiple sulfatase deficiency is characterized by decreased activity of all sulfatases that are coded by different genes. The cause of this LSD is thought to be an error of posttranslational modification of sulfatases.
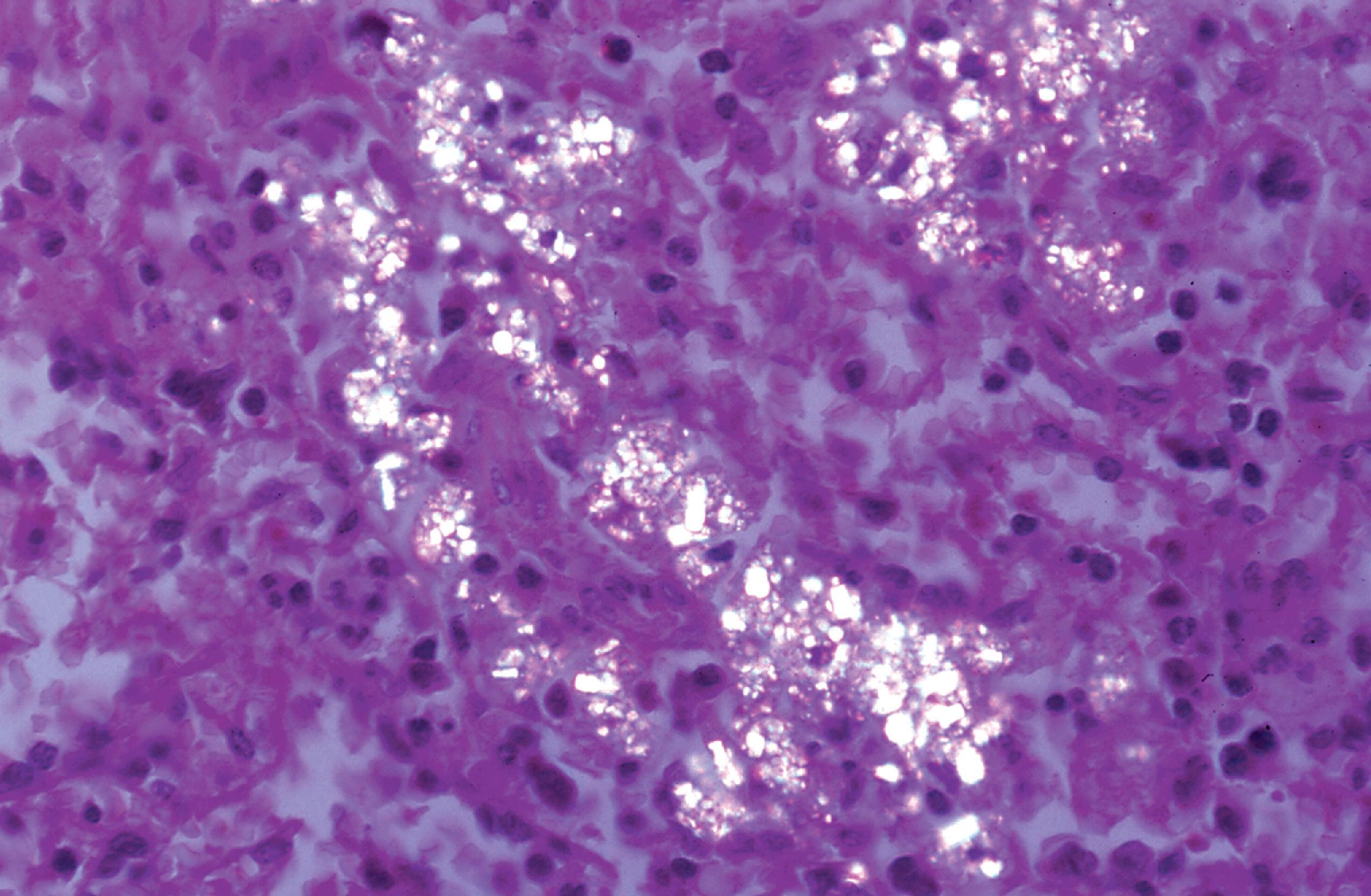
The products of lysosomal degradation are small molecules that either diffuse freely through the lysosomal membrane or are taken out of lysosomes through carrier-mediated transport. Defects of the lysosomal membrane transport system cause sialic acid storage and its milder variant Salla disease, and cystinosis. In cystinosis, birefringent cysteine crystals damage the cornea and glomeruli and accumulate in histiocytes of bone marrow, lymphoid tissues, and other organs ( Fig. 8.2 ). An error of lysosomal transport of cholesterol also causes Niemann-Pick disease type C.
Several drugs impair lysosomal function in a variety of ways, including endolysosomal trafficking, phagocytosis, exocytosis, and lysosomal signaling. Prominent among lysosomotropic drugs are cationic amphiphilic drugs such as amiodarone, chloroquine, chlorpromazine, and haloperidol. These drugs inhibit lysosomal phospholipases through a variety of mechanisms and induce lysosomal storage of phospholipids involving many cells and tissues. Affected cells have a foamy appearance and contain membranous (myelinoid) bodies similar to those that are seen in the sphingolipidoses. In most instances, drug-induced lysosomal storage is reversible when the offending agent is discontinued.
The combined prevalence of all LSDs at birth has been estimated to be between 11.1:100,000 and 14:100,000, and the incidence of Gaucher disease, the single most common LSD, is 1.16-1.75:100,000. The prevalence of the MPS, the most common group of LSDs, has been found to be 4.44:100,000. There is significant variation in the prevalence of individual LSDs among different ethnic and racial groups ( Table 8.1 ). In the Netherlands, the most common LSD is Pompe disease (2:100,000), and the most frequent group of LSDs are the lipidoses (6.2:100,000). Two LSDs, Fabry disease and Hunter syndrome, are X-linked recessive. All the rest are autosomal recessive.
| Disorder | Ethnic group or region |
|---|---|
| GM 2 gangliosidosis (Tay-Sachs disease) | Ashkenazi Jews |
| Gaucher disease | Ashkenazi Jews Sweden * |
| Aspartylglucosaminuria Infantile sialic acid storage and Salla disease Infantile NCL |
Finland |
| Infantile and juvenile NCL | Northern Europe and Scandinavia |
| Neimann-Pick disease | Nova Scotia |
Most LSDs cause one of four basic clinical-pathologic phenotypes: neuronal lipidosis, leukodystrophy, storage histiocytosis, and mucopolysaccharidosis or Hurler phenotype. These phenotypes overlap in several LSDs. Some LSDs (Fabry disease, Niemann-Pick type C) exhibit protean manifestations involving many organ systems. Other LSDs (Farber lipogranulomatosis, Wolman disease, Pompe disease) cause distinct clinical and pathologic changes.
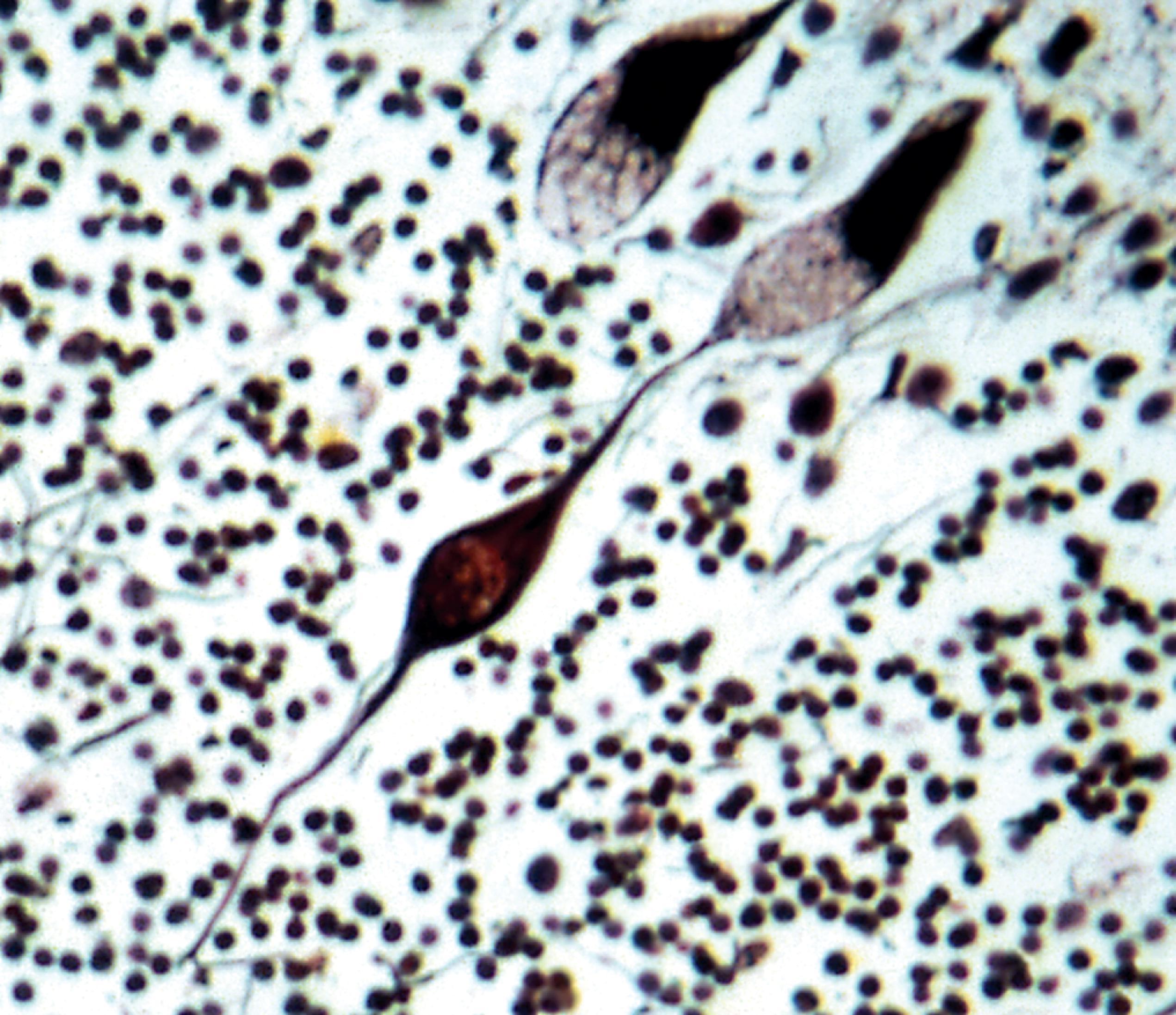
The neuronal lipidosis phenotype is caused by lysosomal storage in the neuronal perikaryon. This phenotype is characterized by slowing of psychomotor development and the eventual loss of acquired motor and perceptual skills, dementia, myoclonus, epilepsy, and visual loss, progressing to a vegetative state. The cherry red spot that is seen in some LSDs with this phenotype is caused by lysosomal storage in the perifoveal ganglion cell layer. While being the principal manifestation of GM 1 and GM 2 gangliosidosis and Niemann-Pick type A and B, neuronal lipidosis occurs also in Niemann-Pick type C and D, the NCLs, the MPS (in which neurons store gangliosides), and most glycoproteinoses.
The leukodystrophy phenotype, seen mainly in metachromatic leukodystrophy and Krabbe disease, is caused by loss of oligodendrocytes and myelin and is characterized clinically by psychomotor retardation, spasticity, ataxia, visual loss, and demyelinative peripheral neuropathy.
The storage histiocytosis phenotype is caused by lysosomal storage in monocyte-macrophage cells and is characterized by hepatosplenomegaly and, occasionally, also by hematopoietic and skeletal abnormalities. This phenotype is seen primarily in Gaucher disease and Niemann-Pick disease.
The MPS phenotype, seen in the MPS, glycoproteinoses, and GM 1 gangliosidosis, is due to visceral, soft tissue, and skeletal changes resulting from lysosomal storage of water-soluble glycoconjugates and consists of coarse facial features, organomegaly, and dysostosis multiplex. The most exaggerated form of these changes is the Hurler phenotype (see Mucopolysaccharidoses further on). Many MPS have secondary neuronal lipidosis, which accounts for the central nervous system (CNS) manifestations.
Combined prevalence 11.1–14:100,000
Two LSDs, Fabry disease and Hunter syndrome, are X linked. All others are autosomal recessive.
Certain LSDs such as Gaucher disease and Tay-Sachs disease (TSD) are frequent among Ashkenazi Jews and other ethnic groups.
Each LSD shows allelic diversity that accounts for its phenotypic variability.
Four core clinical-pathologic phenotypes are recognized: neuronal lipidosis, leukodystrophy, storage histiocytosis, and mucopolysaccharidosis.
Uncleaved substrates accumulate intracellularly in membrane-bound (lysosomal) compartments.
Lamellar products are seen in most sphingolipidoses and reticulogranular material is observed in the MPS.
Accumulation of substrate in the extracellular matrix occurs in the MPS and other LSDs.
Cell damage is caused by the mechanical effects of storage; Impaired recycling and toxicity of the stored materials also play a role.
The phenotype of LSDs depends on which cells and tissues have to function with a defective or absent enzyme.
The phenotype of LSDs depends on which cells and tissues use the defective enzyme. The severity depends on residual enzyme activity, which is determined by the type of mutation.
Some mutations abolish all enzymatic activity, resulting in severe phenotypes. Other mutations leave some residual activity and, therefore, cause milder disease.
The multicatalytic function of some lysosomal enzymes causes overlapping phenotypes.
Enzyme assay of leukocytes and other cells is the standard method.
Assay of amniocytes and cells obtained by chorionic villus sampling (CVS) is used for prenatal diagnosis.
Urine chromatography can be used as a screening test in some LSDs. Molecular analysis can be used to confirm the enzyme assay.
Morphology can contribute to the diagnosis.
Enzyme replacement therapy and substrate replacement therapy are the most commonly used treatment modalities.
The classic clinical profile of LSDs, that is, patients who are normal at birth and develop symptoms at a later time, has some important exceptions. Pathologic changes in the LSDs begin to develop in embryonic and fetal life. Depending on the severity of enzyme deficiency, pathology may be sufficiently advanced to be symptomatic at birth or prenatally ( Box 8.2 ). The clinical manifestations of these LSDs in the newborn period are neurological (hypotonia, seizures, opisthotonus, hydrocephalus), cardiovascular (cardiomegaly, cardiomyopathy, arrhythmias), skeletal, ocular (cataracts, corneal opacity, cherry red spots), among others. Frequent neonatal LSD manifestations are nonimmune hydrops fetalis (NIHF), fetal ascites, and placental hydrops. The incidence of LSDs in NIHF cases specifically worked up for LSDs is 5.2 % and higher in idiopathic cases. The LSDs most frequently associated with NIHF are MPS VII, Gaucher disease, and GM 1 -gangliosidosis, but NIHF occurs also in I-cell disease, sialidosis type II, galactosialidosis, infantile sialic acid storage disease, Salla disease, MPS IV, Niemann-Pick types A and C, Wolman disease, Farber disease, and other LSDs. The placenta in such cases shows diagnostic microscopic and ultrastructural changes. The mechanism of NIHF in these cases is unclear. Several (but not all) of these LSDs involve storage of highly hydrophilic compounds. Increased nuchal translucency, a common finding in aneuploidy and structural fetal abnormalities, has also been reported in LSDs, Zellweger syndrome, and other inherited metabolic disorders.
Sphingolipidoses
Fabry disease
Farber disease
GM 1 gangliosidosis
Gaucher disease type 2
Krabbe disease
Niemann-Pick disease types A and B
Mucopolysaccharidoses
MSP I
MSP IVA
MSP VII
Multiple sulfatase deficiency
Glycoproteinoses
Sialidosis types I and II
Mucolipidosis type 1
Schindler disease
Transport disorders
Infantile sialic storage disease
Salla disease
Niemann-Pick disease type C
Galactosialidosis
cell disease
Mucolipidosis 2
Pompe disease
Wolman disease
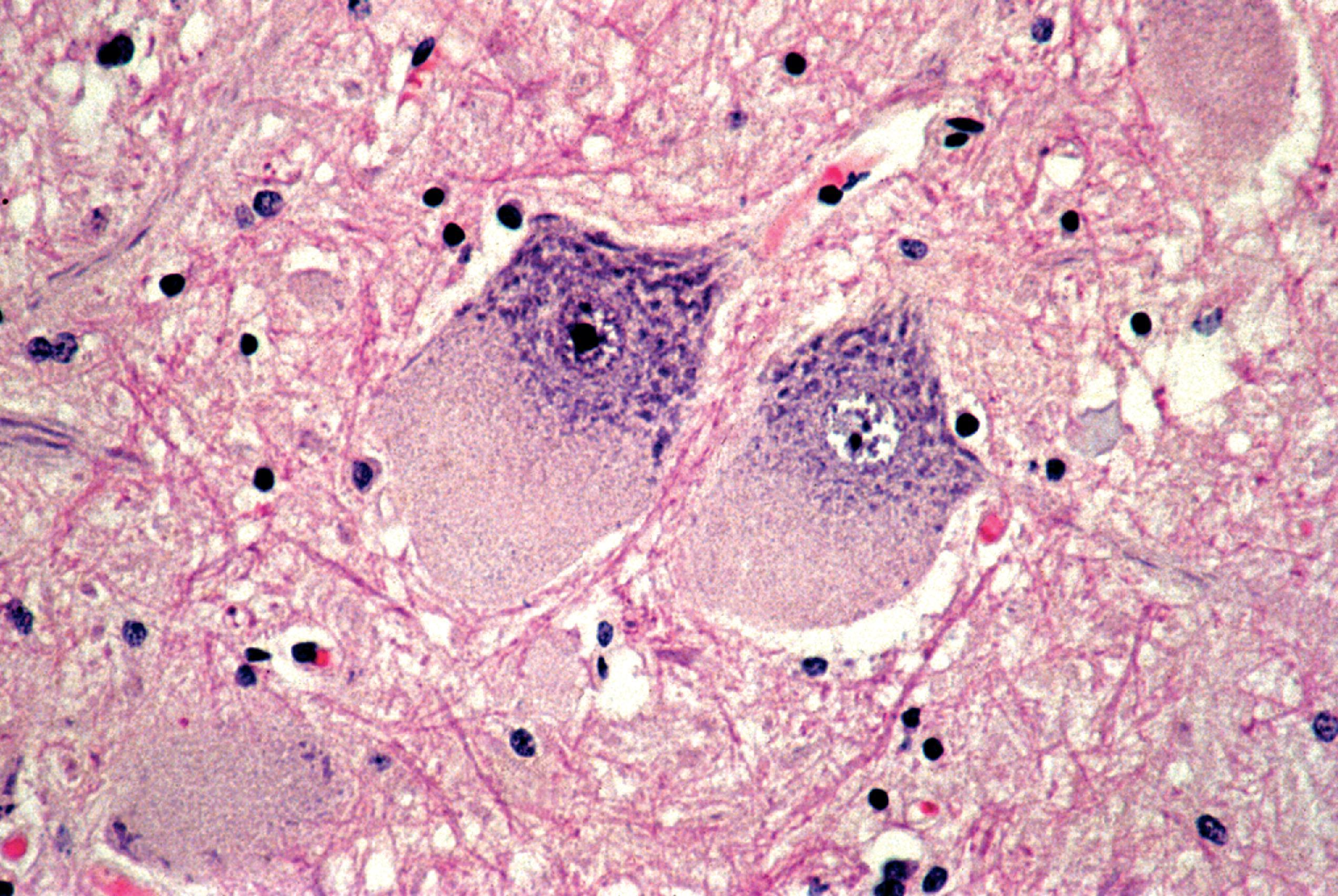
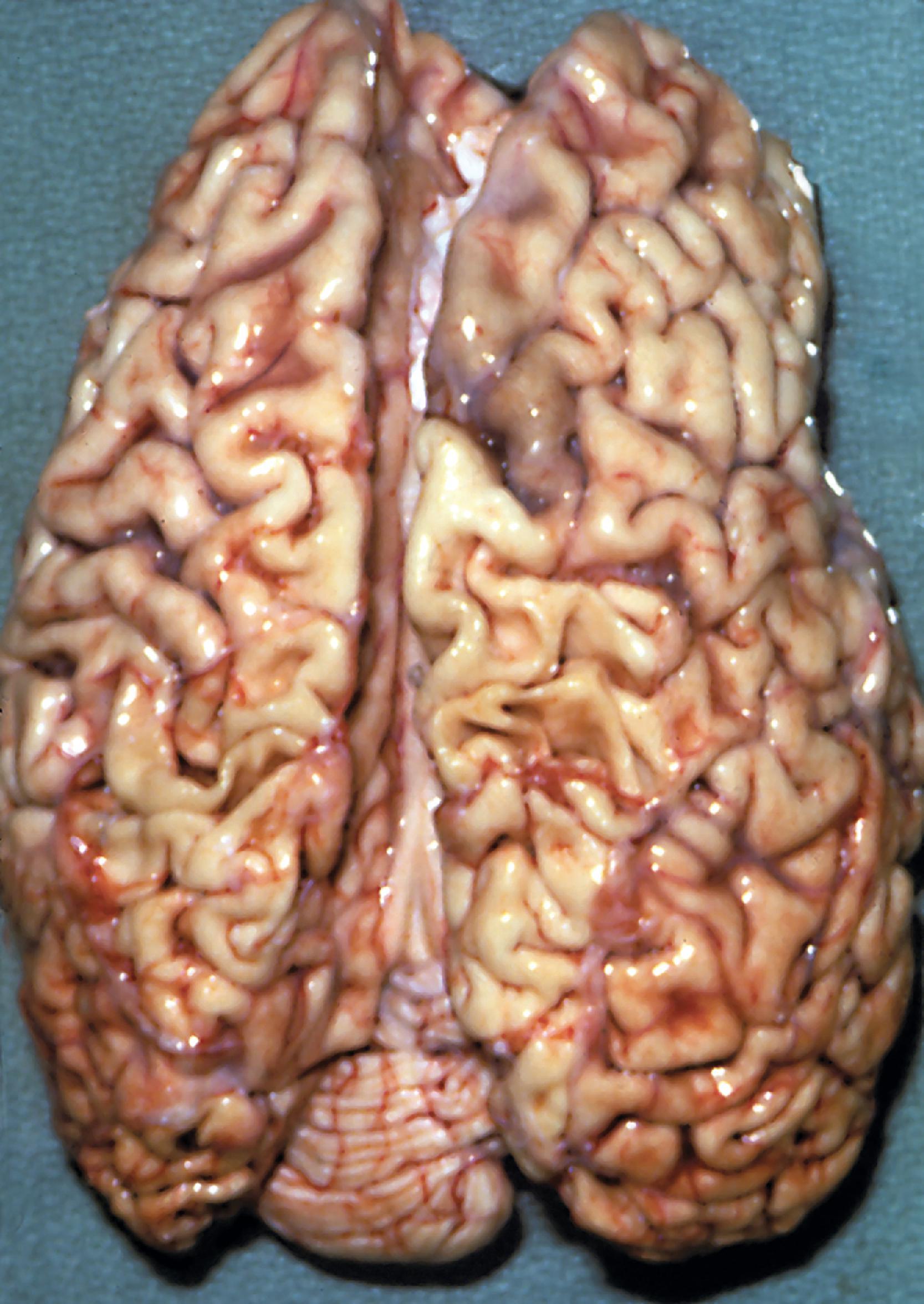
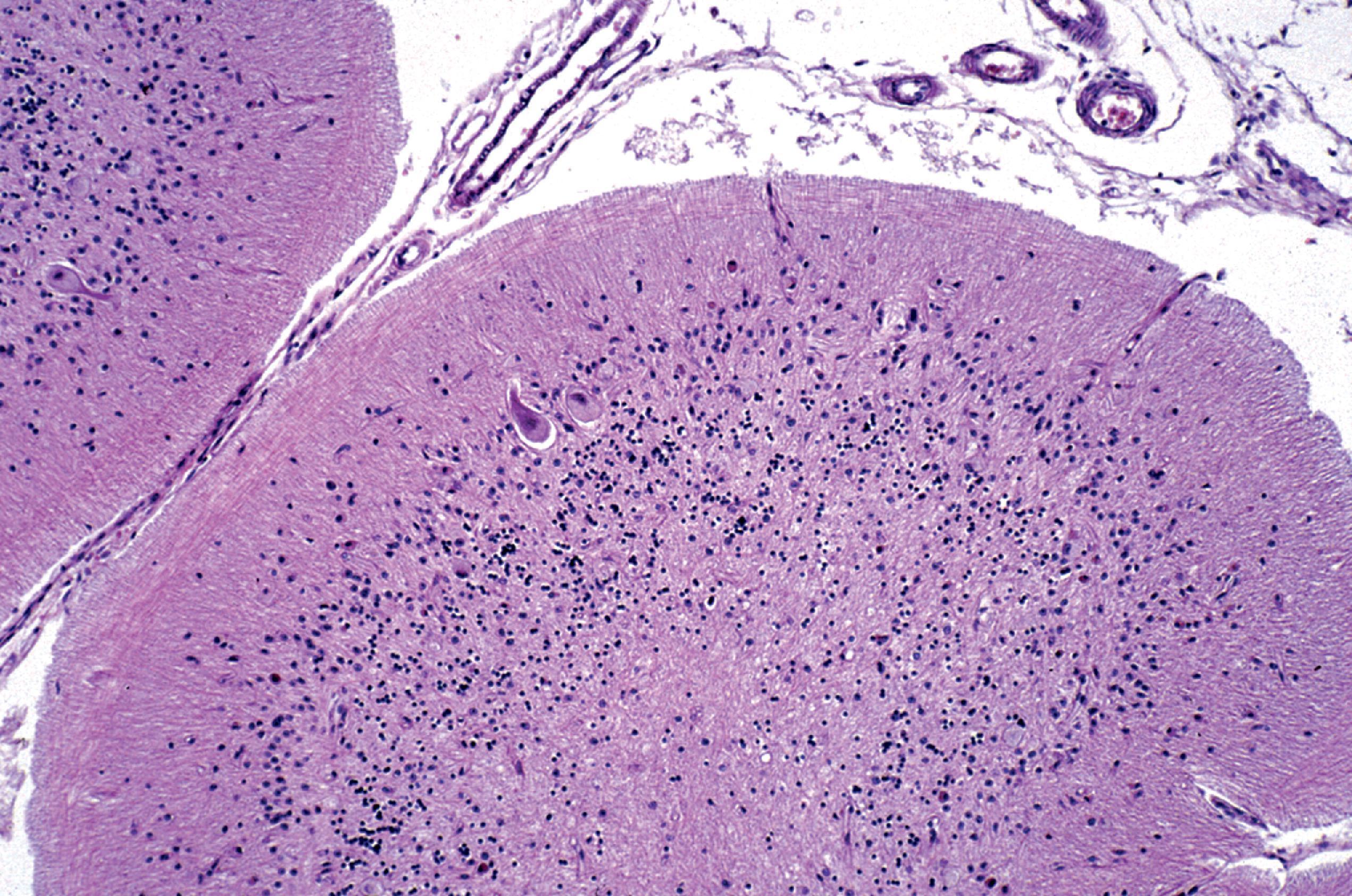
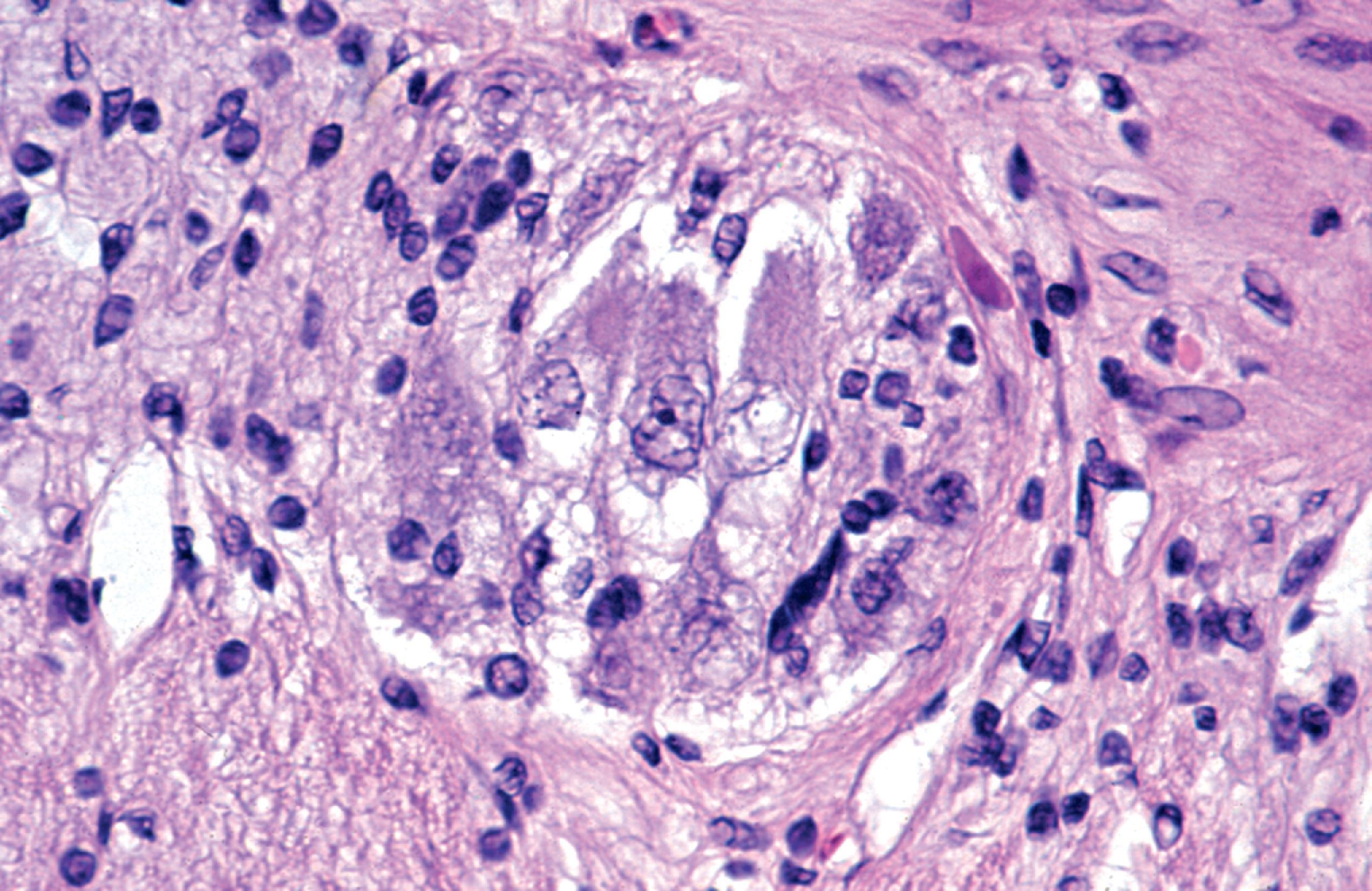
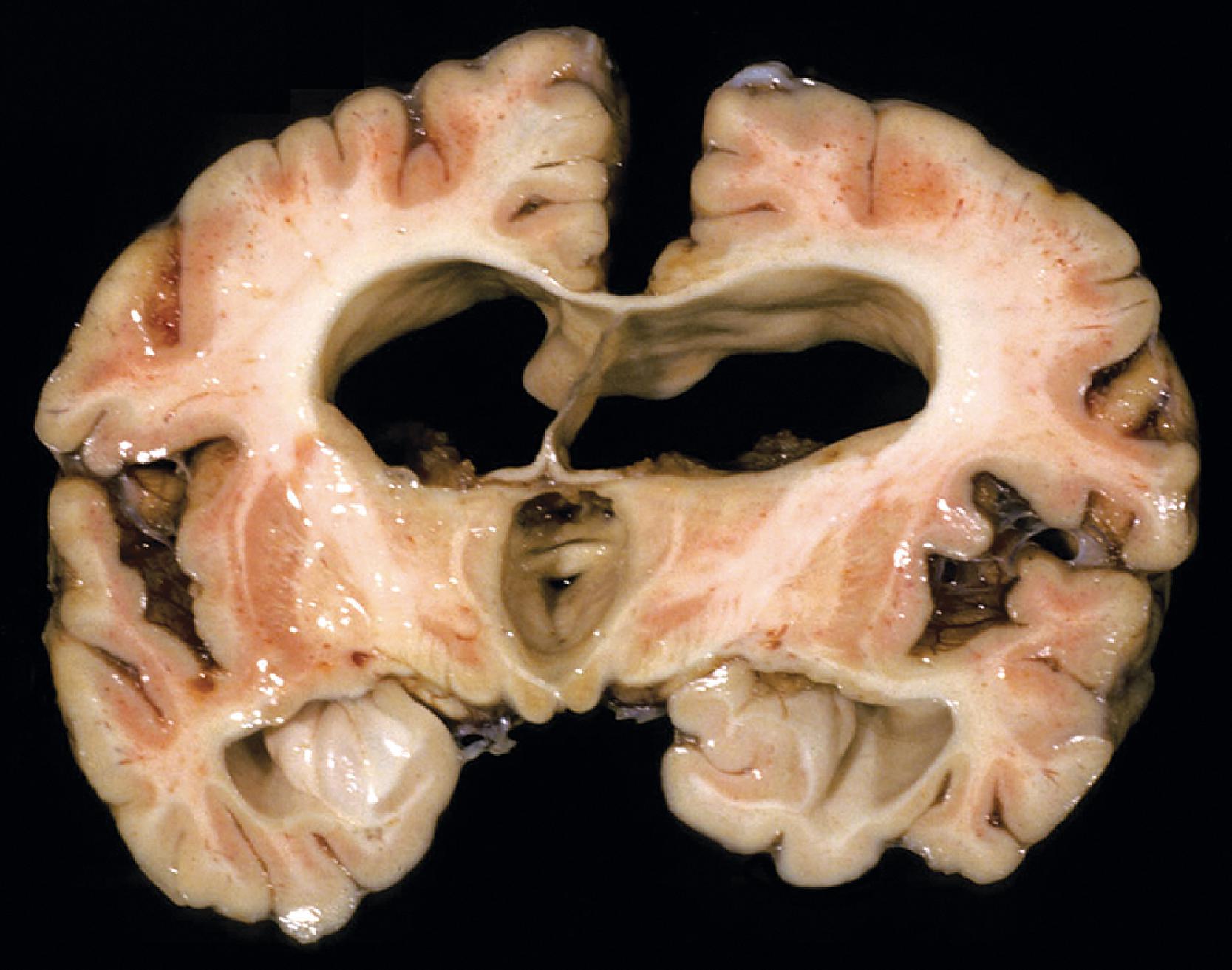
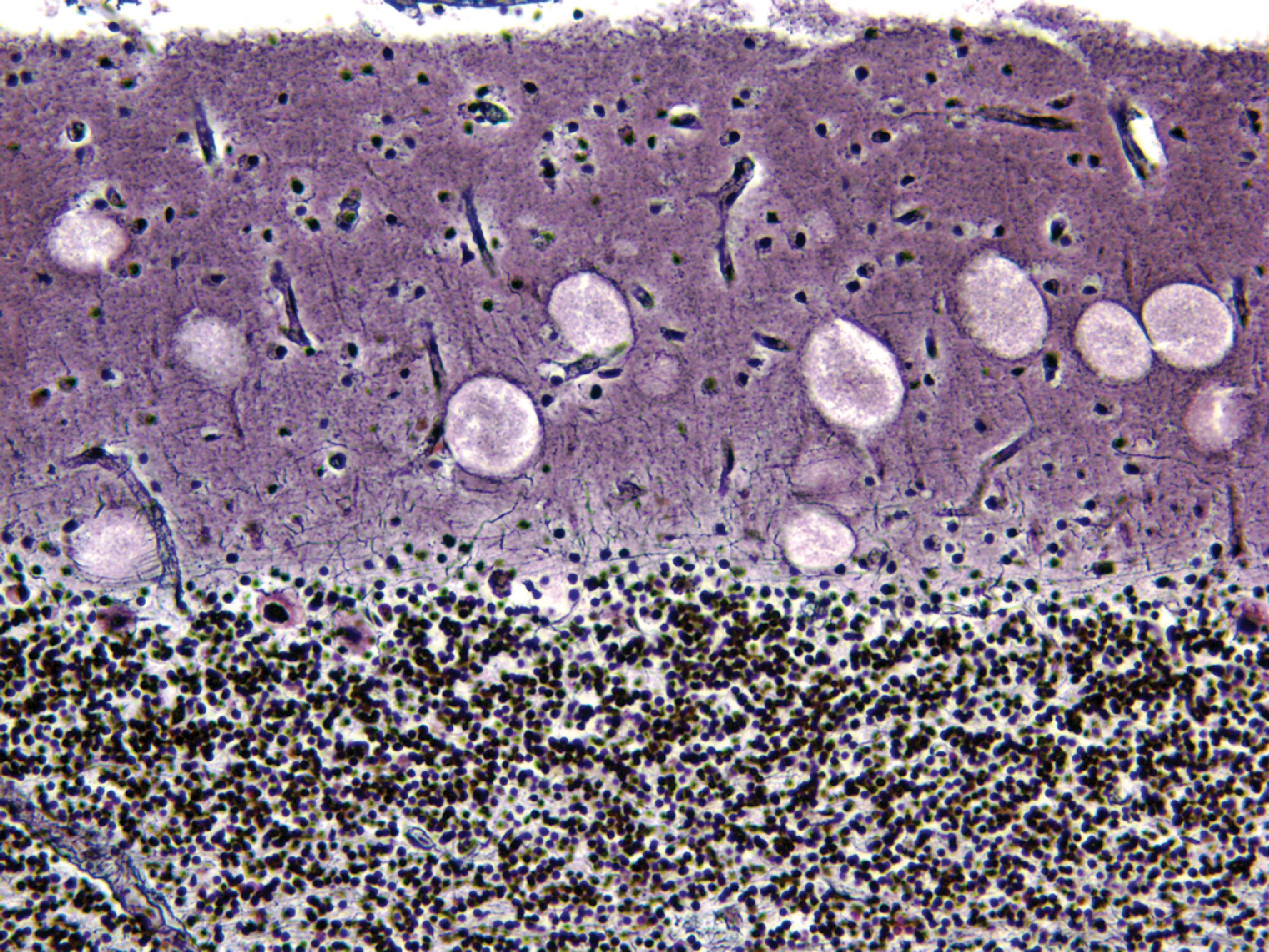
Lysosomal storage distends the cytoplasm. This distension is most impressive when it affects large pyramidal cells ( Fig. 8.3 ). Water-soluble materials are washed out during processing, leaving a clear or vacuolated cytoplasm ( Fig. 8.4 ). Glycolipids have a finely granular or foamy appearance and are sudanophilic and periodic acid-Schiff (PAS)-positive. In the sphingolipidoses and other LSDs with neuronal storage, lysosomes accumulate also in axon hillocks and dendrites causing spherical or torpedo-like swellings ( Fig. 8.5 ). Increased cytoplasmic mass causes organomegaly. Thus, megalencephaly occurs early in the gangliosidoses. However, the end result of the storage is cell death followed by cerebral atrophy and gliosis ( Figs. 8.6 and 8.7 ). These changes are the pathologic substrate of the neuronal lipidosis phenotype. Neuronal lipidosis involves autonomic neurons and retinal ganglion cells. Rectal biopsy with detection of storage in enteric ganglia ( Fig. 8.8 ) was frequently used for the diagnosis of sphingolipidoses before biochemical methods were available. Ganglioside storage in the cellular perifoveal ganglion cell layer creates an opaque, yellowish halo that accentuates the red color of the fovea. The fovea appears as a cherry red spot on fundoscopic examination.
In metachromatic leukodystrophy and Krabbe disease, lipid accumulation involves oligodendroglial cells and Schwann cells. When oligodendroglial cells die, the lipid is picked up by macrophages that accumulate in the white matter. Dysfunction and loss of myelin-producing cells causes loss of myelin. These findings are the substrate of the leukodystrophy phenotype. Storage of water-soluble materials in mesenchymal and epithelial cells and in the extracellular matrix correlates with the MPS phenotype, and accumulation in monocyte-macrophage cells is the substrate of the storage histiocytosis phenotype.
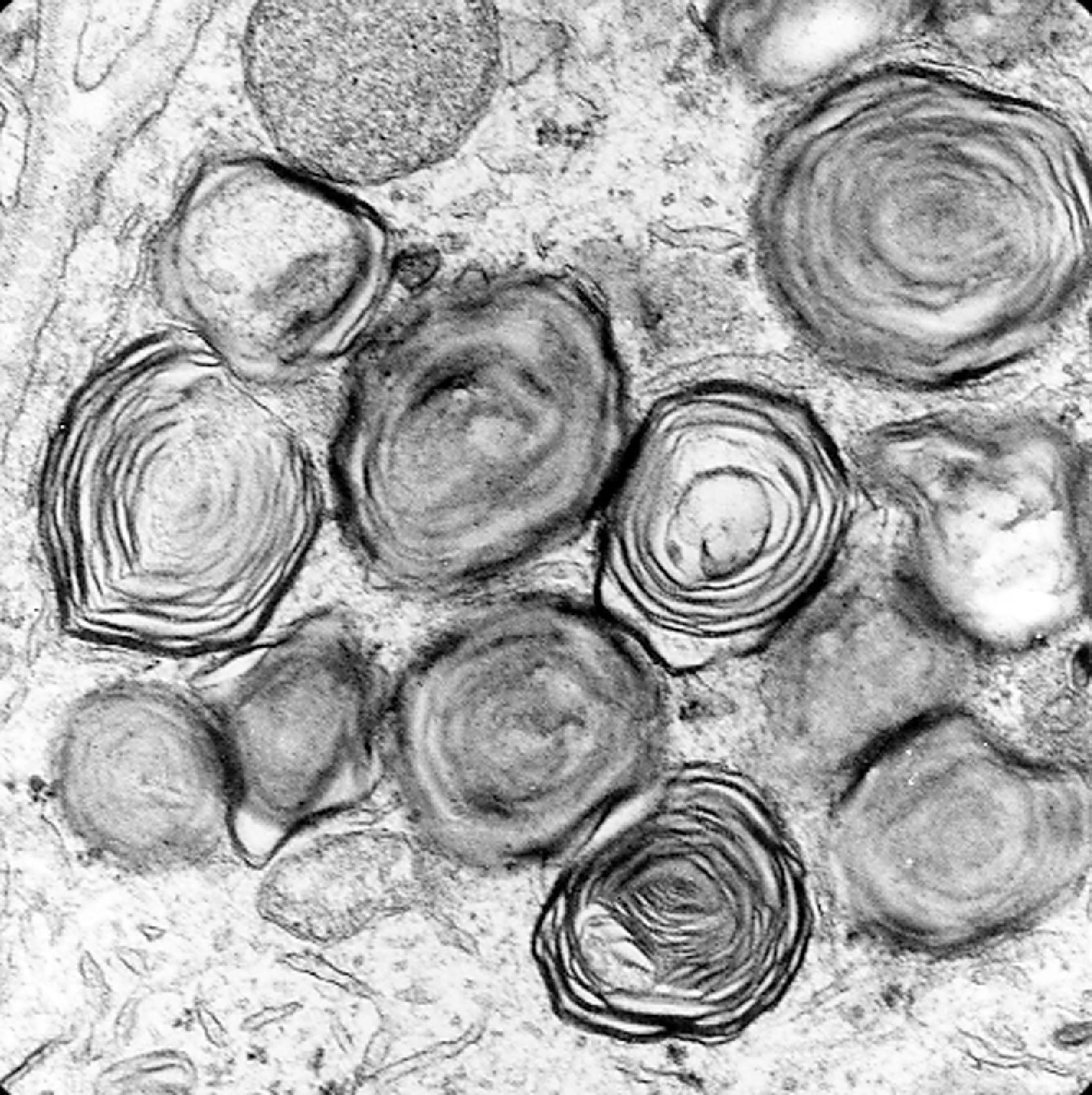
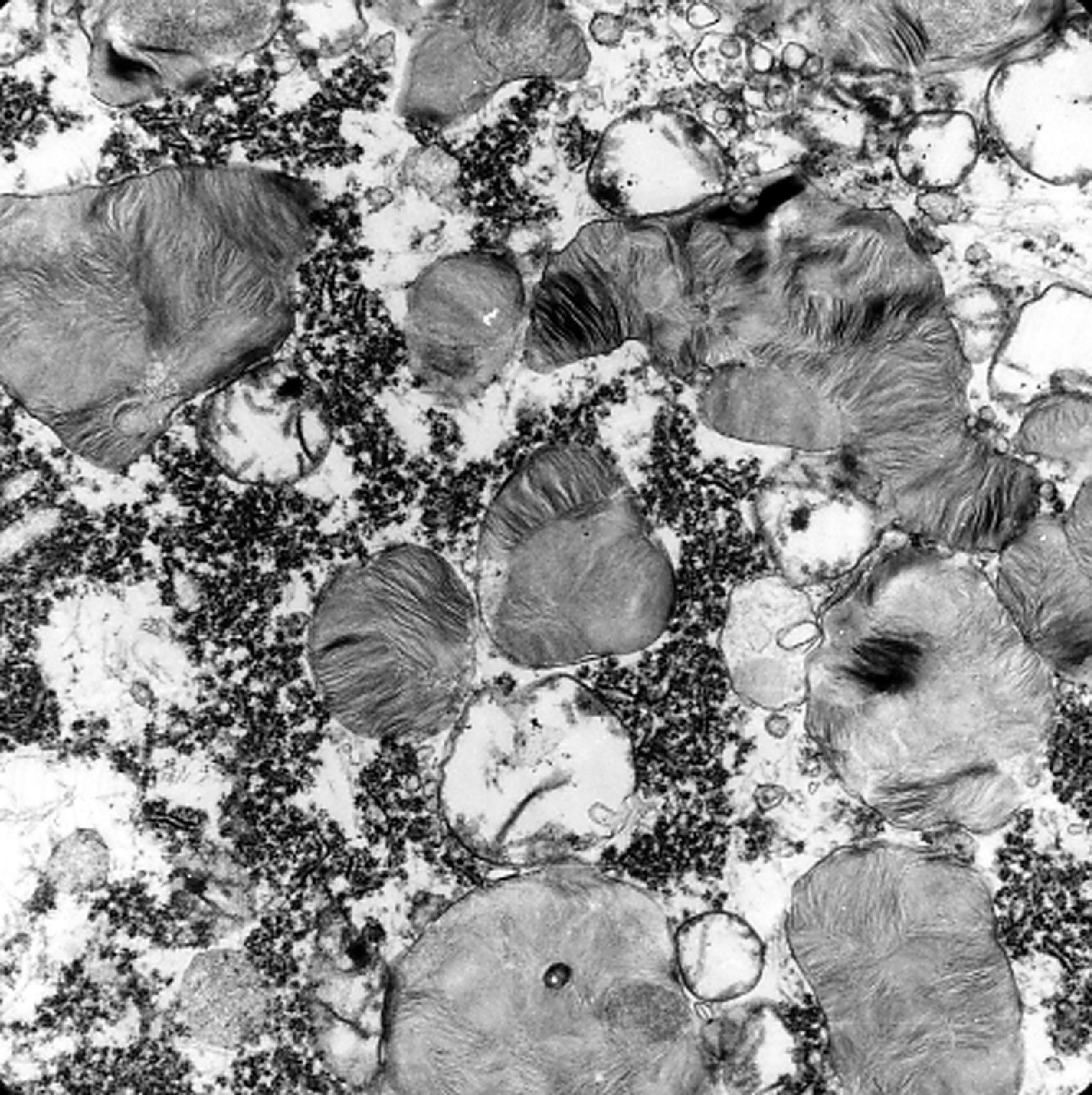
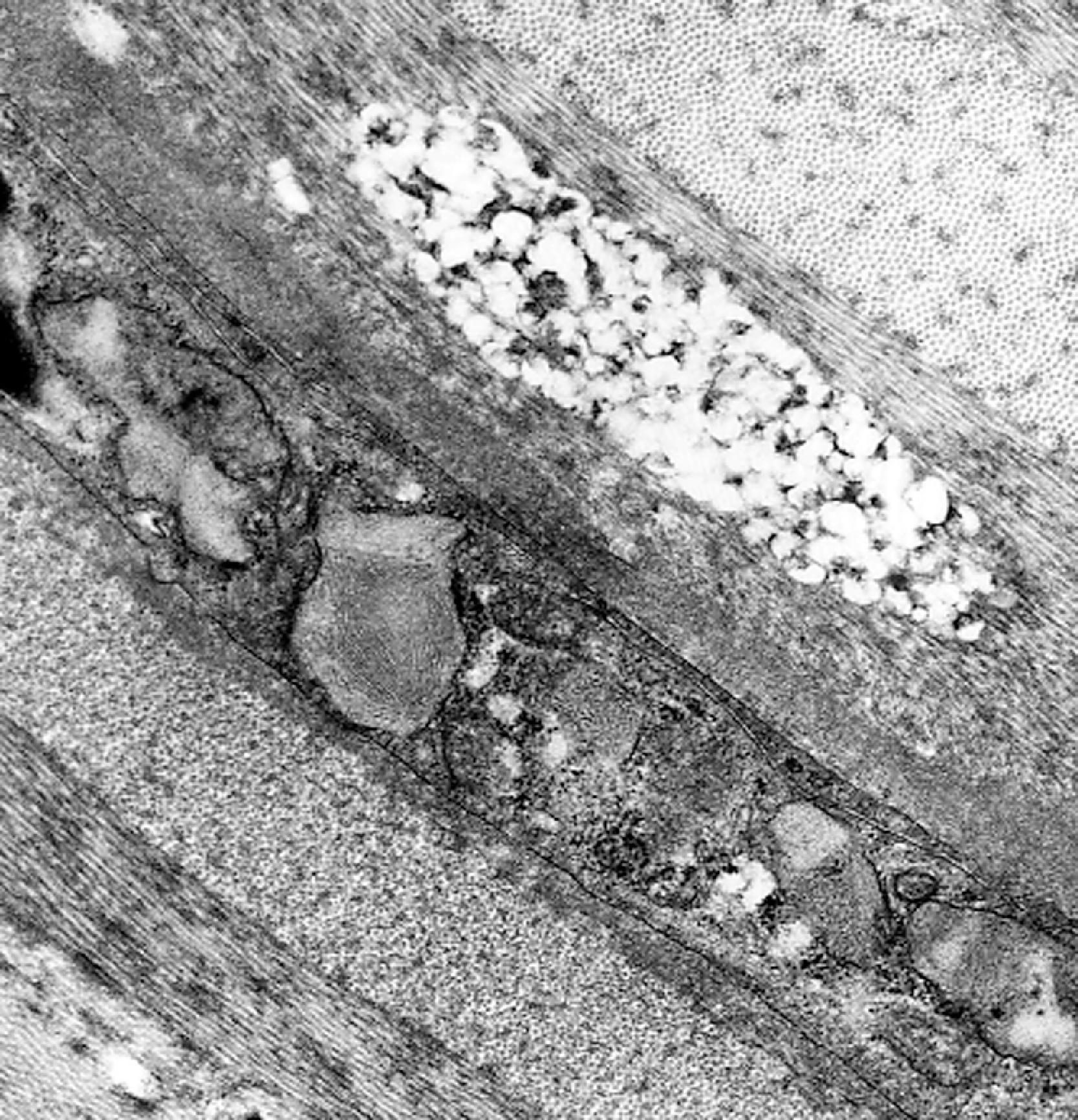
The storage material accumulates in membrane-bound (i.e., intralysosomal) particles ( Fig. 8.9 ) that have acid phosphatase activity ( Fig. 8.10 ). Acid phosphatase has been used as a lysosomal marker since the time lysosomes were discovered by De Duve. The fine structure of the stored material gives a general idea of its chemical composition but is not specific. In general, sphingolipids assume membranous forms that are arranged concentrically or in parallel stacks (membranous cytoplasmic bodies [MCBs], zebra bodies) ( Figs. 8.11 and 8.12 ). Concentric lamellae are more common in the gangliosidoses and zebra bodies in the MPS (in which neurons store gangliosides), but none of these structures are specific for any given type of sphingolipidosis, and they are usually found in various combinations in all of them. Mucopolysaccharides (glycosaminoglycans [GAGs]) are highly soluble in water and are lost in processing, leaving behind clear cells by light microscopy and sparse reticulogranular structures by electron microscopy (see Figs. 8.1 and 8.9 ). Other LSDs with storage of water-soluble compounds have a similar appearance.
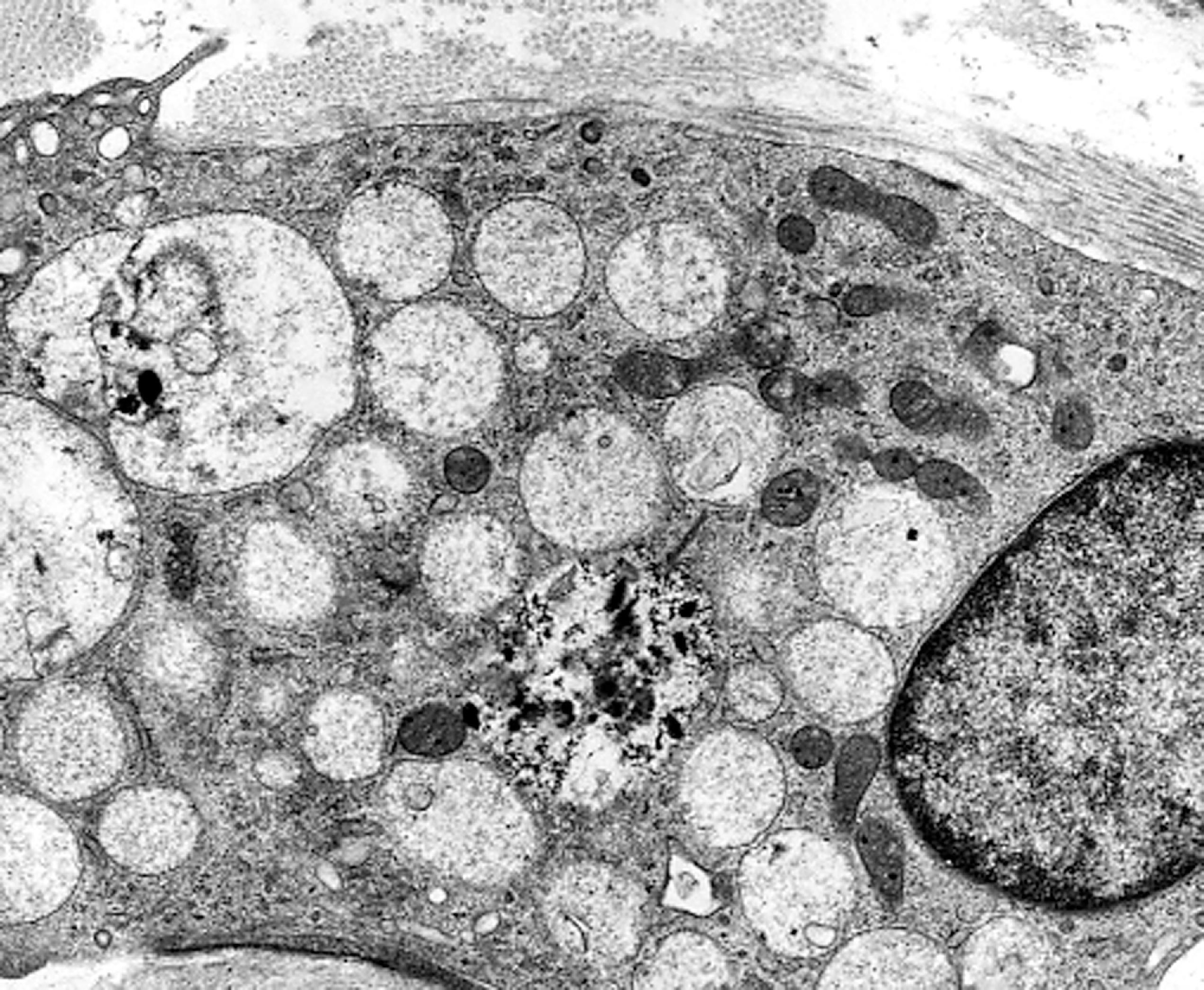
In some instances, such as Gaucher disease and globoid cell leukodystrophy, morphology is diagnostic. For the most part, however, light and electron microscopy are nonspecific. Thus, at the cellular level, all ganglioside-storing LSDs look the same and the MPS are indistinguishable from one another and from the glycoproteinoses.
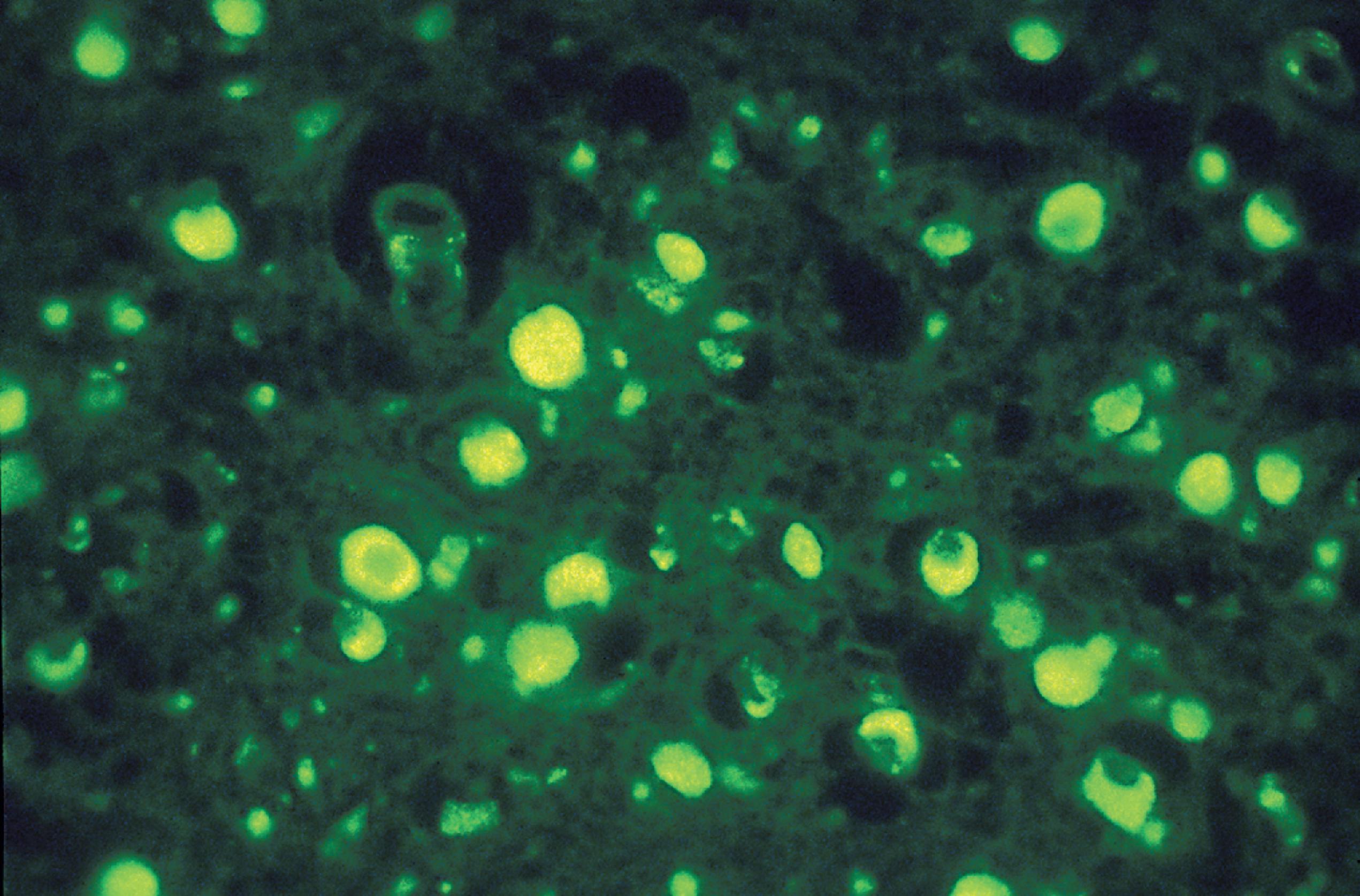
Special techniques supplement light and electron microscopy. Loss of GAGs can be prevented to some extent by adding the cationic dye toluidine blue to the fixatives and buffers used for electron microscopy. Metachromatic stains are diagnostic in MLD and helpful in the MPS. Cystine crystals can be identified under polarized light ( Fig. 8.2 ), and ceroid-lipofuscin is autofluorescent ( Fig. 8.13 ). Because in most LSDs the stored material is a glycoconjugate, lectin histochemistry and immuno-electron microscopy can be used to characterize the storage products.
Cell injury in LSDs is primarily due to the mechanical effects of storage (i.e., displacement of organelles, disruption of cytoplasmic circulation, and, in the case of neurons, impaction of axons and dendrites). This process leads to cellular dysfunction and loss, which is most critical for neurons and oligodendrocytes. Unavailability of ganglioside precursors and other biomaterials due to their sequestration in lysosomes may interfere indirectly with synthetic processes. Impaired recycling and imbalance of membrane lipids in LSDs with ganglioside accumulation cause aberrant dendritic sprouting and abnormal recycling of neurotransmitter receptors (which are embedded in the neuronal membrane). These changes account in part for the seizures, which are common in the neuronal lipidoses, and also adversely affect neurotransmission and other signal transduction activity. Abnormal chemical composition of myelin lipids may play a role in myelin breakdown in the leukodystrophies, but loss of myelin-producing cells is more important.
Several LSDs cause accumulation of materials other than the primary substrate of the mutant enzyme. The prime example of this phenomenon is the MPS in which gangliosides (zebra bodies) accumulate in neurons, but similar changes are seen in several other LSDs. The mechanism of this secondary storage is unclear. One plausible explanation may be that, as lysosomes swell, their proton pumps cannot maintain their acid environment. Lysosomal pH rises, leading to generalized lysosomal exhaustion. The most common secondary storage products are gangliosides, phospholipids, and cholesterol. Secondary storage, especially of gangliosides, is clinically important, and accounts for the diverse fine structure of lysosomal products in some LSDs.
In most LSDs, the stored material is chemically inert. In some gangliosidoses, toxic by-products of the stored gangliosides are also produced and cause additional cell damage. One such example is globoid cell leukodystrophy in which psychosine, a metabolite of galactocerebroside, is toxic to oligodendrocytes. The primary pathology of LSDs is intracellular; however, in many of them, significant changes also develop in the extracellular matrix. Some of these changes represent scarring due to loss of parenchymal elements. In MPS and other LSDs with an MPS phenotype, extracellular accumulation of GAGs and other glycoconjugates causes severe soft tissue changes, which are central to their phenotype.
The type and distribution of substrate in various tissues determine what cells and organ systems are affected in the LSDs. Lysosomal activity also varies between tissues and at different stages of development depending on substrate turnover. For instance, acid maltase deficiency affects most severely tissues with high glycogen turnover, such as liver, heart, and skeletal muscle; hexosaminidase A deficiency primarily affects neurons that have the highest GM 2 content and turnover, especially during the phase of dendritic sprouting; and MPS affect connective tissues and the skeleton, which contain a large number of GAGs. Although LSDs affect all cells and organs, two classes of cells are most vulnerable: (1) neurons because of their large size, high ganglioside content of their membranes, and their inability to regenerate; and (2) phagocytic cells, which are easily overloaded because of their high burden of substrate turnover.
As lysosomes swell in LSDs, their proton pumps cannot maintain the acid environment. Lysosomal pH rises, leading to generalized loss of enzyme activity. This lysosomal exhaustion may also explain the accumulation of diverse products in LSDs (see the section on Mucopolysaccharidoses later in this chapter).
The severity of each LSD depends primarily on residual enzyme activity, which correlates with the genotype. Several mutations of each lysosomal enzyme gene occur. Some of these mutations cause severe enzyme deficiency, while others allow some enzyme to be produced. Because of this allelic diversity and because many patients are compound heterozygotes, no two cases of any given LSD are exactly alike. Normally, cells produce about 10 times the amount of lysosomal enzymes that are needed to carry out cellular function. LSD heterozygotes, with about half normal enzyme activity, are phenotypically normal. In every tissue, there is a threshold of lysosomal enzyme activity, usually 10 % to 20 % of normal, that is required to maintain normal function. Storage develops when enzyme activity falls below this critical level. Some LSDs such as Gaucher disease, show significant clinical variability within the same genotype suggesting that additional genetic and environmental factors contribute to the phenotype.
The molecular defect of LSDs is present from the time of conception. The biochemical defect is expressed in amniocytes, trophoblasts, and fetal tissues, allowing for a prenatal diagnosis in the first trimester by enzyme testing. Typical pathologic changes also develop before the second trimester. Fetal and neonatal tissues have the highest regenerative ability, but by the same token, the period of growth and development challenges the capacity of the lysosomal system with the highest substrate turnover. This is especially true in the developing brain in which large numbers of primitive neurons are eliminated by programmed death. As a consequence, severe enzyme mutations are clinically apparent prenatally or in early childhood. Mutations allowing for higher, (but below threshold) residual activity cause symptoms later in life. For instance, in TSD, severe hexosaminidase deficiency causes diffuse neuronal storage in the first few months of life and is fatal within 1 or 2 years. Mutations resulting in somewhat higher hexosaminidase activity have a later onset, milder symptoms, and a more chronic course, and affect neuronal groups selectively.
Lysosomal enzymes are not substrate-specific. They are specific for certain residues and linkages and break these linkages in whatever molecules they occur. Thus, hexosaminidases cleave β-hexosamine from glycolipids and glycoproteins; enzymes involved in glycoprotein degradation also degrade glycosphingolipids, and GAG-cleaving enzymes participate in the degradation of several classes of GAGs. Deficiency of a single multicatalytic enzyme causes storage of diverse compounds, creating overlapping phenotypes. For example, β-galactosidase deficiency causes GM 1 gangliosidosis and keratan sulfate storage (MPS IV B-Morquio syndrome type B). Conversely, several enzymes participate in the degradation of the same molecule. Deficiency of any of these enzymes may cause storage of similar compounds, resulting in a shared phenotype. Thus, Sanfilippo syndrome phenotype can be caused by four different gene mutations. The Morquio syndrome phenotype can result from either β-galactosidase deficiency or galactosamine-6-sulfatase deficiency.
The gold standard in the laboratory diagnosis of LSDs is measurement of enzyme activity by tandem mass spectrometry or fluorometry. Because the enzyme deficiency is expressed in all tissues, albeit not equally, a diagnosis can be made by testing samples obtained by relatively noninvasive methods, such as serum, plasma, white cells, amniocytes, CVS samples, and cultured skin fibroblasts. Enzyme assay can also be used to detect carriers. Some LSDs can be diagnosed by assaying a leukocyte pellet. In most instances where multiple enzymes need to be evaluated, cultured fibroblasts or amniocytes must be used.
Urine chromatography is a useful screening test for many LSDs. The pattern of glycoconjugates secreted in the urine can identify several MPS and glycoproteinoses, but is not specific because storage of similar compounds may result from different enzyme defects. Because of the great genotypic diversity that characterizes all LSDs, molecular analysis is impractical for primary diagnosis. However, when the mutation is known, molecular analysis can be used for carrier detection and prenatal diagnosis.
Molecular genetic analysis using next-generation sequencing (NGS) and other methods is used to confirm the results of enzyme assays, determine which mutations are involved, and for prenatal diagnosis.
Morphology, combining light microscopy, electron microscopy, and ancillary studies, is an important tool in the diagnosis and investigation of LSDs. Anatomical studies reliably reveal the presence of a storage process and can narrow it down to a presumptive diagnosis, which is important for guiding biochemical, molecular, and genetic studies. Because small amounts of substrates are asymptomatically stored in many cell types, characteristic pathologic changes can be detected by minimally invasive sampling of a variety of cells and tissues such as amniotic fluid, skin, conjunctiva, bone marrow, and circulating leukocytes ( Fig. 8.14 ). In several LSDs, although enzymatic diagnosis is available, morphology can also provide a specific diagnosis ( Table 8.2 ).
| Disorder | Diagnostic finding |
|---|---|
| Gaucher disease | Gaucher cells, characteristic fine structure |
| Globoid cell leukodystrophy | Globoid cells, characteristic fine structure |
| Metachromatic leukodystrophy | Metachromasia, characteristic fine structure |
| Farber lipogranulomatosis | Lipid granulomas, characteristic fine structure |
| Fabry disease | Vascular, renal pathology |
| Pompe disease | Lysosomal glycogen, storage myopathy |
| Wolman disease | Adrenal calcification, lipid droplets, cholesterol crystals |
| Neuronal ceroid lipofuscinoses | Characteristic ultrastructure |
In a few LSDs, morphology is the only diagnostic modality. Morphology is the only means of detecting drug-induced LSDs. It helps in understanding the pathogenesis of cell and tissue injury and is indispensable in evaluating the effects of treatment. The pathology of many LSDs is incompletely described and systematic anatomical study can still make significant contributions to our understanding.
Hematopoietic stem cell transplantation (HSCT), still used for MPS I (Hurler syndrome), was the earliest attempt to treat LSDs. The most common approach to therapy of LSDs presently is enzyme replacement therapy (ERT) using intravenous or intrathecal infusions of recombinant enzymes. ERT is very expensive and does not reverse the neuropathologic changes because enzymes do not cross the blood-brain barrier. Its effectiveness is reduced because some patients develop neutralizing antibodies to the enzymes. Substrate reduction therapy (SRT) aims to reduce the amount of accumulating substrate by inhibiting its synthesis, thus balancing synthesis and catabolism and avoiding the harmful effects of storage. Chaperone therapy helps mutant enzymes regain their function by correcting their conformation. Gene therapy uses viral vectors to introduce genes into organs or infusions of the patient’s own ex vivo genetically corrected hematopoietic stem cells. ERT alone or in combination with SRT has been used to treat Gaucher disease, Fabry disease, MPS, Pompe disease, and other LSDs.
Based on the chemical structure of the stored material, LSDs can be divided into four major groups, the sphingolipidoses, MPS, glycoproteinoses, ceroid lipofuscinoses, and several other individual entities. An account of the core features of each group and brief descriptions of the most common LSDs follows.
This diverse group of LSDs is caused by deficiencies in lysosomal enzymes of sphingolipid degradation. Sphingolipids consist of a backbone of ceramide (N-acylsphingosine) with various attached side chains. They are major constituents of cell membranes, and gangliosides are especially rich in neuronal membranes. Deficiency of enzymes of sphingolipid degradation results in accumulation of undegraded ceramide compounds, especially in the brain. Sphingolipids have a hydrophobic and a hydrophilic side and tend to form bilayers in aqueous moieties. This property is replicated in the storage products of some sphingolipidoses, which form membranous cytoplasmic bodies.
Eight sphingolipidoses are recognized ( Table 8.3 ). Fabry disease is X-linked. All other sphingolipidoses are autosomal recessive. GM 1 gangliosidosis, GM 2 gangliosidosis, and Niemann-Pick disease types A and B cause neuronal storage (neuronal lipidosis). GM 2 gangliosidosis and Niemann-Pick disease types A and B cause also visceral storage (storage histiocytosis). Enlarged, lipid-filled lysosomes balloon the neural soma ( Fig. 8.3 ) and expand axons and dendrites ( Fig. 8.5 ), blocking axoplasmic flow. The axonal swellings are covered by ectopic dendritic spines. Aberrant dendritogenesis is thought to contribute to the neurological dysfunction. Two sphingolipidoses, metachromatic leukodystrophy and Krabbe disease (described elsewhere), cause primarily white matter disease (leukodystrophy) and are described elsewhere. Gaucher, Farber, and Fabry disease cause unique phenotypes and are described separately.
| Disease | Enzyme | Locus | Stored material | Phenotype | ||||
|---|---|---|---|---|---|---|---|---|
| GM 1 gangliosidosis, Morquio type C | β-Galactosidase | 3p21.33 | GM 1 ganglioside Keratan sulfate Glycoprotein |
CRS, MPS, NL | ||||
| GM 2 gangliosidoses | GM 2 gangliosides | CRS, NL | ||||||
| Tay-Sachs disease | Hex A | 15q23-24 | ||||||
| Sandhoff disease | Hex A and B | 5q13 (Hex B) | ||||||
| GM 2 activator deficiency | Hex A and B | 5q31.3-32.1 | ||||||
| Niemann-Pick disease types A and B | Sphingomyelinase | 11p15.1-p15.4 | Sphingomyelin | CRS, NL, SH | ||||
| Gaucher disease | Glucocerebrosidase Glucosylsphingosine |
1q21 | Glucosylceramide | SH | ||||
| Krabbe disease | Galactocerebrosidase Psychosine |
14q24.3-32.1 | Galactosylceramide | LD | ||||
| Metachromatic leukodystrophy | Arylsulfatase A Galactosylsphingosine |
22q13.31-tes | Galactosylsulfatide | LD, PN | ||||
| Fabry disease | α-Galactosidase | Xq22.1 | Trihexosylceramide | AK, HD, NL, PN, SA | ||||
| Farber granulomatosis | Ceramidase | 8p22-p21.3 | Ceramide | CRS, HF, LG, NL |
A group of LSDs caused by deficiencies in enzymes of sphingolipid degradation
Sphingolipids (ceramide compounds)
Gaucher disease is the most common LSD (1.16–1.75:100,000).
Gaucher disease and TSD are frequent in Ashkenazi Jews.
Fabry disease is X-linked recessive; all other sphingolipidoses are autosomal recessive.
Neuronal lipidosis, leukodystrophy, storage histiocytosis, MPS, and other individual entities
Storage of gangliosides in neurons causes initially neuronal ballooning and then neuronal loss. Involvement of oligodendroglia and Schwann cells causes myelin loss.
Storage in monocyte-macrophage cells causes hepatosplenomegaly.
GM 1 gangliosidosis presents in infancy with severe psychomotor retardation and a full-blown Hurler phenotype (see Mucopolysaccharidoses later in this chapter) including coarse facial features ( Fig. 8.15 ), dysostosis multiplex, corneal clouding, and organomegaly. A cherry red spot is seen in 50 % of patients. Patients with infantile onset usually die before 2 years of age. Later onset variants have a milder neurological picture (which includes dystonia and ataxia) and milder somatic and skeletal findings. An allelic variant called Morquio syndrome type B is phenotypically similar to Morquio A (MPS IVA), consisting primarily of skeletal changes (short trunk dwarfism) with normal intelligence and without organomegaly or corneal clouding. The neurological symptoms in Morquio type B develop not as a result of CNS storage but rather as a consequence of atlantoaxial dislocation.
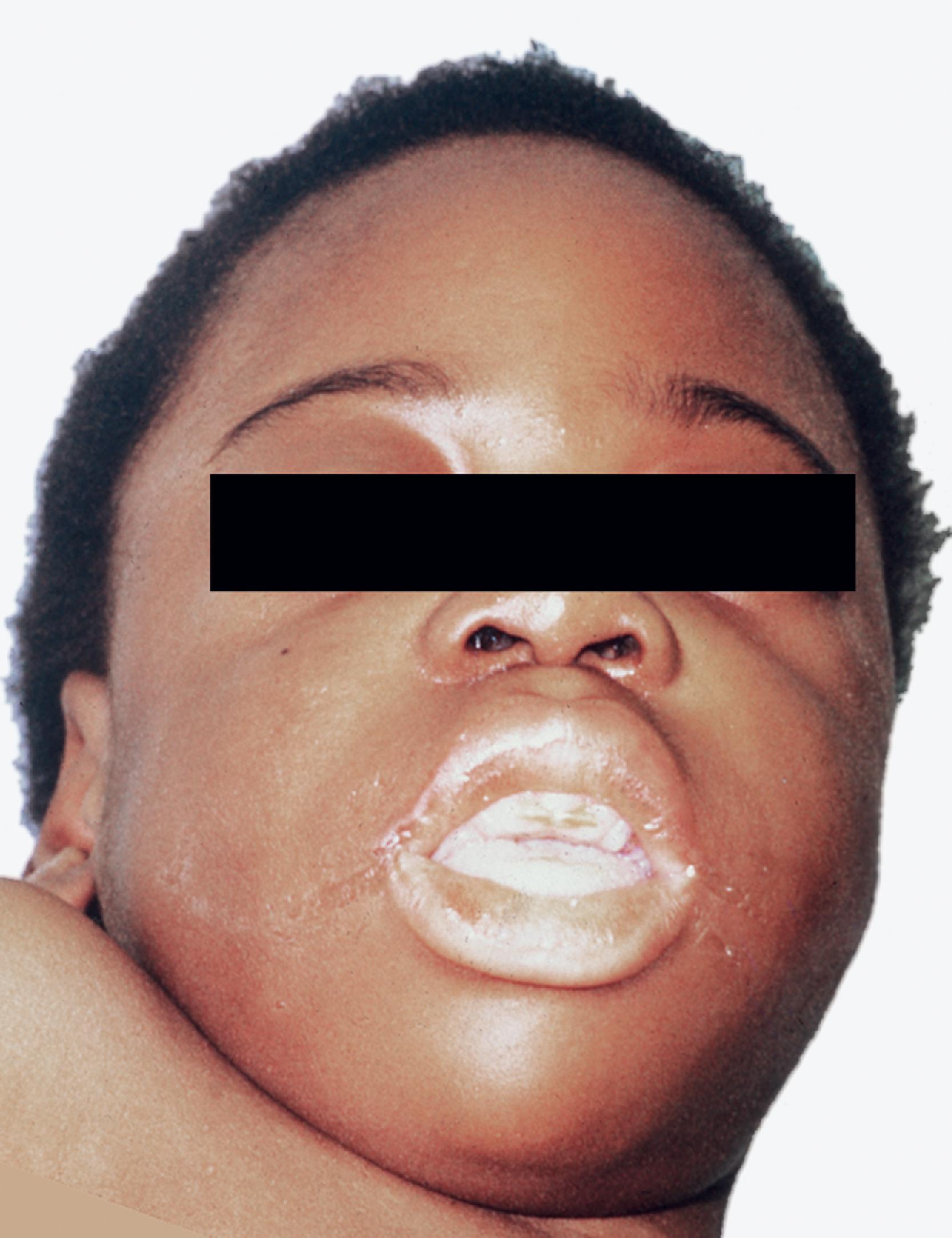
An autosomal recessive LSD caused by deficiency of β-galactosidase and characterized by neuronal lipidosis and mucopolysaccharidosis-like somatic changes
GM 1 ganglioside, keratan sulfate, glycoproteins
Neuronal lipidosis, MPS
Megalencephaly initially followed by cerebral and cerebellar atrophy
Neuronal ballooning, meganeurites; Clear epithelial and mesenchymal cells similar to the MPS
Membranous cytoplasmic bodies in neurons
Sparse reticulogranular material in epithelial and mesenchymal cells
The neuropathologic changes in infantile and late infantile GM 1 gangliosidosis consist of lipid storage in the neuronal soma (neuronal ballooning) (see Fig. 8.3 ) and proximal axon. Storage also occurs in sensory and autonomic ganglionic neurons (see Fig. 8.8 ). On electron microscopic examination, the storage material consists of MCBs (see Fig. 8.11 ). In early phases of the disease, the brain is large. GM 1 ganglioside, normally 20 % of brain gangliosides, increases to 80 %–90 %. As the disease advances, neuronal loss and gliosis occur and cerebral atrophy develops (see Figs. 8.6 and 8.7 ). Storage in retinal ganglion cells results in the cherry red spot. Patients with GM 1 gangliosidosis also have skeletal, soft tissue, and visceral changes similar to Hurler syndrome.
The GM 2 gangliosidoses ( Table 8.4 ) are a prime example of the genotypic complexity and phenotypic diversity of LSDs. GM 2 accumulation can result from a deficiency of hexosaminidase A (Hex A), hexosaminidase B (Hex B), or the GM 2 activator, a polypeptide that forms a complex with GM 2 ganglioside that is important for its degradation. Hex A is made up of two nonidentical subunits, α and ß, that are encoded by the genes HEXA (on 15q23-q24) and HEXB (on 5q13), respectively. Hex B is made up of two identical β subunits. Mutations in HEXA cause deficiency of the Hex A (TSD). Mutations in HEXB cause deficiency of both Hex A and Hex B (Sandhoff disease). Both Hex A and Hex B are multicatalytic and metabolize oligosaccharides, glycolipids, glycoproteins, and GAGs with terminal GlcNAc or GalNAc. Only Hex A catabolizes GM 2 ganglioside.
| Disease | Composition | Effect |
|---|---|---|
| Hex A deficiency | α and β subunits | Catabolizes GM 2 |
| Hex B deficiency | Two β subunits | Catabolizes GM 2 , glycoproteins, glycosaminoglycans |
| Tay-Sachs disease | Hex A deficiency, α-subunit mutation | |
| Sandhoff disease | Hex A and B deficiency, β-subunit mutation | |
| Activator deficiency | GM 2 activator deficiency |
A group of autosomal recessive LSDs caused by deficiencies of hexosaminidase A (TSD), hexosaminidase B (Sandhoff disease), and the GM 2 activator
GM 2 ganglioside
Neuronal lipidosis, blindness, cherry red spots
Megalencephaly initially, followed by cerebral atrophy
Neuronal lipidosis early, neuronal loss and gliosis late
Membranous cytoplasmic bodies
TSD is the prototype of LSDs and was the first LSD to be described. It is caused by an α subunit (and Hex A) deficiency. Patients with classic infantile TSD present in the first year of life and die by 5 years of age. They have profound psychomotor retardation, myoclonus, hypotonia, and cherry red spots. Variants caused by milder mutations begin later and have a subacute or chronic course characterized by seizures, ataxia, spasticity, choreoathetosis, and motor neuron disease with little or no cognitive dysfunction.
The neuropathology of infantile TSD is generalized neuronal lipidosis, indistinguishable from GM 1 gangliosidosis. The brain is larger than normal initially, but by the end of the clinical course, severe neuronal loss and brain atrophy occur. GM 2 ganglioside, normally a minor component of total brain gangliosides, increases to 90 % of brain gangliosides. The few late-onset cases that have been studied show similar but less severe changes throughout the brain, more severe storage in spinal cord neurons and dorsal root ganglia, and more diverse and heterogeneous lysosomal contents.
The clinical profile and pathology of activator deficiency are similar to TSD. Hex A and Hex B deficiencies (Sandhoff disease) are caused by β subunit mutations. The neurological manifestations and neuropathology of Sandhoff disease are similar to TSD, but Sandhoff disease shows also hepatosplenomegaly and visceral storage of glycolipids oligosaccharides, glycoproteins, and glycolipids. No such visceral storage is seen in TSD.
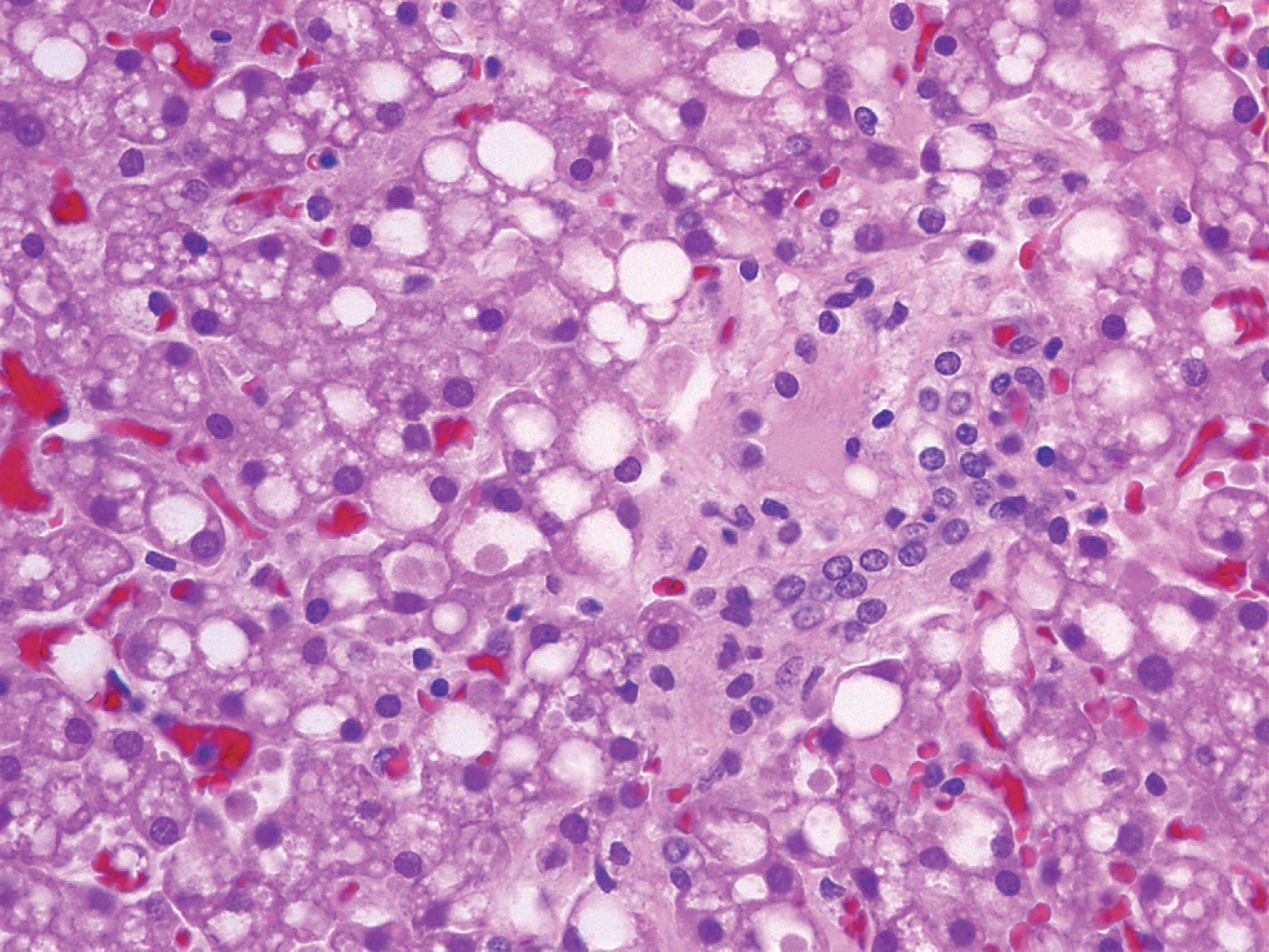
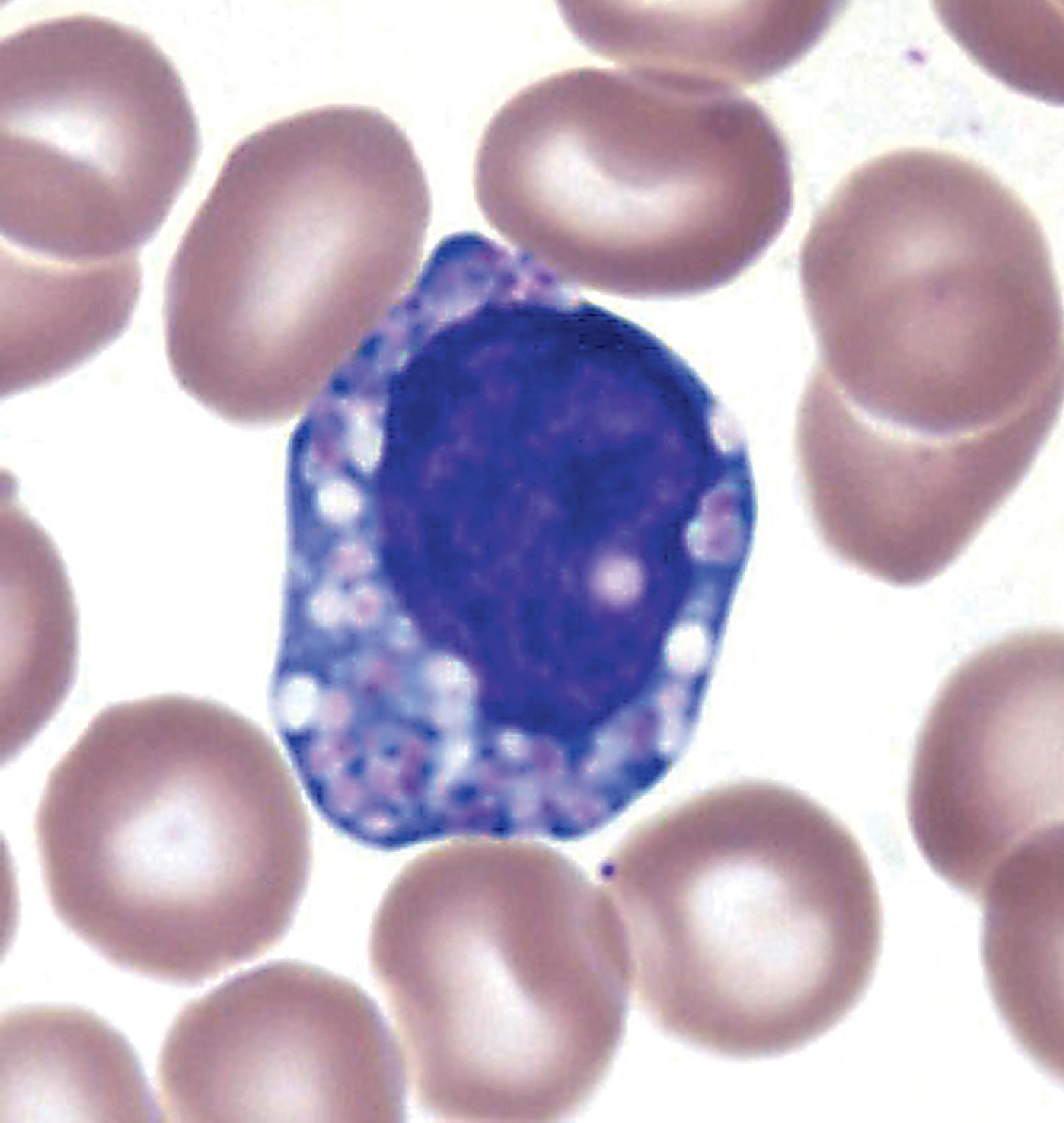
Niemann-Pick types A and B are caused by mutations in the sphingomyelinase gene resulting in lysosomal storage of sphingomyelin. Niemann-Pick type C and its variant D (see further on) are caused by defects of intracellular cholesterol circulation. Niemann-Pick type A has a high incidence among Ashkenazi Jews and presents in infancy with progressive neurological deterioration, cherry red spot, and massive hepatosplenomegaly, and is usually fatal within 2 to 3 years. Niemann-Pick type B presents in infancy or childhood with massive hepatosplenomegaly and pulmonary infiltrates. There is little or no neurological involvement, and patients survive to adulthood. The visceral pathology of Niemann-Pick types A and B consists of foamy histiocytes in the bone marrow ( Fig. 8.16 ), spleen, lymph nodes, hepatic sinusoids, and pulmonary alveoli. The stored sphingomyelin takes the form of small concentric lamellar bodies. Similar materials accumulate in the CNS and autonomic neurons, brain macrophages and microglia, retinal ganglion cells, and other nonneuronal cells. Neuronal storage causes ballooning leading to neuronal degeneration and loss and severe cerebral and cerebellar atrophy.
An autosomal recessive LSD caused by deficiency of sphingomyelinase
Sphingomyelin
Neuronal lipidosis, blindness, cherry red spot, storage histiocytosis
Hepatosplenomegaly, cerebral atrophy
Niemann-Pick cells in hematopoietic tissues, neuronal ballooning, and neuronal loss
Lamellar bodies
Gaucher disease (GD) is an LSD characterized by storage of glucocerebroside (glucosylceramide) in monocyte-macrophage cells due to deficiency of glucocerebrosidase (glucosylceramidase). With an estimated birth frequency of 1:50,000, it is the most common LSD in Caucasians. GD is caused by mutations in the GBA1 gene, located on 1q21. Four mutations account for the vast majority of all forms of GD, although more than 300 mutations have been described. The phenotype is predictable in some mutations. In others, there is a marked phenotypic variability, even among monozygotic twins with GD. In addition to lysosomal deficiency, accumulation of misfolded mutant glucocerebrosidase in the endoplasmic reticulum is thought to have damaging effects that contribute to the pathology.
In addition to having GD, persons with pathogenic GBA1 mutations, including carriers, also have a 20- to 30-fold risk for developing PD, and 70 %–10 % of PD patients have GBA1 mutations. The clinical and pathologic phenotype of PD in GD is similar to idiopathic PD except that it has an earlier onset and causes more cognitive impairment. The mechanism by which GD causes PD is unknown, but it is thought to be a combination of lysosomal impairment, mitochondrial dysfunction, and oxidative and endoplasmic reticulum stress induced by GD. These changes impair α-synuclein degradation, leading to its accumulation.
Three clinical phenotypes are recognized. The most common by far is type 1, the prevalence of which in Ashkenazi Jews is 1:855 and carrier frequency 1:18. Type 1 GD presents from childhood to early adulthood and is characterized by hepatosplenomegaly with occasional splenic infarcts, bone disease (osteopenia, focal lytic or sclerotic lesions, osteonecrosis, pathologic fractures, chronic bone pain), anemia and thrombocytopenia due to hypersplenism, coagulation abnormalities, pulmonary interstitial infiltrates, and other manifestations. Type 1 GD patients may develop neurological manifestations from spinal cord or root compression secondary to bone disease but have no primary CNS involvement by GD.
In type 2 (acute neuronopathic) GD, neurological manifestations (stridor, strabismus and other oculomotor abnormalities, swallowing difficulty, opisthotonus, spasticity) appear before age 2 years and progress rapidly leading to death by 2 to 4 years of age. Some patients present at birth with nonimmune fetal hydrops and ichthyosiform or collodion skin changes. Type 2 GD patients also have hepatosplenomegaly similar to type 1. There is no special ethnic prevalence for type 2 GD. Type 3 (subacute neuronopathic) GD is frequent in Northern Sweden and has similar clinical manifestations to type 2 but is more slowly progressive such that patients may survive into their 20s and 30s. Type 2 and type 3 GD are not distinct entities but rather a spectrum.
An autosomal recessive LSD caused by deficiency of glucocerebrosidase and storage of glucocerebroside in monocyte-macrophage cells
Glucocerebroside
1.16–1.75:100,000; Type 1 GD is the most common LSD.
Type 1: storage histiocytosis causing hepatosplenomegaly, bone disease, anemia, and thrombocytopenia
Type 2: storage histiocytosis and severe neurological abnormalities starting in infancy and causing death by 2–4 years
Type 3: similar to type 2 but milder
Osteopenia, lytic or sclerotic bone lesions, osteonecrosis, and fractures
Hepatosplenomegaly
Gaucher cells: large monocyte-macrophage cells with a “wrinkled tissue paper” appearance. They are found in lymph nodes, bone marrow, spleen, and hepatic sinusoids. In type 2 GD, GCs are present in perivascular CNS spaces.
Tubular inclusions in lysosomes
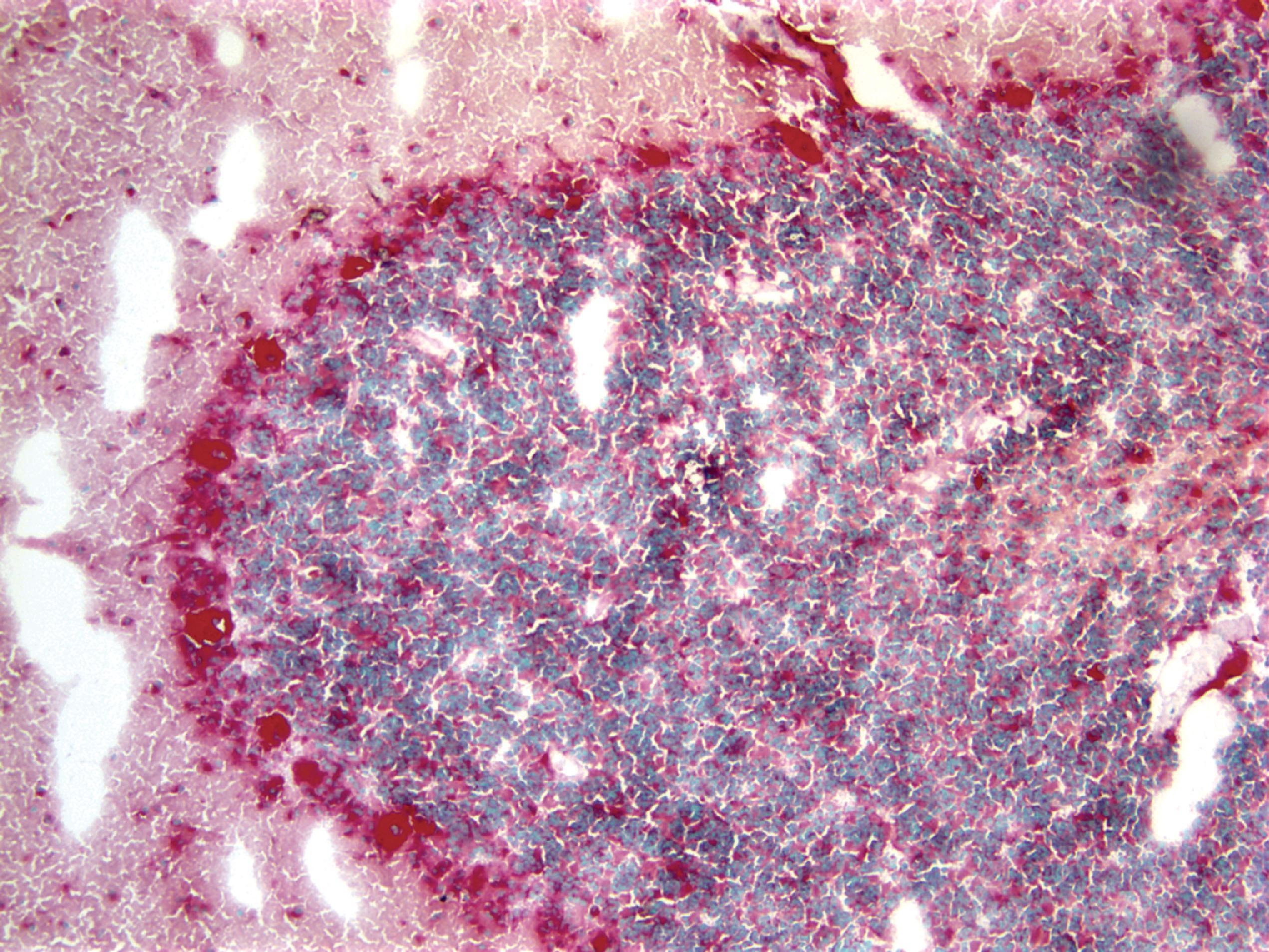
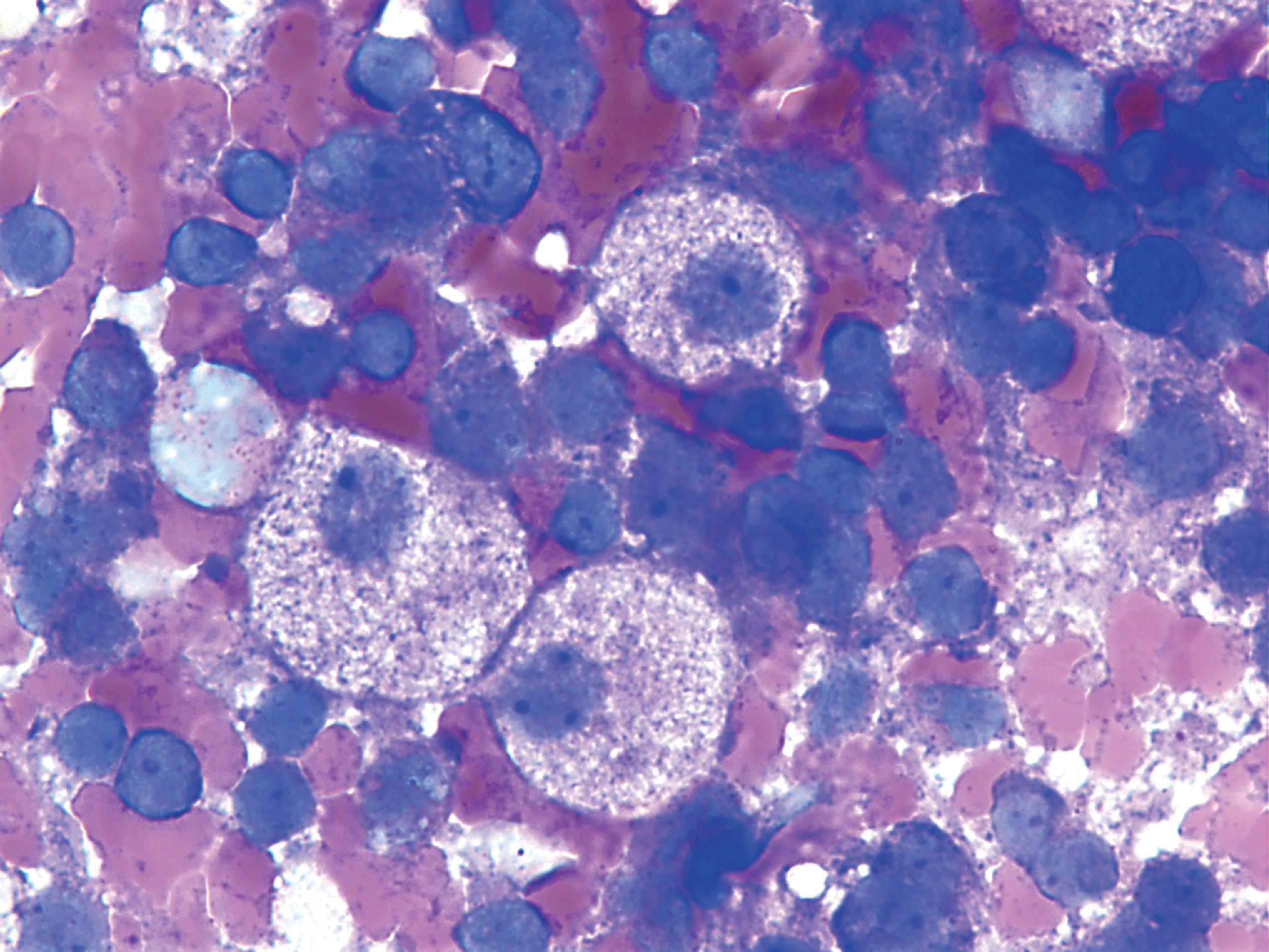
The pathology of GD consists of lysosomal storage of glucocerebroside in cells of the monocyte-macrophage system. This storage leads to a characteristic cellular alteration of these cells–the Gaucher cells (GCs). GCs have a large cytoplasmic mass with a striated appearance that has been likened to wrinkled tissue paper or crumpled silk ( Fig. 8.17 ). This change is caused by storage of twisted bundles of tubules ( Fig. 8.18 ) in large, irregular, branching lysosomal compartments. These inclusions are unique and diagnostic for GD. Biochemically, they consist of glucocerebroside, the main sources of which are thought to be membranes of blood cells phagocytosed by monocytes-macrophages. A similar cellular alteration (pseudo-GC) is sometimes seen in the marrow of patients with chronic myelogenous leukemia.
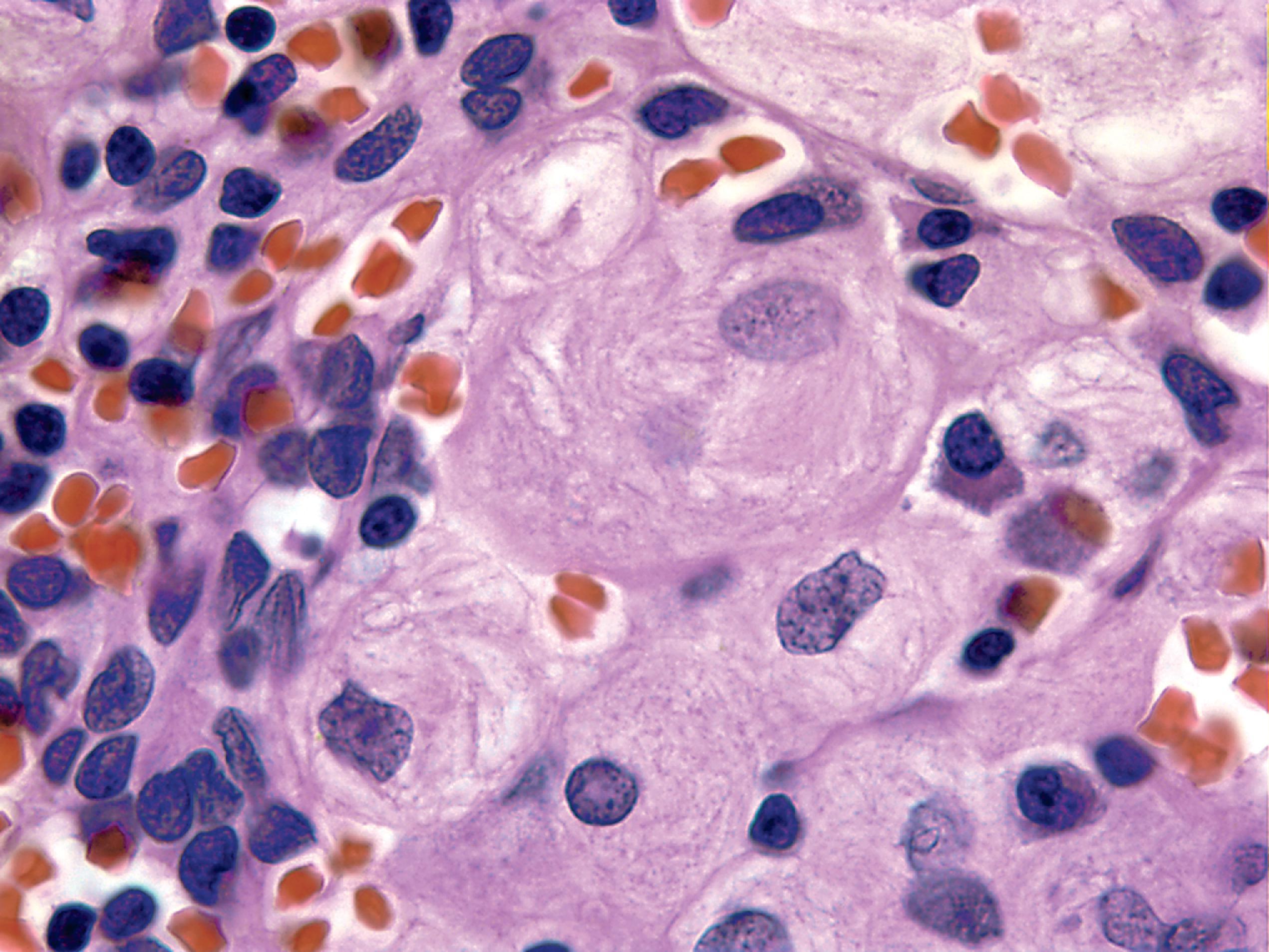
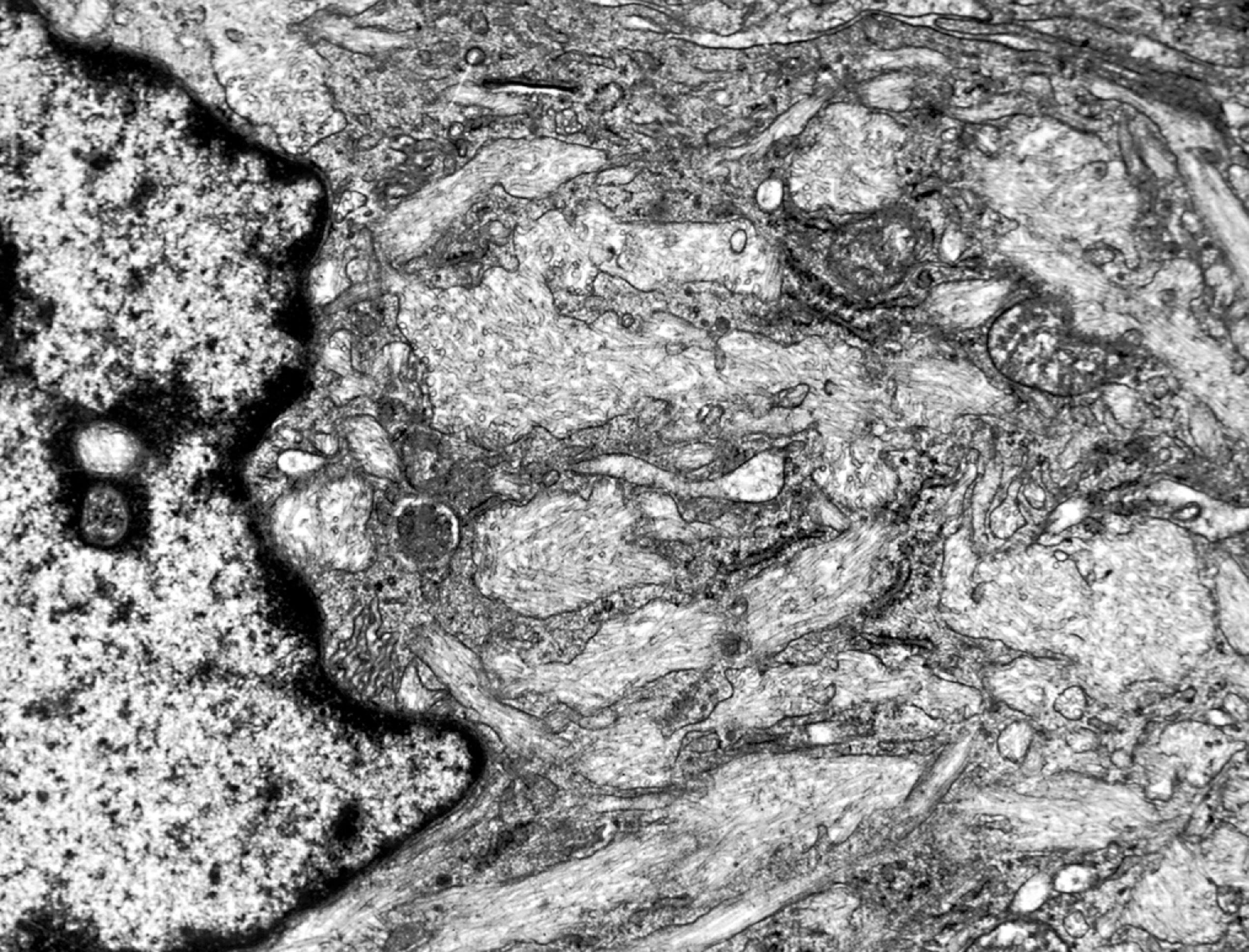
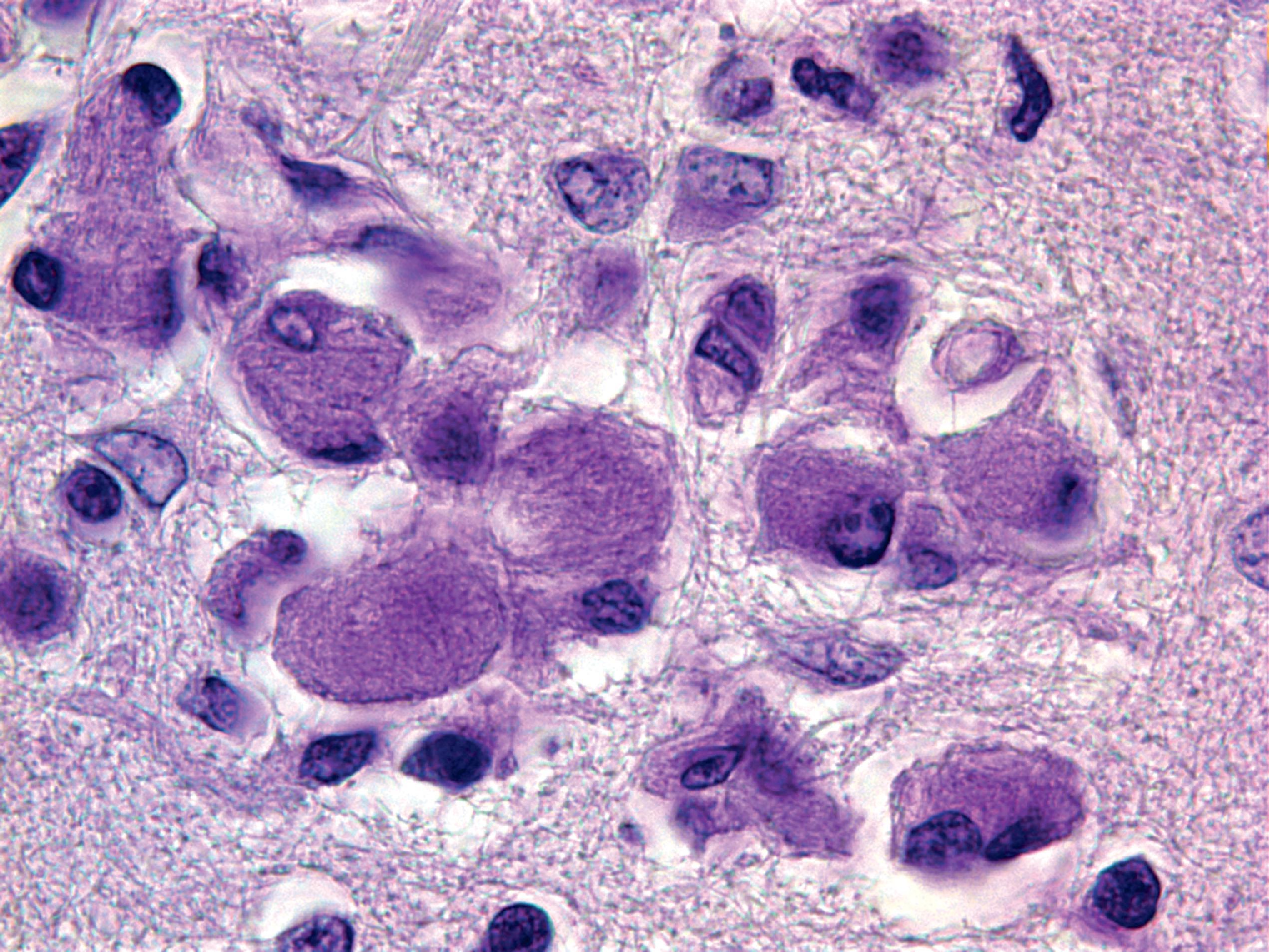
GCs are present in the bone marrow, spleen, lymph nodes ( Fig. 8.18 ), hepatic sinusoids, and other organs and tissues in all forms of GD but account for a small fraction of the mass of the liver and spleen in GD. Cytokines released by GCs induce an inflammatory response that plays a significant role in the organomegaly and systemic manifestations of GD. An increased incidence of cancer including lymphoma, myeloma, and bone tumors has been reported in GD patients. Rare GCs without other pathology also occur in the leptomeninges and Virchow-Robin spaces in type 1 GD.
Types 2 and 3 GD involve the CNS. There are numerous GCs in perivascular spaces and rare GCs in brain parenchyma ( Fig. 8.19 ). No part of the CNS is spared, but the brainstem and deep nuclei are more severely affected than the cortex and account for most neurological deficits. Along with the presence of GCs, there is neuronophagia, neuronal loss, and gliosis. Except for the rare finding of tubular inclusions in neurons, no neuronal storage is seen. Neuronal degeneration and loss have been attributed to the neurotoxic action of glucosyl sphingosine, a by-product of glucocerebroside not normally present in the brain.
The gold standard for the diagnosis of GD is assay of acid β-glucosyl ceramidase activity in blood leukocytes. The diagnosis can also be made by finding GCs in bone marrow aspirates. Enzyme assay of amniocytes and CVS cells can also be used for prenatal diagnosis but is unreliable for carrier detection. Molecular genetic testing (targeted mutation analysis) is available on a clinical basis and is used for carrier detection and for prenatal diagnosis when the disease-causing mutations are known. A panel targeting the 4 or 11 most common mutations detects 89 % and 98 % of all mutations, respectively.
GD is the first LSD that was successfully managed by ERT and its success in GD has opened the road for similar therapies in other LSDs. Current ERT uses imiglucerase, velaglucerase alfa, or taliglucerase alfa. Lifelong ERT is required. ERT reverses the hematologic manifestations and visceromegaly and reduces the bone pathology but has no effect on neuronopathic features because the drugs used for ERT do not cross the blood-brain barrier. SRT using miglustat or eliglustat is used for patients who are unable to receive ERT.
Fabry disease (α-galactosidase [α-gal A] deficiency) is one of two X-linked LSDs, the other one being MPS II (Hunter syndrome). The gene for α-galactosidase, GLA , is located on Xq22.1. Currently, over 900 mutations have been reported. Full-blown Fabry disease becomes clinically apparent in childhood or adolescence with peripheral neuropathy, cutaneous lesions, renal disease, corneal opacities, and cataracts. The peripheral neuropathy (acroparesthesia) is a small fiber neuropathy characterized by intermittent episodes of disabling burning limb pain that are often precipitated by stress, exercise, and increased temperature. No significant weakness or muscle atrophy occurs. Cutaneous telangiectases (angiokeratoma corporis diffusum) cover the body in a bathing trunk distribution. Renal dysfunction progresses to renal failure with hypertension and cardiac and cerebrovascular complications. Heart involvement is manifested by mitral insufficiency, cardiac arrhythmias, cardiomyopathy, angina, and myocardial infarction. Four to 8 % of unselected patients with hypertrophic nonobstructive cardiomyopathy have Fabry disease. Generalized manifestations occur in patients with less than 1 % α-gal A activity. Patients with higher α-gal A activity have milder, later onset disease that affects one organ, usually the heart. Males with full-blown Fabry disease usually die in their 30s or 40s from renal failure, heart disease, or cerebrovascular disease. Because of random X chromosome inactivation, female carriers of Fabry disease may develop any of the manifestations seen in males but usually they have more variable, milder, and late-onset disease than affected males. The only other LSDs that cause severe heart disease are Pompe disease and the MPS (see further on).
ERT using recombinant α-gal A (agalsidase α and agalsidase β), starting as early as possible, slows the development of renal insufficiency and heart disease and decreases neuropathic pain but does not prevent stroke.
An X-linked LSD with multiorgan pathology, caused by deficiency of α-gal A
Trihexosyl ceramide
Neuropathy, angiokeratoma, renal failure, hypertension, heart disease, and corneal opacities
Lipid storage in vascular cells, Schwann cells, neurons, and cardiac myocytes; Ischemic pathology in brain and heart
Membrane-bound lamellar structures
The pathology of Fabry disease consists of widespread lysosomal storage of birefringent lipids in epithelial, mesenchymal, and neural cells ( Fig. 8.20 ). On electron microscope examination, the membrane-bound deposits have a lamellar configuration ( Fig. 8.21 ). Endothelial cells and vascular smooth muscle are most severely affected. Vascular involvement causes endothelial injury, thrombosis, and ischemic changes and weakens vessel walls, leading to telangiectasia of cutaneous, conjunctival, and retinal vessels. The involvement of glomerular cells ( Fig. 8.20 ) and renal tubular epithelium explains the renal complications. Heart disease is due to lipid storage in cardiac myocytes (cardiomyopathy) and ischemic myocardial damage. The CNS complications of Fabry disease are primarily due to ischemia and hypertension, but storage in neurons, glial cells, and in the leptomeninges also occurs. Sural nerve biopsy in Fabry disease shows loss of small myelinated and unmyelinated fibers and lipid deposits in vascular cells, perineurial cells, and Schwann cells. Storage in sensory and autonomic ganglionic neurons also occurs.
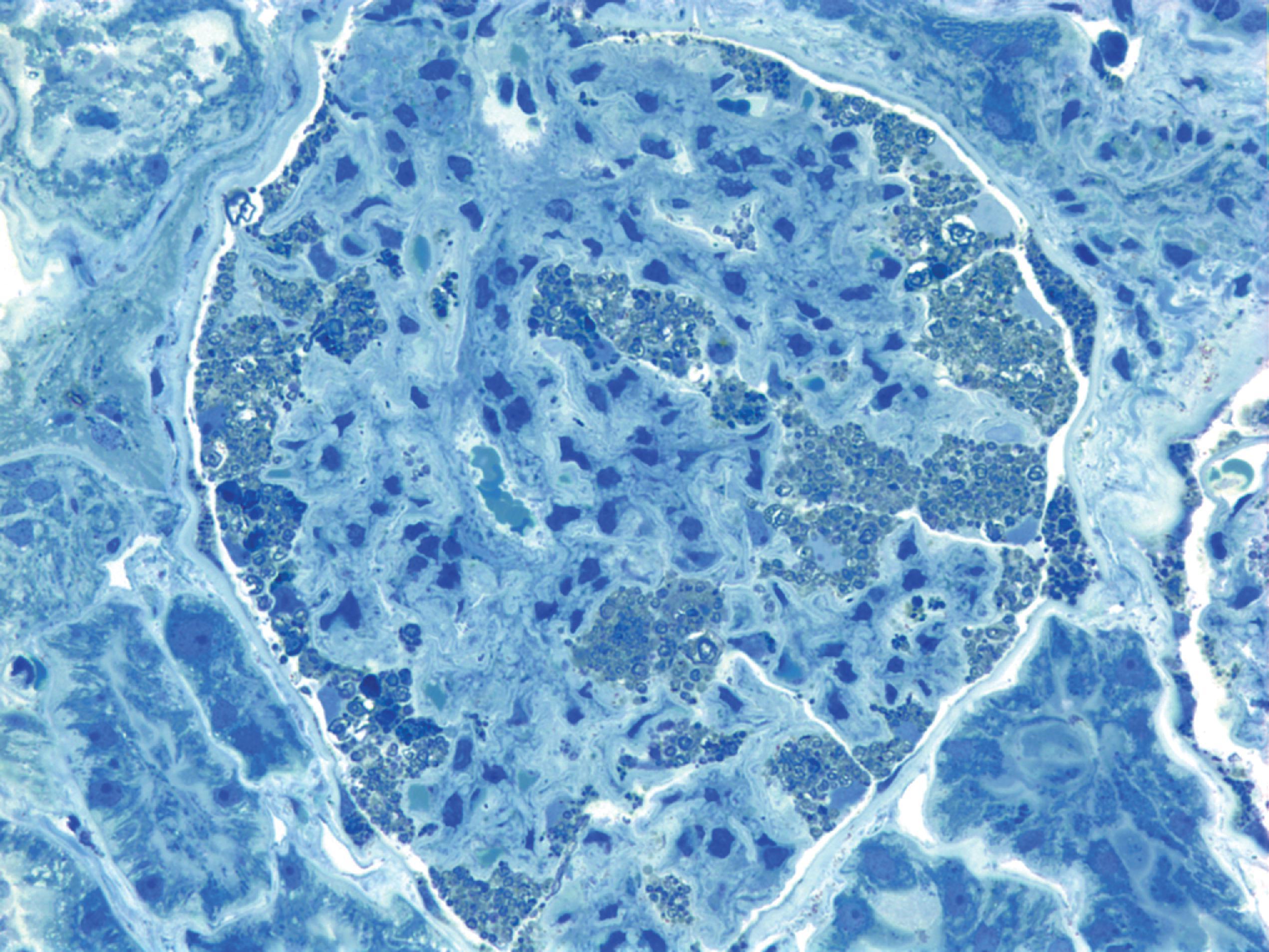
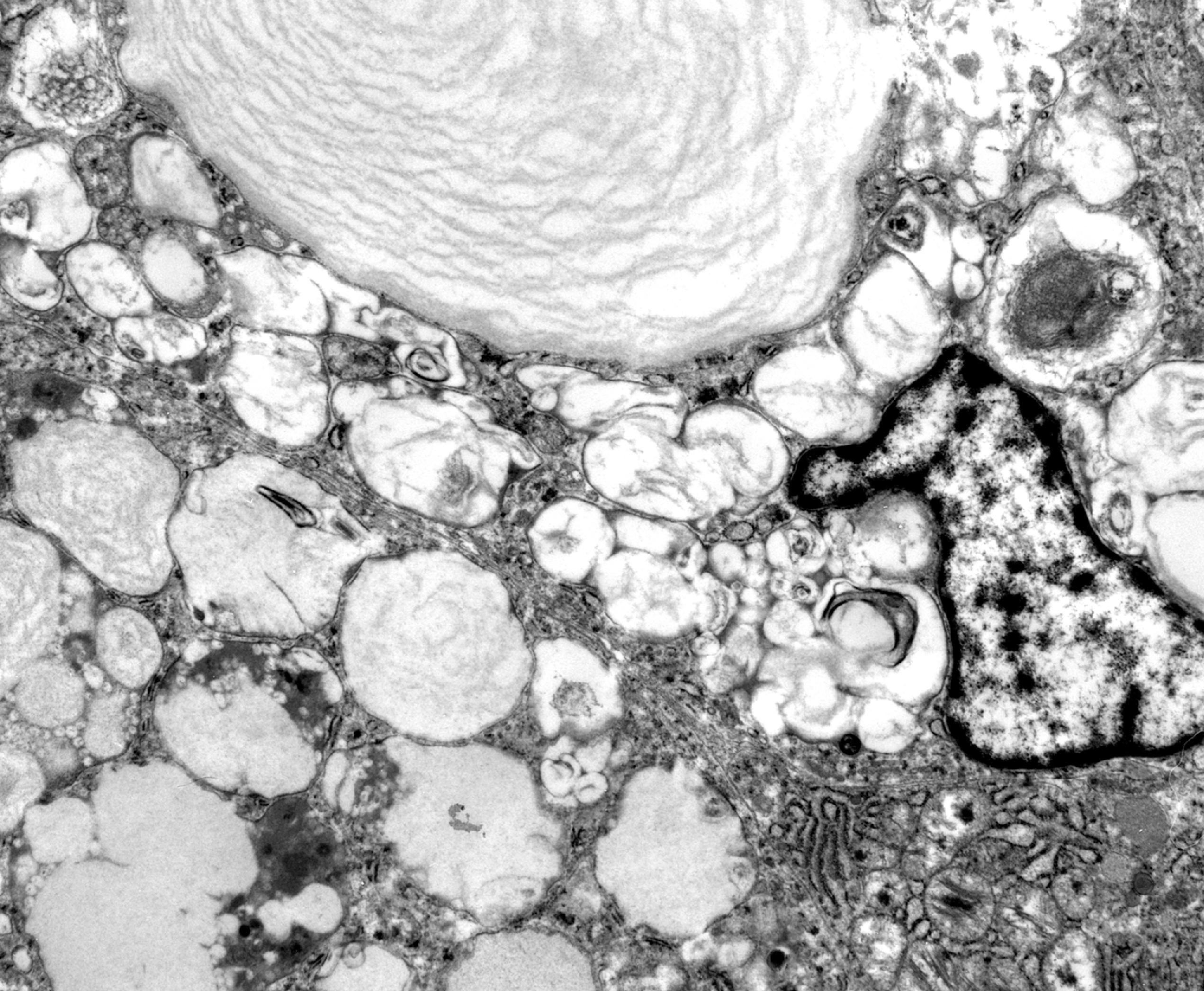
The diagnosis of Fabry disease can be established by finding lower than 25 % of normal α-gal A activity in peripheral blood leukocytes or cultured skin fibroblasts. However, some female carriers have normal α-gal A activity because of random X chromosome inactivation. These carriers can be diagnosed by molecular genetic testing.
Farber lipogranulomatosis, the rare autosomal recessive deficiency of acid ceramidase, encoded by the ASAH1 gene, has a wide phenotypic spectrum. In its classic infantile form, it presents in the first few months of life with a striking characteristic combination of painful, swollen, deformed joints, particularly in the hands and feet; subcutaneous nodules; and hoarseness due to development of granulomas in the larynx. As the disease progresses, granulomas cause skeletal deformities and affect the lungs, liver, heart, and other organs. Patients have psychomotor retardation and usually die in 2 to 3 years. Milder forms have a later onset and longer survival with mild neurological involvement. Cherry red spots associated with progressive neurological symptoms are seen in some patients. A severe rapidly fatal phenotype presents with neonatal hydrops and hepatosplenomegaly. Mutations in the ASAH1 gene also cause a combination of spinal muscular atrophy and progressive myoclonic epilepsy.
A rare autosomal recessive LSD caused by deficiency of ceramidase
Ceramide
Arthropathy, multisystem pathology due to lipid granulomas, and psychomotor retardation
Lipid granulomas in joints and viscera; Lipid storage in neurons
Lamellar (zebra) bodies, banana bodies
The granulomas of Farber disease consist of foamy histiocytes that have accumulated ceramide and are associated with lymphoplasmacytic infiltrates and scarring. They deform and destroy soft tissues, bones, and viscera and cause organomegaly by their sheer volume. The stored material is PAS-positive and consists of diverse structures including curved tubular structures, lamellar profiles (zebra bodies), and banana bodies (electron lucent inclusions with dense rims). Neuronal storage also occurs, especially in large neurons of the spinal cord, brainstem, and cerebellum and, to a lesser extent, in the cerebral cortex. The white matter shows myelin loss and gliosis.
The MPS are inherited metabolic disorders caused by impaired lysosomal degradation of mucopolysaccharides (GAGs). GAGs are long unbranched molecules of repeating disaccharides. They are attached to core proteins forming proteoglycan complexes that consist of 95 % carbohydrate and 5 % protein. GAGs are produced by the brain and most cells and are found mainly on the surface of cells and in the extracellular matrix. They are primarily structural molecules but perform a variety of other biological functions. Proteoglycans form a network with collagen fibers that contributes to structural stability and toughness of connective tissue and cartilage. The large amount of water that GAGs attract cushions tissues from mechanical stress and is important for nutrient circulation. Synthesis of GAGs occurs by stepwise addition of sugar molecules. Degradation of GAGs begins with their internalization by endocytosis followed by stepwise removal of sugar molecules by glycosidases and sulfatases, which occurs in lysosomes. Deficiencies of these enzymes result in the accumulation in lysosomes and in the extracellular matrix of four GAGs: dermatan sulfate, heparan sulfate, keratan sulfate, and chondroitin sulfate.
As a group, the MPS are the most common LSDs. They are autosomal recessive with the exception of MPS II (Hunter syndrome), which is X-linked. As in other LSDs, different enzyme defects can cause the same phenotype (e.g., MPS III [Sanfilippo syndrome] and MPS IV [Morquio syndrome]), and allelic heterogeneity results in a wide phenotypic spectrum (e.g., MPS I [Hurler syndrome], MPS II [Hunter syndrome], and MPS VII [Sly syndrome]).
The MPS share certain core clinical features. They are progressive multisystem disorders characterized by coarse facial features, skeletal and joint abnormalities, organomegaly, corneal clouding, cardiovascular disease, and CNS involvement. MPS I-H (Hurler syndrome), the most severe MPS phenotype, is described in detail as a prototype below. The variation among the other MPS in the expression of key clinical findings is discussed further on and presented in Table 8.5 .
| Type | Syndrome | Chromosomal locus | Enzyme | Stored GAG | Clinical findings | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MPS I | Hurler Scheie Hurler-Scheie |
4p16.3 | α-l-Iduronidase | DS, HS | H, MR, DM, CC, HD | |||||
| MPS II | Hunter | Xq28 | Iduronate sulfatase | DS, HS | H, MR, DM, HD | |||||
| MPS III-A | Sanfilippo A | 17q25.3 | Heparan N -sulfatase | HS | MR | |||||
| MPS III-B | Sanfilippo B | 17q21 | α- N -acetyl glucosaminidase | HS | MR | |||||
| MPS III-C | Sanfilippo C | Chr 14 | Acetyl-CoA: α-glucosaminide acetyltransferase | HS | MR | |||||
| MPS III-D | Sanfilippo D | 12q14 | N -acetylglucosamine-6-sulfatase | HS | MR | |||||
| MPS IV-A | Morquio A | 16q24.3 | Galactose-6-sulfatase | KS, CS | DM, CC, HD | |||||
| MPS IV-B | Morquio B | 3p21.33 | β-Galactosidase | KS | DM, CC, HD | |||||
| MPSVI | Maroteaux-Lamy | 5q13-q14 | Arylsulfatase B | DS | H, DM, CC, HD | |||||
| MPS VII | Sly | 7q21.11 | β-Glucuronidase | DS, HS, CS | H, MR, DM, CC, HF | |||||
| MPS IX | 3p21.2-p21.3 | Hyaluronidase | Hyaluronan |
A group of LSDs caused by deficiencies of enzymes of glycosaminoglycan degradation
Dermatan sulfate, keratan sulfate, chondroitin sulfate
4.4:100,000; The most common group of LSDs
MPS II (Hunter syndrome) is X-linked recessive. All other MPS are autosomal recessive.
MPS I (Hurler phenotype) (see Hurler Syndrome)
GAG storage in viscera, skeleton, and soft tissues; ganglioside storage in neurons (see Hurler Syndrome)
Patients with MPS I-H are normal at birth. Clinical manifestations begin to develop in the first year of life. Most patients are diagnosed by 18 months and die before they reach 10 years. The first abnormality to appear may be inguinal or umbilical hernia followed by coarsening of facial features. Corneal clouding occurs in all patients, and open angle glaucoma and retinal degeneration may cause additional visual impairment. The cardiovascular involvement consists of aortic and mitral insufficiency, hypertrophic cardiomyopathy with arrhythmias, and ischemic heart disease due to coronary artery stenosis. The skeletal manifestations of MPS I-H comprise the syndrome of dysostosis multiplex ( Fig. 8.22 ), which consists of enlarged skull, thick calvarium, J-shaped sella, broad clavicles, oar-shaped ribs, scoliosis, abnormal vertebrae, flared iliac wings, dysplastic acetabula, and shortened long bones with thickened cortices. Thickening of synovium and soft tissues causes progressive joint stiffening and distortion, claw-hand deformity, and carpal tunnel and other nerve entrapment syndromes. Thickening of the tongue, tonsils and adenoids and other soft tissues of the oropharynx causes upper airway obstruction, noisy breathing, and copious nasal discharge. Eustachian tube obstruction causes middle ear infections, which are the main cause of hearing loss seen in patients with severe MPS I-H. The abdomen protrudes due to hepatosplenomegaly. The Hurler phenotype, which has prompted the insensitive term “gargoylism,” consists of short stature, protruding abdomen, and coarse facial features ( Fig. 8.23 ). Delay in neurological development is usually evident by 1 year of age, followed by regression and severe psychomotor retardation. Imaging studies show hydrocephalus and arachnoid cysts. Additional neurological damage is caused by spinal cord compression resulting from spondylolisthesis or thickening of the dura.
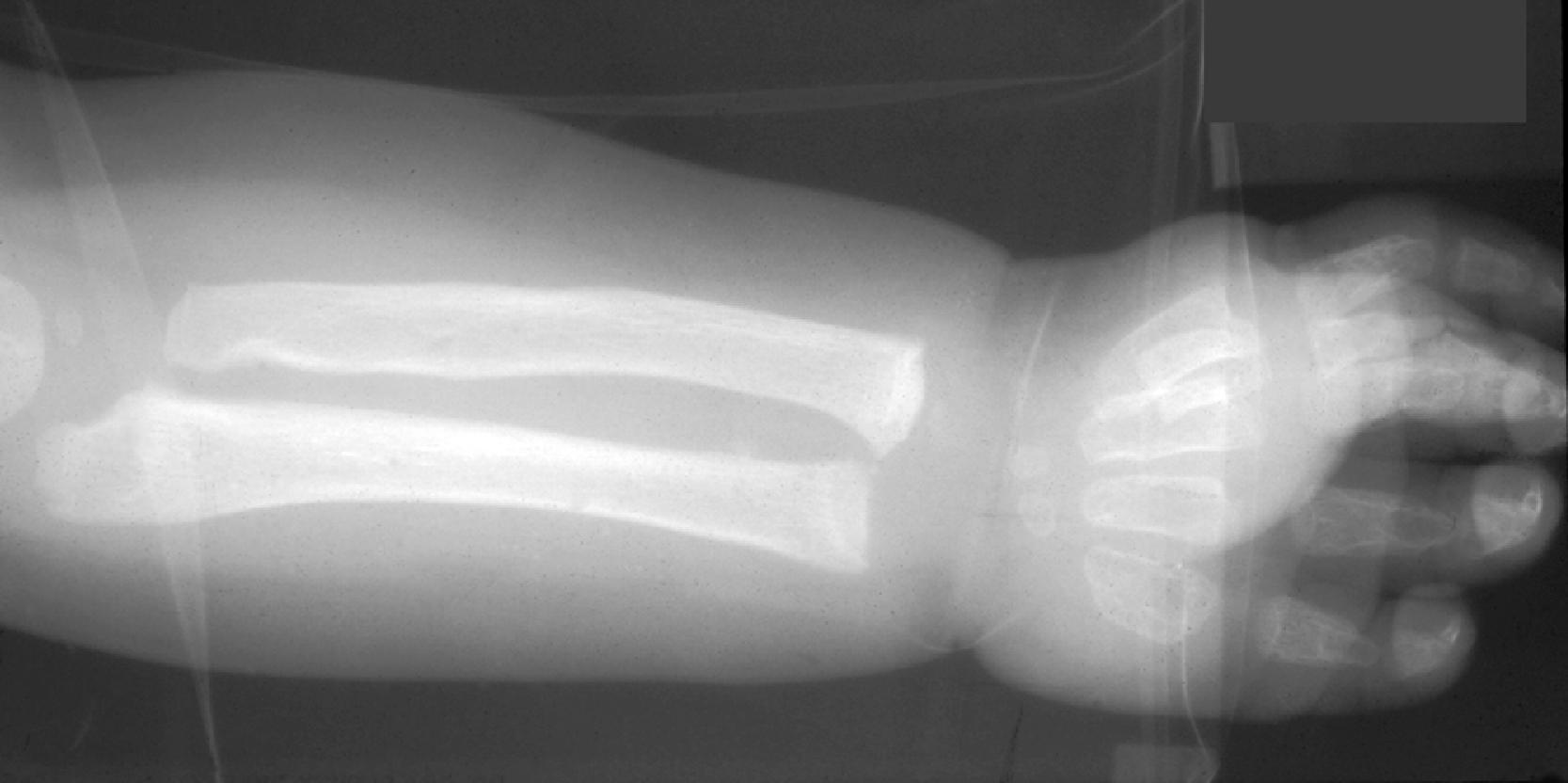
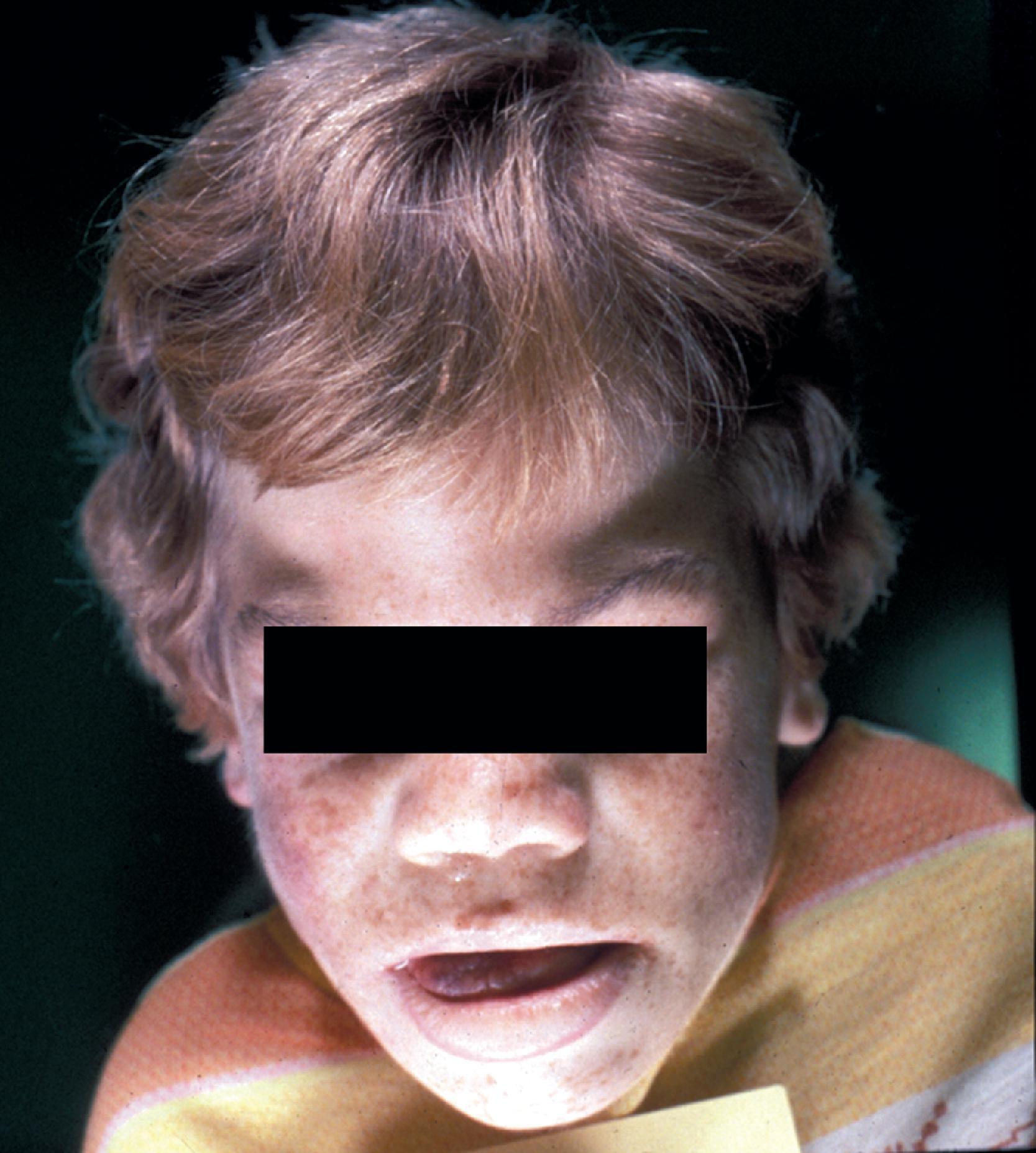
Patients with mild MPS I (Scheie syndrome) and intermediate MPS I (Hurler-Scheie syndrome) have a later onset of the disease and may survive to adulthood. Corneal clouding and all other somatic manifestations develop at a lower pace but may become severe due to prolonged survival. Intellectual impairment is mild or absent.
An LSD with accumulation of GAGs and severe neurological, skeletal, and visceral abnormalities
Psychomotor retardation, coarse facial features, protuberant abdomen, short stature, corneal clouding, and heart disease
Dysostosis multiplex, hydrocephalus
GAGs in urine, vacuolated lymphocytes
Urinary screening for GAGs, enzyme testing (leukocytes, fibroblasts, amniocytes, CVS), DNA testing
Hepatosplenomegaly, myocardial fibrosis, thickening of endocardium and cardiac valves
Thickening of the arachnoid membrane, hydrocephalus, late cortical atrophy
Expansion of perivascular CNS spaces, neuronal lipidosis
Reticulogranular material in epithelial and mesenchymal cells, lamellar material (zebra bodies and MCBs) in neurons
In extraneural tissues, the key cellular pathologyof MPS is cellular swelling with a clear or vacuolated appearance on light microscopic examination (see Fig. 8.4 ). GAGs form a loose branching fibrillary network but are lost in processing because they are highly soluble in water. Electron microscopy shows distended lysosomes that are either empty or contain sparse reticulogranular structures (see Figs. 8.9 and 8.24 ). Accumulation of GAGs in the extracellular matrix, presumably due to deficient recycling and probably also due to discharge from dying cells, is central to the phenotype of MPS I-H and other MPS. Extracellular GAGs are also lost during processing but can be observed in frozen sections with metachromatic stains and in paraffin sections with colloidal iron stains.
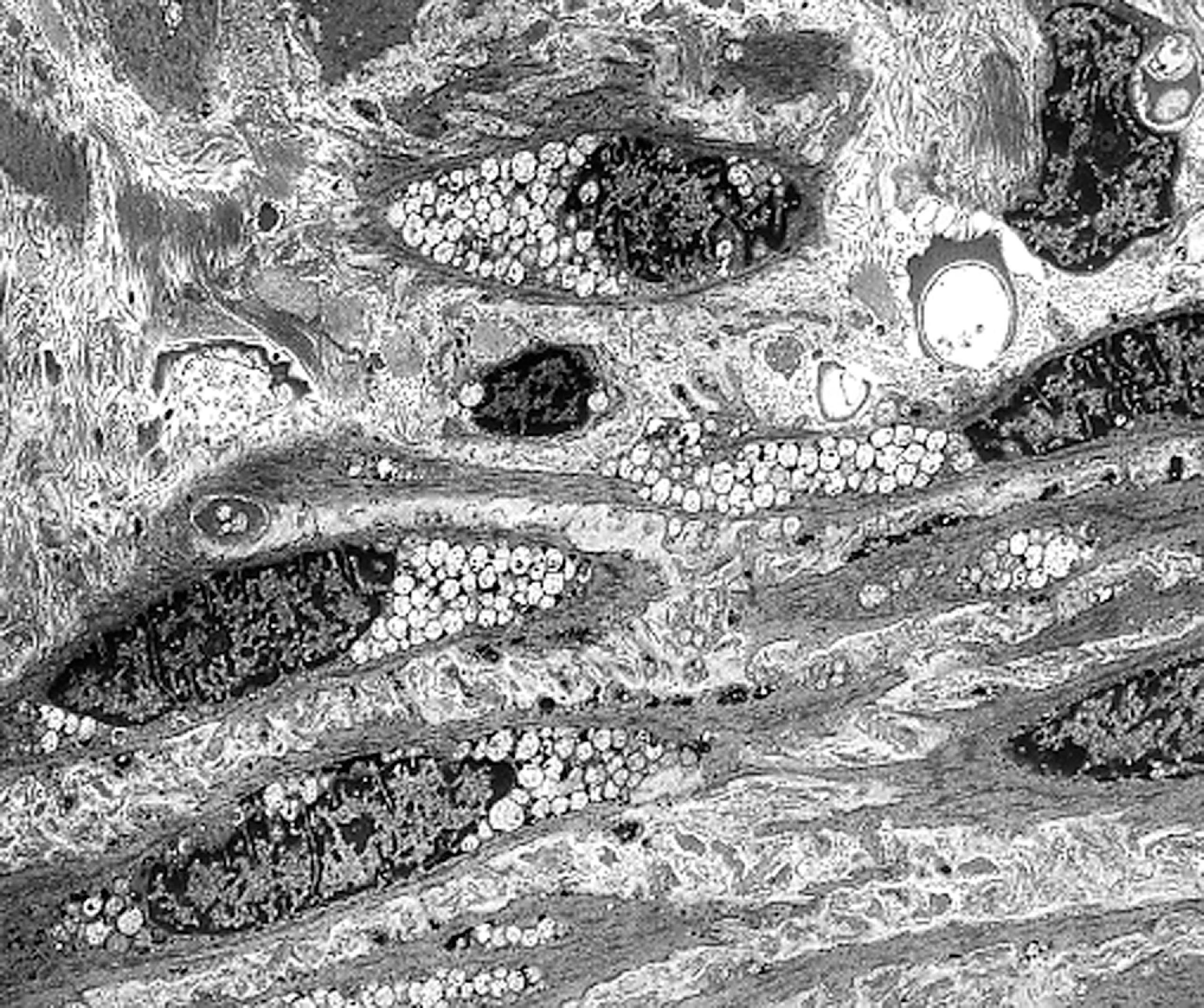
The tissue and organ pathology in the MPS is due to the combined intracellular and extracellular accumulation of GAGs. Cellular swelling is responsible for the organomegaly. GAG storage in connective tissue matrix and fibroblast dysfunction explain the thickening and deformity of connective tissues, the thick swollen skin, coarse facial features, macroglossia, arthropathy, cardiovascular pathology, and corneal opacity. The cardiac pathology of MPS I-H consists of the thickening of heart valves and the endocardium leading to mitral and aortic stiffening and regurgitation ( Fig. 8.25 ). This pathology is compounded by myocardial ischemia due to intimal thickening of the coronary arteries ( Fig. 8.26 ). The cornea is a fibrous structure consisting of fibroblasts in a collagenous stroma. GAG storage in corneal fibroblasts ( Fig. 8.27 ) causes corneal clouding and loss of vision. The skeletal pathology of dysostosis multiplex has not been described. Although there is GAG storage in chondrocytes and cartilage matrix, growth plates appear normal or slightly disorganized. Numerous clear cells are seen in the periosteum, and osteoblasts also store GAGs. Presumably, dysostosis is due to a disturbance of enchondral and periosteal osteogenesis caused by an excess of GAGs in the matrix and osteoblast dysfunction due to intracellular GAG storage.
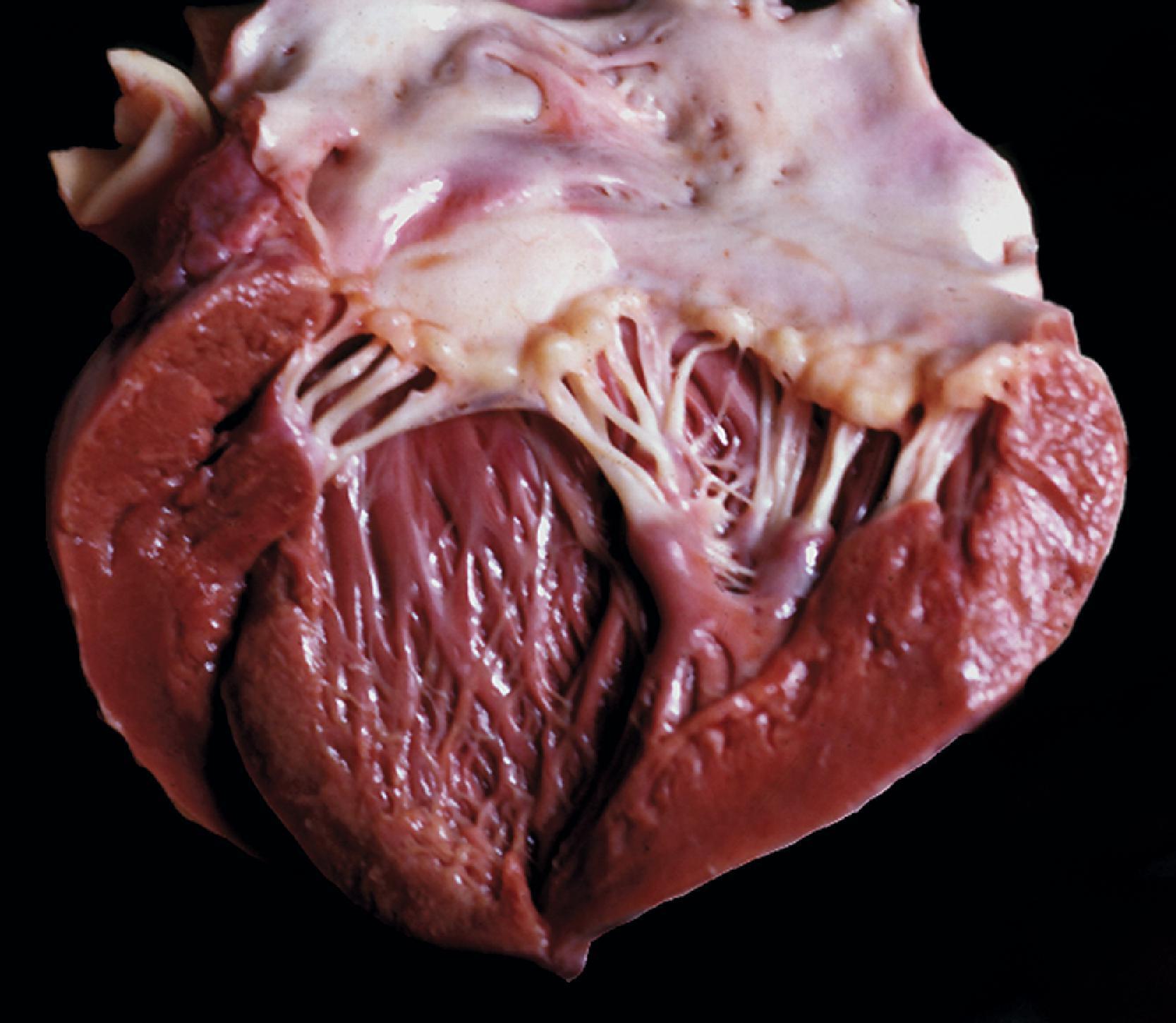
The neuropathologic changes in the MPS can be classified into three categories:
Entrapment neuropathies, optic nerve compression, and compression myelopathy are secondary to the skeletal and soft tissue changes.
GAG deposition causes thickening of the arachnoid membrane and its granulations resulting in impaired CSF circulation and hydrocephalus ( Fig. 8.28 ). The arachnoid membrane is opaque ( Fig. 8.29 ), and there is cystic expansion of perivascular Virchow-Robin spaces ( Fig. 8.30 ).
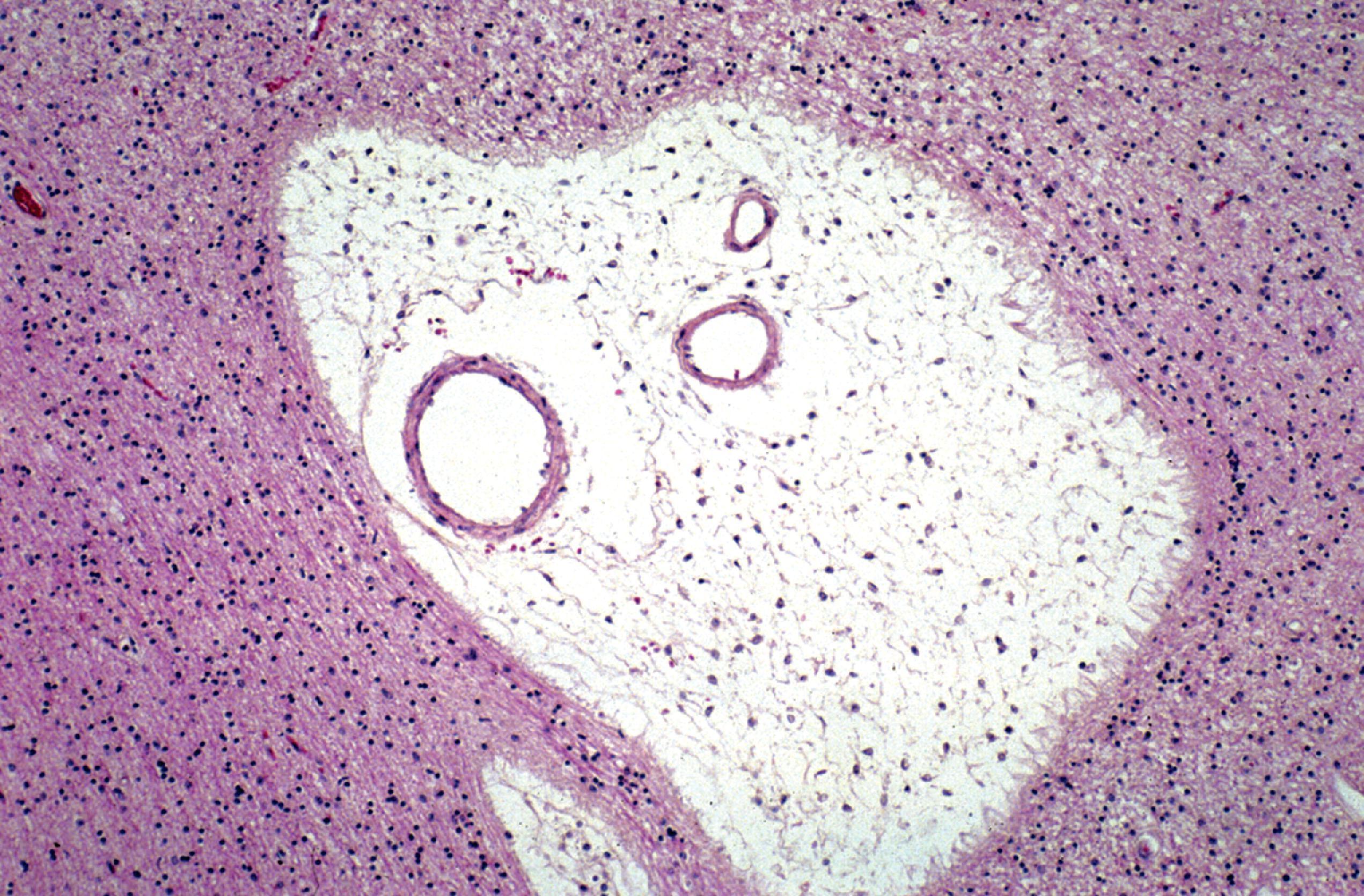
The most devastating CNS pathology of MPS I-H is the process of neuronal lipidosis including axonal and dendritic swellings due to the accumulation of GM 2 and GM 3 gangliosides, cholesterol, and other lipid materials (see Figs. 8.3 and 8.31 ). These products assume the form of concentric or parallel stacks of membranes (zebra bodies) (see Fig. 8.12 ). This pathology, which is seen in all MPS with psychomotor retardation, is indistinguishable from other gangliosidoses. The gangliosides that are deposited in the zebra bodies do not require MPS-cleaving enzymes for their degradation. Ganglioside storage in the MPS has been attributed to the inhibition of neuraminidase and other ganglioside-cleaving enzymes secondary to GAG accumulation in lysosomes. Although neurons process GAGs and GAGs are present in the CNS interstitial space, lysosomal or interstitial GAG storage is not apparent.
Dysostosis in MPS III and VII is milder than in MPS I-H and MPS II. The skeletal dysplasia in MPS IV (Morquio syndrome) most severely affects the vertebral column and causes a short trunk dwarfism and atlantoaxial dislocation due to odontoid hypoplasia and ligamentous laxity. The only neurological problem in MPS IV is compression myelopathy. As a rule, heparan sulfate accumulation correlates with psychomotor retardation and dermatan sulfate with skeletal and mesenchymal abnormalities. In addition to these differences between MPS, within each group, especially MPS I, II, and VII, there is molecular heterogeneity leading to phenotypic variability. MPS IV (Morquio syndrome) and MPS VII cause placental edema and NIHF. The pathogenesis of hydrops in these cases is poorly understood but may be related to the release of hydrophilic glycoconjugates in the matrix (see Table 8.5 ).
Analysis of urinary GAGs can be used as a preliminary screening test. A definitive diagnosis can be made by enzyme assays of leukocytes, cultured fibroblasts, or serum. Enzyme assay of amniotic fluid or CVS cells is used for prenatal diagnosis. Enzyme analysis does not distinguish carriers from normal individuals who are at the lower range of normal enzyme activity. With the exception of Sanfilippo C, the genes causing the MPS have been mapped and sequenced and many mutations have been described. However, the considerable genetic heterogeneity and frequent compound heterozygosity of MPS make DNA analysis impractical for primary diagnosis. When the mutant alleles are known, mutation analysis can be used for carrier detection and prenatal diagnosis.
ERT uses Idursulfase, a recombinant enzyme that was recently approved by the FDA. In patients with MPS II (Hunter syndrome), weekly or every other week infusions of Idursulfase improve physical performance and respiratory function and reduce hepatosplenomegaly and GAG secretion.
The glycoproteinoses (GPs) are a group of LSDs that share features with the MPS and sphingolipidoses ( Table 8.6 ). They are caused by deficiencies of enzymes involved in the degradation of glycoproteins and gangliosides. The stored materials are oligosaccharides, glycopeptides, and glycolipids. All glycoproteinoses are autosomal recessive and are panethnic with the exception of aspartylglycosaminuria, which is largely confined to Finland.
| Disease | Enzyme/protein | Locus | Stored material | Clinical findings | ||||
|---|---|---|---|---|---|---|---|---|
| Sialidosis | Acid sialidase | 6p21.3 | Sialyloligosaccharides | H, MR, M, DM, CRS, HF, NS | ||||
| α-Mannosidosis | α-Mannosidase | 19p13.2q12 | α-Mannose oligosaccharides | H, MR, DM | ||||
| β-Mannosidosis | β-Mannosidase | 4q22-q25 | β-Mannose oligosaccharides | |||||
| Fucosidosis | α-Fucosidase | 1p34 | Fucose oligosaccharides, glycolipids | H, MR, DM, AK | ||||
| Aspartylglucosaminuria | Aspartylglucosaminidase | 4q32-q33 | Glycoasparagines Aspartylglucosamine |
H, MR, M | ||||
| Schindler disease | α- N -acetylgalactosaminidase | 22q13 | α- N -acetylgalactosamine | MR |
The multicatalytic action of the deficient lysosomal enzymes in the glycoproteinoses explains the diversity of storage products and their hybrid phenotypes, which include elements of MPS and sphingolipidoses. In particular, the role of glycoproteins as structural molecules and components of mucinous secretions explains the connective tissue matrix and skeletal pathology that resembles that of MPS.
Like other LSDs, the glycoproteinoses are progressive disorders. They resemble the MPS in that most of them have core clinical components of coarse facial features, skeletal abnormalities, and psychomotor retardation. However, even these core components may be absent or very mild in some glycoproteinoses. For instance, mannosidosis and fucosidosis have mild kyphosis and no true dysostosis multiplex, and Schindler disease has severe neurological deterioration without skeletal abnormalities. Other features such as corneal opacity, organomegaly, and heart disease are even more variable. Variations in severity within each group further widen the phenotypic spectrum. Diverse neurological manifestations including myoclonus are seen in sialidosis and Schindler disease. Moreover, several glycoproteinoses present unique and distinctive clinical features such as cherry red spot (sialidosis), angiokeratoma (β-mannosidosis and fucosidosis), and nephrotic syndrome (sialidosis).
The most significant phenotypic variation is seen in sialidosis. Three sialidosis phenotypes are distinguished. Sialidosis I is characterized by juvenile to adult onset, cherry red spot, and myoclonus without dysostosis or psychomotor retardation; sialidosis II (mucolipidosis I, childhood dysmorphic sialidosis) is characterized by severe neurological and somatic abnormalities; and severe neonatal sialidosis characterized by fetal and neonatal hydrops, nephrotic syndrome, and early death.
A group of autosomal recessive LSDs caused by deficiencies of enzymes that are involved in the degradation of glycoproteins and gangliosides
Glycoproteinoses combine phenotypic features of mucopolysaccharidoses and gangliosidoses
Psychomotor retardation, coarse facial features, skeletal abnormalities, myoclonus, cherry red spot, angiokeratoma, nonimmune fetal hydrops
Urine thin layer chromatography or electrophoresis
Enzyme assay on leukocytes and cultured fibroblasts, amniocytes, and CVS cells
Hepatosplenomegaly
Clear vacuoles in leukocytes, mesenchymal and epithelial cells, neurons and glial cells. Neuroaxonal swellings in Schindler disease
Reticulogranular material, membranous cytoplasmic bodies, lipofuscin-like products
The core pathology of the GPs consists of neurovisceral storage of highly water-soluble oligosaccharides. This storage results in vacuolization of multiple cell types including leukocytes, mesenchymal cells, epithelial cells, neurons, and glial cells. By light microscopy, the vacuoles are clear or contain a small amount of metachromatic material. On electron microscopic examination, the vacuoles are membrane-bound and contain sparse reticulogranular or floccular material which represents oligosaccharides leftover after tissue processing. In fucosidosis, stored glycolipids appear in neurons as reticulogranular material, membranous bodies, and lipofuscin-like products. The pathology of Schindler disease is unique and consists of neuroaxonal dystrophy in which tubular, vesicular, and lamellar materials are stored in axons. No other neurovisceral storage is seen. However, type 2 Schindler disease, a milder form of this entity reported in Japan (Kanzaki disease), shows clear cytoplasmic vacuoles in somatic cells and angiokeratoma. In advanced disease, neuronal storage in the GPs leads to neuronal loss, gliosis, and cerebral atrophy.
Urinary screening by thin layer chromatography or electrophoresis reveals an abnormal pattern of oligosaccharides and glycopeptides. Vacuolated lymphocytes and membrane-bound vacuoles with reticulogranular material in skin, conjunctiva, and other biopsies provide additional evidence of lysosomal storage. A definitive diagnosis can be made by enzyme assay on leukocytes or cultured fibroblasts, and prenatal diagnosis can be made by enzyme testing of amniocytes and chorionic villous samples.
Lipofuscin is an undegradable material that is formed in the free radical-rich environment of lysosomes by peroxidation of membrane phospholipids. It appears in several tissues with advancing age and is an indicator of free radical injury. The NCLs are a group of disorders characterized clinically by progressive psychomotor retardation, seizures, and blindness, and pathologically by lysosomal accumulation in neurons and other cells of PAS-positive, acid fast, autofluorescent (see Fig. 8.13 ) lipopigments, similar to ceroid and lipofuscin. With an estimated incidence of 1.6–2.4:100,000 in the United States and higher in Scandinavian countries, they are the most frequent hereditary neurodegenerative diseases in childhood. Batten reported the first case of NCL in 1903 and described the clinical and pathologic findings in juvenile NCL in 1914. The name Batten disease is used for juvenile NCL (JNCL) and sometimes for all NCLs.
Although the NCLs have been known for 100 years, their genetic and molecular basis has only been recently clarified, and their classification and nomenclature are confusing and beyond the scope of this review (see Anil B. Mukherjee et al. in Suggested Reading). Mutations in thirteen genes, designated CLN1 - CLN14 ( CLN9 has been redesignated as CLN4 ), have been identified. The proteins encoded by most of these genes have been also characterized, but the functions of several of these proteins are unknown or poorly understood. Mutations in the same gene can cause different NCL phenotypes. The two best known genes, CLN1 and CLN2 , code for the lysosomal enzymes palmitoyl protein thioesterase 1 (PPT1) and tripeptidyl peptidase 1 (TPP1) respectively. PPT1 removes long-chain fatty acid chains from proteins, and TPP1 cleaves tripeptides from small proteins before their further degradation by other proteases. The in vivo substrates of these enzymes are not known with certainty. These genes are also expressed in extra-lysosomal locations such as synapses, and their intracellular location in extraneural cells is not the same as it is in neurons. The storage products in the NCLs are of two main types. Saposins A and D are stored in infantile and adult NCL. Subunit C of mitochondrial ATP synthase is stored in most other NCLs. It is not clear how PPT1 and TPP1 deficiency leads to accumulation of these products. CLN11 (GRN) encodes progranulin and granulins. Homozygous GRN mutations cause childhood-onset NCL. Heterozygous mutations cause frontotemporal lobar degeneration.
Clinically and pathologically, the NCLs are indistinguishable from other LSDs that present with the neuronal lipidosis phenotype. Fourteen NCL entities, designated CLN1-CLN14 disease, have been identified (CLN9 and CLN4 are the same disease). The main NCL clinical phenotypes are infantile NCL (INCL), late infantile NCL (LINCL), JNCL (Batten disease), and adult NCL (ANCL [Kufs disease]) ( Table 8.7 ). INCL is most common in Finland. The other NCLs occur worldwide. The light microscopic changes are similar in all NCLs, but the fine structure of the storage material is different and is important for their pathologic recognition. Neuronal storage leads to neuronal loss and cerebral and cerebellar atrophy, which may be mild or extreme depending on the type of NCL and duration of illness. In addition to the retina and the CNS, subclinical storage with the same fine structure occurs in a variety of other tissues, including endothelial cells, Schwann cells, smooth and skeletal muscle, sweat glands, and lymphocytes. Examination of these tissues and cells may be used for diagnosis. Vacuolated lymphocytes are present in JNCL-CLN3.
| NCL | Gene | Encoded protein | EM | Storage | ||||
|---|---|---|---|---|---|---|---|---|
| Infantile NCL (Santavuori-Haltia disease) | CLN1 | PPT1 | GROD | S-acetylated proteins, including saposin D | ||||
| Late infantile NCL | CLN2, CLN5, CLN6 | TPP1 | CLB | SCMAS | ||||
| Juvenile NCL (Batten disease) | CLN3, CLN5 | CLN3 | FP | SCMAS | ||||
| Northern epilepsy | CLN8 | CLN8 | ||||||
| Adult NCL (Kufs disease) | CLN4, CLN5, CLN 6, CLN11/PRGN, CLN13 | PPT1 | Mixed | SCMAS |
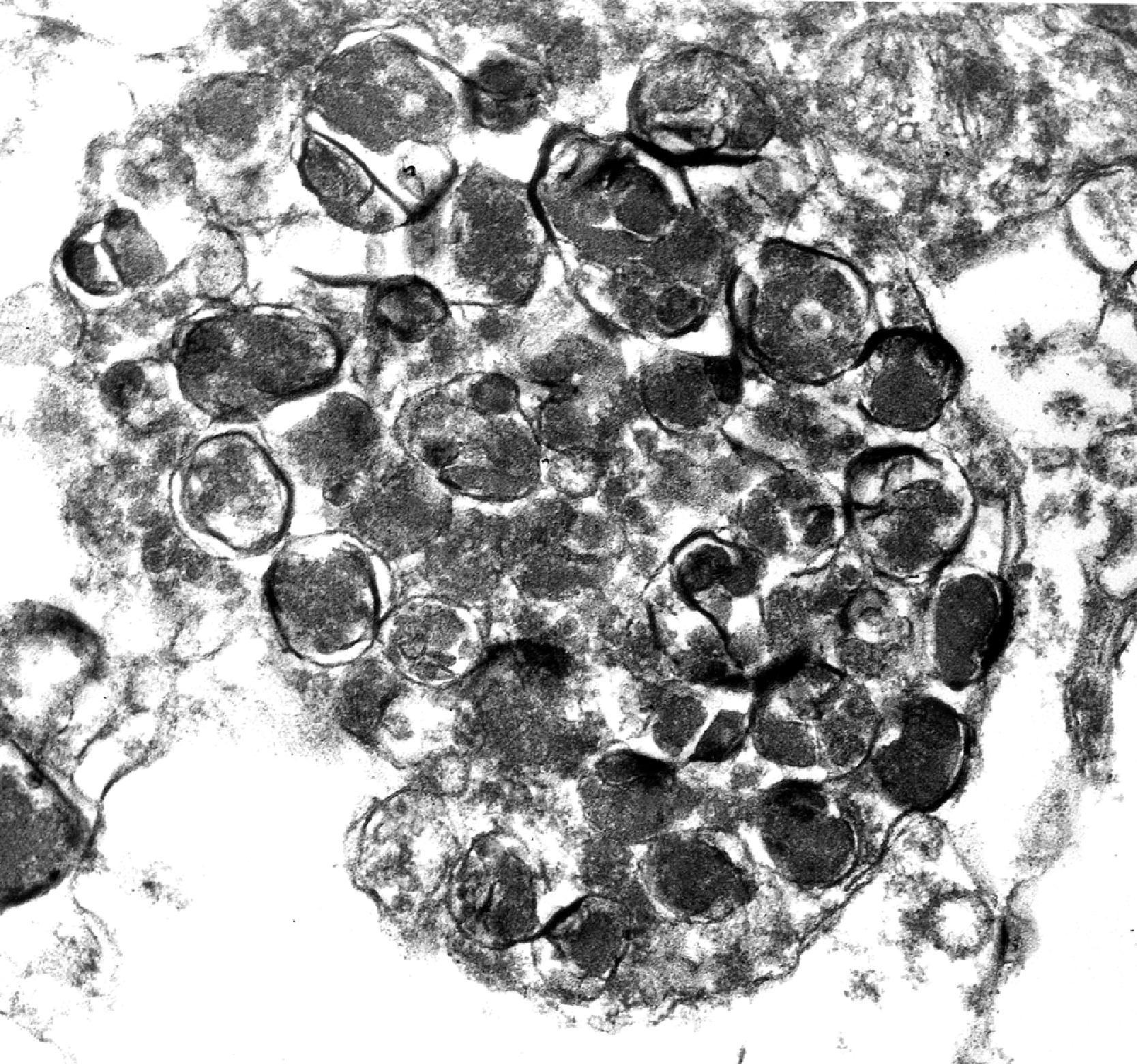
INCL (CLN1 disease) is caused by mutations in the CLN1 gene, which encodes PPT1. Patients are normal at birth and develop normally for a few months but, within 1 year of age, they present with hypotonia, myoclonus, seizures, ataxia, progressive psychomotor retardation, and blindness and die between 8 and 13 years of age. Neurons and glial cells in INCL contain membrane-bound granular osmiophilic deposits ( Fig. 8.32 ). In patients surviving beyond 3 to 4 years of age, virtually all cortical neurons are lost and there is striking cerebellar atrophy. MRI shows cortical atrophy and thalamic hypointensity.
LINCL (CLN2 disease) is caused by mutations in the CLN2 gene, which encodes TTP1. Patients present between 2 and 4 years of age with seizures, myoclonus, and ataxia. Psychomotor regression and blindness ensue and death occurs between 10 and 30 years of age. The storage material in LINCL consists of curvilinear bodies ( Fig. 8.33 ). Patients with advanced disease have severe cerebral and cerebellar atrophy ( Fig. 8.34 ). MRI shows cerebral and cerebellar atrophy.
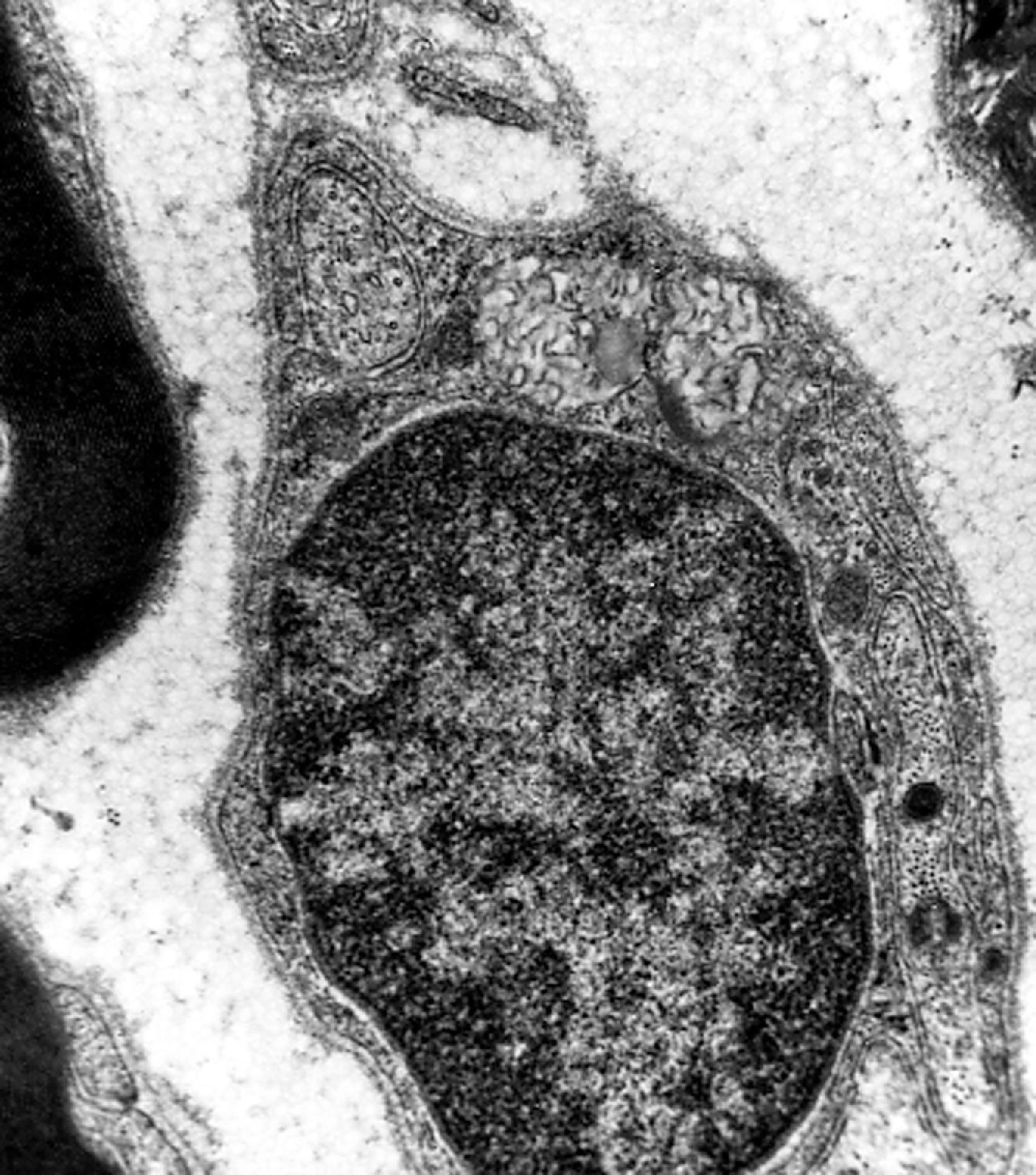
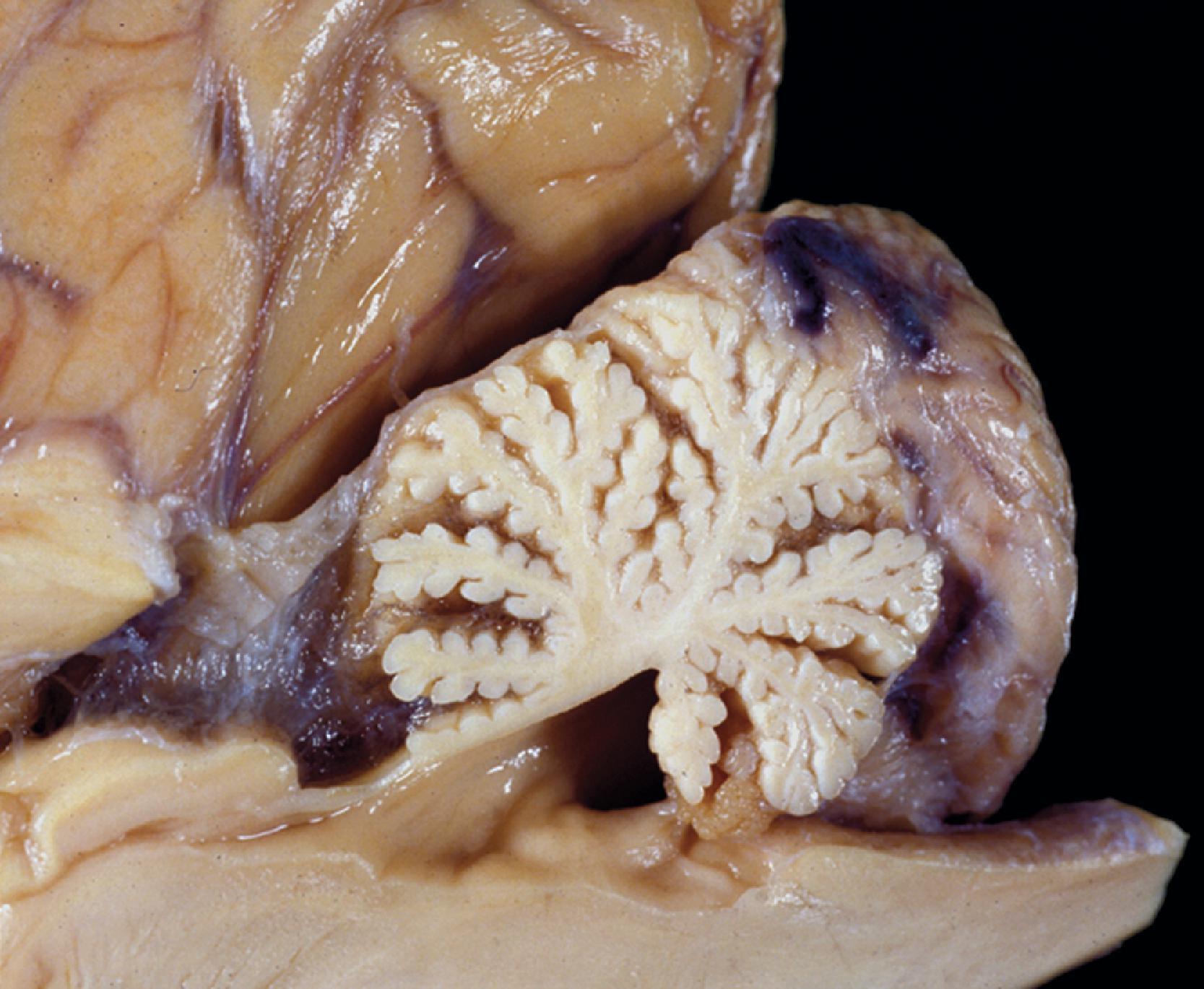
JNCL (CLN3 disease, Batten disease) is caused by mutations in the CLN3 gene, which encodes CLN3 protein (Battenin). It begins between 4 and 10 years of age with rapidly progressive visual loss, which leads to blindness in 2 to 4 years. Seizures and myoclonus appear later, and progressive psychomotor retardation and focal neurologic deficits develop, leading to death by 20 to 30 years of age. The storage material takes the form of fingerprint bodies, sometimes mixed with curvilinear and other inclusions ( Fig. 8.35 ). The brain shows neuronal loss and cortical atrophy but not of the extreme degree seen in INCL and LINCL. MRI shows cerebral and cerebellar atrophy.
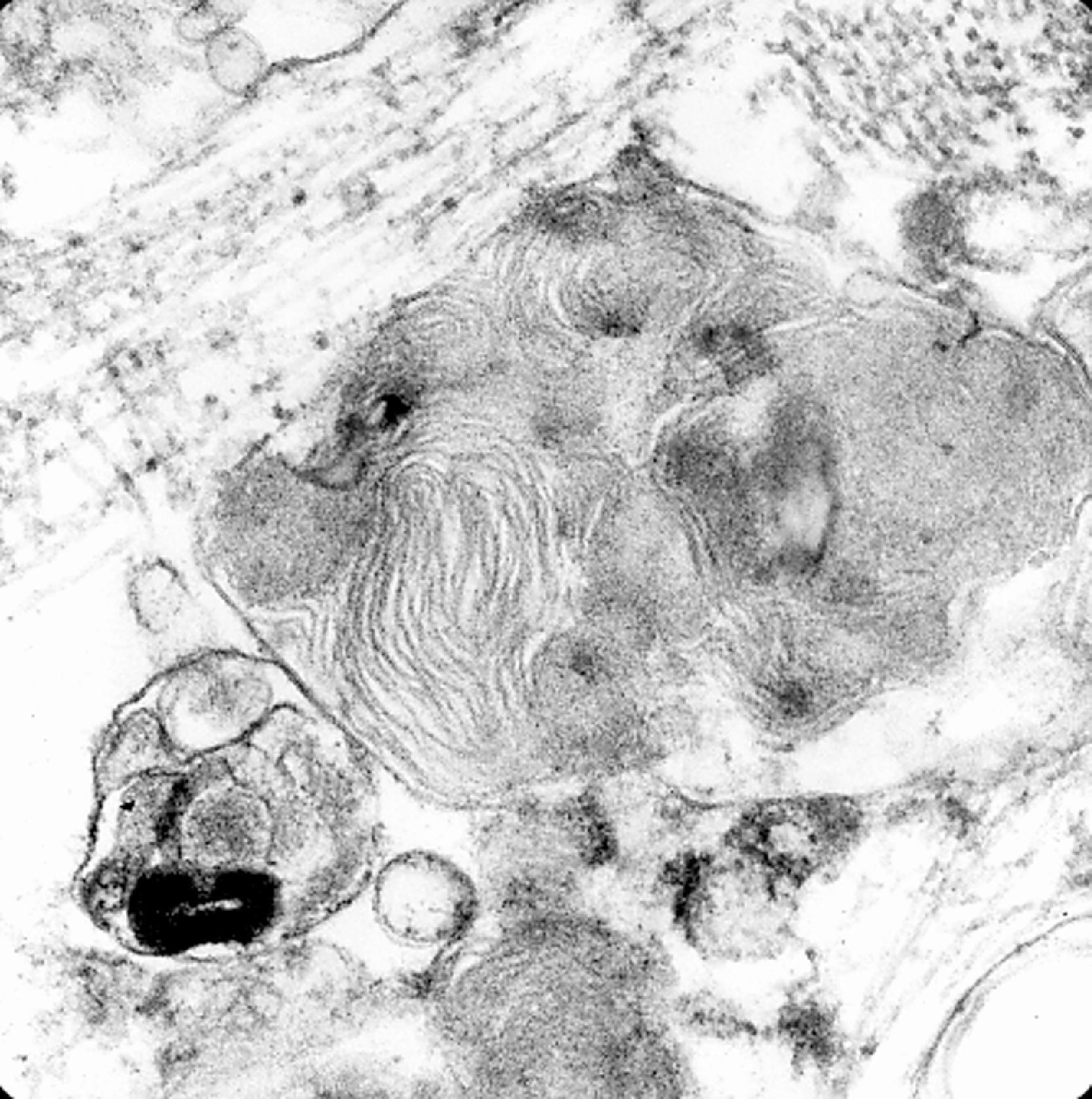
ANCL (CLN4 disease, Kufs disease) is caused by mutations in the CLN4 / DNAJC5 gene, which encodes cysteine-string protein alpha (CSPa). It presents, on the average, by 30 years of age. Some patients present with myoclonus, ataxia, and dementia and others with behavioral abnormalities and dementia. Progressive neurodegeneration leads to death in 10 years. The storage material in ANCL is a mixture of granular osmophilic deposits and other inclusions ( Fig. 8.36 ). Neuronal loss and cortical atrophy are not as pronounced as in other NCLs.
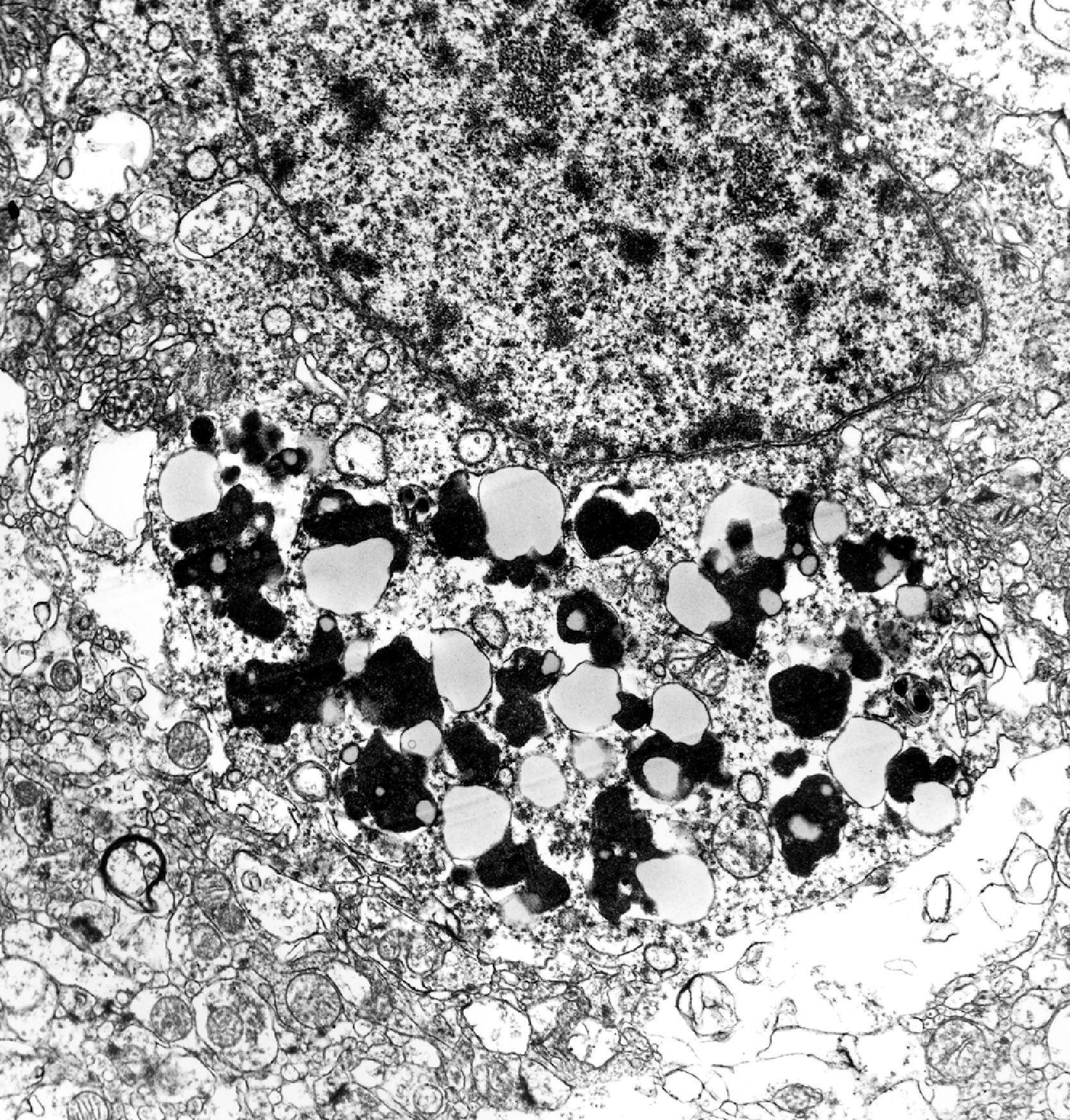
It should be noted, however, that phenotypes similar to INCL, LINCL, JNCL, and ANCL are sometimes caused by a variety of mutations in other CLN genes. Moreover, the fine structure of the stored material is prevalent in the major forms of NCLs as described above but is not entirely disease-specific, and some NCLs contain diverse inclusions that may vary in different tissues.
A group of disorders characterized clinically by neurological deterioration and pathologically by accumulation of lipofuscin-like products in neurons and other cells
1:12,500 to 1:25,000; The most common neurodegenerative disorders in children
Autosomal recessive except for ANCL, which can be either autosomal recessive or autosomal dominant. Thirteen genes causing NCLs have been identified.
Lysosomal proteases in some NCLs, unknown function proteins in most
Four clinical phenotypes, INCL, LINCL, JNCL, and ANCL were traditionally recognized. Now there are 13.
Hypotonia, myoclonus, seizures, ataxia, psychomotor retardation, blindness
Dementia in ANCL
Enzyme testing, electron microscopy of lymphocytes and biopsies of skin, conjunctiva and other tissues, and genetic testing
Cerebral and cerebellar atrophy in advanced stages of NCLs
Neuronal ballooning; Neuronal loss and gliosis in advanced disease
Granular osmiophilic deposits in INCL, curvilinear bodies in INCL, fingerprint and mixed profiles in JNCL, granular osmiophilic deposits in ANCL (see Table 8.7 )
The diagnosis of the NCLs is based on the clinical findings and electron microscopy of blood lymphocytes or of biopsies of skin, conjunctiva, and other tissues. Enzyme activity of PPT1 and TPP1 in leukocytes, skin fibroblasts, amniocytes, and CVS cells can be used for diagnosis, prenatal diagnosis, and carrier detection. Molecular genetic testing for some NCLs is also available on a clinical basis.
Niemann-Pick type C (NPC) is not due to a deficiency of a lysosomal hydrolase. It is caused by a defect in intracellular cholesterol circulation that results in lysosomal storage of phospholipids and glycolipids (including GM 2 ganglioside) and derangement of glycolipid metabolism. Recycled cholesterol from the plasma membranes and endocytosed low-density lipoprotein (LDL) cholesterol particles are normally hydrolyzed in endosomes-lysosomes, and cholesterol is then transported back to the plasma membrane. In NPC cells, uptake and hydrolysis are normal, but subsequent cholesterol transport is arrested leading to accumulation of cholesterol in perinuclear lysosomes and deficiency of cholesterol in cell membranes. This transport abnormality affects also other lysosomal contents, which accumulate because they cannot be moved out of lysosomes. NPC is a panethnic disease with protean clinical manifestations and may present from fetal life to adulthood.
NPC is autosomal recessive. Ninety-five percent of cases, designated as NPC1, are caused by mutations in the NPC1 gene on 18q11-q12. The product of this gene is a membrane protein in the endoplasmic reticulum that is thought to be involved in intracellular sorting of cholesterol and glycosphingolipids. Approximately 380 mutations in NPC1 have been reported. Most affected individuals are compound heterozygotes. Five percent of NPC cases, designated as NPC2, are caused by mutations in the NPC2 gene on 14q24.3, which codes for a cholesterol-binding protein. At an estimated prevalence of 1:150,000 in Europe, NPC is much more frequent than Niemann-Pick A and B combined. Niemann-Pick type D refers to a genetic cluster of this disease in Nova Scotia.
Infantile-onset NPC may present with fetal ascites, neonatal hepatitis, hypotonia, and delay in psychomotor development. Neurological symptoms predominate when the onset is in childhood. In addition to psychomotor retardation, the neurological presentation includes impaired vertical gaze, dystonia, speech abnormality, and seizures. Similar neurological manifestations developing at a slower pace and psychiatric illness characterize adolescent and adult presentations.
An autosomal recessive disorder of intracellular cholesterol processing
1:150,000 in Europe; Panethnic
Cholesterol, sphingolipids
Neuronal lipidosis, storage histiocytosis, liver disease, hypotonia, fetal ascites
Filipin test
Hepatosplenomegaly; normal brain initially, cortical atrophy in advanced cases
Niemann-Pick cells in viscera, ballooned neurons in the brain
Membranous cytoplasmic bodies
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here